Abstract
Animal‐based research and drug safety studies are essential to understanding the mysteries of nature and the long‐term survival of humans. Due to the rapid increase in the global human population, conflict‐ and economically driven human migration, tourism‐related activities, densely populated metropolitan areas, and local policies, humans will be affected by a multitude of novel disease‐causing microorganisms and civilizational diseases. Despite disparities among countries, recent and planned changes in regulations concerning animal research and drug safety studies could have detrimental effects on both the animal research community and nations lacking sufficient social support systems. Based on existing scientific literature, I argue that we need animal research encompassing aspects such as animal development, behavior, drug safety studies, and for the understanding of future civilizational diseases. Depending on the nature of the research questions and local challenges, a suitable animal model organism should be made mandatory.
Keywords: animal model, drug safety studies, research, zebrafish
If animal research is completely replaced with cell cultures‐ and other alternative model systems‐based research, our next generation of scientists may not be able to understand the mysteries of nature, the ontogeny of diseases, and may probably be incapable of developing safe and affordable therapeutics.
1. RACE TO APPLY ALTERNATIVES TO ANIMAL RESEARCH AND DRUG SAFETY STUDIES
Historical texts have several variations on the parable of the blind men and an elephant. 1 , 2 The issue with the story is that every blind man’s perception and description of the elephant was true. What was lacking was the completeness of the truth. We are all alive today because of tremendous technological progress and advancements. 3 This includes the drugs available for treating diseases and vaccines for disease prevention. 4 , 5 One could argue that early Chinese physicians and Peruvian shamans recognized the value of wormwood plants and cinchona trees as treatments for symptoms of malaria. 6 , 7 , 8 , 9 Yet animal research was necessary to identify novel drugs and vaccines against drug‐resistant plasmodium that causes malaria. 10 , 11 , 12 Developing countries whose infrastructure, local habitat, and weather were well suited for the spread of the disease benefited the most from antimalarial drugs. However, despite the imbalance of problems faced due to lack of adequate social and infrastructure support, there are discussions in the European Union parliament and actions undertaken by the United States Food and Drug Administration (FDA) for stricter rules for animal research, drug safety studies, or steps to abolish animal‐based drug safety tests. 13 , 14 , 15 While many researchers have expressed concerns about the risks and limitations, several other countries (Canada, India, and South Korea) have already introduced or amended their existing laws. 16 , 17 , 18 Therefore, there is a need to stress the importance of animal research and animal‐based drug safety studies over alternative systems and its implications for our future.
2. FEW EXAMPLES OF ANIMAL RESEARCH–BASED DRUGS
Animal research has led to the identification of numerous drugs, including levodopa for treating Parkinson's disease patients, 19 , 20 , 21 Pitocin administered to millions of pregnant women to induce labor, 22 , 23 , 24 insulin to treat diabetes mellitus, 25 , 26 and lithium to treat mania. 27 Animal research has also identified the cause of mysterious Parkinson‐like symptoms in relatively young adults who consumed illicit heroin‐like drugs manufactured using sloppy practices. 28 , 29 , 30 , 31 Moreover, animal research has identified bench‐to‐bedside drugs. A recent example relates to the treatment of children with Dravet syndrome, a genetic condition in which affected children experience fever‐associated seizures. 32 Researchers have used behavioral assays on larval zebrafish models of Dravet syndrome to identify several drugs that could potentially be used to treat affected children, with one such drug being lorcaserin. 33 However, lorcaserin, a weight‐loss drug previously approved by the FDA, has been withdrawn from the U.S. market due to increased risks of developing cancer, so it is likely that we still need better and safer drugs for the affected children. 34
3. ANIMAL RESEARCH IS REQUIRED TO UNDERSTAND CIVILIZATIONAL DISEASES
We are now aware of the risks of fast‐food consumption in increasing cholesterol levels and the associated risks of developing diseases such as atherosclerosis that can lead to stroke or heart attack. 35 Without basic research on animal model organisms, we will be unaware of the role of cholesterol in atherosclerosis. 36 , 37 Similarly, even though we are aware of common practices of bullying in schools and workplaces, and the associated risks of developing mental health issues, 38 , 39 , 40 technological advances have led to an unprecedented increase in the use of social media irrespective of the age of the users despite imbalances in resilience to handle bullying. 41 With the invention of easily accessible graphic design and artificial intelligence tools, we already see the adverse effects of deepfake‐based fake information on naive individuals and on the society permitted by uncontrolled social media use. 42 , 43 The invincibility of certain groups of professional Internet users has resulted in a lack of clarity regarding policies, systemic solutions to tackle cyberbullying and to help the affected individuals and their families. 44 , 45 But only using animal models, we are understanding the role of genetics and environment in resilience and susceptibility to stress. 46 If we have still not completely understood the factors that make an animal susceptible to stress, how can one trust drugs based on research using alternate model systems to treat cyberbullying victims?
Without animal research, patients with peptic ulcers will continue to receive treatment with antacids and proton‐pump blockers. 47 Despite the ridicule of the scientific community, the unwavering persistence and work of Australian researchers ‐ Barry Marshall and Robin Warren challenged decades of medical dogma and proved that bacterium Helicobacter pylori was the causative agent of peptic ulcers using animal models. 48 Convincing the medical community to prescribe antibiotics capable of eliminating the bacterium still took time. Helicobacter pylori is one of the bacteria in uncooked vegetables, which are quite common in street foods. 49 If atherosclerosis, mental health issues, and peptic ulcer are considered civilizational diseases, we still do not know what other civilizational diseases humans are yet to face and which alternate model systems will be sufficient and reliable enough to understand them and to identify safe and affordable drugs in a timely manner.
4. LIMITATIONS OF ANIMAL RESEARCH
To some extent, animal research has its limitations. For instance, the genetic background of mice can influence whether morphine is addictive or not. 50 Similarly, the rat park experiments challenged conventional beliefs on animal‐based drug addiction studies. 51 , 52 Fialuridine, a drug considered safe through both cell culture studies and animal studies, led to the death of 5 of 15 patients in clinical trials due to liver injury. 53 To a certain extent, the researchers in these studies and their data interpretations are like the parable of the blind men and an elephant. According to their data, they were right. But they lacked complete truth at the beginning of their research, which is how science evolves. But that doesn't mean we should stop animal research, we actually need to understand why that particular model and questions asked were not sufficient. We need to ask the bigger questions about the origin of diseases and if the model we use can allow to address such questions with minimal limitations. We need to ask how to address those limitations that will render our answers closer to the true interpretation of the elephant. By asking such questions, as in the history of science, we may have the opportunity to make gigantic leap, at least in our imaginations if not in practice. It is usually imagination, followed by persistent and well‐controlled scientific experiments using the appropriate model organisms, that will change scientific dogmas and help find new drugs that can improve the lives of millions of people.
5. LIMITATIONS OF ANIMAL MODELS USED BY PHARMACEUTICAL COMPANIES
Although animal‐based studies are important, the craze of pharmaceutical companies to use imperfect animal models for drug discovery should also not be left unrestricted. The use of personalized mice models of cancer in hope of finding drugs to delay or cure cancer in certain group of patients is also an injustice to the animals. 54 , 55 , 56 , 57 Typically, these animals are immunocompromised to accept human cancer cells, and this immune makeup does not accurately represent the immune response in humans or the tumor microenvironment. 58 Despite 20 years of drug screening using animal research for Alzheimer's disease, an affordable cure has not yet been found. 59 , 60 Therefore, despite awareness of the limitations of these animal models, the reasons why pharmaceutical companies continue to use such models for drug screening need to be reinvestigated. 61
By switching to non‐animal model systems, we are not solving the problem; we are only diverting our attention. Instead of increasing the cooperation among blind men and/or bringing more blind men who can interpret the elephant differently from others, the change in policies seems to indicate that we put our trust in a handful of blind men, who believe and are able to lobby that their interpretation of the elephant is the only correct interpretation and that interpretation is sufficient to make safe drugs and to treat human diseases. If we are led to beleive that with artificial intelligence‐based tools, we are ready to tackle all the human diseases, one of the first AI‐designed drug EXS‐21546 recently failed in clinical trials. 122 , 123 Whether it failed because of the tool or the incomplete data that was used to built the tool is still unclear. The identity of the data used to model AI tools is derived solely from the knowledge of basic research, which is also dependent on advancements in technology. With such tools, we are merely combining and extrapolating the interpretations of the blind men. However, without seeing the elephant, these interpretations will probably never be perfect. Moreover, majority of the western pharmaceutical companies largely ignore the knowledge from indigenous medicines that were in practical application even before the first patented drug. 125 , 126 Only 4 years ago, serotonin, a neurotransmitter ‐ well known for its role in depression, was identified to be a new player in epigenetics. 124 It is still unclear what are the implications of this finding on the 80+ years of research on serotonin. 127 , 128
6. IMPLICATIONS OF ANIMAL MODEL ALTERNATIVES FOR DEVELOPING NATIONS
In 2023, we cannot imagine a world without safe transportation, as it promotes the socioeconomic aspects of every nation. Similarly, we cannot imagine a world without safe drugs or novel vaccines to treat and prevent diseases. A global pandemic that disrupts normal human activities can severely impact the socioeconomic prospects and growth of nations. 62 Developing countries are often the hardest hit, with populations largely dependent on daily income and cash transactions. 63 , 64
More than a century ago, the first animal research–based drug discovery was carried out by Ehrlich and Hata to treat syphilis, a sexually transmitted disease. 65 If syphilis were a new disease in 2023, it is true that with the technologies available now, diagnosticians and microbiologists can quickly identify the bacterium (Treponema pallidum) and determine the antibiotic sensitivity for individual patients, recommending the appropriate antibiotic treatment. Despite the challenges in identifying drugs against antibiotic‐resistant bacteria, millions of people in developing countries have limited access to advanced diagnostic facilities and physicians due to their reliance on daily wages. 66 , 67 , 68 Let us imagine a hypothetical situation where all these people will have magical access to such facilities and, unsurprisingly, we encounter antibiotic‐resistant syphilis‐causing bacteria. As we have seen recently with the COVID‐19 pandemic, typically, researchers and companies in developed countries with extensive resources, along with access to advanced computational tools, techniques, and non‐animal‐based automated drug screening technologies, have the capability to investigate the antibiotic resistance mechanisms and provide a list of potential hits that can be tested for toxicity in non‐animal models. 69 , 70 , 71 , 72 , 73 However, the risk will still be present in the clinical trial stage.
In the case of either genocides or drug safety, we must remember the lessons learned from the past. 74 , 75 One example of drugs that did not undergo animal testing was the drug thalidomide. Pregnant mothers who consumed it gave birth to children with birth defects. With several thousands of such children, the thalidomide scandal is still one of the “biggest man‐made medical disasters”. 76 , 77 It was only after the scandal that thalidomide was identified as a teratogenic agent though animal research. 78 , 79 , 80 Why was thalidomide, a drug consumed in almost 50 countries, not approved by the U.S. FDA at that time? It was only with the sheer determination and resistance of Frances Oldham Kelsey, a reviewer for FDA, that the thalidomide disaster left the United States untouched. She was determined in her requests for scientifically reliable evidence and did not succumb to pressure. 81
Now, if animals are not used for drug safety studies, how should researchers obtain scientific evidence on future thalidomide‐like drugs? Although alternative models, such as in silico toxicity predictions, cell cultures, organoids, and organ‐on‐chips, are available for drug safety studies, these systems are not perfect. 82 , 83 , 84 If these alternate models were already flawless, then why do we still have the antibiotic crisis or a lack of affordable drugs for Alzheimer's disease? 59 , 85 , 86 , 87 , 88 With such imperfect systems, untested drugs identified using them will pose a risk to patients, with pregnant patients being particularly vulnerable. 89 , 90 Even if a child is born with defects, in contrast to countries where social healthcare system and infrastructure are designed to support people with disabilities, the children most affected will be those in countries lacking such resources. 91 , 92 , 93
Accessible infrastructure in developing countries for a person receiving an average national salary will be a major barrier to the proper upbringing of the affected child. Moreover, in such countries, the average salary is a paradoxical number and does not reflect the harsh reality of poverty. 94 , 95 We have already required the assistance of billionaire philanthropists and nongovernmental organization–based activists to ensure high vaccination rates for children and access to education in developing countries. Considering this reality for vaccination and education, it is not difficult to imagine the challenges of providing accessible social support for affected children. Therefore, in the absence of a system in place to address the consequences and provide support to affected families and children, drug approval agencies must require animal‐based drug safety tests prior to clinical trials in humans.
Another issue is the current cost of patented organ‐on‐chips; it is unclear how many countries can afford these expensive chips for drug screening. 96 If mammalian models are completely removed in drug safety studies, then, at the very least, testing in vertebrate model organisms like zebrafish larvae should be mandated. Such model organisms allow for drug safety tests at an organism level and are potentially more relevant and less resource consuming. 97 , 98 The maintenance of zebrafish for drug screening is relatively more cost‐effective than the use of expensive fetal bovine serum required for cell culture–based alternative models, and which is commonly extracted via cardiac puncture from bovine fetuses performed without anesthesia. 99 , 100 , 101 , 102 Therefore, in the process of replacing animals for drug safety, we may inadvertently increase the demand for cardiac punctures on bovine fetuses to extract fetal bovine serum.
Finally, it is also worthy to point out the struggles for ensuring access to antiretroviral therapies to patients. The lobbying power of pharmaceutical companies in protecting patents prevented millions of patients from accessing antiretroviral therapies. The altruistic proposal from the Indian pharmaceutical giant Cipla, bearing the motto “No one shall be denied,” and its offer of antiretroviral treatment for less than $1 per patient per day, compared to $33 per patient per day in developed countries, was largely ignored. It was only after the Anthrax scare in the United States that policies were relaxed to allow access to antiretroviral therapies. 103 , 104 The benefit of the existing system of intellectual property rights for patients in developing countries remains unclear, particularly when the establishment of alternative strategies, such as patent pooling, proves to be a challenging process. 105 , 106
7. OTHER LIMITATIONS OF CELL CULTURE–BASED ALTERNATIVES
There are other limitations in using cell culture–based systems. Apart from our genetic makeup, our gut microbiome plays an important role in our health. 107 , 108 Only with recent advances in sequencing technologies have we discovered the role of the microbiome in proper animal development, behavior, and drug resistance. 107 , 108 It is still unclear what the mechanisms are by which the gut microbiome can translocate to distant tissues and the implications of drug discovery. 109 It is still unclear whether, for effective disease treatment, we need to target only the affected tissues or also the local tissue microbiome. 109 , 110 And the effect of host genetics on the gut microbiome has been addressed only recently. 111 With respect to drug safety, studies using mouse models have revealed that interpersonal variation in drug toxicity can be due to the gut microbiome makeup. 112 In cell culture–based drug screening, the use of antibiotics and antimycotics in such systems often leads to drug screens being performed under conditions that ignore the toxic effects of metabolic by‐products of the gut microbiome. 113 Therefore, using such systems, our search for drugs will be like the blind men’s interpretation of the elephant. Only that, with such incomplete interpretation, we will be risking the health and lives of patients, and the future of our students who want to ask big questions in science.
8. ENDING ARGUMENTS
Drugs are a curse and a boon. For instance, without antimalarial drugs, African colonization and enslavement would have probably been limited. 114 From the perspective of the African population at that time, antimalarial drugs could have been seen as a curse. However, the same drug is now a boon for them, allowing them to move beyond malaria and address other challenges. 115 , 116 Rather than progressing to address other civilizational challenges, 117 without animal models in research and drug safety studies, we are forced to abandon our preexisting solutions and be stuck with facing challenges of the past.
With all the scientific literature cited and arguments listed in this article, in my personal view, we are not ready to replace animals for drug safety studies or basic research at least for processes that are also regulated by physiology, gasotransmitter‐mediated endocrine (gasocrine) signaling, or related to addressing other fundamental questions. Animal research must be made a mandatory requirement to validate and reproduce the findings in other non‐animal‐based systems before clinical trials. Instead of barriers against basic research using animal models, we require more support for basic research. This will not only help reveal the mysteries of nature but also help train our students to become the next generation of scientists who will be addressing the big questions. 118 , 119 , 120 , 121 If animals are completely replaced with cell cultures and other systems, our students might find themselves in a similar scenario of blind men and an elephant (Figure 1).
FIGURE 1.
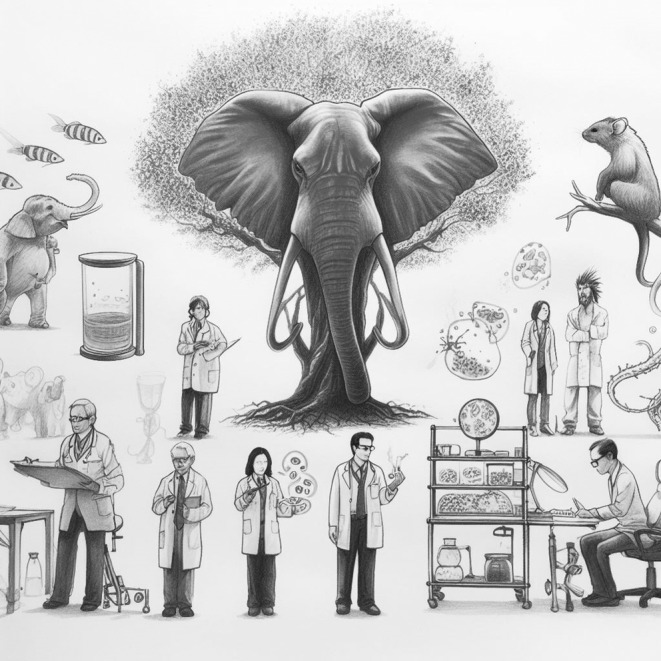
Need for animal‐based research : If animal research is completely replaced with cell cultures‐ and other alternative model systems‐based research, our students may not be able to understand the mysteries of nature (such as the gasocrine signaling), 129 ontogeny of diseases and may be probably uncapable of developing safe and affordable therapeutics.
AUTHOR CONTRIBUTIONS
Savani Anbalagan: conceptualization, writing of the original draft, and review and editing.
CONFLICT OF INTEREST STATEMENT
Savani Anbalagan is an assistant professor in Adam Mickiewicz University, Poznań, Poland. He is the principal investigator for two zebrafish‐based research grants awarded by the National Science Centre, Poland.
ACKNOWLEDGMENTS
I thank Iwona Kanonik‐Jędrzejak and Arleta Kucz for technical and administrative support. I thank IBMIB for the unconditional support. I thank my funding organization National Science Centre for the grants (SONATA‐BIS 2020/38/E/NZ3/00090 and SONATA 2021/43/D/NZ3/01798). The funding agency was not involved in the design of this manuscript. I thank Microsoft Image creator tool team and DALL.E 3 team, whose tool allowed the creation of Figure 1.
Anbalagan S. “Blind men and an elephant”: The need for animals in research, drug safety studies, and understanding civilizational diseases. Anim Models Exp Med. 2023;6:627‐633. doi: 10.1002/ame2.12364
REFERENCES
- 1. The Blind Men and the Elephant. 1992. (Scholastic Incorporated).
- 2. Encyclopedia of Perception. SAGE Publications Inc; 2023. https://us.sagepub.com/en‐us/nam/encyclopedia‐of‐perception/book229708 [Google Scholar]
- 3. Harari YN. Sapiens: A Brief History of Humankind. Harper Collins; 2015. [Google Scholar]
- 4. Merchant C, and Merchant C. The Origin of Disease: the War Within. AuthorHouse; 2018. [Google Scholar]
- 5. Rees AR. A New History of Vaccines for Infectious Diseases: Immunization—Chance and Necessity. Academic Press; 2022. [Google Scholar]
- 6. Honigsbaum M. The Fever Trail: In Search of the Cure for Malaria. Picador; 2003. [Google Scholar]
- 7. Rocco F. The Miraculous Fever‐Tree: Malaria and the Quest for a Cure that Changed the World. HarperCollins; 2003. [Google Scholar]
- 8. Zhang W, Shao Y, Li D, et al. Tu Youyou's Journey in the Search for Artemisini. World Scientific; 2018. [Google Scholar]
- 9. Packard RM. The origins of antimalarial‐drug resistance. N Engl J Med. 2014;371:397‐399. doi: 10.1056/NEJMp1403340 [DOI] [PubMed] [Google Scholar]
- 10. Tyagi BK. Dr Ronald Ross Mosquito, Malaria, India and the Nobel Prize. Scientific Publishers; 2020. [Google Scholar]
- 11. Baird JK. Effectiveness of antimalarial drugs. N Engl J Med. 2005;352:1565‐1577. doi: 10.1056/NEJMra043207 [DOI] [PubMed] [Google Scholar]
- 12. Alonso PL, O'Brien KL. A malaria vaccine for Africa—an important step in a century‐long quest. N Engl J Med. 2022;386:1005‐1007. doi: 10.1056/NEJMp2116591 [DOI] [PubMed] [Google Scholar]
- 13. Hazekamp A. Motion for a resolution on a coordinated Union‐level Action Plan to facilitate the transition to innovation without the use of animals in research, regulatory testing and education | B9‐0427/2021 | European Parliament. https://www.europarl.europa.eu/doceo/document/B‐9‐2021‐0427_EN.html
- 14. FDA no longer needs to require animal tests before human drug trials. https://www.science.org/content/article/fda‐no‐longer‐needs‐require‐animal‐tests‐human‐drug‐trials
- 15. Sen Paul R. [R‐K. (2022). S.5002—117th Congress (2021‐2022): FDA Modernization Act 2.0. http://www.congress.gov/bill/117th‐congress/senate‐bill/5002
- 16. India takes first step to remove animals from drug‐testing process. 2023. The Hindu.
- 17. Han JJ. FDA modernization act 2.0 allows for alternatives to animal testing. Artif Organs. 2023;47:449‐450. doi: 10.1111/aor.14503 [DOI] [PubMed] [Google Scholar]
- 18. HSI/Korea Celebrates New Bill to Promote Non‐Animal Testing in South Korea. Humane Society International; 2023. https://www.hsi.org/news‐resources/hsi‐korea‐celebrates‐new‐bill‐to‐promote‐non‐animal‐testing‐in‐south‐korea/ [Google Scholar]
- 19. Calne DB. Drugs for the Treatment of Parkinson's Disease. Springer Science & Business Media; 2012. [Google Scholar]
- 20. Abbott A. Levodopa: the story so far. Nature. 2010;466:S6‐S7. doi: 10.1038/466S6a [DOI] [PubMed] [Google Scholar]
- 21. Ovallath S, Sulthana B. Levodopa: history and therapeutic applications. Ann Indian Acad Neurol. 2017;20:185‐189. doi: 10.4103/aian.AIAN_241_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. den Hertog CE, de Groot AN, van Dongen PW. History and use of oxytocics. Eur J Obstet Gynecol Reprod Biol. 2001;94:8‐12. doi: 10.1016/s0301-2115(00)00311-0 [DOI] [PubMed] [Google Scholar]
- 23. Leng G, Pineda R, Sabatier N, Ludwig M. 60 years of neuroendocrinology: the posterior pituitary, from Geoffrey Harris to our present understanding. J Endocrinol. 2015;226:T173‐T185. doi: 10.1530/JOE-15-0087 [DOI] [PubMed] [Google Scholar]
- 24. Page K, McCool WF, Guidera M. Examination of the pharmacology of oxytocin and clinical guidelines for use in labor. J Midwifery Womens Health. 2017;62:425‐433. doi: 10.1111/jmwh.12610 [DOI] [PubMed] [Google Scholar]
- 25. Bliss M. The Discovery of Insulin. University of Toronto Press; 2017. [Google Scholar]
- 26. Bankston J. Frederick Banting and the Discovery of Insulin. Mitchell Lane; 2002. [Google Scholar]
- 27. Draaisma D. Lithium: the gripping history of a psychiatric success story. Nature. 2019;572:584‐585. doi: 10.1038/d41586-019-02480-0 [DOI] [Google Scholar]
- 28. Lewin R. Trail of ironies to Parkinson's disease. Science. 1984;224:1083‐1085. doi: 10.1126/science.6426059 [DOI] [PubMed] [Google Scholar]
- 29. Lewin R. Parkinson's disease: an environmental cause? Science. 1985;229:257‐258. doi: 10.1126/science.3925554 [DOI] [PubMed] [Google Scholar]
- 30. Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic parkinsonism in humans due to a product of meperidine‐analog synthesis. Science. 1983;219:979‐980. doi: 10.1126/science.6823561 [DOI] [PubMed] [Google Scholar]
- 31. Javitch JA, D'Amato RJ, Strittmatter SM, Snyder SH. Parkinsonism‐inducing neurotoxin, N‐methyl‐4‐phenyl‐1,2,3,6 ‐tetrahydropyridine: uptake of the metabolite N‐methyl‐4‐phenylpyridine by dopamine neurons explains selective toxicity. Proc Natl Acad Sci USA. 1985;82:2173‐2177. doi: 10.1073/pnas.82.7.2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bureau M, Genton P, Dravet C, et al. Epileptic Syndromes in Infancy, Childhood and Adolescence. 6th ed. John Libbey Eurotext; 2019. [Google Scholar]
- 33. Griffin A, Hamling KR, Knupp K, Hong S, Lee LP, Baraban SC. Clemizole and modulators of serotonin signalling suppress seizures in Dravet syndrome. Brain. 2017;140:669‐683. doi: 10.1093/brain/aww342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sharretts J, Galescu O, Gomatam S, Andraca‐Carrera E, Hampp C, Yanoff L. Cancer risk associated with lorcaserin—the FDA's review of the CAMELLIA‐TIMI 61 trial. N Engl J Med. 2020;383:1000‐1002. doi: 10.1056/NEJMp2003873 [DOI] [PubMed] [Google Scholar]
- 35. Stein N. Public Health Nutrition. Jones & Bartlett Publishers; 2014. [Google Scholar]
- 36. Deming QB, Mosbach EH, Bevans M, et al. Blood pressure, cholesterol content of serum and tissues, and atherogenesis in the rat. J Exp Med. 1958;107:581‐598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Preclinical animal studies in ischemic stroke: Challenges and some solutions. doi: 10.1002/ame2.12166 [DOI] [PMC free article] [PubMed]
- 38. Cobb EP. Workplace Bullying and Harassment: New Developments in International Law. Taylor & Francis; 2017. [Google Scholar]
- 39. Cowie H, Myers C‐A. School Bullying and Mental Health: Risks, Intervention and Prevention. Routledge; 2017. [Google Scholar]
- 40. Westhues K. The Envy of Excellence: Administrative Mobbing of High‐Achieving Professors. Tribunal for Academic Justice; 2005. [Google Scholar]
- 41. Gennaro S, Miller B. Young People and Social Media: Contemporary Children's Digital Culture. Vernon Press; 2021. [Google Scholar]
- 42. Grothaus M. Trust No One: Inside the World of Deepfakes. Hachette UK; 2021. [Google Scholar]
- 43. Yuval Noah Harari argues that AI has hacked the operating system of human civilisation. The Economist.
- 44. Jacobs TA. Cyberbullying Law. American Bar Association; 2020. [Google Scholar]
- 45. Leslie RS. Handbook of Research on Cyberbullying and Online Harassment in the Workplace. IGI Global; 2020. [Google Scholar]
- 46. Hoffman KL. Modeling Neuropsychiatric Disorders in Laboratory Animals. Woodhead Publishing; 2015. [Google Scholar]
- 47. Thompson WG. The Ulcer Story. Da Capo Press; 1996. [Google Scholar]
- 48. Marshall B. Helicobacter Pioneers: Firsthand Accounts from the Scientists Who Discovered Helicobacters 1892–1982. Wiley; 2002. [Google Scholar]
- 49. Monno R, De Laurentiis V, Trerotoli P, Roselli AM, Ierardi E, Portincasa P. Helicobacter pylori infection: association with dietary habits and socioeconomic conditions. Clin Res Hepatol Gastroenterol. 2019;43:603‐607. doi: 10.1016/j.clinre.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 50. Elmer GI, Pieper JO, Hamilton LR, Wise RA. Qualitative differences between C57BL/6J and DBA/2J mice in morphine potentiation of brain stimulation reward and intravenous self‐administration. Psychopharmacology. 2010;208:309‐321. doi: 10.1007/s00213-009-1732-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gage SH, Sumnall HR. Rat Park: how a rat paradise changed the narrative of addiction. Addiction. 2019;114:917‐922. doi: 10.1111/add.14481 [DOI] [PubMed] [Google Scholar]
- 52. Hadaway PF, Alexander BK, Coambs RB, Beyerstein B. The effect of housing and gender on preference for morphine‐sucrose solutions in rats. Psychopharmacology. 1979;66:87‐91. doi: 10.1007/BF00431995 [DOI] [PubMed] [Google Scholar]
- 53. Institute of Medicine (US) Committee to Review the Fialuridine (FIAU/FIAC) Clinical Trials . In: Manning FJ, Swartz M, eds. Review of the Fialuridine (FIAU) Clinical Trials. National Academies Press (US); 1995. [PubMed] [Google Scholar]
- 54. Hoffman RM. Patient‐Derived Mouse Models of Cancer: Patient‐Derived Orthotopic Xenografts (PDOX). Springer; 2017. [Google Scholar]
- 55. Uthamanthil R, Tinkey P, de Stanchina E. Patient Derived Tumor Xenograft Models: Promise, Potential and Practice. Academic Press; 2016. [Google Scholar]
- 56. Ice RJ, Chen M, Sidorov M, et al. Drug responses are conserved across patient‐derived xenograft models of melanoma leading to identification of novel drug combination therapies. Br J Cancer. 2020;122:648‐657. doi: 10.1038/s41416-019-0696-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tentler JJ, Tan AC, Weekes CD, et al. Patient‐derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012;9:338‐350. doi: 10.1038/nrclinonc.2012.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Giacobbe A, Abate‐Shen C. Modeling metastasis in mice: a closer look. Trends Cancer. 2021;7:916‐929. doi: 10.1016/j.trecan.2021.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Panza F, Lozupone M, Logroscino G, Imbimbo BP. A critical appraisal of amyloid‐β‐targeting therapies for Alzheimer disease. Nat Rev Neurol. 2019;15:73‐88. doi: 10.1038/s41582-018-0116-6 [DOI] [PubMed] [Google Scholar]
- 60. Jönsson L, Wimo A, Handels R, et al. The affordability of lecanemab, an amyloid‐targeting therapy for Alzheimer's disease: an EADC‐EC viewpoint. Lancet Reg Health Eur. 2023;29:100657. doi: 10.1016/j.lanepe.2023.100657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mayer B, Tuckermann J, Muche R. Could a phase model help to improve translational animal research? Animal Model Exp Med. 2022;5(6):550‐556. doi: 10.1002/ame2.12284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Susskind D, Vines D. The economics of the COVID‐19 pandemic: an assessment. Oxf Rev Econ Policy. 2020;36:S1‐S13. doi: 10.1093/oxrep/graa036 [DOI] [Google Scholar]
- 63. Karl P, Jenny S, James T. The Short‐Run Economic Costs of COVID‐19 in Developing Countries in 2020: A Synthesis of Results from a Multi‐Country Modeling Exercise. Intl Food Policy Res Inst; 2021. [Google Scholar]
- 64. Varshney D, Kumar A, Mishra AK, Rashid S, Joshi PK. India's COVID‐19 social assistance package and its impact on the agriculture sector. Agr Syst. 2021;189:103049. doi: 10.1016/j.agsy.2021.103049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ehrlich P, Hata S. Die Experimentelle Chemotherapie der Spirillosen. Springer; 1910. doi: 10.1007/978-3-642-64926-4 [DOI] [Google Scholar]
- 66. Peters DH, Garg A, Bloom G, Walker DG, Brieger WR, Rahman MH. Poverty and access to health care in developing countries. Ann N Y Acad Sci. 2008;1136:161‐171. doi: 10.1196/annals.1425.011 [DOI] [PubMed] [Google Scholar]
- 67. Brown RE. Medical problems of the developing countries. Science. 1966;153:271‐275. doi: 10.1126/science.153.3733.271 [DOI] [PubMed] [Google Scholar]
- 68. Low quality healthcare is increasing the burden of illness and health costs globally. https://www.who.int/news/item/05‐07‐2018‐low‐quality‐healthcare‐is‐increasing‐the‐burden‐of‐illness‐and‐health‐costs‐globally
- 69. Liu G, Catacutan DB, Rathod K, et al. Deep learning‐guided discovery of an antibiotic targeting Acinetobacter baumannii . Nat Chem Biol. 2023;1–9:1342‐1350. doi: 10.1038/s41589-023-01349-8 [DOI] [PubMed] [Google Scholar]
- 70. Callaway E, Ledford H, Viglione G, Watson T, Witze A. COVID and 2020: an extraordinary year for science. Nature. 2020;588:550‐552. doi: 10.1038/d41586-020-03437-4 [DOI] [PubMed] [Google Scholar]
- 71. Wadman M. The long shot. Science. 2020;370:649‐653. doi: 10.1126/science.370.6517.649 [DOI] [PubMed] [Google Scholar]
- 72. Baggen J, Vanstreels E, Jansen S, Daelemans D. Cellular host factors for SARS‐CoV‐2 infection. Nat Microbiol. 2021;6:1219‐1232. doi: 10.1038/s41564-021-00958-0 [DOI] [PubMed] [Google Scholar]
- 73. Schultze JL, Aschenbrenner AC. COVID‐19 and the human innate immune system. Cell. 2021;184:1671‐1692. doi: 10.1016/j.cell.2021.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Totten S. Teaching and Learning about Genocide and Crimes Against Humanity: Fundamental Issues and Pedagogical Approaches. IAP; 2019. [Google Scholar]
- 75. Naimark NM. Genocide: A World History. Oxford University Press; 2017. [Google Scholar]
- 76. Johnson M. Thalidomide Catastrophe (Onwards and Upwards). 2018.
- 77. Magazanik M. Silent Shock: the Men behind the Thalidomide Scandal and an Australian Family's Long Road to Justice. Text Publishing Company; 2015. [Google Scholar]
- 78. Brent RL. Drug testing in animals for teratogenic effects. Thalidomide in the pregnant rat. J Pediatr. 1964;64:762‐770. doi: 10.1016/s0022-3476(64)80626-0 [DOI] [PubMed] [Google Scholar]
- 79. Loosli B. Induction of fetal malformations in rabbits with thalidomide. Pathol Microbiol (Basel). 1964;27:1003‐1011. [PubMed] [Google Scholar]
- 80. Staples RE, Holtkamp DE. Effects of parental thalidomide treatment on gestation and fetal development. Exp Mol Pathol Suppl. 1963;2:81‐106. [PubMed] [Google Scholar]
- 81. Watts G. Frances Oldham Kelsey. Lancet. 2015;386:1334. doi: 10.1016/S0140-6736(15)00339-6 [DOI] [PubMed] [Google Scholar]
- 82. Benfenati E. In Silico Methods for Predicting Drug Toxicity. Springer; 2022. [Google Scholar]
- 83. Tang X‐Y, Wu S, Wang D, et al. Human organoids in basic research and clinical applications. Signal Transduct Target Ther. 2022;7:168. doi: 10.1038/s41392-022-01024-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ma C, Peng Y, Li H, Chen W. Organ‐on‐a‐chip: a new paradigm for drug development. Trends Pharmacol Sci. 2021;42:119‐133. doi: 10.1016/j.tips.2020.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. OECD, and World Health Organization . Challenges to Tackling Antimicrobial Resistance Economic and Policy Responses: Economic and Policy Responses. OECD Publishing; 2020. [Google Scholar]
- 86. Sagar S, Kaistha S, Das AJ, Kumar R. Antibiotic Resistant Bacteria: A Challenge to Modern Medicine. Springer Nature; 2019. [Google Scholar]
- 87. Watkins RR, Bonomo RA. The Ongoing Challenge of Antimicrobial Resistance, an Issue of Infectious Disease Clinics of North America. EBook Elsevier Health Sciences; 2020. [Google Scholar]
- 88. Stadler M, Dersch P. How to Overcome the Antibiotic Crisis: Facts, Challenges, Technologies and Future Perspectives. Springer; 2016. [Google Scholar]
- 89. Dathe K, Schaefer C. Drug safety in pregnancy: the German Embryotox institute. Eur J Clin Pharmacol. 2018;74:171‐179. doi: 10.1007/s00228-017-2351-y [DOI] [PubMed] [Google Scholar]
- 90. Schaefer C. Drug safety in pregnancy‐a particular challenge. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2018;61:1129‐1138. doi: 10.1007/s00103-018-2798-8 [DOI] [PubMed] [Google Scholar]
- 91. Barrientos A. Social Assistance in Developing Countries. Cambridge University Press; 2013. [Google Scholar]
- 92. Barnard KE, Brandt PA, Raff BS, Carroll P. Social Support and Families of Vulnerable Infants. March of Dimes Birth Defects Foundation; 1984. [Google Scholar]
- 93. Kar A. Birth Defects in India: Epidemiology and Public Health Implications. Springer Nature; 2021. [Google Scholar]
- 94. Gordon D. Child Poverty in the Developing World. Policy Press; 2003. [Google Scholar]
- 95. Brooks G. Poverty and Our Future. The Rosen Publishing Group, Inc; 2021. [Google Scholar]
- 96. Towards a body‐on‐a‐chip. The Economist.
- 97. MacRae CA, Peterson RT. Zebrafish as tools for drug discovery. Nat Rev Drug Discov. 2015;14:721‐731. doi: 10.1038/nrd4627 [DOI] [PubMed] [Google Scholar]
- 98. Patton EE, Zon LI, Langenau DM. Zebrafish disease models in drug discovery: from preclinical modelling to clinical trials. Nat Rev Drug Discov. 2021;20:611‐628. doi: 10.1038/s41573-021-00210-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kim S, Carlson R, Zafreen L, Rajpurohit SK, Jagadeeswaran P. Modular, easy‐to‐assemble, low‐cost zebrafish facility. Zebrafish. 2009;6:269‐274. doi: 10.1089/zeb.2009.0587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Tangara A, Paresys G, Bouallague F, et al. An open‐source and low‐cost feeding system for zebrafish facilities. bioRxiv 2019. doi: 10.1101/558205 [DOI]
- 101. Paige C, Hill B, Canterbury J, Sweitzer S, Romero‐Sandoval EA. Construction of an affordable and easy‐to‐build zebrafish facility. J Vis Exp. 2014;51989:e51989. doi: 10.3791/51989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Jochems CEA, van der Valk JBF, Stafleu FR, Baumans V. The use of fetal bovine serum: ethical or scientific problem? Altern Lab Anim. 2002;30:219‐227. doi: 10.1177/026119290203000208 [DOI] [PubMed] [Google Scholar]
- 103. Bower JL, Leonard HB, Paine LS. Capitalism at Risk: Rethinking the Role of Business. Harvard Business Press; 2011. [Google Scholar]
- 104. McBride D. Bioterrorism: the History of a Crisis in American Society: 2 Volume Set. Taylor & Francis; 2022. [Google Scholar]
- 105. Satyanarayana K, Srivastava S. Patent pooling for promoting access to antiretroviral drugs (ARVs)—a strategic option for India. Open AIDS J. 2010;4:41‐53. doi: 10.2174/1874613601004020041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. WHO initiative signs new licensing agreements on COVID‐19 technologies. https://www.who.int/news/item/29‐08‐2023‐who‐initiative‐signs‐new‐licensing‐agreements‐on‐covid‐19‐technologies
- 107. Haller D. The Gut Microbiome in Health and Disease. Springer; 2018. [Google Scholar]
- 108. Sanna S, Kurilshikov A, van der Graaf A, Fu J, Zhernakova A. Challenges and future directions for studying effects of host genetics on the gut microbiome. Nat Genet. 2022;54:100‐106. doi: 10.1038/s41588-021-00983-z [DOI] [PubMed] [Google Scholar]
- 109. Thapa M, Kumari A, Chin C‐Y, et al. Translocation of gut commensal bacteria to the brain. bioRxiv. 2023. doi: 10.1101/2023.08.30.555630 [DOI]
- 110. Riquelme E, Zhang Y, Zhang L, et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell. 2019;178:795‐806.e12. doi: 10.1016/j.cell.2019.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Cahana I, Iraqi FA. Impact of host genetics on gut microbiome: take‐home lessons from human and mouse studies. Animal Model Exp Med. 2020;3:229‐236. doi: 10.1002/ame2.12134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zimmermann M, Zimmermann‐Kogadeeva M, Wegmann R, Goodman AL. Separating host and microbiome contributions to drug pharmacokinetics and toxicity. Science. 2019;363:eaat9931. doi: 10.1126/science.aat9931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Freshney RI. Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications. John Wiley & Sons; 2015. [Google Scholar]
- 114. Curtin PD. Disease and Empire: the Health of European Troops in the Conquest of Africa. Cambridge University Press; 1998. [Google Scholar]
- 115. Goujon A, Haller M, Kmet BM. Higher Education in Africa: Challenges for Development, Mobility and Cooperation. Cambridge Scholars Publishing; 2017. [Google Scholar]
- 116. Grah, JPA . The Contemporary Challenges of African Development: The Problematic Influence of Endogenous and Exogenous Factors. AuthorHouse; 2020. [Google Scholar]
- 117. Avery JS. Civilization's Crisis: A Set of Linked Challenges. World Scientific; 2017. [Google Scholar]
- 118. Archer L, DeWitt J. Understanding Young People's Science Aspirations: How Students Form Ideas about ‘Becoming a Scientist’. Routledge; 2016. [Google Scholar]
- 119. Thompson GR. Pioneers of Medicine without A Nobel Prize. World Scientific; 2014. [Google Scholar]
- 120. McConville M. Open questions: microbes, metabolism and host‐pathogen interactions. BMC Biol. 2014;12:18. doi: 10.1186/1741-7007-12-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Saunders TE, Ingham PW. Open questions: how to get developmental biology into shape? BMC Biol. 2019;17:17. doi: 10.1186/s12915-019-0636-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Arnold C. Inside the nascent industry of AI‐designed drugs. Nature Medicine. 2023;29(6):1292‐1295. doi: 10.1038/s41591-023-02361-0 [DOI] [PubMed] [Google Scholar]
- 123. First Wave of AI‐Designed Drugs Faces Major Clinical Setbacks. https://medicaltrend.org/2023/10/21/first‐wave‐of‐ai‐designed‐drugs‐faces‐major‐clinical‐setbacks
- 124. Farrelly LA, Thompson RE, Zhao S, Lepack AE, Lyu Y, Bhanu NV, Zhang B, Loh Y‐HE, Ramakrishnan A, Vadodaria KC, Heard KJ, Erikson G, Nakadai T, Bastle RM, Lukasak BJ, Zebroski H, Alenina N, Bader M, Berton O, … Maze I. Histone serotonylation is a permissive modification that enhances TFIID binding to H3K4me3. Nature. 2019;567(7749):535‐539. doi: 10.1038/s41586-019-1024-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chinese medicine in the treatment of autoimmune hepatitis: Progress and future opportunities. Animal Model Exp Med. 2022;5(2):95‐107. doi: 10.1002/ame2.12201 [DOI] [PMC free article] [PubMed]
- 126.Oldenlandia affinis (R&S) DC. A plant containing uteroactive peptides used in African traditional medicine. J Ethnopharmacol. 2000;70(3):197‐203. doi: 10.1016/s0378-8741(99)00175-0 [DOI] [PubMed]
- 127. Anastas JN, Shi Y. Histone Serotonylation: Can the Brain Have “Happy” Chromatin? Molecular Cell. 2019;74(3):418–420. doi: 10.1016/j.molcel.2019.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Whitaker‐Azmitia P. The Discovery of Serotonin and its Role in Neuroscience. Neuropsychopharmacology. 1999;21(2):2S–8S. doi: 10.1016/s0893-133x(99)00031-7 [DOI] [PubMed] [Google Scholar]
- 129. Anbalagan S. Arguments for Gasocrine Signaling. OSF Preprints. 2023. doi: 10.31219/osf.io/fe85j [DOI] [Google Scholar]


