Highlights
-
•
We present the largest French, multicenter, retrospective study on oligometastatic patients treated with SBRT for an adrenal metastasis.
-
•
A total of 110 patients treated for 121 adrenal lesions were included. Non-small-cell lung cancer was the predominant histologic type (55.4 %). 82 % of patients had at least 2 metastatic sites. The median PTV was 70 cm3 with a median prescription dose of 40 Gy. The mean Biologically Effective Dose (BED)10 dose was 74.2 Gy. Local control at 1 and 2 years was 85.9 % and 72.5 % respectively. The median Overall survival and Progression free survival were 31.6 and 8.5 months respectively. Local control was significantly improved by systemic treatment one month before or after SBRT (p = 0.009) and by a BED10 greater than or equal to 50 Gy (p = 0.003).
-
•
Tolerance was excellent, no grade 3 and 4 toxicities were described due to SBRT.
Keywords: Stereotactic body radiation therapy, Oligometastasis, Adrenal gland, Local control, Survival, Prognostic factors
Abstract
Aim
The adrenal gland is a common site of metastasis with a rate of up to 27% in autopsy series. The incidence of these metastases is increasing due to greater use of Positron Emission Tomography scans and improved overall survival of patients with metastatic cancers. Stereotactic body radiation therapy (SBRT) is a non-invasive treatment option for metastasis. The aim of this study is to assess prognostic factors influencing local control, progression-free and overall survival in oligometastatic patients treated with SBRT for an adrenal metastasis.
Methods
In this multicentric retrospective study, we included patients with adrenal metastases treated with SBRT between 2010 and 2021 in eleven french centers. All primary tumors were included.
Results
A total of 110 patients treated for 121 adrenal lesions were included. Non-small-cell lung cancer was the predominant histologic type (55.4 %). Eighty-two percent of patients had at least 2 metastatic sites. The median Planning Target Volume was 70 cm3 with a median prescription dose of 40 Gray (Gy). The mean Biologically Effective Dose (BED) 10 dose was 74.2 Gy. Local control at 1 and 2 years was 85.9 % and 72.5 % respectively. The median overall survival and progression-free survival were 31.6 and 8.5 months respectively. Local control was significantly improved by systemic treatment one month before or after SBRT (p = 0.009) and by a BED10 greater than or equal to 50 Gy (p = 0.003).
In multivariate analysis, oligometastatic presentation (p = 0.009) and a metachronous metastatic presentation (p = 0.008) were independent factors for progression-free survival.
Tolerance was excellent, no grade 3 and 4 toxicities were described due to SBRT.
Conclusion
Stereotactic radiotherapy of adrenal metastases makes possible a local control of more than 85% at one year and was well tolerated. The factors influencing survival in oligometastatic patients still need to be found in order to better select those who benefit the most from this type of treatment.
Introduction
The theory of the oligometastatic state was proposed in the 1990 s by the radiation oncologists Hellman and Weichselbaum as an intermediate stage in the evolution of tumors, with a metastatic disease limited in number and location, supporting the presumption of temporal evolution [1] where oligometastatic tumors may not have acquired the broad array of genetic changes required to develop widespread metastases [2], [3].
This concept is used to refer to patients with a limited disease burden, based on the absence of polymetastatic disease in the patient’s history, indicating cancer with low metastatic capacity.
This paradigm shift indicates a state of the disease in which a contingent of patients could benefit from local ablative treatment (surgery, radiotherapy, thermal ablation) at the metastatic sites.
The adrenal glands are a common site for metastatic spread from various primary cancer, most commonly from lung, gastric and renal tumors. In autopsy analyses, metastases are found in the adrenal glands in up to 27 % of patients with cancer [4].
The improved survival of patients with metastatic disease leads to a situation in which the oligometastatic presentation is increasingly common. Similarly, the improved quality of imaging and its systematic use in the follow-up of patients may increase the incidence of discovery of adrenal metastases, especially with the use of Positron Emission Tomography (PET) scans.
Laparoscopic adrenalectomy remains the gold standard in this indication, with a local control (LC) rate ranging from 77 to 88 %. Nevertheless, with a postoperative complication rate of 22 %, including 7 % of grade 3 to 5, the surgical approach requires strict patient selection [5].
Technological progress has made it possible to develop SBRT which delivers ablative doses to tumors while sparing surrounding normal tissue. It can be proposed as an alternative to surgical treatment for oligometastases as it is less invasive and thus a viable option for patients who are not eligible for surgery.
The objective of this retrospective multi-institutional French study is to present the outcomes following SBRT for adrenal metastases in the oligometastatic setting. This includes assessing LC, progression-free survival (PFS), overall survival (OS), safety, and identifying clinical factors that impact these outcomes. Additionally, the study aims to refine the patient selection criteria for this treatment approach..
Methods
Patient characteristics
Between January 2010 and September 2021, 110 patients with a total of 121 adrenal lesions were treated (11 patients irradiated for metastases in both adrenal glands) in eleven centers: eight centers from the COLIB network and three university centers in France (Creil, Bordeaux, Reims, Le Mans, Tours, Angers, Nantes, Valenciennes, Lyon, Dijon, Rennes). Standard demographic and clinico-pathologic data were collected for each patient. Patients were included if the radiotherapy was delivered with ablative intent defined as less than 10 fractions and more than four Gy per fraction. Patients were excluded from the analysis if they had prior surgery or radiotherapy on the adrenal glands.
To identify prognostic factors for local response and survival after SBRT, we categorized three groups of patients treated for adrenal metastases:
- Oligorecurrence is defined as a solitary metastatic relapse in the adrenal gland, with controlled primary tumors [6], [7].
- Oligopersistence is defined as patients who continue to exhibit either a partial or no response in their adrenal disease at least three months after receiving systemic treatment [6], [7].
- Oligoprogressive is defined as at least one other metastatic site not being controlled during the adrenal SBRT but with most other metastases responding or stable while on a systemic treatment strategy [6], [7].
A time interval of 6 months was used to describe a metachronous metastasis after the treatment of the primary cancer.
The study complies with the reference methodology adopted by the French Data Protection Authority and patients did not object to the use of their clinical data for the research purposes.
Treatment planning
The treatment techniques varied among different centers in the study. The routine use of intravenous contrast was not common. To account for respiratory motion, 4D- computed tomography (CT) scans were employed whenever feasible. Tumor motion management was performed by various techniques, respiratory gating, spine or tumor tracking and abdominal compression. Treatment planning was carried out using various approaches, including 3D planning, static Intensity-Modulated Radiation Therapy (IMRT), or Volumetric Modulated Arc Therapy (VMAT). Cone beam image guidance was utilized when needed based on the discretion of the treating physician. The specific dose and fractionation scheme used for each patient were determined by the treating physician.
Target volume delineation depended on the imaging modalities used for diagnosis. Whenever feasible, the gross tumor volume (GTV) encompassed the macroscopic tumor observed on FDG-PET-CT and MRI scans. An Internal Target Volume (ITV) was delineated in 70 % of treatment plans, using a series of CT scans acquired throughout the respiratory cycle, from maximum inhalation to maximum exhalation. The planning target volume (PTV) was created by applying a symmetric margin expansion of 3–5 mm.
Total dose and fractionation schemes were at the discretion of the treating physicians and was dependent on the adjacent normal tissue. Dose constraints were based on published guidelines and varied depending on the number of fractions [8].
Outcome evaluation of the Response to SBRT
Local control was defined as the absence of tumor growth in the treated adrenal gland. Response evaluation was most often planned three months after the end of SBRT and repeated every three or four months, usually for the two first years, and consisted of a clinical evaluation and imaging (CT, MRI or PET scan), at the physician’s discretion. Local relapse assessments were conducted centrally by the coordinating center overseeing the study. If imaging reports did not indicate adrenal progression, they were reviewed by an experienced radiation oncologist. In cases of uncertainty, a radiologist reviewed the images. This process was carried out for each surveillance imaging until an adrenal relapse was documented. The assessments for relapse were based on established criteria, namely Response Evaluation Criteria In Solid Tumors (RECIST) [8], the immune RESIST [9], and the PET Response Criteria in Solid Tumors PERCIST [10].
PFS was defined as the time from the first day of the radiotherapy to any in- or out-of-field disease progression or mortality. If the patient was treated for bilateral metastases at separate times, the date of the first day of the first SBRT was considered.
OS was calculated from the end of radiotherapy to either the date of death from any cause or the date of the last follow-up examination. To evaluate the effects of different treatment protocols with various fraction sizes and total doses for the PTV, a BED was used in a linear-quadratic model using an α/β ratio of 10 for the tumor, in accordance with standard practice.
Statistical analysis
Descriptive statistics were used to summarize overall cohort characteristics.
The primary analysis focused on 1- and 2-year LC rates, while secondary analyses included OS and PFS, all calculated using the Kaplan-Meier method. Toxicity data were evaluated and recorded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v4.0.
To identify the prognostic factors associated with the outcomes, univariate and multivariate Cox proportional hazard models were used to assess the potential influence of different parameters on LC, PFS and OS, to estimate the odds ratio (OR), 95 % confidence interval (95 % CI) and p-value. Subgroups were compared using log-rank statistics. Values of p < 0.05 were considered statistically significant.
All statistical analyses were performed using version 9.3 of SAS/STAT software (SAS institute Inc., Cary, NC).
Results
Characteristics of the overall study population
Patients and tumor characteristics
Patient and adrenal metastasis characteristics are summarized in Table 1.
Table 1.
Patient and tumor characteristics.
| Variables | N (%) |
|---|---|
|
Gender Male Female |
70 (63.6)40 (36.4) |
|
ECOG PS 0 1 2 3 |
11 (10.0)90 (81.8)8 (7.3)1 (0.9) |
|
Primary Tumor NSCLC Renal cell carcinoma SCLC Breast Esophagus Hepatocellular carcinoma Others |
67 (55.4)15 (13.6)11 (9.1)6 (5.5)6 (5.5) 4 (3.3)12 (9.9) |
|
Metastasis and primitive tumor Synchronous Metachronous |
68 (61.8)42 (38.2) |
|
Number of organ sites with metastases at the time of SBRT1 (adrenal only) 2–3 4–5 > 5 |
20 (18.2)70 (63.6)18 (16.4)2 (1.8) |
|
Number of treatment lines before SBRT 0 1 2 3 |
7 (6.4)79 (71.8)22 (20.0)2 (1.8) |
|
Metastatic presentation at SBRT Oligopersistence Oligorecurrent Oligoprogressive |
29 (26.4)47 (42.7)34 (30.9) |
|
Primary treated No Yes Unknown |
27 (24.6)81 (73.6)2 (1.8) |
|
Tumor location Right adrenal Left adrenal Bilateral |
50 (45.4)53 (48.2)7 (6.4) |
A total of 121 adrenal metastases were treated, in 110 patients. Eleven patients were treated for bilateral adrenal metastases. Median follow-up was 22.1 months (IC95%: 0.9–118.6). The median age was 66 years (range 42–93). The most frequent primary tumors were non-small-cell lung cancer (NSCLC) in 67 patients (55.4 %), renal cell carcinoma in 15 (13.6 %) and small cell lung cancer (SCLC) in 11 (9.1 %) patients. The primary tumor and adrenal metastasis occurred synchronously in 68 patients (61.8 %).
The median time interval between the diagnosis of the adrenal metastasis and the primary tumor was 11 months. The adrenal metastasis treated was the only metastatic site in 18.2 % of the patients, 63.6 % of patients had an oligometastatic disease with 2 to 3 organs with metastatic disease.
Twenty-nine patients (26.4 %) were treated in a state of oligopersistence, 47 patients (42.7 %) of the patients were classified as having an oligorecurrence tumor state. Thirty-four patients (30.9 %) were categorized as oligoprogressive. At the time of SBRT, the primary tumor had been treated locally (by radiation or surgery) in 73.6 % of patients.
Treatment characteristics
Treatment and dosimetry characteristics are shown in Table 2, Table 3.
Table 2.
Treatment characteristics.
| Variables | N (%) |
|---|---|
|
Diagnosis mode Biopsy CT Scan PET |
4 (3.6)65 (59.1)41 (37.3) |
|
Tumor motion management Respiratory gating Spine tracking Tumor tracking Abdominal compression |
85 (70.2)25 (20.7)8 (6.7)3 (2.4) |
|
Systemic treatment (4 weeks prior to or after SBRT) None Chemotherapy Immunotherapy Other |
41 (37.3)26 (23.6)26 (23.6)17 (15.5) |
|
Dose/number of fractions 45/5 35/5 45/3 36/6 30/5 Other |
14 (11.5)12 (9.9)12 (9.9)11 (9.0)9 (7.4)63 (52) |
|
BED10 ≥ 50 Gy Yes No |
103 (85.2)18 (14.8) |
Table 3.
Dosimetry characteristics.
| Variables | Mean | Median (Min; max) |
|---|---|---|
| Tumor largest diameter (mm) | 31.5 | 28.3 (9.0; 71.0) |
| Number of fractions | 5.5 | 5.0 (3.0; 10.0) |
| Dose per fraction | 7.9 | 7 (3.0; 15.0) |
| GTV (cm3) | 50.8 | 36 (0.5; 183) |
| PTV (cm3) | 65.3 | 70 (4.7; 148.1) |
| Total dose (Gy) | 40 | 40.0 (21.0; 60.0) |
| Maximum dose (Gy) | 47.2 | 46.5 (23.0; 78.0) |
| Mean dose (Gy) | 43.3 | 42.6 (22.5; 68.5) |
| Minimum dose (Gy) | 32.1 | 32.0 (8.5; 51.0) |
| BED10 (Gy) | 74.2 | 72.0 (28.4; 115.5) |
| Isodose line | 91.8 | 95 (70;100) |
Tumor motion management was performed by respiratory gating in 85 of the treatment (71.9 %).
A total of 25 different radiotherapy treatment plans were used for the 121 adrenal metastases. The five most frequently used plans were as follows: 45 Gy in 5 fractions (11.5 %), 35 Gy in 5 fractions (9.9 %), 45 Gy in 3 fractions (9.9 %), 36 Gy in 6 fractions (9.0 %), and 30 Gy in 5 fractions (6.6 %). For detailed information on each of these plans, refer to the supplementary data section. Median tumor size was 28.3 mm, with a median PTV of 70 cm3.
SBRT was delivered with a median radiation dose of 40 Gy (range: 21–60 Gy) and with a median fraction dose of 7.5 Gy (range: 4–15 Gy).
Because there was diversity in the total dose and fraction size, we used the BED for analysis and found that the median BED10 was 72.0 Gy (range: 28.4–115.5 Gy). 85.2 % of treatments were delivered with a BED10 ≥ 50 Gy,
47.1 % received a BED10 of more than 73.2 Gy and 24.7 % a BED10 superior or equal to 100 Gy. The median isodose of prescription was 95 %.
Tumor Response and survival outcomes
Local control
The univariate and multivariate analysis of LC are summarized in Table 4.
Table 4.
Univariate and multivariate analysis for local control.
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| OR (95 % CI) | p-value | OR (95 % CI) | p-value | ||
| Age | ≤65 years >65 years |
10.89 (0.34; 2.33) |
0.82 | - | - |
| Laterality | Right Left |
10.36 (0.13; 1.10) |
0.13 | – | – |
| Systemic treatment, 4 weeks prior to or after SBRT |
No Yes |
1 0.33 (0.11; 0.93) |
0.03 |
1 0.24 (0.08; 0.70) |
0.009 |
| Primary Tumor | Lungs (NSCLC + SCLC) Kidney Liver Breast Esophagus Other |
2.88 (0.91; 9.07)0.17 (0.02; 1.39)2.73 (0.34; 21.92)1.7 (0.33;68.01)1.02 (0.13; 8.01)0.33 (0.04; 2.60) |
0.49 | - - - - - - |
– |
| Metastatic presentation at SBRT |
Oligopersistence Oligorecurrence Oligoprogressive |
11.42 (0.36; 5.49)1.61 (0.38; 6.72) |
0.80 | – - |
– - |
| Number of lines before SBRT | Increase 1 | 1.21 (0.45; 3.26) | 0.71 | – | – |
| PTV, cc | Increase of 10 cc | 0.99 (0.94; 1.05) | 0.91 | – | – |
| Dose, Gy | Increase of 5 Gy | 0.72 (0.52; 1.01) | 0.05 | – | – |
| BED10 ≥ 50 Gy |
>=50 Gy <50 Gy |
1 3.57 (1.26; 10.09) |
0.01 |
1 5.11 (1.73; 15.04) |
0.003 |
| BED10 ≥ 80 Gy | >=80 Gy <80 Gy |
1 2.50 (0.70; 8.89) |
0.15 | – | – |
| BED10 ≥ 100 Gy | >=100 Gy <100 Gy |
1 3.77 (0.49; 28.73) |
0.20 | – | – |
The rates of adrenal metastasis local control at 1 and 2 years were 85.9 % (95 %CI: 75.6 %-92.0 %) and 72.5 % (95 %CI: 56.9 %-83.3 %) with 17 patients (15 %) presenting a local relapse (Fig. 1a). Among the 17 cases of local adrenal metastasis relapse, 14 patients (82.3 %) also exhibited out-field progression, indicating disease advancement beyond the adrenal region.
Fig. 1.
Kaplan-Meier curves showing a) local control, b) progression-free survival and c) overall survival in patients treated with SBRT for adrenal metastases.
In the univariate analysis, systemic treatment (p < 0.03) and a BED10 superior or equal to 50 Gy (p < 0.01) were factors for better LC.
At multivariate analysis, a systemic treatment four weeks prior or after the SBRT (OR 0.24 (95 %CI: 0.08–0.70; p = 0.009) and a BED10 Gy of more than or equal to 50 Gy (OR 5.11 (95 %CI: 1.7–15.0; p = 0.003) remained significant independent factors for LC.
The 1-year local control rate was 91.5 % for patients with a BED10 greater than 50 Gy and 59.7 % for those with a BED10 lower than 50 Gy (Fig. 2).
Fig. 2.
Probability of local control divided into BED10Gy</≥ 50 Gy.
Increasing the cut-off of BED10Gy at 80 and 100 Gy did not affect the impact on local control in our study.
No correlation was found between the total prescribed dose or PTV size. Patients with different oligometastatic setting or different primary tumors had similar LC.
The number of systemic treatment lines before the adrenal SBRT was not a prognostic factor for local failure.
Progression-free survival
The PFS at 1 and 2 years was respectively 35.4 % and 22.3 %. The median time to progression was 8.5 months (95 % CI: 7.0–10.7; Fig. 1b).
In total, 71 patients experienced disease progression. Among these patients, 14 (19.7 %) exhibited both adrenal and out-field progression concurrently. In contrast, only three patients (4.2 %) experienced progression solely within the adrenal gland treated by SBRT.
The metachronous state (p = 0.02) and metastatic presentation at SBRT (p = 0.008) were prognostic factors for better PFS in the univariate analysis (Table 5).
Table 5.
Univariate and multivariate analysis for progression-free survival.
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| OR (95 % CI) | p-value | OR (95 % CI) | p-value | ||
| Age | ≤65 years >65 years |
10.98 (0.61; 1.57) |
0.93 | - | - |
| ECOG PS | < 2 ≥2 |
11.13 (0.46; 2.82) |
0.78 | – | – |
| Number of organ sites with metastases at the time of SBRT | Adrenal only 2–3 ≥ 4 |
11.75 (0.83; 3.72)2.54 (1.07; 6.03) |
0.10 |
- - - |
– |
| Metachronous adrenal metastasis | 6 months | 0.95 (0.91; 0.99) | 0.02 | 0.99 (0.98; 0.99) | 0.009 |
| Primary Tumor | Lungs (NSCLC + SCLC) Kidney Liver Breast Esophagus Other |
10.80 (0.40; 1.61)1.63 (0.50; 5.29)0.88 (0.34; 2.24)0.77 (0.24; 2.49)0.78 (0.36; 1.66) |
0.88 | - - - - - - |
– |
| Metastatic presentation at SBRT |
Oligopersistence Oligorecurrence Oligoprogressive |
1 1.19 (0.63; 2.26) 2.39 (1.25; 4.58) |
0.008 |
1 1.45 (0.75; 2.78) 2.87 (1.47; 5.59) |
0.003 |
| Number of lines before SBRT | 0 1 2 3 |
10.65 (0.20; 2.08)0.58 (0.17; 2.03)0.85 (0.14; 5.14) |
0.83 | - - - - |
– |
| Primary tumor treated | No yes |
10.79 (0.45; 1.38) |
0.40 | – | – |
| Local control of the adrenal metastasis | No Yes |
11.41 (0.78; 2.53) |
0.25 | – |
– |
In the multivariate analysis, the oligometastatic presentation and metachronous state remained significant independent factors for PFS (Table 5).
A longer interval of time between the diagnosis of the primary tumor and the first metastasis was associated with a better PFS (OR 0.99 (95 %CI: 0.98–0.99; p = 0.009).
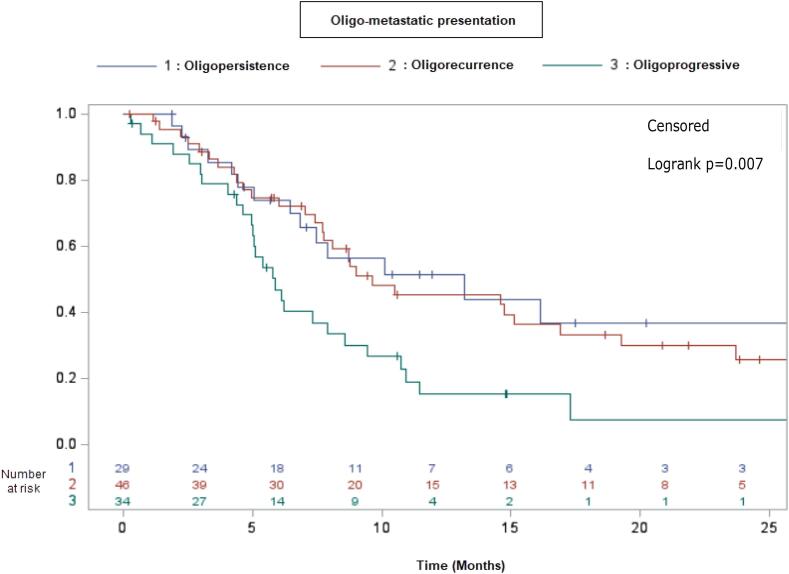
The median progression-free survival within the oligometastatic presentation varied, with a duration of 12.8 months observed in the oligopersistence state, 9.2 months in cases of oligorecurrence, and 5.7 months among those classified as oligoprogressive (Fig. 3).
Fig. 3.
Probability of progression-free survival based on the oligometastatic presentation.
They were no difference between oligopersistence and oligorecurrent adrenal metastasis. The risk of relapse was greater for patients with other non-controlled metastases or primary tumor (OR 2.87 (95 %CI: 1.47; 5.59, p = 0.003).
The local control of the metastasis of the adrenal gland was not a predictive factor for PFS.
The type of primary tumor, Eastern Cooperative Oncology Group (ECOG) Performance Status, the number of metastatic sites were not prognostic factors for relapse.
Overall survival
The median OS time was 31.6 months (95 %CI: 19.8; Not reached; Fig. 1c).
At the time of analysis, 35 of the 110 (31.8 %) patients had died, none of the deaths were related to the SBRT. All patients who died presented progression, and 7 (20 %) presented local relapse.
The OS rates for all 110 patients at 1 and 2 years were 76.0 % and 55.8 % respectively.
In the univariate analysis, the number of metastatic sites at the time of the adrenal SBRT (p = 0.01) and the metastatic presentation during the adrenal SBRT (p = 0.01) were prognostic factors for OS. In the oligometastatic presentation, only oligorecurrent adrenal metastasis was associated with better OS on the univariate analysis (p = 0.03) (Table 6).
Table 6.
Univariate and multivariate analysis for overall survival.
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| OR (95 % CI) | p-value | OR (95 % CI) | p-value | ||
| Age | ≤65 years >65 years |
11.32 (0.67; 2.58) |
0.42 | - | - |
| ECOG PS | < 2 ≥2 |
11.90 (0.67; 5.42) |
0.22 | – | – |
| Number of organ sites with metastases at the time of SBRT | Adrenal only 2–3 ≥ 4 |
11.60 (0.48; 5.39) 4.50 (1.24; 16.35) |
0.01 | 11.50 (0.42; 5.28) 4.20 (1.10; 16.0) |
0.01 |
| Metachronous adrenal metastasis | 6 months |
0.93 (0.87; 1.00) |
0.06 | 0.98 (0.97; 0.99) |
0.03 |
| Primary Tumor | Lungs (NSCLC + SCLC) Kidney Liver Breast Esophagus Other |
10.75 (0.28; 2.05)5.34 (1.53; 18.88)0.88 (0.20; 3.78)2.16 (0.63; 7.35)0.77 (0.22; 2.61) |
0.08 | - - - - - - |
– |
| Metastatic presentation at SBRT |
Oligopersistence Oligorecurrence Oligoprogressive |
1 1.01(0.37; 2.7) 2.43 (0.96; 6.14) |
0.03 | 11.13 (0.40; 3.21)2.39 (0.92; 6.21) |
0.07 |
| Number of lines before SBRT | 0 1 2 3 |
10.49 (0.06; 3.76)0.63 (0.07; 5.19)1.03 (0.06; 17.05) |
0.75 | - - - - |
– |
| Primary tumor treated | No Yes |
10.98 (0.42; 2.28) |
0.97 | – | – |
In the multivariate analysis, OS tended to be shorter for patients presenting more than one metastatic site, with a decrease in OS associated with four or more metastatic sites at time of treatment (OR 4.1 (95 % CI: 1.10–16.0); p = 0.01) (Table 6).
The ECOG, type of primary tumor, control of the primary disease and metachronous metastasis did not influence OS.
Safety
In this study, SBRT was well tolerated, with no acute toxicity exceeding grade II observed. Acute toxicity data were available for nine patients, representing 8.1 % of the total cohort. Among these cases, the most prevalent toxicity reported was fatigue, with grade II fatigue noted in three patients and grade I in two patients. Additionally, two patients experienced grade I nausea. Notably, all of the eleven patients who underwent bilateral SBRT were documented to have adrenal dysfunction during follow-up.
Discussion
The present study is the largest French series to report the outcome of 121 adrenal gland metastases treated with SBRT in eleven centers. With a median follow-up of 11.8 months from the time of SBRT, we observed LC rates of 85.9 % at 1 year and 72.5 % at 2 years, comparable with other published SBRT [11], [12], [13], [14], [15] and surgical reports [16].
Several studies have assessed potential prognostic factors for local control with SBRT for adrenal metastases. It has been shown that one of the most important prognostic factors for local control is a higher prescribed dose.
A meta-analysis conducted by Chen et al. [12], including 36 studies and 1006 patients, reported a correlation between a higher median BED10 and LC. Based on a meta-regression model, a dose of 80 Gy, and 100 Gy predicted 1-year LC of 84.8 %, and 92.9 %, and 2-year LC of 70.1 %, and 85.6 %, respectively.
Stumpf et al. [13] created a tumor control probability model for adrenal gland metastasis, which incorporates different studies on SBRT with dosimetry and local control information. This model suggests that at 1 year, LC of more than 95 % can be achieved with a BED10 of 116.4 Gy.
The study by Buergy et al. [14] is the largest analysis to determine dose/volume cut-points using the data from 218 lesions in 196 patients. They demonstrated that when the cut-points for the PTV-D50% dose of 73.2 Gy (BED10), GTV-D50% dose of 74.2 Gy (BED10), GTV-mean dose of 73.0 Gy (BED10) were surpassed, the risk of local recurrence during the patient's follow-up was significantly lower compared to the lower-dose group (p < 0.05).
In our study, 47.1 % of the adrenal metastases treated received a BED10 of more than 73.2 Gy and 24.7 % received a BED10 equal to or greater than 100 Gy. This was achieved by using 55 Gy in 5 fractions, 45 Gy in 3 fractions, 60 Gy in 8 fractions, and 50 Gy in 5 fractions (prescribed BED10: 115.5, 112.5, 105, 100 Gy, respectively). Only a cut-off with a BED10 equal to or greater than 50 Gy had a lower risk of local relapse (p = 0.003). This cut-point is lower compared to those of the other studies, probably because of the high variety of dose and fractionation schemes used.
There is no clear correlation between large tumors and propensity for treatment failure. However, the size of the metastasis is often correlated with a suboptimal dose delivered because of the proximity of healthy tissue.
According to our findings and those of Buergy et al. [14], there was no difference in the probability of LC based on GTV and PTV size. This suggests that large volume adrenal metastasis should not be excluded from SBRT, provided that the treatment plan meets healthy tissue constraint.
We did not observe a significant correlation between primary tumor, histology, and LC. Instead, there was a reduced failure rate in patients with an active systemic treatment, four weeks before or after the SBRT, which remained significant in the adjusted analysis (p = 0.009). Systemic therapy paired with local consolidative therapy may have synergistic effects on local control.
The selection process of oligometastatic patients benefiting from a local curative treatment remains challenging.
We divided adrenal metastasis into three categories: oligopersistent, oligorecurrent, and oligoprogressive because we hypothesized that adrenal tumors resistant to systemic therapy were more likely to experience local relapse and have worse PFS and OS. Nevertheless, we did not find better local responses between those three states; adrenal tumors remained just as radiosensitive.
Recent studies have looked for factors predictive of long-term survival after ablative therapies and identified prognostic factors that tend to include: age, metastatic site, primary tumor, prior chemotherapy for primary disease, slow-growing disease (metachronous metastases or a long disease-free interval), and low disease burden [17], [18], [19]. The metachronous state was significant only for PFS, with patients who developed adrenal metastasis more than 6 months after the primary tumor having a better disease-free survival (p = 0.008).
The safety of adrenal SBRT has already been described in other retrospective series: Franzese et al. [16] reported 14.7 % grade 1 toxicities, 2.1 % grade 2. The meta-analysis by Chen et al. [12] reported toxicity of grade 3 or more in only 18 of the 1006 patients analyzed (1.8 %).
In our study, the treatment was well tolerated, with mild fatigue, nausea, and vomiting, and no grade 3, 4, or 5 toxicities were described. All patients treated for bilateral adrenal metastasis were placed under hydrocortisone at the end of the second radiotherapy.
While the study highlights a low rate of toxicity associated with SBRT, it is crucial to acknowledge the potential limitation of under-reporting due to the retrospective design of our study.
This study is subject to several limitations, including its retrospective design and the heterogeneity of the patient cohort. Variations in histology, the use and type of systemic therapy, and variability in radiation treatment doses are notable factors contributing to the study's limitations.
Conclusion
The present study is the largest French series of patients treated with SBRT for adrenal metastases series and confirms the efficacy of this treatment as a safe and beneficial method for obtaining local curative treatment. In our opinion, the dose delivered should depend off the oligometastic context: for patients with a low tumor burden, a dose allowing a BED10 of 100 Gy is a desirable goal. In patients with a more aggressive metastatic disease, a dose delivering a BED10 higher than 50 Gy allows a local control higher than 90 % at 1 year while respecting the tolerance to organs at risk.
Although some patients achieve long-term survival after ablative treatment, it remains unclear whether the treatment of their metastases extends their survival, or whether their long survival is merely due to the presence of slow-growing disease. Further prospective studies are needed to better clarify selection criteria, which require better predictive and prognostic bio markers.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2023.100708.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Weichselbaum R.R., Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. June 2011;8(6):378–382. doi: 10.1038/nrclinonc.2011.44. [DOI] [PubMed] [Google Scholar]
- 2.Reyes D.K., Pienta K.J. The biology and treatment of oligometastatic cancer. Oncotarget April 13. 2015;6(11):8491–8524. doi: 10.18632/oncotarget.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. [DOI] [PubMed]
- 4.Abrams H.L., Spiro R., Goldstein N. Metastases in carcinoma. Analysis of 1000 autopsied cases. Cancer. 1950;3(1):74–85. doi: 10.1002/1097-0142(1950)3:1<74::aid-cncr2820030111>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Gunjur A., Duong C., Ball D., Siva S. Surgical and ablative therapies for the management of adrenal ‘oligometastases’ – A systematic review. Cancer Treat Rev. August 2014;40(7):838–846. doi: 10.1016/j.ctrv.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Niibe Y., Hayakawa K. Oligometastases and Oligo-recurrence: The New Era of Cancer Therapy. Jpn J Clin Oncol. February 2010;40(2):107–111. doi: 10.1093/jjco/hyp167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guckenberger M., Lievens Y., Bouma A.B., et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020 Jan;21(1):e18–e28. doi: 10.1016/S1470-2045(19)30718-1. [DOI] [PubMed] [Google Scholar]
- 8.Eisenhauer,, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Seymour L., Bogaerts J., Perrone A., Ford R., Schwartz L.H., Mandrekar S., et al. iRECIST: Guidelines for Response Criteria for Use in Trials Testing Immunotherapeutics. 2017. The Lancet. Oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tirkes T., Hollar M.A., Tann M., Kohli M.D., Akisik F., Sandrasegaran K., et al. Radiographics : a review publication of the Radiological Society of North America. Inc. 2013;33(5):1323–1341. doi: 10.1148/rg.335125214. [DOI] [PubMed] [Google Scholar]
- 11.Noël G., Antoni D. Organs at risk radiation dose constraints. Cancer/radiothérapie. February 2022;26(1–2):59–75. doi: 10.1016/j.canrad.2021.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Chen W.C., Baal J.D., Baal U., Pai J., Gottschalk A., Boreta L., et al. Stereotactic Body Radiation Therapy of Adrenal Metastases: A Pooled Meta-Analysis and Systematic Review of 39 Studies with 1006 Patients. Int J Radiat Oncol Biol Phys. 2020 May 1;107(1):48–61. doi: 10.1016/j.ijrobp.2020.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stumpf P.K., Yorke E.D., El Naqa I., Cuneo K.C., Grimm J., Goodman K.A. Modeling of Tumor Control Probability in Stereotactic Body Radiation Therapy for Adrenal Tumors. Int J Radiat Oncol. 2021 May;110(1):217–226. doi: 10.1016/j.ijrobp.2020.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buergy D., Würschmidt F., Gkika E., Hörner-Rieber J., Knippen S., Gerum S., et al. Stereotactic or conformal radiotherapy for adrenal metastases: Patient characteristics and outcomes in a multicenter analysis. Int J Cancer. 2021;149(2):358–370. doi: 10.1002/ijc.33546. [DOI] [PubMed] [Google Scholar]
- 15.Corbin K.S., Ranck M.C., Hasselle M.D., Golden D.W., Partouche J., Wu T., et al. Feasibility and toxicity of hypofractionated image guided radiation therapy for large volume limited metastatic disease. Pract Radiat Oncol. 2013 Oct;3(4):316–322. doi: 10.1016/j.prro.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Franzese C., Nicosia L., Facondo G., Lo Faro L., Cuccia F., Vullo G., et al. Stereotactic body radiation therapy for adrenal gland metastases: outcome and predictive factors from a multicenter analysis. Clin Exp Metastasis. 2021 Dec;38(6):511–518. doi: 10.1007/s10585-021-10124-9. [DOI] [PubMed] [Google Scholar]
- 17.Gunjur A., Duong C., Ball D., Siva S. Surgical and ablative therapies for the management of adrenal ‘oligometastases’ – A systematic review. Cancer Treat Rev. 2014 Aug;40(7):838–846. doi: 10.1016/j.ctrv.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Hong J.C., Ayala-Peacock D.N., Lee J., Blackstock A.W., Okunieff P., Sung M.W., et al. Classification for long-term survival in oligometastatic patients treated with ablative radiotherapy: A multi-institutional pooled analysis. PLoS One. 2018 Apr 12;13(4):e0195149. doi: 10.1371/journal.pone.0195149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitroda S.P., Khodarev N.N., Huang L., et al. Integrated molecular subtyping defines a curable oligometastatic state in colorectal liver metastasis. Nat Commun. 2018;9:1793. doi: 10.1038/s41467-018-04278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.