Abstract
Nipah virus (NiV), a highly pathogenic member of the family Paramyxoviridae, encodes the surface glycoproteins F and G. Since internalization of the NiV envelope proteins from the cell surface might be of functional importance for viral pathogenesis either by regulating cytopathogenicity or by modulating recognition of infected cells by the immune system, we analyzed the endocytosis of the NiV F and G proteins. Interestingly, we found both glycoproteins to be internalized in infected and transfected cells. As endocytosis is normally mediated by tyrosine- or dileucine-dependent signals in the cytoplasmic tails of transmembrane proteins, all potential internalization signals in the NiV glycoproteins were mutated. Whereas the G protein appeared to be constitutively internalized with the bulk flow during membrane turnover, uptake of the F protein was found to be signal mediated. F endocytosis clearly depended on a membrane-proximal YXXΦ motif and was found to be of functional importance for the biological activity of the protein.
Nipah virus (NiV), a member of the genus Henipavirus within the family Paramyxoviridae, is highly pathogenic for many species (5, 12, 25, 27, 28). Virions have an envelope with two integral membrane proteins, the viral attachment protein G and the fusion (F) protein. The fact that NiV infects a variety of different cell types of different species in vivo and in vitro strongly suggests that the cellular receptor recognized by the G protein is widely expressed and that proteolytic activation of the NiV F protein is not restricted (4, 12). Interestingly, activation of the F protein appears to depend on a novel type of proteolytic cleavage that has not yet been described for any other paramyxoviral fusion protein. Cleavage of the precursor F protein into the F1 and F2 subunits, which releases the hydrophobic fusion peptide at the N terminus of F1, is mediated by a ubiquitous protease that does not require a basic amino acid at the cleavage site and therefore differs from the furin- or trypsin-like proteases known to activate other paramyxoviral F proteins (17). Active G/F1,2 complexes on the surface of virions are required for virus penetration into host cells, and glycoprotein complexes expressed on the surface of infected or transfected cells induce fusion with adjacent cells, resulting in multinucleated syncytia. Glycoprotein-mediated cell-to-cell fusion significantly influences the cytopathogenicity of NiV.
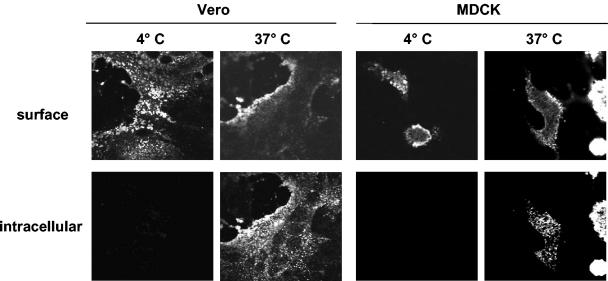
Since internalization of envelope components may be an important posttranslational regulatory mechanism to downregulate the surface expression of viral glycoproteins, we wanted to examine whether NiV glycoproteins undergo endocytosis. For this purpose, Vero and MDCK cells were infected with NiV under biosafety level 4 (P4) conditions. At 24 h postinfection (p.i.), the infected cells were washed and incubated without prior fixation with a polyclonal anti-NiV serum from an infected guinea pig. After antibody incubation at 4°C, the cells were either kept on ice or incubated for 30 min at 37°C to allow endocytosis to occur. Then, the cells were fixed with 4% paraformaldehyde. Surface-bound primary antibodies were detected by incubation with a fluorescein isothiocyanate (FITC)-conjugated secondary antibody (dilution, 1:50; Dianova). After permeabilization with methanol-acetone (1:1) for 5 min, internalized primary antibodies were stained with a rhodamine-conjugated secondary antibody (dilution, 1:500; Dianova). The results of this antibody uptake experiment are shown in Fig. 1. Irrespective of the fact that cell-to-cell fusion was already very advanced in Vero cells whereas almost no syncytium formation could be detected in NiV-infected MDCK cells at 24 h p.i., the NiV glycoproteins were internalized in both cell lines. After incubation at 37°C, NiV-infected cells showed both surface fluorescence and numerous fluorescent intracellular vesicles throughout the cell.
FIG. 1.
Confocal microscopy analysis of NiV glycoprotein endocytosis in infected cells. Vero and MDCK cells were infected with NiV at a multiplicity of infection of 0.1. At 24 h p.i., cells were incubated with an NiV-specific guinea pig antiserum at 4°C for 30 min without prior fixation. Then, cells were either kept on ice (4°C) or warmed to 37°C to allow endocytosis to occur (37°C). After 30 min, cells were fixed with 4% paraformaldehyde for 16 h, and surface-bound antibodies were detected by incubation with an FITC-conjugated secondary antibody (surface). After permeabilization of the cells with methanol-acetone, internalized antibodies were stained with a rhodamine-conjugated secondary antibody (intracellular). The images shown represent the central sections from the cells analyzed by laser scanning confocal microscopy.
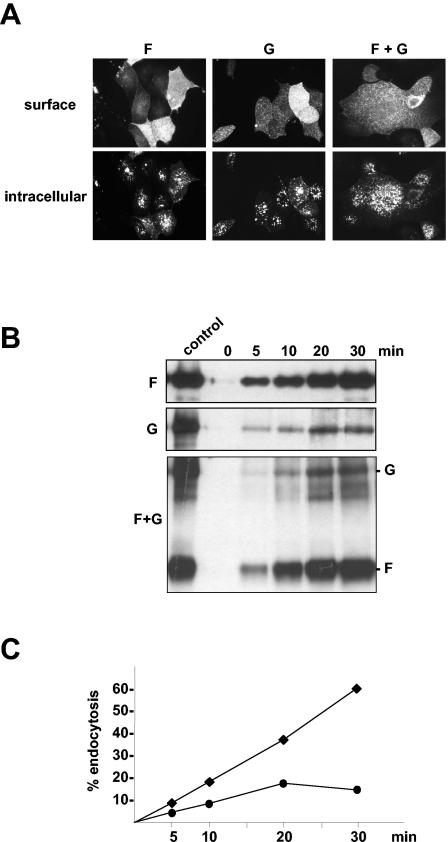
Because the polyclonal anti-NiV guinea pig serum binds to both F and G proteins, it had to be elucidated whether only one or both glycoproteins undergo endocytosis. To answer this question, the internalization of F and G proteins in singly transfected cells was analyzed. cDNA fragments spanning the F or G genes of the NiV genome (GenBank accession number AF212302) were cloned into the pczCFG5 vector as described previously (16). To study endocytosis of transiently expressed proteins, F- and G-encoding plasmids were transfected into MDCK cells using Lipofectamine 2000 (Gibco BRL). The antibody uptake experiment, results for which are shown in Fig. 2A, clearly demonstrated that both proteins, expressed either singly (panels F and G) or together (panel F+G), were markedly endocytosed within 30 min. As with infected cells, intracellular staining displayed multiple fluorescent vesicles. Since it has been reported that endocytosis of viral glycoproteins can be induced by an antibody-mediated mechanism (8, 24), the internalization of NiV proteins G and F was also analyzed in the absence of antibodies. For this analysis, we performed a biotin endocytosis assay, which allows the determination of the rate at which viral glycoproteins are internalized. Essentially as described previously (18), cells transiently expressing either F or G proteins alone or in combination were surface labeled with cleavable disulfide-linked NHS-SS-biotin [sulfosuccinimidyl 2-(biotinamido)ethyl-1,3′-dipropionate; Pierce] at 4°C. Then, cells were incubated for 0, 5, 10, 20, or 30 min at 37°C to allow endocytosis to occur. After rapid cooling to 4°C, biotin still exposed on the cell surface was cleaved by incubation with 50 mM MESNA (2-mercaptoethanesulfonic acid; Sigma). To determine the total amount of biotinylated protein, one sample was neither incubated at 37°C nor reduced with MESNA (control). After immunoprecipitation with the anti-NiV antiserum, biotinylated proteins were separated on a sodium dodecyl sulfate-10% polyacrylamide gel under nonreducing conditions, transferred to nitrocellulose, and detected with peroxidase-conjugated streptavidin (Amersham) and a chemiluminescent substrate (Amersham). The results shown in Fig. 2B clearly demonstrate that the internalizations of the NiV F and G proteins are antibody independent and are obviously not linked. The uptake of F and G was essentially the same in singly expressing (Fig. 2B, panels F and G) or coexpressing (Fig. 2B, panel F+G) cells. The amount of intracellular F protein increased with increasing time of incubation at 37°C. After 30 min, more than 50% of the protein had been internalized. The endocytosis rate determined by densitometric quantification revealed that the F protein was endocytosed at a rate of about 2%/min (Fig. 2C). In contrast, the G protein is constitutively internalized at the lower rate of 1%/min, more characteristic of bulk uptake during membrane turnover than of active clustering into endocytic vesicles. The finding that the amount of intracellular G increased only over the first 20 min of incubation at 37°C suggests that the endocytosed G protein does not accumulate intracellularly but is slowly degraded or recycled to the plasma membrane.
FIG. 2.
Endocytosis of singly expressed or coexpressed NiV F and G proteins. (A) MDCK cells were transfected with pczCFG5 plasmids encoding either the NiV F or G proteins or were cotransfected with both plasmids (F + G). At 24 h posttransfection, the cells were incubated with the NiV-specific antiserum at 4°C for 30 min and then warmed to 37°C to allow endocytosis to occur. Surface-bound antibodies were visualized by FITC-conjugated secondary antibodies (surface), and internalized primary antibodies were stained after permeabilization with methanol-acetone by rhodamine-conjugated secondary antibodies (intracellular). (B) MDCK cells expressing F, G, or F and G (F+G) proteins were surface labeled with cleavable NHS-SS-biotin at 4°C. Then, the cells were warmed to 37°C for the times indicated to allow endocytosis of the biotinylated proteins to occur. Subsequently, cell surface proteins were reduced with MESNA. To determine the total amount of surface-biotinylated proteins, the cells were compared to cells that were neither warmed to 37°C nor treated with MESNA (control). After cell lysis, F and G proteins were immunoprecipitated with the NiV-specific antiserum. The samples were separated on a sodium dodecyl sulfate-10% polyacrylamide gel under nonreducing conditions and transferred to nitrocellulose. Biotinylated proteins were then detected with peroxidase-conjugated streptavidin. The control lane represents 50% of the total amount of biotinylated F or G proteins. (C) To determine the rate of internalization, the percentages of internalized F (♦) and G (•) proteins measured in the experiment shown in Fig. 2B (panels F and G) were plotted as a function of the time that cells were incubated at 37°C.
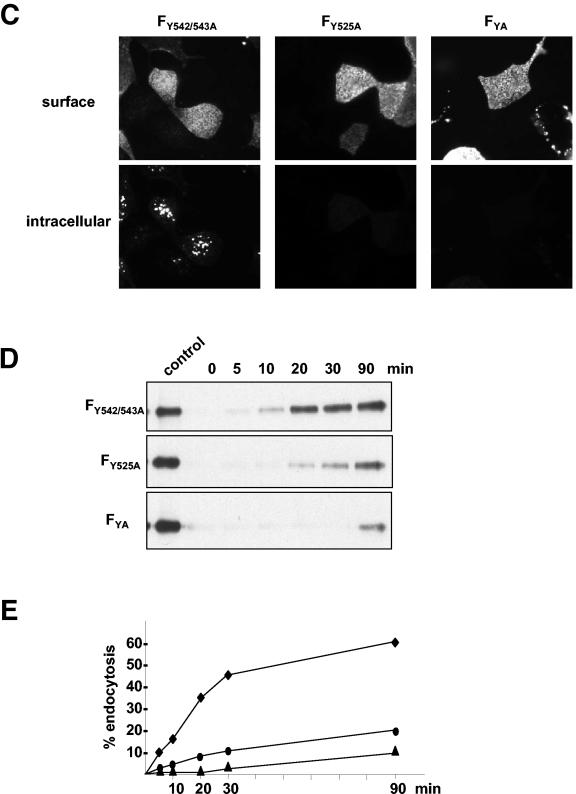
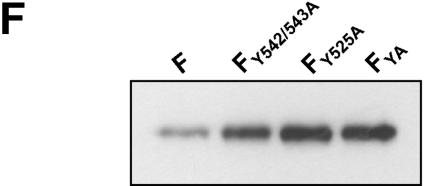
Endocytosis, namely, the sorting of transmembrane proteins into the endosomal and lysosomal compartments, is mediated by signals present in the cytosolic tails of the proteins. The most widely used signals are referred to as tyrosine-based motifs, which constitute a family of degenerate motifs minimally defined by the presence of a critical tyrosine residue (2). Most of the tyrosine-based signals conform to the consensus motif YXXΦ (where Y is tyrosine, X is any amino acid, and Φ is an amino acid with a bulky hydrophobic group) or NPXY (where N is asparagine and P is proline). Other signals are known as dileucine (LL)-based motifs. Characteristic tyrosine or dileucine motifs are recognized by cytosolic adaptor complexes (AP), in particular AP-2, which selectively concentrate proteins within clathrin-coated vesicles. Further transport after clathrin-mediated endocytosis to recycling or lysosomal compartments is complexly regulated by interaction with different other cytosolic recognition molecules, by phosphorylation, or by conjugation with ubiquitin (for reviews, see references 3, 10, and 11 and references therein). Interestingly, the 28-residue cytoplasmic tail of the NiV F protein contains a membrane-proximal tyrosine (position 525) that perfectly fits into the YXXΦ-type endocytosis signals and two additional tyrosines (dityrosine motif) at positions 542 and 543. The 45-residue cytoplasmic tail of the G protein has a membrane-proximal dileucine motif at positions 41 and 42 and also a dityrosine motif (positions 28 and 29). To evaluate the importance of the potential endocytosis signals in the cytoplasmic domains of the NiV glycoproteins, the internalization of different tail mutants was analyzed. The F and G mutants depicted in Fig. 3A were generated by introducing site-specific mutations into the double-stranded pczCFG5 plasmids by using a QuikChange mutagenesis kit (Stratagene) as described previously (16). By using complementary oligonucleotide primers, tyrosine or leucine residues in the cytoplasmic tails of F and G were changed to alanines. The endocytosis of the mutated glycoproteins was analyzed after transient expression in MDCK cells. In the G protein, neither disruption of the dityrosine motif (mutant GY28/29A) nor mutation of the dileucine motif (GL41/42A) influenced internalization of the protein (Fig. 3B). This result again indicates that G is internalized with the bulk of the plasma membrane and that the tyrosines and leucines in the cytoplasmic tail of the G protein do not function as signals for active clustering of the protein in clathrin-coated vesicles. The F protein in which the dityrosine motif was mutated (FY542/543A) was still efficiently internalized, whereas the F protein with the disrupted YXXΦ motif (FY525A) and the FYA mutant with all tyrosines replaced by alanines were no longer endocytosed to a detectable degree (Fig. 3C). Quantification of the endocytosis with the biotinylation assay (Fig. 3D and E) revealed that the internalization of FY542/543A was slightly reduced compared to that of wild-type F (1.6%/min versus 2%/min). Furthermore, the uptake rate of the mutant lacking all tyrosines in the cytoplasmic tail (FYA) was lower than that of FY525A (0.1%/min versus <0.2%/min). The uptake rate of FYA was not increased in the presence of the G protein, demonstrating again that the internalizations of the two NiV glycoproteins are not linked (data not shown). By quantitatively analyzing the surface expression levels of the F mutants, we expectedly found that the less the protein was endocytosed, the larger the amount of protein expressed on the surface (Fig. 3F). Together, these results clearly show that an intact YXXΦ motif is the main prerequisite for the constitutive endocytosis of the F protein. Nevertheless, mutation of the dityrosine motif had a moderately negative effect on F uptake in either the presence or absence of a functional YXXΦ internalization motif.
FIG. 3.
Internalization of F and G proteins with mutations in the potential endocytosis signals. (A) Sequences of the cytoplasmic domains of wild-type and mutant G and F proteins. The numbers above the sequences indicate the amino acid positions. Amino acid sequences are shown in a single-letter code; boldface letters indicate exchanged amino acid residues. The vertical lines separate the transmembrane domains from those predicted to be in the cytoplasm. (B and C) Antibody uptake analysis of mutant G (GY28/29A and GL41/42A) (B) and mutant F (FY542/543A, FY525A, and FYA) (C) proteins was performed as described in the legend to Fig. 2A. (D) Endocytosis of mutant F proteins (FY542/543A, FY525A, and FYA) was determined after surface biotinylation and MESNA reduction as described in the legend to Fig. 2B. (E) The rates of internalization were determined by plotting the percentages of endocytosed FY542/543A (⧫), FY525A (•), and FYA (▴) proteins as functions of the times that the cells were incubated at 37°C. (F) The total amount of surface-expressed wild-type (F) and mutant F (FY542/543A, FY525A, and FYA) proteins was determined by the surface biotinylation of 2 × 106 cells at 30 h posttransfection. After cell lysis, the F proteins were immunoprecipitated and detected as described in the legend to Fig. 2A.
To analyze the functional activity of the G and F mutants, fusion assays were performed as described previously (17). Only cells coexpressing both functionally active NiV glycoproteins can fuse with adjacent cells, resulting in the formation of multinucleated syncytia. Figure 4 shows that coexpression of F with either wild-type or mutant G proteins resulted in efficient cell-to-cell fusion. As expected, wild-type G, GY28/29A, and GL41/42A, which are endocytosed at almost identical rates, showed no differences in their fusion promotion activities. In contrast, the fusion activities of the F mutants clearly differed. Whereas FY542/543A induced syncytium formation almost as efficiently as the wild-type F protein did, the size of the syncytia formed by cells coexpressing G and either FY525A or FYA was clearly reduced. This finding shows that F endocytosis is obviously essential for biological activity.
FIG. 4.
Biological activity of mutant G and F proteins. MDCK cells were cotransfected with plasmids encoding either wild-type or mutant G or F proteins as indicated. To visualize syncytium formation, the cells were fixed at 24 h posttransfection with ethanol and stained with Giemsa staining solution. Representative microscopic fields were photographed.
Internalization of the NiV F protein depends on a characteristic YXXΦ motif, a requirement also reported for many cellular proteins such as lamp-1, the transferrin receptor, CD63, the epidermal growth factor (reviewed in reference 2), and several viral glycoproteins, such as the varicella-zoster gE and gH proteins and the surface glycoproteins of human immunodeficiency virus (HIV) and simian immunodeficiency virus (1, 19, 20, 23). Therefore, the functionality of the YXXΦ motif in the context of the NiV F protein is not too surprising. Nevertheless, there are cases in which this signal is not functional, such as that of the envelope protein of the murine leukemia virus (6, 9). Interestingly, whereas none of the other paramyxovirus F proteins contains a classical YXXΦ motif, the YSRL endocytosis signal in the NiV F protein is also found in the cytoplasmic tail of the Hendra virus F protein. This fact, together with our finding that the fusion activity of the NiV F protein is clearly downregulated in the absence of a functional endocytosis signal, strongly suggests that constitutive endocytosis due to a conserved internalization signal is a particular property of critical biological importance of Henipavirus fusion proteins.
In contrast to that of the NiV F protein, internalization of the G protein depends on neither a tyrosine- nor a dileucine-based signal, indicating that G is endocytosed at a rate of 1%/min together with the bulk of the plasma membrane during basal membrane turnover. The finding that the uptake rates of FYA and FY525A (≤0.2%/min) are significantly below the bulk uptake rate suggests that these proteins are excluded from coated pits, as has been described for the influenza virus HA protein (13).
It has been proposed that endocytosis of viral envelope proteins from the cell surface as well as any other mechanism to downmodulate glycoproteins from the cell surface may play a significant role in viral pathogenesis by limiting cytopathogenicity as well as recognition of infected cells by the immune system (15). Disruption of endocytosis signals, resulting in higher expression levels of the viral glycoproteins on the cell surface, can have noticeable effects, such as upregulation of virus-mediated cell-to-cell fusion, as has recently been shown for varicella-zoster virus (21). In contrast, mutation of the endocytosis signal in the NiV F protein led to the downregulation of cell-to-cell fusion. Mutations of endocytosis signals can also affect virus assembly. Whereas enhanced surface expression of an endocytosis-deficient simian immunodeficiency virus Env protein correlated strictly with increased levels of Env content in virions and enhanced infectivity, mutation of endocytosis signals in the HIV Env protein did not increase the total amount of glycoprotein incorporated into infectious virions, although fusogenicity and infectivity were enhanced (26, 29). Although it must be assumed that glycoprotein endocytosis also has an effect on the assembly of NiV, this effect cannot be studied unless a reverse genetics system is established and recombinant NiV encoding glycoproteins which are no longer endocytosed can be generated.
Even if many viral glycoproteins are known to undergo endocytosis upon single expression, the uptake of these glycoproteins is not always observed in infected cells. Whereas internalization of HN protein in simian virus 5-infected cells is undisturbed (14), none of the F and H glycoproteins in measles virus (MV)-infected cells is endocytosed, although both proteins possess tyrosine-dependent internalization signals in their cytoplasmic tails (18). In MV-infected cells, interaction of the endocytosis signals with cellular adaptor complexes responsible for the uptake of F and H upon single expression is very likely prevented by the matrix protein which binds to the glycoprotein tails, thereby initiating the assembly of new virions (A. Maisner, unpublished observation). Similar mechanisms to regulate the expression of viral envelope proteins by interaction with viral core proteins have been proposed for Sendai virus and HIV (7, 22). Our finding that the NiV surface glycoproteins are efficiently endocytosed in infected cells suggests that, in contrast to what is observed for MV, Sendai virus, and HIV, expression of other viral proteins does not interfere principally with glycoprotein uptake. Given the importance of F internalization for the biological activity of the protein, it must be assumed that endocytosis of this NiV glycoprotein not only regulates fusion in cells transiently expressing both NiV glycoproteins but also influences virus spread by cell-to-cell fusion in NiV-infected cells.
.
Acknowledgments
We thank Markus Czub, Hana Weingartl, and Mary Jane Cardosa for providing the cloned NiV F and G genes, the NiV-specific guinea pig antiserum, and the Nipah virus isolate.
This work was supported by a grant from the German Research Foundation (DFG) to A.M. (MA 1886/5-1).
REFERENCES
- 1.Berlioz-Torrent, C., B. L. Shacklett, L. Erdtmann, L. Delamarre, I. Bouchaert, P. Sonigo, M. C. Dokhelar, and R. Benarous. 1999. Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adaptor complexes modulate intracellular and cell surface expression of envelope glycoproteins. J. Virol. 73:1350-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonifacino, J. S., and E. C. Dell'Angelica. 1999. Molecular bases for the recognition of tyrosine-based sorting signals. J. Cell Biol. 145:923-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonifacino, J. S., and L. M. Traub. 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72:395-447. [DOI] [PubMed] [Google Scholar]
- 4.Bossart, K. N., L.-F. Wang, M. N. Flora, K. B. Chua, S. K. Lam, B. T. Eaton, and C. C. Broder. 2002. Membrane fusion tropism and heterotypic functional activities of the Nipah virus and Hendra virus envelope glycoproteins. J. Virol. 76:11186-11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chua, K. B., K. J. Goh, K. T. Wong, A. Kamarulzaman, P. S. Tan, T. G. Ksiazek, S. R. Zaki, G. Paul, S. K. Lam, and C. T. Tan. 1999. Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. Lancet 354:1257-1259. [DOI] [PubMed] [Google Scholar]
- 6.Danis, C., J. Deschambeault, S. Do Carmo, E. A. Cohen, E. Rassart, and G. Lemay. 2004. The tyrosine-based YXXO targeting motif of murine leukemia virus envelope glycoprotein affects pathogenesis. Virology 324:173-183. [DOI] [PubMed] [Google Scholar]
- 7.Egan, M. A., L. M. Carruth, J. F. Rowell, X. Yu, and R. F. Siliciano. 1996. Human immunodeficiency virus type 1 envelope protein endocytosis mediated by a highly conserved intrinsic internalization signal in the cytoplasmic domain of gp41 is suppressed in the presence of the Pr55gag precursor protein. J. Virol. 70:6547-6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Favoreel, H. W., H. J. Nauwynck, P. Van Oostveldt, and M. B. Pensaert. 2000. Role of anti-gB and -gD antibodies in antibody-induced endocytosis of viral and cellular cell surface glycoproteins expressed on pseudorabies virus-infected monocytes. Virology 267:151-158. [DOI] [PubMed] [Google Scholar]
- 9.Grange, M.-P., V. Blot, L. Delamarre, I. Bouchaert, A. Rocca, A. Dautry-Varsat, and M. C. Dokhélar. 2000. Identification of two intracellular mechanisms leading to reduced expression of oncoretrovirus envelope glycoproteins at the cell surface. J. Virol. 74:11734-11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heilker, R., M. Spiess, and P. Crottet. 1999. Recognition of sorting signals by clathrin adaptors. Bioessays 21:558-567. [DOI] [PubMed] [Google Scholar]
- 11.Hicke, L., and R. Dunn. 2003. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu. Rev. Cell Dev. Biol. 19:141-172. [DOI] [PubMed] [Google Scholar]
- 12.Hooper, P., S. Zaki, P. Daniels, and D. Middleton. 2001. Comparative pathology of the diseases caused by Hendra and Nipah viruses. Microbes Infect. 3:315-322. [DOI] [PubMed] [Google Scholar]
- 13.Lazarovits, J., H. Y. Naim, A. C. Rodriguez, R. H. Wang, E. Fire, C. Bird, Y. I. Henis, and M. G. Roth. 1996. Endocytosis of chimeric influenza virus hemagglutinin proteins that lack a cytoplasmic recognition feature for coated pits. J. Cell Biol. 134:339-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leser, G. P., K. J. Ector, and R. A. Lamb. 1996. The paramyxovirus simian virus 5 hemagglutinin-neuraminidase glycoprotein, but not the fusion glycoprotein, is internalized via coated pits and enters the endocytic pathway. Mol. Biol. Cell 7:155-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marsh, M., and A. Pelchen-Matthews. 2000. Endocytosis in viral replication. Traffic 1:525-532. [DOI] [PubMed] [Google Scholar]
- 16.Moll, M., A. Kaufmann, and A. Maisner. 2004. Influence of N-glycans on processing and biological activity of the Nipah virus fusion protein. J. Virol. 78:7274-7278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moll, M., S. Diederich, H.-D. Klenk, M. Czub, and A. Maisner. 2004. Ubiquitous activation of the Nipah virus fusion protein does not require a basic amino acid at the cleavage site. J. Virol. 78:9705-9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moll, M., H. D. Klenk, G. Herrler, and A. Maisner. 2001. A single amino acid change in the cytoplasmic domains of measles virus glycoproteins H and F alters targeting, endocytosis, and cell fusion in polarized Madin-Darby canine kidney cells. J. Biol. Chem. 276:17887-17894. [DOI] [PubMed] [Google Scholar]
- 19.Olson, J. K., and C. Grose. 1997. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail. J. Virol. 71:4042-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasieka, T. J., L. Maresova, and C. Grose. 2003. A functional YNKI motif in the short cytoplasmic tail of varicella-zoster virus glycoprotein gH mediates clathrin-dependent and antibody-independent endocytosis. J. Virol. 77:4191-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasieka, T. J., L. Maresova, K. Shiraki, and C. Grose. 2004. Regulation of varicella-zoster virus-induced cell-to-cell fusion by the endocytosis-competent glycoproteins gH and gE. J. Virol. 78:2884-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roux, L., P. Beffy, and A. Portner. 1985. Three variations in the cell surface expression of the haemagglutinin-neuraminidase glycoprotein of Sendai virus. J. Gen. Virol. 66:987-1000. [DOI] [PubMed] [Google Scholar]
- 23.Sauter, M. M., A. Pelchen-Matthews, R. Bron, M. Marsh, C. C. LaBranche, P. J. Vance, J. Romano, B. S. Haggarty, T. K. Hart, W. M. Lee, and J. A. Hoxie. 1996. An internalization signal in the simian immunodeficiency virus transmembrane protein cytoplasmic domain modulates expression of envelope glycoproteins on the cell surface. J. Cell Biol. 132:795-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tirabassi, R. S., and L. W. Enquist. 1998. Role of envelope protein gE endocytosis in the pseudorabies virus life cycle. J. Virol. 72:4571-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang, L.-F., M. Yu, E. Hansson, L. I. Pritchard, B. Shiell, W. P. Michalski, and B. T. Eaton. 2000. The exceptionally large genome of Hendra virus: support for creation of a new genus within the family Paramyxoviridae. J. Virol. 74:9972-9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.West, J. T., S. K. Weldon, S. Wyss, X. Lin, Q. Yu, M. Thali, and E. Hunter. 2002. Mutation of the dominant endocytosis motif in human immunodeficiency virus type 1 gp41 can complement matrix mutations without increasing Env incorporation. J. Virol. 76:3338-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong, K. T., I. Grosjean, C. Brisson, B. Blanquier, M. Fevre-Montange, A. Bernard, P. Loth, M. C. Georges-Courbot, M. Chevallier, H. Akaoka, P. Marianneau, S. K. Lam, T. F. Wild, and V. Deubel. 2003. A golden hamster model for human acute Nipah virus infection. Am. J. Pathol. 163:2127-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong, K. T., W. J. Shieh, S. Kumar, K. Norain, W. Abdullah, J. Guarner, C. S. Goldsmith, K. B. Chua, S. K. Lam, C. T. Tan, K. J. Goh, H. T. Chong, R. Jusoh, P. E. Rollin, T. G. Ksiazek, and S. R. Zaki. 2002. Nipah virus infection: pathology and pathogenesis of an emerging paramyxoviral zoonosis. Am. J. Pathol. 161:2153-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuste, E., J. D. Reeves, R. W. Doms, and R. C. Desrosiers. 2004. Modulation of Env content in virions of simian immunodeficiency virus: correlation with cell surface expression and virion infectivity. J. Virol. 78:6775-6785. [DOI] [PMC free article] [PubMed] [Google Scholar]