Abstract
Background and Purpose
To identify anatomic prognostic factors and their potential roles in refining M1 classification for de novo metastatic nasopharyngeal carcinoma (M1‐NPC).
Materials and Methods
All M1‐NPC treated with chemotherapy and/or radiotherapy between 2010 and 2019 from two centers (training and validation cohort) were included. The prognostic value of metastatic disease extent and involved organs for overall survival (OS) were assessed by several multivariable analyses (MVA) models. A new M1 classification was proposed and validated in a separate cohort who received immuno‐chemotherapy.
Results
A total of 197 M1‐NPC in the training and 307 in the validation cohorts were included for M1 subdivision study with median follow‐up of 46 and 57 months. MVA model with “≤2 organs/≤5 lesions” as the definition of oligometastasis had the highest C‐index (0.623) versus others (0.606–0.621). Patients with oligometastasis had better OS versus polymetastasis (hazard ratio [HR] 0.47/0.63) while liver metastases carried worse OS (HR 1.57/1.45) in MVA in the training/validation cohorts, respectively. We proposed to divide M1‐NPC into M1a (oligometastasis without liver metastases) and M1b (liver metastases or polymetastasis) with 3‐year OS of 66.5%/31.7% and 64.9%/35.0% in the training/validation cohorts, respectively. M1a subset had a better median progress‐free survival (not reach vs. 17 months, p < 0.001) in the immuno‐chemotherapy cohort (n = 163).
Conclusion
Oligometastasis (≤2 organs/≤5 lesions) and liver metastasis are prognostic for M1‐NPC. Subdivision of M1‐NPC into M1a (oligometastasis without liver metastasis) and M1b (liver metastasis or polymetastasis) depicts the prognosis well in M1‐NPC patients who received immuno‐chemotherapy.
Keywords: immunotherapy, M1 categories, nasopharyngeal carcinoma, oligometastatic disease, radiotherapy
1. INTRODUCTION
De novo metastatic nasopharyngeal carcinoma (M1‐NPC) accounts for 5%–10% of newly diagnosed NPC cases. 1 M1‐NPC generally has a poor long‐term survival, with 3‐year overall survival (OS) of 20%–30%. 2 , 3 , 4 However, heterogeneity exist. With contemporary treatment, a subset of M1‐NPC patients is able to enjoy long‐term survival or even cure. 5 , 6 , 7 , 8
Current M classification of the eighth edition TNM (TNM‐8) has classified all M1 NPC as one stage group without further sub‐categorization. Emerging data showed that M1‐NPC patients with different metastatic extent or site of metastasis may have different outcomes and may require different treatment approaches. 8 , 9 , 10 , 11 , 12 OMD is reported as an important prognostic indicator for M1‐NPC. 4 , 6 , 7 , 13 However, the definition of OMD remains unsettled. Some studies divide OMD and polymetastatic disease (PMD) according to number of metastatic lesions, 7 , 14 , 15 while others also included number of metastatic organs. 16 , 17 In addition, various metastatic organs appear to have differential prognostic importance. It has been reported that liver metastases carried worse OS versus other metastases, while NPC patients with lung metastases have a better prognosis. 8 , 12 These data suggest that OMD/PMD and the presence/absence of liver metastasis have the potential to refine current M1 classification, and to further guide treatment in M1‐NPC.
Retrospective and prospective clinical studies have confirmed that locoregional radiotherapy (LR‐RT), that is, RT to primary tumor and cervical nodal areas, in M1‐NPC can significantly improve progression‐free survival (PFS) and OS. 18 , 19 However, previous retrospective studies have confirmed that not all patients with M1‐NPC benefit from LR‐RT and that OMD status may be an excellent way to differentiate the benefiting population. 4 , 13 , 20 In addition, systemic treatment of M1‐NPC has entered a new era of immunotherapy. Anti‐PD‐1 monoclonal antibody (mAb) combined with chemotherapy is now considered the first‐line standard‐of‐care. 21 , 22 , 23 Therefore, deriving M1 subcategories that can effectively depict prognosis following immuno‐chemotherapy is worthy of exploration.
Here, we used data from two academic centers in NPC‐endemic areas (one served as the training and the other as the validation cohort) to assess the prognostic value of metastatic disease extent (OMD vs. PMD) and involved organ(s) (liver vs. lung vs. bone vs. other) in M1‐NPC. We hope to derive a prognostically most appropriate OMD definition to identify a subset who may benefit from LR‐RT, and to propose a refined M classification that could depict prognosis well in M1‐NPC following contemporary immuno‐chemotherapy.
2. MATERIALS AND METHODS
2.1. Patient cohorts
Patients with M1‐NPC diagnosed and treated at Jiangxi Cancer Hospital from January 2010 to December 2019 were used as the training cohort while validation cohort was derived by randomly selection of 25% of M1‐NPC patients (n = 1400) treated at the Sun Yat‐sen University Cancer Center from January 2010 to December 2019. The inclusion criteria of the two cohorts were as follows: (i) pathologically‐proven NPC, (ii) pathological or imaging diagnosis of de novo distant metastatic disease at diagnosis, and (iii) received at least two cycles of first‐line chemotherapy. The exclusion criteria were as follows: (i) prior radiotherapy in the head and neck region, and (ii) history of other malignancies in the past 5 years.
In addition, an immuno‐chemotherapy cohort was assembled which included M1‐NPC patients treated with anti‐PD‐1 mAb combined with chemotherapy as the first‐line treatment between January 2018 and July 2021 at four cancer centers (Sun Yat‐Sen University Cancer Center, Jiangxi Cancer Hospital, Fujian Cancer Hospital, and Hubei Cancer Hospital). The inclusion criteria were as follows: (i) pathologically proven NPC, (ii) pathological or imaging diagnosis of distant metastasis, and (iii) received at least two cycles of chemotherapy plus anti‐PD‐1 mAb. The exclusion criteria were: history of other malignant tumors in the past 5 years.
To complete the staging diagnosis, flexible fiberoptic endoscopy, MRI of the head and neck, basic serum chemistry, EBV‐DNA, chest CT, bone scan and ultrasound/CT of liver and abdomen, or positron emission tomography/computed tomography (PET/CT) were included. If liver metastasis was found by B‐ultrasound, supplementary examinations with enhanced CT or enhanced MRI was conducted. If bone metastases are possible on ECT, enhanced MRI was generally completed. Each institution's ethics review board approved the study, and exempted informed consent for the collection of clinical data from patients.
2.2. Treatment
Patients received at least two cycles of platinum‐based chemotherapy (once in every 3 weeks). After chemotherapy, LR‐RT was considered according to the treatment plan. Intensity‐modulated radiation therapy (IMRT) was used to treat nasopharyngeal lesions and regional metastatic lymph nodes using a cumulative dose of 66–70 Gy/30–35 fractions. Patients received anti‐PD‐1 monoclonal antibodies, including camrelizumab, toripalimab, tislelizumab, pembrolizumab, penpulimab, sintilimab, and nivolumab (all 200 mg intravenously on Day 1, except toripalimab, which was 240 mg intravenously on Day 1). All anti‐PD‐1 monoclonal antibodies were administered every 3‐week cycle for at least two cycles until tumor progression, intolerable side effects, or the doctor's decision. Detailed information and the dose of chemotherapy regimens for patients are provided in the Supplementary Methods.
2.3. Recording metastatic organs and metastatic lesions
Liver, lung, bone, distant lymph nodes (excluding cervical lymph node metastases), spleen, adrenal glands, and meningeal metastases were counted as one metastatic organ each. Number of lesions was counted based on imaging findings while distant metastatic lymph nodes were counted as one lesion per each individual lymphatic drainage area (axillary, mediastinum, retroperitoneal, and inguinal).
2.4. Statistical analyses
The study comprised 6 steps:
Step 1. Identifying potential anatomic prognostic factors in univariable analysis (UVA)
Step 2. Deriving an appropriate definition of OMD versus PMD based on highest C‐index among various multivariable analysis (MVA) models
Step 3. Assessing the prognostic importance of liver versus other organ metastases
Step 4. Proposing a refined M classification that further subcategorized M1‐NPC into M1a and M1b
Step 5. Confirming the value of M1a versus M1b subdivision in predicting who might benefit from LR‐RT
Step 6. Validating the ability of M1a and M1b subdivision in depicting outcomes in a separate multi‐center cohort of M1‐NPC treated with immuno‐chemotherapy
Chi‐squared or Fisher's exact tests were used to compare categorical variables, and the Mann–Whitney U‐test was used to compare continuous variables. Actuarial rates of OS (any cause of death as an event) and PFS (disease progression or death as an event) were calculated using the Kaplan–Meier method with log‐rank test for comparison of different groups. All time‐to events were calculated from the first diagnosis of M1‐NPC. UVA and MVA were performed using the Cox proportional hazard model to assess the prognostic value of metastatic organs (bone, liver, lung, or other metastases), number of metastatic organs, and number of the metastatic lesions, together with gender, age, ECOG performance status, T categories, N categories, LR‐RT, local treatment for metastatic lesions, Chemotherapy, Epstein–Barr virus (EBV) DNA (Low vs. High), and lactate dehydrogenase (LDH) (Normal vs. Abnormal). Harrell's C‐index (C‐index) was used to compare the predictability of OS for MVA models included various definition of OMD. All tests were two‐sided with a p < 0.05 as statistically significance.
3. RESULTS
3.1. Baseline characteristics
Of 340 M1‐NPC patients treated in Jiangxi Cancer Hospital during the study period, 79 did not complete two cycles of palliative chemotherapy, 24 lacked complete radiological data, 37 received 2D routine radiotherapy, and 3 had other primary malignancies. The remaining 197 patients were included as the training cohort while 307 cases of M1‐NPC from the Sun Yat‐sen University Cancer Center were included as the validation cohort (Figure S1).
Bone, liver, lung, and distant lymph nodes or metastases at other sites were identified in 64.5%, 36.0%, 24.4%, and 15.7% of patients in the training cohort, and 76.9%, 30.6%, 23.8%, and 21.8% of patients in the validation cohort, respectively. Moreover, 64.0% of patients in the training cohort received LR‐RT, and 155 (50.5%) in the validation cohort received LR‐RT. Detailed information is shown in Table 1.
TABLE 1.
Clinical characteristics of patients in training and validation cohorts.
| Variables | Training cohort, n (%) | Validation cohort, n (%) | p |
|---|---|---|---|
| Case Number | 197 | 307 | |
| Gender | |||
| Male | 164 (83.2) | 258 (84.0) | 0.815 |
| Female | 33 (16.8) | 49 (16.0) | |
| Age, median (IQR; years) | 52 (43–59) | 46 (39–54) | <0.001 |
| ECOG | |||
| 0–1 | 189 (95.9) | 298 (97.1) | 0.493 |
| 2 | 8 (4.1) | 9 (2.9) | |
| T categories a | |||
| T1‐3 | 123 (62.4) | 191 (62.2) | 0.960 |
| T4 | 74 (37.6) | 116 (37.8) | |
| N categories a | |||
| N0‐1 | 36 (18.3) | 67 (21.8) | 0.281 |
| N2‐3 | 161 (81.7) | 240 (78.2) | |
| Bone metastases | |||
| None | 70 (35.5) | 84 (27.4) | 0.052 |
| Yes | 127 (64.5) | 223 (72.6) | |
| Liver metastases | |||
| None | 122 (64.0) | 213 (69.4) | 0.084 |
| Yes | 75 (36.0) | 94 (30.6) | |
| Lung metastases | |||
| None | 149 (75.6) | 234 (76.2) | 0.880 |
| Yes | 48 (24.4) | 73 (23.8) | |
| Other metastases | |||
| None | 166 (84.3) | 240 (78.2) | 0.920 |
| Yes | 31 (15.7) | 67 (21.8) | |
| LR‐RT | |||
| None | 71 (36.0) | 152 (49.5) | 0.003 |
| Yes | 126(64.0) | 155 (50.5) | |
| Local treatment for metastatic lesions | |||
| None | 158 (80.2) | 257 (83.7) | 0.313 |
| Yes | 39 (19.8) | 50 (16.3) | |
| Local treatment method for metastatic lesions | |||
| None | 158 (80.2) | 257 (83.7) | 0.148 |
| Radiotherapy for metastatic | 36 (18.3) | 43 (14.0) | |
| Radiofrequency ablation for liver metastases | 3 (1.5) | 7 (2.3) | |
| Chemotherapy | |||
| <6 cycles | 124 (62.9) | 107 (34.9) | <0.001 |
| ≥6 cycles | 73 (37.1) | 200 (65.1) | |
| EBV DNA | |||
| Undetectable | 44 (22.3) | 37 (12.1) | 0.006 |
| Detectable | 143 (72.6) | 258 (84.0) | |
| NA | 10 (5.1) | 12 (3.9) | |
| EBV‐DNA(Copies/mL) c | |||
| Best cut‐off | 4700 | 11,800 | 0.001 |
| Low | 90 (48.1) | 98 (33.2) | |
| High | 97 (51.9) | 197 (66.8) | |
| LDH(IU/L) | |||
| Normal | 131 (66.5) | 187 (60.9) | 0.205 |
| Abnormal b | 66 (33.5) | 120 (39.1) | |
| No. of metastatic organ | |||
| 1 | 135 (68.5) | 204 (66.4) | 0.299 |
| 2 | 41 (20.8) | 56 (18.2) | |
| ≥3 | 21 (10.7) | 47 (15.3) | |
| No. of metastatic lesion | |||
| 1 | 48 (24.4) | 83 (27.0) | 0.228 |
| 2 | 20 (10.1) | 37 (12.1) | |
| 3 | 17 (8.6) | 32 (10.4) | |
| 4 | 23 (11.7) | 17 (5.5) | |
| 5 | 11 (5.6) | 19 (6.2) | |
| >5 | 78 (39.6) | 119 (38.8) | |
According to the 8th edition of the American Joint Committee on Cancer/ Union for International Cancer Control cancer staging manual.
Abnormal threshold, >250 U/L.
Detectable thresholds: 0 copy/mL.
Abbreviations: EBV DNA, Epstein–Barr virus deoxyribonucleic acid; IQR, interquartile range; LDH, Lactate dehydrogenase; LR‐RT, locoregional radiotherapy.
3.2. Potential anatomic prognostic factors in UVA
The median follow‐up of the training and validation cohort was 45 and 57 months, and the 3‐years OS of the training and validation cohort was 47.1% and 49.0%, respectively (p = 0.791, Figure 1A). UVA showed that liver metastases [training: Hazard ratio (HR) 1.83, (95% confidence interval: 1.24–2.72), p = 0.003; validation: HR 1.75 (1.29–2.36), p < 0.001], number of metastatic organs [training: HR 1.97 (1.31–2.95), p = 0.001; validation: HR 1.53 (1.13–2.06), p = 0.006], number of metastatic lesions [training: HR 2.56 (1.72–3.81), p < 0.001; validation: HR 2.02 (1.51–2.71), p < 0.001] were potential anatomic prognostic factors, together with EBV DNA [training: HR 1.76 (1.07–2.88), p = 0.025; validation HR 1.69 (1.21–2.36, p = 0.002], LDH [training HR 2.11 (1.41–3.14), p < 0.001; validation: HR 1.39 (1.03–1.87), p = 0.029], and LR‐RT [training: HR 0.44 (0.30–0.66, p < 0.001; validation: HR 0.54 (0.35–0.83), p < 0.001] (Table S1).
FIGURE 1.
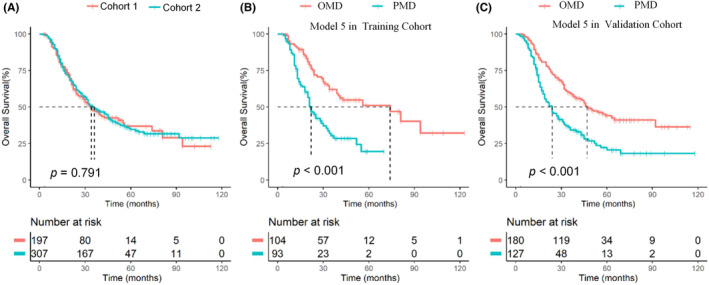
OS in the training versus validation cohorts (A), and stratified by oligometastasis versus polymetastasis (Model 5) in the training (B) and validation cohorts (C). OS, overall survival; OMD, oligometastatic disease; PMD, polymetastatic disease.
3.3. Derivation of a most appropriate definition of oligometastasis with MVA
The following six MVA models, based on different combination of the number of metastatic organs and lesions commonly used in literature for OMD definition, were constructed to derive a prognostically most appropriate differentiation of OMD versus PMD: Model 1 (one metastatic lesion), Model 2 (one metastatic organ and ≤3 metastatic lesions), Model 3 (one metastatic organ and ≤5 metastatic lesions), Model 4 (≤2 metastatic organs and ≤3 metastatic lesions), Model 5 (≤2 metastatic organs and ≤5 metastatic lesions), and Model 6 (≤3 metastatic organs and ≤5 metastatic lesions). OMD had higher OS versus PMD in all six models (all p < 0.001; Figure 1B,C and Figures S2 and S3). The C‐indices of the six models were 0.613/0.606/0.618/0.617/0.623/0.621 and 0.563/0.585/0.592/0.575/0.593/0.591 in the training and validation cohorts, respectively (Table 2). Model 5 (≤2 metastatic organs and ≤5 metastatic lesions) had the highest performance in OS prediction, and we consider it as the most appropriate OMD definition for M1‐NPC. The median OS of patients with OMD was significantly better than that of patients with PMD in both the training and validation cohorts (both p < 0.001; Figure 1B,C and Figure S4).
TABLE 2.
The C‐index and AIC of various MVA models to define OMD.
| OMD Models | Training cohort | Validation cohort |
|---|---|---|
| Model 1 (one metastatic lesion) | 0.613 | 0.563 |
| Model 2 (one metastatic organ and ≤3 metastatic lesions) | 0.606 | 0.585 |
| Model 3 (one metastatic organ and ≤5 metastatic lesions) | 0.618 | 0.592 |
| Model 4 (≤ 2 metastatic organs and ≤3 metastatic lesions) | 0.617 | 0.575 |
| Model 5 (≤ 2 metastatic organs and ≤5 metastatic lesions) | 0.623 | 0.593 |
| Model 6 (≤ 3 metastatic organs and ≤5 metastatic lesions) | 0.621 | 0.591 |
Abbreviations: C‐index, concordance index; OMD, oligometastatic disease.
After adjusting for sex, age, ECOG status, T category, N category, number of chemotherapy cycles, LR‐RT, local treatment for metastatic lesions, EBV DNA, and LDH, MVA showed that OMD was an independent prognostic factor in the training cohorts (HR 2.11 [1.37–3.25], p = 0.001), which was further verified in the validation cohort (HR 1.60 [1.17–2.18], p = 0.003) (Table 3). Note that liver metastasis status and OMD were not simultaneously included in the MVA model, given their certain collinearity.
TABLE 3.
Multivariable analysis of OS in M1‐NPC patients.
| Variables | Training cohort | Validation cohort | ||
|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | |
| MAV analyses include OMD | ||||
| OMD | 0.47 (0.31–0.73) | 0.001 | 0.63 (0.46–0.85) | 0.003 |
| EBV DNA | 1.68 (1.08–2.61) | 0.021 | 1.44 (1.02–2.03) | 0.037 |
| LR‐RT | 0.69 (0.44–1.08) | 0.106 | 0.57 (0.42–0.77) | <0.001 |
| MAV analyses included metastatic organ | ||||
| Liver metastases | 1.57 (1.04–2.38) | 0.032 | 1.45 (1.05–2.01) | 0.023 |
| EBV DNA | 1.66 (1.07–2.58) | 0.024 | 1.51 (1.07–2.12) | 0.018 |
| LR‐RT | 0.60 (0.39–0.94) | 0.025 | 0.57 (0.42–0.78) | <0.001 |
Abbreviations: M1‐NPC, de novo metastatic nasopharyngeal carcinoma; OMD, oligometastatic disease; LR‐RT, locoregional radiotherapy; EBV DNA, Epstein–Barr virus deoxyribonucleic acid.
3.4. Confirmation of the prognostic value of liver metastasis in MVA
Compared to other organ metastasis, liver metastases had a significantly worse 3‐year OS (35.2% vs. 55.0%, p < 0.001 and 35.8% vs. 54.6%, p < 0.001, Figure S3A,B). MVA showed that among the metastatic organs, only liver metastasis was an independent prognostic factor for M1‐NPC in the training (HR 1.57 [1.04–2.38], p = 0.032) and validation cohorts (HR 1.45 [1.05–2.01], p = 0.023) (Table 3).
3.5. Proposal of subdivision of M1 in to M1a and M1b subcategories
Considering that OMD status and liver metastases were both important prognostic factors for M1‐NPC, we combined data from both cohorts to refine the M1 subgroup. We constructed a subcategory model to classify the patients into three groups: A (OMD without liver metastases), B (OMD with liver metastases), and C (PMD). Kaplan–Meier survival analysis showed that the 3‐year OS of A, B, and C in the training cohort were 65.6%, 42.2%, and 33.1%, respectively (p < 0.001, Figure 2A). In group A, 3‐year OS was significantly better in patients who received LR‐RT than in those without LR‐RT (71.4% vs. 50.5%, p = 0.001, Figure 2B), while in groups B and C, there was no significant difference in the 3‐year OS between those who received LR‐RT and those without LR‐RT (44.2% vs. 38.6%, p = 0.389, Figure 2C; 33.8% vs. 27.9%, p = 0.116, Figure 2D).
FIGURE 2.
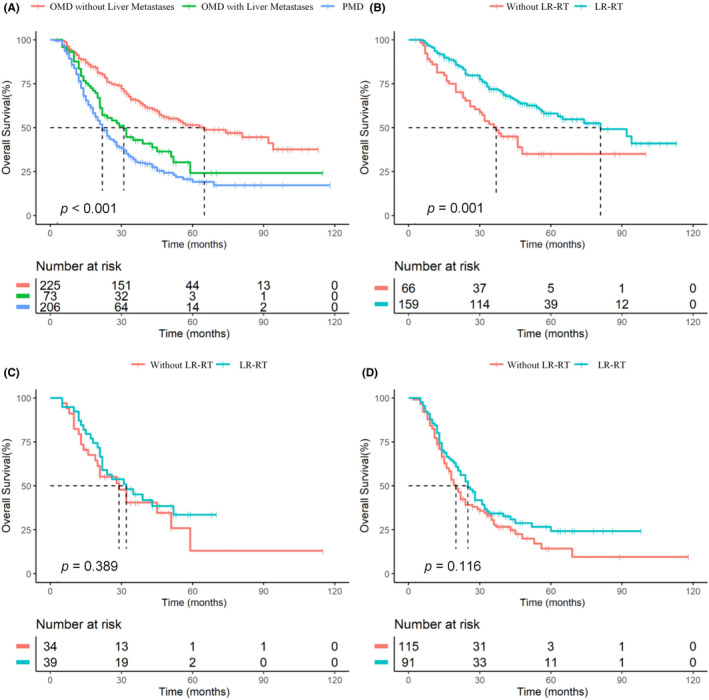
OS stratified by OMD with versus OMD without liver metastases versus PMD Groups (A); OS stratified by with versus without LR‐RT in OMD without liver metastasis (B), OMD with liver metastases (C), and PMD (D) subgroups. OS, overall survival; OMD, oligometastatic disease; PMD, polymetastatic disease; LR‐RT: locoregional radiotherapy.
Considering that the B and C groups had similar 3‐year OS and both did not benefit from LR‐RT, we combined them and revised the M1 classification proposal as M1a (OMD without liver metastases) and M1b (liver metastases or PMD). The 3‐year OS of M1a was better than that of M1b in both the training and validation cohorts (66.5% vs. 31.7%, p < 0.001; 64.9% vs. 35.0%, p < 0.001; Figure 3A,B). The C‐index of our refining M1 subcategory model for prognostic evaluation in the training and validation cohorts was 0.6258 and 0.6011, respectively, and time‐dependent receiver operating characteristic (ROC) curve analysis showed that the area under the curve (AUC) of the 3‐ and 5‐year OS in the training and validation cohorts were 0.691 and 0.659, and 0.766 and 0.644, respectively (Figure S5A,B). In the M1a group, patients who received LR‐RT had a higher 3‐year OS than those who did not (71.4% vs. 50.5%, p = 0.001), whereas those in the M1b group who received LR‐RT and did not receive LR‐RT showed no significant difference in OS (37.4% vs. 30.8%, p = 0.053, Figure S6).
FIGURE 3.
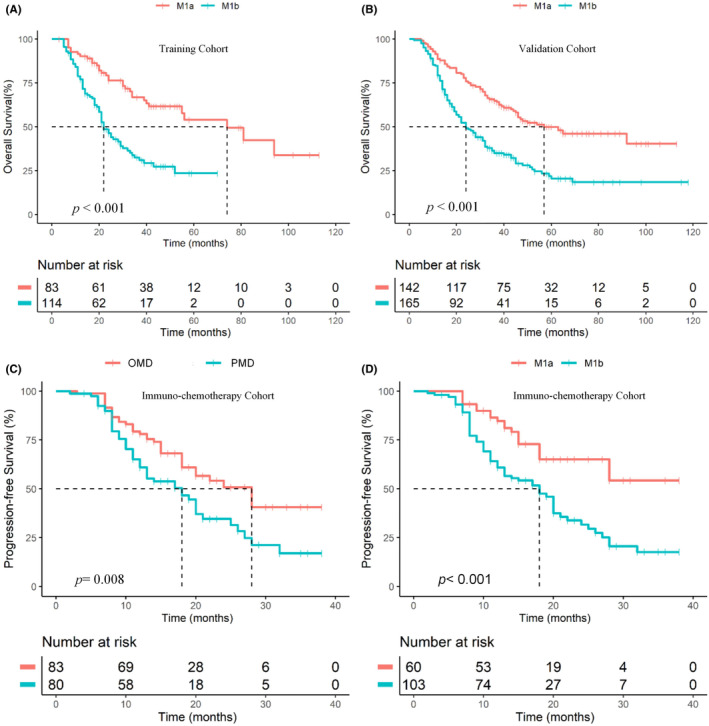
OS of M1a versus M1b subcategories in the training (A) and validation (B) cohorts, and PFS Stratified by OMD versus PMD (C) and by M1a versus M1b (D) in the immuno‐chemotherapy group. OS, overall survival; PFS, progression‐free survival; OMD, oligometastatic disease; PMD, polymetastatic disease.
3.6. Validation the usefulness of M1 subdivision in a separate immuno‐chemotherapy cohort
In order to verify the prognostic performance of the refining OMD model and M1 subcategories in era of immunotherapy, 163 patients with M1‐NPC who received chemotherapy plus anti‐PD‐1 in four centers were included. The median follow‐up time was 22 (range: 2–38) months (Table S2). Kaplan–Meier survival curves showed that patients with OMD had a superior median PFS compared to those with PMD (24 months vs. 17 months, p = 0.008, Figure 3C). In addition, patients with M1a had a significantly better median PFS than those with M1b (not reach vs. 17 months, p < 0.001, Figure 3D).
4. DISCUSSION
Current TNM‐8 has uniformly grouped M1‐NPC as one category, which lacks the ability to differentiate the prognosis of patients with M1‐NPC. Further subdivision of M1‐NPC has clinical value to better depict prognosis following contemporary treatment and may potentially guide treatment. Our study identified anatomic prognostic factors and proposed a clinical useful M1 subdivision in M1‐NPC. We found that metastasis involved ≤2 organs with ≤5 total metastatic lesions had the best distinction of OMD and PMD for both training and validation cohorts. Patients with liver metastasis also carried the worst outcomes versus other organ involvement. Based on OMD and liver metastases status, we proposed to subdivide M1 into M1a (OMD without liver metastases) and M1b (liver metastases or PMD). Our results also showed that patients in M1a could benefit from LR‐RT besides systemic therapy, whereas those in M1b could not. In addition, the subdivision of M1 into M1a and M1b could stratify the prognosis well in a separate cohort of M1‐NPC who received immuno‐chemotherapy.
Although the theory of OMD was refining in 1995, 24 there remains no consensus definition in NPC. Although studies have found that the number of metastatic organs and lesions in NPC are important prognostic factors for M1‐NPC, few have systematically analyzed the prognostic value of the number of metastatic organs and lesions. 25 The ESTRO‐ASTRO consensus also defines OMD as having 1–5 metastatic lesions but does not provide a specific definition for metastatic organs. 14 By comparing the MVA model performance, we found that metastasis involved ≤2 metastatic organs with ≤5 metastatic lesions in total had the best ability to differentiate OMD versus PMD in our study. Our findings are consistent with the definition of OMD in metastatic colorectal cancer according to the ESMO consensus guidelines, 26 although it is slightly different form a single‐center study from Singapore which showed that the OMD model with a single organ and ≤5 lesions had the best performance in OS prediction. 14 However, their study also found that the OMD model with ≤2 organs and ≤5 metastatic lesions had similar performance to that of OMD with a single organ and ≤5 lesions (C‐index: 0.6152 and 0.6225). Recently, two retrospective studies from our center and other center found that the OMD model with ≤2 organs and ≤5 metastatic lesions could not only effectively evaluate the prognosis of patients with M1‐NPC, but could also help to screen populations benefit from LR‐RT. 4 , 13 Therefore, we believe that OMD (≤2 metastatic organs and ≤5 lesions) may be an appropriate definition in M1‐NPC.
Our results also confirmed that liver metastasis is an independent prognostic factor for M1‐NPC, which is consistent with previously published reports. 20 , 27 The C‐index of refining M1 subcategories model was higher than that of the OMD model in our study, suggesting the liver metastasis should also be considered in risk stratification of M1‐NPC. Importantly, the refining subcategories can also effectively distinguish beneficiaries from LR‐RT treatment. M1a patients can benefit from LR‐RT, whereas M1b patients do not. Although prospective and multiple retrospective studies have directly demonstrated that LR‐RT can provide survival benefits to patients with M1‐NPC, 18 , 19 , 28 , 29 , 30 recent retrospective studies, including our previous study, have found that patients with OMD were the optimal candidates for LR‐RT. 4 , 13 , 20 , 27 However, current study confirmed that neither PMD patients nor OMD patients with liver metastases could benefit from LR‐RT. Local consolidative therapy of metastatic lesions has achieved a good therapeutic effect in many kinds of cancers, 9 , 31 , 32 and our MVA results showed that local treatment of metastatic lesions did not improve the OS of NPC patients. This is probably because our local treatment were not consolidative treatments for all metastatic lesions. Currently, two ongoing phase III trials are investigating the role of consolidative by radiotherapy directed to all metastatic lesions in metastatic NPC (NCT05128201 and NCT04421469), and we are eagerly awaiting these results.
With the approval of anti‐PD‐1 mAbs for RM‐NPC in the first‐line setting, the era of immunotherapy for NPC is coming. It is important to determine whether refining OMD definition and M1 subcategory are still effective for M1‐NPC patients received immuno‐chemotherapy. As shown in our study, the refining OMD and M1 subcategories effectively depicted the prognosis of immune‐chemotherapy cohorts, indicating clinical relevance of the current definition of the OMD and M1 subcategories.
Several limitations of this study warrant discussion. First, the data were derived from multiple academic cancer centers in an endemic jurisdiction and whether the findings can be generalized to other patient populations remains to be determined. Second, despite our study showed that patients with liver metastasis or PMD status did not benefit from LR‐RT, the treatment is not randomly assigned and the findings should be validated. In addition, although the refined OMD definition and M1 subcategories in our study can effectively assess the PFS of patients treated with immuno‐chemotherapy, whether they can effectively assess OS remains to be determined. Owing to the short follow‐up time of the immunotherapy cohort, OS cannot be accurately assessed at present, and subsequent long‐term follow‐up verification remains necessary. Finally, we did not include EBV DNA in the M1 subdivision since this is an anatomic grouping since this is an anatomic grouping. It is conceivable that EBV DNA has a potential to be included for prognostic grouping to enhance outcome prediction after consistency in EBV DNA testing is demonstrated. 27 , 28
5. CONCLUSION
The definition of ≤2 metastatic organs and ≤5 metastatic lesions is the most appropriate separation of OMD versus PMD for M1‐NPC in our study. Liver metastasis is also a strong adverse prognostic factor. Patients with OMD without liver metastasis have better OS and can be considered as M1a while the remaining as M1b. The M1a (OMD without liver metastasis) and M1b (liver metastasis or PMD) subdivision provides better prognostic evaluation for patients with M1‐NPC receiving immuno‐chemotherapy, and have the potential to screen the population regarding who will gain the greatest benefit from LR‐RT. Further external cohorts, especially multicenter studies, are warranted to validate the prognostic value of the refining M1 subcategories.
AUTHOR CONTRIBUTIONS
Tian‐Zhu Lu: Conceptualization (equal); formal analysis (equal); funding acquisition (equal); writing – original draft (equal); writing – review and editing (equal). Fu‐juan Zeng: Conceptualization (equal); data curation (equal); formal analysis (equal); writing – original draft (equal); writing – review and editing (equal). Yu‐Jun Hu: Conceptualization (equal); data curation (equal); formal analysis (equal); writing – original draft (equal); writing – review and editing (equal). Min Fang: Data curation (equal); investigation (equal); writing – review and editing (equal). Fang‐yan Zhong: Data curation (equal); formal analysis (equal); methodology (equal); writing – review and editing (equal). Bi‐juan Chen: Investigation (equal); resources (equal). Hao Zhang: Investigation (equal); resources (equal). Qiao‐juan Guo: Data curation (supporting); investigation (supporting); writing – review and editing (supporting). Jian‐ji Pan: Project administration (equal); supervision (equal); writing – review and editing (equal). Xiao‐chang Gong: Data curation (equal); formal analysis (equal); resources (equal); supervision (equal). Shao Hui Huang: Supervision (equal); writing – review and editing (equal). Zhao‐hui Liao: Resources (equal); supervision (equal). Yunfei Xia: Conceptualization (equal); project administration (equal); writing – review and editing (lead). Jin‐gao Li: Conceptualization (equal); data curation (equal); funding acquisition (equal); project administration (equal); supervision (equal); writing – original draft (lead); writing – review and editing (lead).
FUNDING INFORMATION
This work was supported by the National Natural Science Foundation of China (grant 82103478, 82160710), the Open Fund for Scientific Research of Jiangxi Cancer Hospital (grant 2021 J13, 2021K01), Non‐profit Central Research Institute Fund of Chinese Academy of Medical Sciences (grant 2020‐PT320‐004), and the Jiangxi Province Key R&D Program (Key Program) (grant 20232BBG70025).
CONFLICT OF INTEREST STATEMENT
All authors have no conflicts of interest to declare.
ETHICS APPROVAL STATEMENT
The study was approved institution's ethics review board of Jiangxi Cancer Hospital (No. 2021ky164).
Supporting information
Figure S1.
Lu T‐Z, Zeng F‐j, Hu Y‐J, et al. Anatomic prognostic factors and their potential roles in refining M1 classification for de novo metastatic nasopharyngeal carcinoma. Cancer Med. 2023;12:22091‐22102. doi: 10.1002/cam4.6816
Tian‐Zhu Lu, Fu‐juan Zeng, Yu‐Jun Hu, and Min Fang contributed equally to this work.
Yunfei Xia and Jin‐gao Li senior authors contributed equally to this work.
Contributor Information
Yunfei Xia, Email: xiayf@sysucc.org.cn.
Jin‐gao Li, Email: lijingao@hotmail.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64‐80. doi: 10.1016/s0140-6736(19)30956-0 [DOI] [PubMed] [Google Scholar]
- 2. Lin M, Yang Q, You R, et al. Metastatic characteristics associated with survival of synchronous metastatic nasopharyngeal carcinoma in non‐epidemic areas. Oral Oncol. 2021;115:105200. doi: 10.1016/j.oraloncology.2021.105200 [DOI] [PubMed] [Google Scholar]
- 3. Hong S, Zhang Y, Yu G, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin as first‐line therapy for recurrent or metastatic nasopharyngeal carcinoma: final overall survival analysis of GEM20110714 phase III study. J Clin Oncol. 2021;39(29):3273‐3282. doi: 10.1200/jco.21.00396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zeng F, Lu T, Xie F, et al. Effects of locoregional radiotherapy in de novo metastatic nasopharyngeal carcinoma: a real‐world study. Transl Oncol. 2021;14(11):101187. doi: 10.1016/j.tranon.2021.101187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fandi A, Bachouchi M, Azli N, et al. Long‐term disease‐free survivors in metastatic undifferentiated carcinoma of nasopharyngeal type. J Clin Oncol. 2000;18(6):1324‐1330. doi: 10.1200/jco.2000.18.6.1324 [DOI] [PubMed] [Google Scholar]
- 6. Tian YH, Zou WH, Xiao WW, et al. Oligometastases in AJCC stage IVc nasopharyngeal carcinoma: a subset with better overall survival. Head Neck. 2016;38(8):1152‐1157. doi: 10.1002/hed.24345 [DOI] [PubMed] [Google Scholar]
- 7. Chee J, Liu X, Eu D, et al. Defining a cohort of oligometastatic nasopharyngeal carcinoma patients with improved clinical outcomes. Head Neck. 2020;42(5):945‐954. doi: 10.1002/hed.26061 [DOI] [PubMed] [Google Scholar]
- 8. Hui EP, Leung SF, Au JS, et al. Lung metastasis alone in nasopharyngeal carcinoma: a relatively favorable prognostic group. A study by the Hong Kong nasopharyngeal carcinoma study group. Cancer. 2004;101(2):300‐306. doi: 10.1002/cncr.20358 [DOI] [PubMed] [Google Scholar]
- 9. Gomez DR, Tang C, Zhang J, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with Oligometastatic non‐small‐cell lung cancer: long‐term results of a multi‐institutional, phase II, randomized study. J Clin Oncol. 2019;37(18):1558‐1565. doi: 10.1200/jco.19.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trovo M, Furlan C, Polesel J, et al. Radical radiation therapy for oligometastatic breast cancer: results of a prospective phase II trial. Radiother Oncol. 2018;126(1):177‐180. doi: 10.1016/j.radonc.2017.08.032 [DOI] [PubMed] [Google Scholar]
- 11. Moretto R, Rossini D, Zucchelli G, et al. Oligometastatic colorectal cancer: prognosis, role of locoregional treatments and impact of first‐line chemotherapy‐a pooled analysis of TRIBE and TRIBE2 studies by Gruppo Oncologico del Nord Ovest. Eur J Cancer. 2020;139:81‐89. doi: 10.1016/j.ejca.2020.08.009 [DOI] [PubMed] [Google Scholar]
- 12. Zeng L, Tian YM, Huang Y, et al. Retrospective analysis of 234 nasopharyngeal carcinoma patients with distant metastasis at initial diagnosis: therapeutic approaches and prognostic factors. PLoS One. 2014;9(9):e108070. doi: 10.1371/journal.pone.0108070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu H, Lu L, Lu T, et al. Identifying the optimal candidates for locoregional radiation therapy in patients with de novo metastatic nasopharyngeal carcinoma. Head Neck. 2021. 43(9):2602‐2610. doi: 10.1002/hed.26726 [DOI] [PubMed] [Google Scholar]
- 14. Lievens Y, Guckenberger M, Gomez D, et al. Defining oligometastatic disease from a radiation oncology perspective: an ESTRO‐ASTRO consensus document. Radiother Oncol. 2020;148:157‐166. doi: 10.1016/j.radonc.2020.04.003 [DOI] [PubMed] [Google Scholar]
- 15. Wang H, He F, Wang X, et al. Investigation of the definition of De novo Oligometastatic nasopharyngeal carcinoma: a retrospective study. J Oncol. 2021;2021:9977455. doi: 10.1155/2021/9977455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shen L, Dong J, Li S, et al. M1 stage subdivision and treatment outcome of patients with bone‐only metastasis of nasopharyngeal carcinoma. Oncologist. 2015;20(3):291‐298. doi: 10.1634/theoncologist.2014-0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chan SK, Lin C, Huang SH, et al. Refining TNM‐8 M1 categories with anatomic subgroups for previously untreated de novo metastatic nasopharyngeal carcinoma. Oral Oncol. 2022;126:105736. doi: 10.1016/j.oraloncology.2022.105736 [DOI] [PubMed] [Google Scholar]
- 18. You R, Liu YP, Huang PY, et al. Efficacy and safety of Locoregional radiotherapy with chemotherapy vs chemotherapy alone in De novo metastatic nasopharyngeal carcinoma: a multicenter phase 3 randomized clinical trial. JAMA Oncol. 2020;6(9):1345‐1352. doi: 10.1001/jamaoncol.2020.1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen MY, Jiang R, Guo L, et al. Locoregional radiotherapy in patients with distant metastases of nasopharyngeal carcinoma at diagnosis. Chin J Cancer. 2013;32(11):604‐613. doi: 10.5732/cjc.013.10148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zou X, You R, Liu H, et al. Establishment and validation of M1 stage subdivisions for de novo metastatic nasopharyngeal carcinoma to better predict prognosis and guide treatment. Eur J Cancer. 2017;77:117‐126. doi: 10.1016/j.ejca.2017.02.029 [DOI] [PubMed] [Google Scholar]
- 21. Mai HQ, Chen QY, Chen D, et al. Toripalimab or placebo plus chemotherapy as first‐line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Nat Med. 2021;27(9):1536‐1543. doi: 10.1038/s41591-021-01444-0 [DOI] [PubMed] [Google Scholar]
- 22. Yang Y, Qu S, Li J, et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first‐line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN‐1st): a multicentre, randomised, double‐blind, phase 3 trial. Lancet Oncol. 2021;22(8):1162‐1174. doi: 10.1016/s1470-2045(21)00302-8 [DOI] [PubMed] [Google Scholar]
- 23. Tang LL, Chen YP, Chen CB, et al. The Chinese Society of Clinical Oncology (CSCO) clinical guidelines for the diagnosis and treatment of nasopharyngeal carcinoma. Cancer Commun (Lond). 2021;41(11):1195‐1227. doi: 10.1002/cac2.12218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13(1):8‐10. doi: 10.1200/jco.1995.13.1.8 [DOI] [PubMed] [Google Scholar]
- 25. Tian YM, Huang WZ, Lan YH, Zhao C, Bai L, Han F. Prognostic model and optimal treatment for patients with stage IVc nasopharyngeal carcinoma at diagnosis. Sci Rep. 2019;9(1):19272. doi: 10.1038/s41598-019-55586-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386‐1422. doi: 10.1093/annonc/mdw235 [DOI] [PubMed] [Google Scholar]
- 27. Zheng WH, He XJ, Chen FP, et al. Establishing M1 stage subdivisions by incorporating radiological features and Epstein‐Barr virus DNA for metastatic nasopharyngeal carcinoma. Ann Transl Med. 2020;8(4):83. doi: 10.21037/atm.2020.01.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang JH, Sun XS, Xiao BB, et al. Subdivision of de‐novo metastatic nasopharyngeal carcinoma based on tumor burden and pretreatment EBV DNA for therapeutic guidance of locoregional radiotherapy. BMC Cancer. 2021;21(1):534. doi: 10.1186/s12885-021-08246-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shuang H, Feng J, Caineng C, et al. The value of radical radiotherapy in the primary tumor of newly diagnosed oligo‐metastatic nasopharyngeal carcinoma patients. Clin Transl Oncol. 2019;21(2):213‐219. doi: 10.1007/s12094-018-1911-7 [DOI] [PubMed] [Google Scholar]
- 30. Liao W, He J, Gou Q, et al. Synchronous metastatic nasopharyngeal carcinoma: characteristics and survival of patients adding definitive nasopharyngeal‐neck radiotherapy to systematic chemotherapy. Cancer Manag Res. 2020;12:10211‐10219. doi: 10.2147/cmar.S276286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu Y, Verma V, Liang F, et al. Local consolidative therapy versus systemic therapy alone for metastatic non‐small cell lung cancer: a systematic review and meta‐analysis. Int J Radiat Oncol Biol Phys. 2022;114(4):635‐644. doi: 10.1016/j.ijrobp.2022.02.023 [DOI] [PubMed] [Google Scholar]
- 32. Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of Oligometastatic cancers: long‐term results of the SABR‐COMET phase II randomized trial. J Clin Oncol. 2020;38(25):2830‐2838. doi: 10.1200/jco.20.00818 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


