Abstract
The effect of human cytomegalovirus (HCMV) gene expression on beta interferon (IFN-β) expression was examined. We demonstrate that the HCMV immediate-early 2 (IE2) gene product IE86 can effectively block the induction of IFN-β during HCMV infection. IE86 also efficiently blocked the induction of IFN-β following Sendai virus infection, demonstrating that IE86's ability to block induction of IFN-β is not limited to HCMV infection, identifying IE2 as an IFN-β antagonist.
Virus-infected cells respond to infection by inducing antiviral programs that limit viral replication. This response is characterized by the induction of cellular signaling pathways that lead to the transcription and production of alpha and beta interferons (IFN-α and IFN-β). These newly synthesized IFNs are secreted from the host cell and act in an autocrine and paracrine fashion to activate a global antiviral response within the infected cell and the surrounding tissue (20). The IFN-induced antiviral state is characterized by the transcriptional activation of a plethora of cellular genes involved in blocking virus replication. However, as viruses have evolved, they have developed mechanisms to block this antiviral response induced by IFNs, thereby allowing for viral persistence within the infected host. In fact, many viruses encode proteins that specifically target the production of IFNs (11, 18, 20).
Microarray studies have demonstrated that human cytomegalovirus (HCMV) infection regulates a number of genes involved in the host antiviral response (6, 19). Interestingly, the expression of a number of these genes was enhanced significantly when viral gene expression was inhibited, suggesting that viral proteins may actively block the expression of these genes. One of the genes regulated in this fashion was that for IFN-β. Infection with transcriptionally inactive UV-irradiated virus or infection with wild-type virus in the presence of cycloheximide resulted in a dramatic induction of IFN-β RNA compared to that for wild-type infection (6). These results suggested that de novo viral gene expression is required to block the induction of IFN-β and that expression of this viral gene product(s) may attenuate the host antiviral response during HCMV infection. However, the newly synthesized HCMV gene product responsible for blocking the induction of IFN-β has not been identified. In this study we examine the role of HCMV gene expression on the inhibition of IFN-β and demonstrate that the HCMV immediate-early 2 (IE2) gene product IE86 can efficiently block the expression of IFN-β during HCMV and Sendai virus infection.
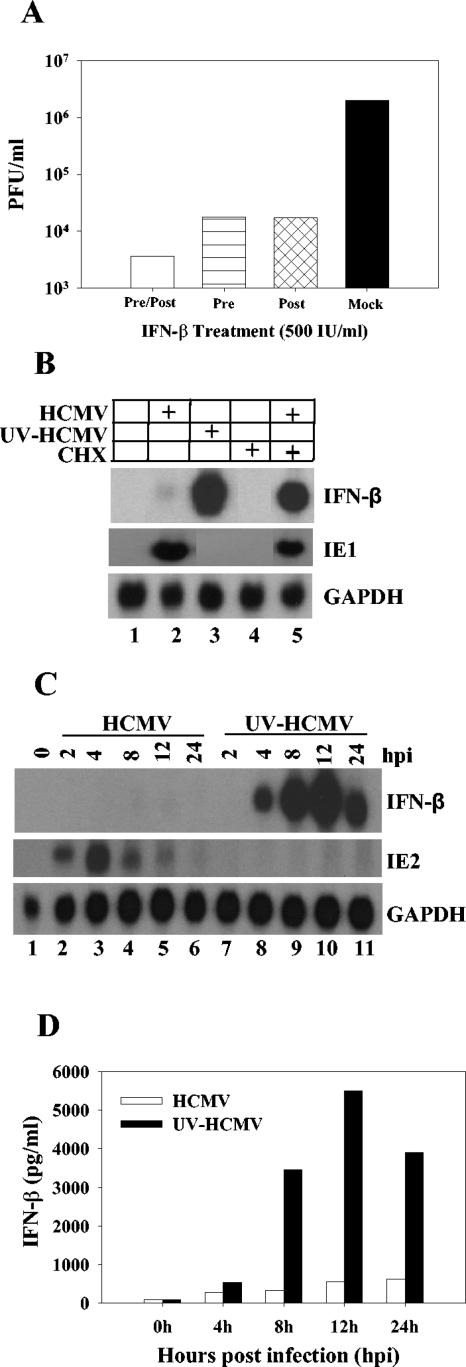
To assess the biological significance of suppressing the expression of IFN-β during HCMV infection, we examined what effect IFN-β has on HCMV replication. Human foreskin fibroblasts (HFF) were either pretreated with 500 IU of IFN-β/ml for 24 h, treated with IFN-β after HCMV infection, or pre- and posttreated with IFN-β during HCMV infection. Cells were infected with wild-type HCMV at a multiplicity of 0.1 PFU/cell. Ten days postinfection, cultures were harvested and infectious virus was quantified by plaque assay. As shown in Fig. 1A, preinfection (horizontally hatched bar) or postinfection (cross-hatched bar) treatment of HFF cells with IFN-β inhibited HCMV virus production by greater than 99% compared to untreated (black bar) control cells. Virus production was inhibited by greater than 99.9% if cells were both pretreated and treated during HCMV infection (open bar) with IFN-β. These results demonstrate that IFN-β can efficiently block HCMV replication in HFF cells and suggest that the ability to block the production of IFN-β would be advantageous for viral replication.
FIG. 1.
HCMV and IFN-β expression. (A) HCMV replication is inhibited by IFN-β treatment. HFF cells were mock treated or treated with 500 IU of IFN-β/ml. Cells were then infected with HCMV at a multiplicity of 0.1 PFU/cell. After 1 h of incubation, the inoculum was replaced with fresh medium either with or without 500 IU of IFN-β/ml. Virus was harvested 10 days postinfection and quantified by plaque assay on HFF cells. Conditions were mock treatment (black bar), pretreatment alone (horizontally striped bar), posttreatment alone (cross-hatched bar), and continuous treatment (open bar). (B) HFF cells were either mock infected; infected with HCMV, UV-inactivated HCMV, or wild-type HCMV in the presence of 100 μg of cycloheximide (CHX)/ml; or treated with cycloheximide alone. RNA was isolated 6 h posttreatment and analyzed for IFN-β, IE1, and GAPDH transcript by Northern blotting. (C) HFF cells were infected at a multiplicity of 5 PFU/cell with either HCMV or UV-inactivated HCMV. RNA was harvested at various times postinfection and assayed for IFN-β, IE2, and GAPDH by Northern blotting. (D) Supernatants from cells infected with either wild-type HCMV (open bars) orUV-inactivated HCMV (black bars) were harvested and assayed for IFN-β secretion by ELISA. Data represent the averages of two independent experiments.
We examined IFN-β expression following infection with wild-type HCMV and UV-inactivated HCMV. To remove exogenous cytokines and growth factors from our virus stocks, virus was purified by ultracentrifugation in an SW28 rotor for 1 h at 20,000 rpm. Purified virus was resuspended in conditioned medium and used for infection. Life-extended HFF cells (4) were infected at a multiplicity of 5 PFU/cell with either wild-type virus, UV-irradiated virus (360 mJ/cm2 in a Stratalinker), or wild-type virus in the presence of cycloheximide (100 μg/ml). RNA was harvested 6 h postinfection. Total RNA (6 μg) was separated by electrophoresis on a 1% formaldehyde gel and transferred to nitrocellulose membranes with a Turboblotter (Schleicher & Schuell) according to the manufacturer's instructions. Membranes were cross-linked and probed with 32P-labeled IFN-β cDNA. Figure 1B demonstrates the robust IFN-β induction following infection with UV-inactivated HCMV or infection with wild-type virus in the presence of cycloheximide (lanes 3 and 5). The IE1 transcript was also probed for to demonstrate that our UV irradiation protocol effectively blocks viral transcription, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was included as a loading control (Fig. 1B). We next determined the kinetics of IFN-β induction following infection with UV-inactivated virus. Cells were infected with either purified wild-type virus or UV-inactivated virus at a multiplicity of 5 PFU/cell. RNA was harvested at various times postinfection and used for Northern blot analysis. As shown in Fig. 1C, IFN-β RNA was barely detectable at any time after wild-type HCMV infection. However, IFN-β transcript levels were induced by 4 h and reached maximal levels by 12 h postinfection with UV-inactivated virus. Blots were also probed for expression of the HCMV IE2 transcript to demonstrate that our UV irradiation protocol effectively blocks viral transcription (Fig. 1C). These results demonstrate that IFN-β RNA levels are induced following infection with UV-inactivated virus. To determine if IFN-β was secreted during infection with wild-type virus or UV-inactivated virus, we performed a quantitative IFN-β-specific enzyme-linked immunosorbent assay (ELISA) on the supernatants from infected cells. Figure 1D shows that IFN-β protein is synthesized and secreted from the infected cells and that there is a significant increase in the amount of IFN-β produced in response to infection with UV-inactivated virus compared to wild-type infection. Taken together, these results demonstrate that IFN-β can efficiently block HCMV replication and that, following infection, viral gene expression is required to efficiently block the induction of IFN-β.
Since infection with UV-inactivated virus induced the expression of IFN-β within the first 4 h after infection, and this induction is blocked during wild-type infection, we hypothesized that the viral gene product responsible for inhibiting IFN-β may be an immediate-early protein. To test this hypothesis, we utilized replication-defective adenovirus vectors that express the HCMV IE1 (IE72) and IE2 (IE86) proteins to determine if either gene product could block the induction of IFN-β following infection with UV-inactivated HCMV. HFF cells were transduced with adenoviral vectors encoding either IE1, IE2, green fluorescent protein (GFP), UL83 (pp65), or UL82 (pp71) at a multiplicity of 3 PFU/cell in the presence of 1 μl of Lipofectamine (Invitrogen). Twenty-four hours after transduction, the cells were infected with UV-inactivated HCMV (5 PFU/cell), and RNA was harvested 8 h postinfection. As shown in Fig. 2A, wild-type HCMV infection or transduction with adenovirus alone did not induce IFN-β expression (lanes 2 and 3), but infection with UV-inactivated HCMV alone resulted in a robust induction of IFN-β RNA levels (lane 4). Expression of IE1, pp71, pp65, or GFP prior to infection with UV-inactivated virus had little effect on IFN-β RNA induction (compare lane 4 with lanes 5, 7, 8, and 9). However, expression of IE2 prior to infection with UV-inactivated virus efficiently blocked the induction of IFN-β (compare lanes 4 and 6). Interestingly, prior expression of IE2 had no effect on the induction of the IFN-stimulated gene ISG56 or 6-16 following infection with wild-type HCMV (data not shown). Western blots are included in Fig. 2A to demonstrate protein expression of the various genes at the time of infection with UV-HCMV. The level of secreted IFN-β from transduced cells infected with UV-inactivated HCMV was also evaluated. As shown in Fig. 2B, expression of IE2 prior to infection with UV-inactivated HCMV dramatically reduced the secretion of IFN-β. There was little or no inhibition of IFN-β secretion in cells expressing IE1, pp65, pp71, or GFP prior to infection with UV-inactivated HCMV compared to UV-HCMV alone.
FIG. 2.
Expression of IE86 blocks the induction of IFN-β during HCMV infection. (A) HFF cells were transduced with replication-defective adenoviruses expressing IE1, IE2, pp65, pp71, or GFP for 24 h. Transduced cells were then infected with UV-HCMV at a multiplicity of 5 PFU/cell. RNA was harvested 6 h postinfection with UV-HCMV and analyzed for IFN-β and GAPDH transcript. Expression of IE72, IE86, pp71, pp65, and GFP expressed from the adenoviruses is also shown. (B) Supernatants from infected samples as described for panel A were assayed for IFN-β secretion by ELISA. Data represent the averages of two independent experiments. WT, wild type. (C) HFF cells were transduced with replication-defective adenoviruses expressing either IE2 or GFP for 24 h. Transduced cells were then infected with HCMV in the presence of 100 μg of cycloheximide (CHX)/ml or treated with CHX alone. RNA was harvested 6 h postinfection and assayed for IFN-β and GAPDH by Northern blotting.
We also determined if IE2 could block the induction of IFN-β RNA expression following infection with wild-type HCMV in the presence of cycloheximide. HFF cells were transduced with replication-defective adenoviruses that express either IE2 or GFP 24 h prior to infection with wild-type HCMV in the presence of cycloheximide. As shown in Fig. 2C, infection with HCMV or the addition of cycloheximide alone to uninfected cells had no effect on IFN-β induction (lanes 2 and 4). However, cells infected with wild-type HCMV in the presence of cycloheximide resulted in a robust induction of IFN-β RNA (lane 3). Expression of IE2 prior to infection with wild-type HCMV in the presence of cycloheximide efficiently blocked the induction of IFN-β (lane 6), whereas prior expression of GFP had no effect on the induction of IFN-β (lane 5). These results demonstrate that the HCMV IE2 gene product can block the induction of IFN-β RNA and protein secretion during HCMV infection.
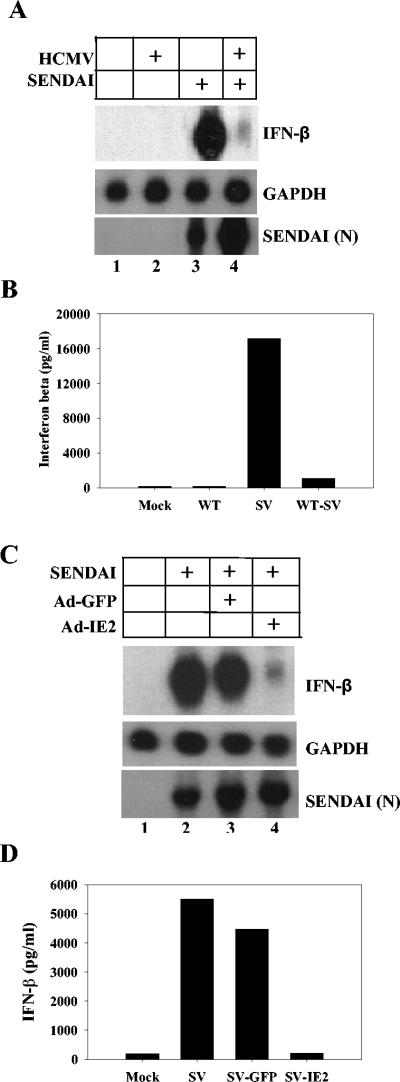
We next determined whether IE2's ability to block induction of IFN-β expression was specific to HCMV or if it could also block induction of IFN-β by other viruses. To address this, an experiment was performed using wild-type Sendai virus (Charles River Labs), which has previously been shown to be a strong inducer of IFN-β (21). HFF cells were infected for 6 h with purified HCMV at a multiplicity of 5 PFU/cell. The cells were then washed with phosphate-buffered saline and superinfected with Sendai virus (100 hemagglutinin units/ml). RNA and medium were harvested 16 h after Sendai virus infection, and Northern blotting and ELISA were performed for IFN-β expression. As shown in Fig. 3A and B, Sendai virus infection alone dramatically induced IFN-β RNA accumulation (Fig. 3A, lane 3) and IFN-β secretion (Fig. 3B). However, preinfection with wild-type HCMV blocked the induction of IFN-β transcript (Fig. 3A, lane 4) and the protein secretion (Fig. 3B) that is induced during wild-type Sendai virus infection. To confirm that HFF cells infected with HCMV were still susceptible to Sendai virus infection, blots were also probed for the Sendai virus N transcript. Both mock-infected and HCMV-infected cells that were infected with Sendai virus efficiently expressed the Sendai virus N transcript (Fig. 3A). These results suggest that the HCMV gene product that is blocking the IFN response during HCMV infection is also capable of inhibiting the response induced by Sendai virus. To determine if IE2 could block the induction of IFN-β during Sendai virus infection, HFF cells were transduced with adenoviruses that express either IE2 or GFP. Twenty-four hours after transduction, the cells were infected with Sendai virus. RNA and supernatants were harvested 6 h post-Sendai virus infection and assayed for IFN-β. As shown in Fig. 3C and D, Sendai virus infection alone resulted in a dramatic induction of IFN-β expression. Expression of GFP prior to Sendai virus infection had no effect on the induction of IFN-β following Sendai virus infection (Fig. 3C and D). However, prior expression of IE2 efficiently blocked accumulation of IFN-β transcript and the secretion of IFN-β induced by Sendai virus infection (Fig. 3C and D). IE2 was also able to block the induction of IFN-β following infection with vesicular stomatitis virus (data not shown). Blots were also probed for the Sendai virus-specific N transcript to eliminate the possibility that adenovirus transduction prevents infection by Sendai virus (Fig. 3C).
FIG. 3.
IE86 blocks the induction of IFN-β expression following Sendai virus infection. (A) HFF cells were either mock infected or infected with HCMV at a multiplicity of 5 PFU/cell. Six hours postinfection the cells were superinfected with Sendai virus (100 hemagglutinin units/ml). RNA was isolated 16 h after Sendai virus infection and assayed for IFN-β, GAPDH, and Sendai virus N transcript by Northern blotting. (B) Supernatants from infected samples described for panel A were assayed for IFN-β secretion by ELISA. Data represent the averages of two independent experiments. WT, wild type; SV, Sendai virus. (C) HFF cells were either mock transduced or transduced with replication-defective adenoviruses for 24 h that express either IE2 or GFP. Transduced cells were then infected with Sendai virus. RNA was isolated 6 h post-Sendai virus infection and assayed for IFN-β, GAPDH, and Sendai virus N transcript by Northern blotting. (D) Supernatants from infected samples described for panel C were assayed for secretion of IFN-β by ELISA. Data represent the averages of two independent experiments.
Our results demonstrate that HCMV gene expression is required to efficiently block the induction of IFN-β following HCMV infection and that the HCMV IE2 gene product IE86 is at least in part responsible for this inhibition. We also show that IE2 can block the induction of IFN-β following Sendai virus infection, demonstrating that this function of IE2 is not limited to a productive HCMV infection.
Induction of IFN-β transcription is a tightly regulated process that involves the activation of a number of signal transduction cascades and the recruitment of transcription factors including NF-κB, IFN regulatory factor 3 (IRF-3), and ATF/c-jun to the IFN-β promoter to form an enhanceosome that facilitates rapid preinitiation complex formation (1, 12, 13, 22). IFN-β enhanceosome formation is also dependent upon the activity of chromatin modifiers including CBP and P/CAF (13), which are recruited to the promoter in a complex with RNA polymerase II.
A number of viral proteins have been shown to disrupt these activation pathways and block enhanceosome formation. The Ebola virus VP35 protein and the hepatitis C virus NS3/4A protease interfere with the phosphorylation of IRF-3 and therefore block its ability to translocate to the nucleus and activate transcription (2, 8). The rotavirus NSP1 protein and the human papillomavirus E6 protein directly bind to IRF-3 and inhibit its ability to transactivate (9, 16). Other viral proteins have been shown to interact with CBP/p300 and alter its interaction with IRF-3 (10). The mechanism by which IE86 blocks IFN-β expression has yet to be defined. However, a number of groups have reported the phosphorylation and nuclear translocation of IRF-3 following HCMV infection (3, 5, 14, 15). We have also demonstrated that expression of IE2 prior to infection with UV-inactivated HCMV or wild-type Sendai virus infection does not block IRF-3 phosphorylation, dimerization, or nuclear translocation (data not shown). Taken together, these data suggest that HCMV and, specifically, IE2 do not inhibit IFN-β expression by blocking IRF-3 activation. Recently, Browne and Shenk reported that the HCMV UL83 gene product pp65 was involved in regulating the activation of NF-κB (5) and subsequent expression of IFN-responsive genes. Interestingly, we also observed a slight reduction in IFN-β RNA abundance when pp65 was expressed prior to infection with UV-HCMV (Fig. 2A, lane 7). However, this reduction by pp65 was not as dramatic as that observed with IE2 (Fig. 2A, lane 6). We are currently examining if IE2 and pp65 cooperate to block IFN-β expression by regulating the activation of NF-κB during virus infection or if the expression of IE2 is in some way influenced by pp65. IE2 has previously been shown to interact with other components of the IFN-β enhanceosome including CBP and P/CAF (7, 17), suggesting that IE2's interaction with these proteins may play a role in blocking IFN-β enhanceosome formation and activation. Studies are under way to determine the mechanism by which IE2 blocks the expression of IFN-β.
Acknowledgments
We are grateful to Jay Nelson (Oregon Health Sciences University) and Robert Kalejta (University of Wisconsin—Madison) for reagents and Michael Gale, Jr. (UT Southwestern), and Stacy Cantrell for technical help and for critically reading the manuscript.
This work was supported in part by NIH grant AI53838 (to W.A.B.). R.T.T. was supported by NIH training grant T32 AI07520-06.
REFERENCES
- 1.Agalioti, T., S. Lomvardas, B. Parekh, J. Yie, T. Maniatis, and D. Thanos. 2000. Ordered recruitment of chromatin modifying and general transcription factors to the IFN-beta promoter. Cell 103:667-678. [DOI] [PubMed] [Google Scholar]
- 2.Basler, C. F., A. Mikulasova, L. Martinez-Sobrido, J. Paragas, E. Muhlberger, M. Bray, H. D. Klenk, P. Palese, and A. Garcia-Sastre. 2003. The Ebola virus VP35 protein inhibits activation of interferon regulatory factor 3. J. Virol. 77:7945-7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boehme, K. W., J. Singh, S. T. Perry, and T. Compton. 2004. Human cytomegalovirus elicits a coordinated cellular antiviral response via envelope glycoprotein B. J. Virol. 78:1202-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bresnahan, W. A., G. E. Hultman, and T. Shenk. 2000. Replication of wild-type and mutant human cytomegalovirus in life-extended human diploid fibroblasts. J. Virol. 74:10816-10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Browne, E. P., and T. Shenk. 2003. Human cytomegalovirus UL83-coded pp65 virion protein inhibits antiviral gene expression in infected cells. Proc. Natl. Acad. Sci. USA 100:11439-11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browne, E. P., B. Wing, D. Coleman, and T. Shenk. 2001. Altered cellular mRNA levels in human cytomegalovirus-infected fibroblasts: viral block to the accumulation of antiviral mRNAs. J. Virol. 75:12319-12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryant, L. A., P. Mixon, M. Davidson, A. J. Bannister, T. Kouzarides, and J. H. Sinclair. 2000. The human cytomegalovirus 86-kilodalton major immediate-early protein interacts physically and functionally with histone acetyltransferase P/CAF. J. Virol. 74:7230-7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foy, E., K. Li, C. Wang, R. Sumpter, Jr., M. Ikeda, S. M. Lemon, and M. Gale, Jr. 2003. Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science 300:1145-1148. [DOI] [PubMed] [Google Scholar]
- 9.Graff, J. W., D. N. Mitzel, C. M. Weisend, M. L. Flenniken, and M. E. Hardy. 2002. Interferon regulatory factor 3 is a cellular partner of rotavirus NSP1. J. Virol. 76:9545-9550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juang, Y. T., W. Lowther, M. Kellum, W. C. Au, R. Lin, J. Hiscott, and P. M. Pitha. 1998. Primary activation of interferon A and interferon B gene transcription by interferon regulatory factor 3. Proc. Natl. Acad. Sci. USA 95:9837-9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katze, M. G., Y. He, and M. Gale, Jr. 2002. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2:675-687. [DOI] [PubMed] [Google Scholar]
- 12.Merika, M., and D. Thanos. 2001. Enhanceosomes. Curr. Opin. Genet. Dev. 11:205-208. [DOI] [PubMed] [Google Scholar]
- 13.Munshi, N., M. Merika, J. Yie, K. Senger, G. Chen, and D. Thanos. 1998. Acetylation of HMG I(Y) by CBP turns off IFN beta expression by disrupting the enhanceosome. Mol. Cell 2:457-467. [DOI] [PubMed] [Google Scholar]
- 14.Navarro, L., K. Mowen, S. Rodems, B. Weaver, N. Reich, D. Spector, and M. David. 1998. Cytomegalovirus activates interferon immediate-early response gene expression and an interferon regulatory factor 3-containing interferon-stimulated response element-binding complex. Mol. Cell. Biol. 18:3796-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preston, C. M., A. N. Harman, and M. J. Nicholl. 2001. Activation of interferon response factor-3 in human cells infected with herpes simplex virus type 1 or human cytomegalovirus. J. Virol. 75:8909-8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ronco, L. V., A. Y. Karpova, M. Vidal, and P. M. Howley. 1998. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev. 12:2061-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz, R., B. Helmich, and D. H. Spector. 1996. CREB and CREB-binding proteins play an important role in the IE2 86-kilodalton protein-mediated transactivation of the human cytomegalovirus 2.2-kilobase RNA promoter. J. Virol. 70:6955-6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sen, G. C. 2001. Viruses and interferons. Annu. Rev. Microbiol. 55:255-281. [DOI] [PubMed] [Google Scholar]
- 19.Simmen, K. A., J. Singh, B. G. Luukkonen, M. Lopper, A. Bittner, N. E. Miller, M. R. Jackson, T. Compton, and K. Fruh. 2001. Global modulation of cellular transcription by human cytomegalovirus is initiated by viral glycoprotein B. Proc. Natl. Acad. Sci. USA 98:7140-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 21.Wathelet, M. G., C. H. Lin, B. S. Parekh, L. V. Ronco, P. M. Howley, and T. Maniatis. 1998. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-beta enhancer in vivo. Mol. Cell 1:507-518. [DOI] [PubMed] [Google Scholar]
- 22.Yie, J., K. Senger, and D. Thanos. 1999. Mechanism by which the IFN-beta enhanceosome activates transcription. Proc. Natl. Acad. Sci. USA 96:13108-13113. [DOI] [PMC free article] [PubMed] [Google Scholar]