Highlights
-
•
Pu'er tea is co-fermented by Saccharomyces, Rhizopus and Aspergillus niger.
-
•
A new type of Pu'er tea with fruity and floral aroma was fermented.
-
•
The fermentation method has a significant effect on the aroma of Pu'er tea.
-
•
The key aroma compounds in different types of Pu'er tea were elucidated.
Keywords: Pu'er tea, Key aroma components, Synergistic fermentation of beneficial microorganisms, Floral and fruity Pu'er tea
Abstract
Aroma is a key indicator of the quality and value of Pu'er tea. A total of 36 aroma components were detected,which Saccharomyces, Rhizopus, and Aspergillus niger, were in the ratios of 2:1:2, 2:2:2, and 2:3:2 inoculated to ferment Pu’er tea, comparing with natural fermentation. In addition, 12 key aroma compounds were identified by analysing ROAVs. Methoxyphenyl compounds and β-ionone were the primary contributors to the formation of aged and woody aroma when fermenting Pu’er tea naturally or using Rhizopus, while linalool and its oxides, benzyl alcohol, hexanal, and limonene were the primary contributors to the formation of floral and fruity aroma when fermenting Pu’er tea using synergistic fermentation with Saccharomyces, Rhizopus, and Aspergillus niger. This study identified the key aroma components of the Pu’er tea fermented using five methods, which revealed and demonstrated the potential application of synergistic effects of different microorganisms in the changes of aroma of Pu’er tea.
1. Introduction
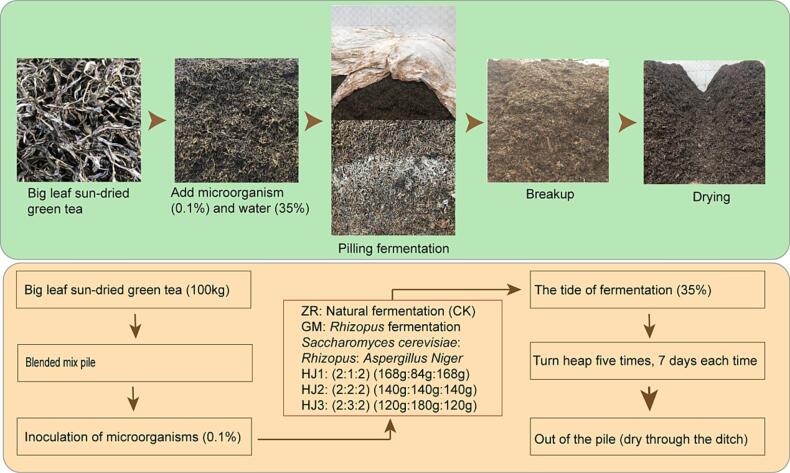
Fermented Pu'er tea is manufactured by a special pile fermentation technique of the sun-dried green tea of the Yunnan large-leaf tea (Camellia sinensis [Linn] var. assamica [Masters] Kitamura) leaves (Li et al., 2022a). Microorganisms, artificially inoculated or naturally infecting during the tea fermentation process, secret various extracellular enzymes, which make a type of tea with red and bright soup, mellow tasty, as well as flavored with a characteristic aged fragrance resulting from moist-heat action and microbial enzymatic action (Wang, Qiu, Gan, & Zhu, 2022c). The type of tea has a lot benefits, such as nourishing the stomach, regulating gut microbiota, preventing obesity, reducing lipids, resisting oxidation, scavenging free radical, etc. (Shang, Li, Zhou, Gan, & Li, 2021). The right processing ways make tea give off better aroma because the type and content of volatile compounds in tea has changed by processing (Zheng, Li, Xiang, & Liang, 2016). Regulating and improving the quality of Pu'er tea by artificially inoculating microorganisms make it helpful to understand the mechanism of action of microorganism, as well as the mechanism of formation of volatile aroma compounds (Deng et al., 2021). See (Fig. 1).
Fig. 1.
Flow chart of the synergistic fermentation process using beneficial microorganisms.
Aroma is one of the important factors affecting the quality of Pu'er tea, and it is also a key indicator for evaluating its value (Liu et al., 2021). Microorganisms are the key factors in the aroma formation during the Pu'er tea fermentation process, which is found that Aspergillus, Penicllium, Rhizopus, Mucor, Saccharomyces and bacteria are the microbial groups in the process of Pu'er tea fermentation (Ji, Gong, Peng, Liu, Zeng, & Yan, 2016). Exogenous addition of microorganisms to ferment Pu'er tea can improve the tea aroma and shorten the fermentation time (Piao, Zhang, & Chen, 2022). Pu'er tea fermented with Saccharomyces and Aspergillus niger can promote the production of more alcohols in the fermentation process, which gives it with unique floral and fruity aromas (Zhang et al., 2010). Moreover, mixed fermentation with microorganisms can reduce the types and contents of methoxy compounds in Pu'er tea while increasing the contents of alcohols and terpenes (Zheng, Zhang, Ren, Bai, Li, Wang, Shan, Dong, & Yi, 2023). These findings suggest that using different microorganisms and synergistic effects can shorten the fermentation time and increase the diversity and content of aroma components, improving the aroma and quality of Pu'er tea.
More than 1,000 volatile compounds have been identified in Pu’er tea to date (Wang et al., 2022a). The higher content of methoxyphenols and their alkane aroma substances in Pu'er tea is crucial to the aroma formation, which gives tea off primarily the aged tea aroma. In contrast, the relatively high content of linalool and its oxides, phenylethanol, rosinol and other alcohols in pu'er tea formed distinct floral and fruity aromas (Nian et al., 2020). The Pu’er tea with floral and fruity aromas exists currently a market gap in some degree, compared with the Pu’er tea with an aged aroma that is dominated in the market.
To reveal the key aroma components of Pu'er tea that has been synergistic fermented with three types of beneficial microorganisms (Saccharomyces: Rhizopus: Aspergillus niger in ratios of 2:1:2, 2:2:2, and 2:3:2), fermented with Rhizopus, and through natural fermentation without synergistic fermentation. We used SDE and GC–MS techniques to extract aroma components from Pu'er tea The key aroma components were analyzed using clustering partial least squares discriminant (PLS-DA), relative odor activity value (ROAV), principal component analysis (PCA), and a partial least squares regression (PLSR) to attempt to describe a basis for the scientific evaluation and in-depth exploration of the quality of Pu'er tea aromas following fermentation by different microorganisms.
2. Materials and methods
2.1. Fermentation strains
Aspergillus niger (strain ZL200510010941.4), Saccharomyces cerevisiae Hansen (strain ZL200510010940.X), and Rhizopus arrhizus (strain 200710065787.X) were used in this experiment, which were obtained by the project research team of the National Natural Science Foundation (Yunnan, China), stored in the China General Microbiological Culture Collection Center (CGMCC) and granted a patent.
2.2. Tea samples
Fresh leaves of the Mengku big leaf species (Camellia sinensis var. assamica [Masters] kitamure cv. Mengku Davecha) in Lincang tea region, of which grade was one bud, three leaves, and four leaves, were collected for fermentation after being processed based on the Technical Regulations for the Production of Yunnan Big Leaf Sun-cured Green Tea (Q/T PCX01-2007).
2.3. Chemicals and materials
Anhydrous sodium sulfate, ethanol, and dichloromethane were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). 2,4,6-Trimethylpyridine, hexanal, nonanal, (E)-2-nonenal, 1-penten-3-ol, 3-octanone, 2-pentylfuran, 1-butanol, octanal, (E)-2-octenal, 2,3-octanedione, (E,Z)-2,6-nonadienal, (E)-2-pentenal, 1-octen-3-ol, (E,E)-2,4-heptadienal, heptanal, benzaldehyde, lactic acid (85 %), (E,E)-2,4-nonadienal, (E)-2-hexenal, (E)-2-decenal, 2,4-di-tert-butylphenol, (E,E)-2,4-decadienal, acetic acid and n-Alkane mixed standard (C7-C30) of chromatographic grade was purchased from Sigma Aldrich (St. Louis, MO, USA).
This study employed GC–MS model GC-14B (Shimadzu, Kyoto, Japan), SDE device model HB-TSZL (Anhui Great Wall Glass Instrument Factory (Xuancheng, China), and the thermoelectric thermostatic water bath model HWS-24 from the Shanghai Yiheng Scientific Instrument Co., LTD (Shanghai, China).
2.4. Fermentation process
The screened and cleaned tea leaves were evenly placed into a pile and a pile, weighed 420 kg from each pile and transferred into the fermentation site where was located at the Pu'er Zuxiang Tea Co., Ltd. (Pu'er, Yunnan Province). Tea leaves were fermented using 5 different treatments. One treatment was natural fermentation (ZR, no add strain), one treatment was fermented using Rhizopus (GM), the other treatments were fermented using the mixture of Saccharomyces, Rhizopus and Aspergillus niger in different ratios (HJ1 2:1:2, HJ2 2:2:2, and HJ3 2:3:2). The microorganisms, of which weight was 0.1 % of the weight of tea sample, were mixed with the water, of which weight was 35 % of the weight of tea sample. The mixture was sprayed on the tea leaves pile for inoculation, mixing well. The treated tea leaves were pile up 1–1.5 m of height for fermentation.
For fermented tea pile, temperature was 55 ℃, humidity was 95 %. For fermentation ambient, temperature was 25 ℃, humidity was 90 %. The piles were turned every 7 days since the 7th day of fermentation. Samples were taken on the 35th day of fermentation according to the 5-point sampling method, using technical replicates, mixed and divided into three equal parts for the detection of aromatic compounds.
2.5. Aroma analytical method
SDE was used to extract the aromatic compounds, as described by Li et al. (2022b). Briefly, 50.0 g of tea was accurately weighed and transferred onto simultaneous distillation and extraction equipment. The resultant solution obtained following a two hour-extraction with 30 mL of dichloromethane was concentrated to 0.5 mL, and the compounds were evaluated by GC–MS (Li et al., 2022b).
The analytical conditions for GC–MS included a 30 m × 0.2 mm (id) × 0.5 μm (film thickness) HP-5 column (Agilent, USA). The carrier gas was > 99.999 % pure helium at a 1 mL/min flow rate. The column temperature was originally set at 60 ℃ for 2 min, and then increased to 200 ℃ at a heating rate of 3 ℃/ min for 25 min. The inlet temperature was 250 ℃. The ionization mode was EI (electron ionization), and the ionization energy was 70 EV. The ion source temperature was 250 ℃. The GC–MS was scanned throughout the full spectrum range of 40 ∼ 400 m/z. The sample size was 1 µL.
The mass spectra libraries available from Wiley and the National Institute of Standards and Technology (NIST) were searched for compounds that corresponded to each chromatographic peak in the series, and the resulting mass spectra were manually analyzed by combining the data with a photographic examination of the values described in the literature (Yin, Huang, Huang, Wu, Tong, Liu, Zhou, Liu, & Zhang, 2022). The parameters of actual composition, mass spectrometry, retention index (RI), and retention time were used to generate the retention time value of tea aroma components and the comparison and identification of components (Wang, Sun, Lassabliere, Yu, & Liu, 2022b). The reference materials of some components were compared, and the peak area was used to standardize the quantification of the total ion chromatogram (TIC) to determine the relative content of each component (Lv et al., 2012).
The relative content (%) of the volatile compounds was calculated as follows:
where Ci and Ai denote the mass fraction (%) and peak area of compound i, respectively, with the total peak area of IS is As. Data are expressed as relative content means ± SD (n = 3).
Furthermore, RI value was compared with those reported in the literature (Wang et al., 2022b) and was used to verify selected compounds. The RI value was calculated using the following equation:
where Rt (X) denotes the retention time of the chromatographic peak of the analyzed compound, Rt (N) and Rt (N + 1) are the retention time of the chromatographic peaks of n-alkanes, with carbon number N and N + 1, before and after the outflow of the target compound (X).
2.6. Relative odor activity value
To evaluate the contribution of aroma components to the synergistic fermentation of Pu’er tea by different microorganisms, the ROAV method (Zhang et al., 2020b) was used to evaluate the amount of the components. ROAVmax = 100 and the ROAV of other aroma components were calculated using the following equation:
where Ti and Tmax are the corresponding thresholds of each flavor component and the corresponding thresholds of the largest contribution component in μg/kg, respectively. Ci and Cmax denote the relative content of each flavor component and the relative percentage of the largest contributing component, respectively. ROAV ≥ 1.0, this substance contributed more to the volatile flavor and was a key aroma component.
2.7. Sensory evaluation
The standard of the “Tea Sensory Evaluation Method” (GB/T23776-2018) (General Administration of Quality Supervision and Administration, 2018) was used to evaluate the characteristics and quality of Pu'er tea samples aromas fermented by different microorganisms. Five trained tea reviewers (3 males and 2 females, aged 20–35) assessed the aroma. Five sensory descriptors (fermented, aged, floral, fruity, and sweet) were used to describe the characteristics of Pu'er tea samples quantitatively. An intensity scale of 0–7 (0–1 was very weak, 1–2 weak, 2–3 weak, 3–4 medium, 4–5 strong, 5–6 strong, and 6–7 extremely strong) was used for sensory evaluation. The final score was determined by discussion and calculations.
2.8. Statistical analysis
Microsoft Excel 2010 (Redmond, WA, USA) was used for data processing, SPSS 24.0 (IBM, Inc., Armonk, NY, USA) was used for ANOVA, and GraphPad Prism 9.0 (GraphPad Software, Inc.) was used to plot the bar charts. TBtools (https://github.com/CJ-Chen/TBtools) was used to analyze the heat maps and Venn plots. MetaboAnalyst5.0 (https://www.metaboanalyst.ca) was employed for the PLS-DA and VIP analyses. SIMCA 14.1 (Umetrics, Umea, Sweden) was used for PCA, while The Unscrambler X 10.4 software was used for PLSR. Each measurement was repeated three times, and all the data were presented as averages. All data were presented as the mean value ± SD. P < 0.05 denoted significant differences between groups.
3. Results and discussion
3.1. Analysis of aroma types and the relative contents of different microorganisms during the synergistic fermentation of Pu'er tea
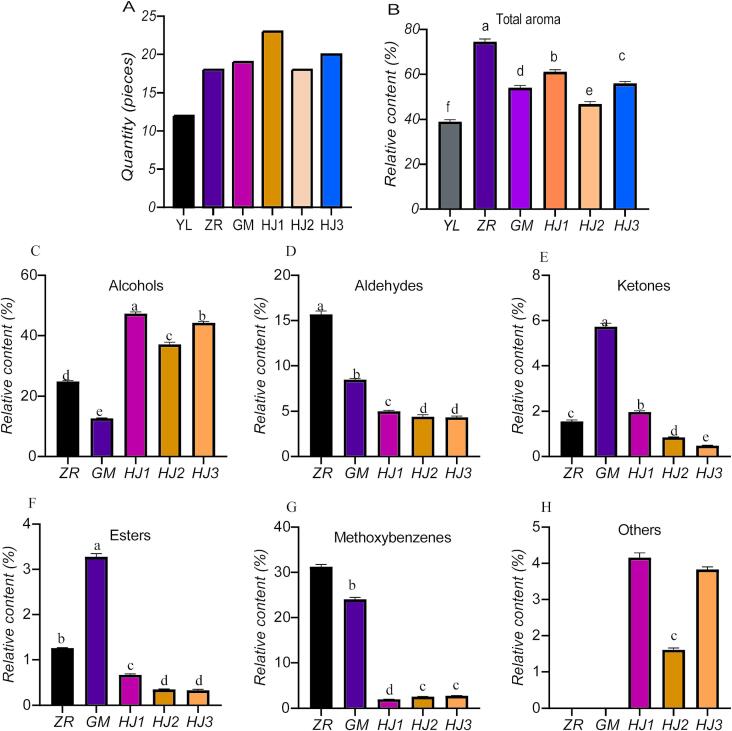
The SDE method was used to extract the aroma components from raw materials and all fermented treatments. A qualitative GC–MS analysis revealed 36 aromatic compounds that are alcohols, methoxybenzenes, ketones, esters, aldehydes, and phenolics were detected in the samples in total, including 12 aromatic compounds in YL, 18 romatic compounds in ZR, 19 aromatic compounds in GM, 23 aromatic compounds in HJ1, 18 aromatic compounds in HJ2, and 20 aromatic compounds in HJ3. (Fig. 2A).
Fig. 2.
Types of aromas and the relative contents of different microorganisms during the synergistic fermentation of Pu'er tea. A: Aroma quantity. B: Total aroma relatively content. C: The relative content difference of aroma components in alcohols. D: The relative content difference of aldehyde aroma components. E: The relative content difference of ketone aroma components. F: Differences in the relative content of esters. G: The relative content of methoxybenzenes. H: The relative contents of aroma components were different in others (phenol, β-pinene, longifolene, limonene). YL: Raw materials. ZR: Traditional natural fermentation. GM: Inoculated with Rhizopus for fermentation. HJ1: Synergistic fermentation following inoculation with Saccharomyces: Rhizopus: Aspergillus niger in a ratio of 2:1:2. HJ2: Synergistic fermentation following inoculation with Saccharomyces: Rhizopus: Aspergillus niger in a ratio of 2:2:2. HJ3: Synergistic fermentation following inoculation with Saccharomyces: Rhizopus: Aspergillus niger in a ratio of 2:3:2.
There were significant differences among the samples for the number of aroma components, the relative content of aroma components and the types of aromas, and there was the greatest change (P < 0.05) for total aroma in naturally fermented tea (ZR) (Fig. 2B).
The following natural fermentation of Pu'er tea: (1) The relative content of alcohol aroma components (47.32 %) in Pu'er tea was the highest in HJ1, which was significantly higher than that in the other four fermentation methods (Fig. 2C). The next highest relative content was found in HJ3 (44.28 %) and HJ2 (37.12 %). However, the relative content of Pu'er tea inoculated with Rhizopus for fermentation (12.67 %) was significantly the lowest (P < 0.05). It was found that microbial fermentation increased the content of the aromatic compounds linalool with its oxides and other alcohol compounds in some studies (Wang, Sun, Lassabliere, Yu, & Liu, 2020). Similarly, mixed inoculation microbial fermentation more significantly increased the number of alcohol aromatic compounds that were alcohols (Wang et al., 2022b).
(2) The relative content of aldehydes was the highest ZR (15.7 %) (Fig. 2D), followed by GM (8.47 %), HJ1 (5.01 %), HJ2 (4.42 %), HJ3 (4.34 %). Aldehydes are primarily obtained owing to oxidative decomposition of lipids and higher alcohols during the fermentation process (Barra et al., 2007, Wang et al., 2022a). After microbial inoculation, both kiwi fruit (Actinida deliciosa Chev) juice (Barra et al., 2007, Wang et al., 2022a) and jujube (Zizyphus jujuba Mill) (Pan, Zhang, Xu, Lao, & Wu, 2022) showed a decrease in the aroma components of aldehydes, which could be attributed to the microorganisms inhibited the oxidation of alcohols.
(3) There were higher relative contents of ketones (5.73 %) and esters (3.28 %) in GM (Fig. 2E, F), which were significantly higher than the other fermentation methods (P < 0.05). Interestingly, the contents of ketones and esters in mixed microbial fermentation of plum (Prunus spp.) wine increased (Zhang, Zhong, Heygi, Wang, & Du, 2022), which was contrasted with the findings of this study. I However, it was found that fermentation with Rhizopus could induce the biosynthesis of many aromatic ketones and esters (Liu et al., 2019), which could be the reason why there were significantly higher amounts of ketone aromatic components in the Pu'er tea fermented by Rhizopus.
(4) The relative content of methoxybenzene aroma components in the traditional natural fermentation of Pu'er tea ZR (31.24 %) was significantly higher than in the other four fermentation methods (P < 0.05) (Fig. 2G). Studies have shown that methoxybenzenes were produced by epigallocatechin and epigallocatechate esters under the action of esterase and tannase to produce gallic acid (GA), which is produced by microbial methylation (Lv et al., 2014). This is consistent with the results of Deng et al. (2021) and Liu et al. (2022). During the late period of traditional natural fermentation, the content of methoxybenzenes in tea increased significantly (Deng et al., 2021, Liu et al., 2022).
(5) The other aroma components were primarily limonene, which was only detected in the beneficial microbial synergistic fermentation of Pu'er tea. The relative content of limonene in Pu'er tea (4.16 %) was significantly higher following the synergistic fermentation of inoculated Saccharomyces, Rhizopus, and Aspergillus niger in a ratio of 2:1:2 (P < 0.05) (Fig. 2H). The fermentation of Nanfeng tangerine (Citrus reticulata cv. Kinokuni) wines by Saccharomyces mixed with non-Saccharomyces increased the content of limonene significantly (Xu et al., 2022), which is consistent with the results of this study.
3.2. Assessment of the characteristic substances of fermented Pu’er tea using different microorganisms
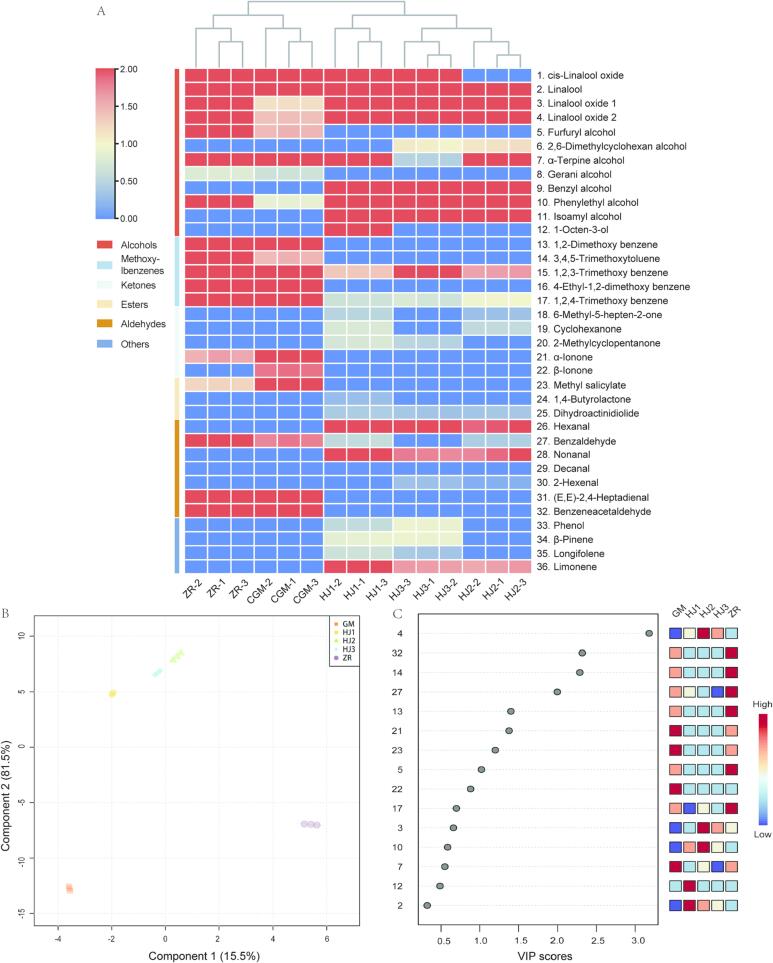
The aroma components in Pu'er tea are complex. To distinguish the differences of key aroma components in fermented tea more effectively and intuitively, the relative contents of aroma compounds fermented by the five methods were analyzed using a clustering heat map, and the rules of distribution of 36 types of aroma compounds in five samples of Pu'er tea were studied in more detail (Fig. 3A).
Fig. 3.
Aroma component clustering heat map analysis and least squares discriminant analysis of Pu'er tea co-fermented by different microorganisms. A: Aroma component clustering heat map, B: Aroma component partial least squares discriminant analysis (PLS-DA), C: Aroma component VIP analysis. ZR: Traditional natural fermentation. GM: Rhizopus inoculated for fermentation. HJ1: Saccharomyces: Rhizopus: Aspergillus niger inoculated for synergistic fermentation in a ratio of 2:1:2. HJ2: Saccharomyces: Rhizopus: Aspergillus niger inoculated for synergistic fermentation in a ratio of 2:2:2. HJ3: Saccharomyces: Rhizopus: Aspergillus niger inoculated for synergistic fermentation in a ratio of 2:3:2. VIP: variable importance in projection.
A total of 18 aroma components were detected in naturally fermented Pu'er tea, with the highest relative contents identified as 1,2,3-trimethoxybenzene (10.35 %) and 1,2-dimethoxybenzene (8.66 %), which mainly defined aged aroma (Table 1). This was followed by phenylacetaldehyde (7.08 %), linalool oxidized2 (5.54 %), benzaldehyde (5.39 %), and 3,4,5-trimethoxytoluene (5.33 %). A total of 19 aroma components were detected in Pu'er tea fermented after inoculation with Rhizopus. The highest relative levels were also found for 1,2,3-trimethoxybenzene (10.39 %) and 1,2-dimethoxybenzene (6.30 %). These compounds mainly characterized by aged fragrance (Table 1). These were followed by β-ionone (3.88 %), (E,E)-2,4-heptadienal (3.51 %), 4-ethyl-1,2-dimethoxybenzene (3.43 %), and methyl salicylate (3.28 %). A total of 23 aroma components were detected in Pu'er tea co-fermented by inoculation with Saccharomyces: Rhizopus: Aspergillus niger in a ratio of 2:1:2. The highest relative contents were found for cis-linalool oxide (13.52 %) and phenylethyl alcohol (9.66 %), which were mainly characterized by floral and fruity aromas (Table 1). These were followed by linalool-2-oxide (6.99 %) and linalool (5.25 %). A total of 23 aroma components were detected in Pu'er tea that had been synergistic fermented by inoculation with Saccharomyces: Rhizopus: Aspergillus niger in a ratio of 2:2:2. Phenylethyl alcohol (11.01 %) and linalool oxide 2 (10.59 %) were the highest relative contents found in Pu'er tea, mainly characterized by floral and fruity aromas (Table 1). These were followed by linalool alcohol (4.27 %) and benzyl alcohol (3.14 %). A total of 20 aroma components were detected in Pu'er tea synergistic fermented by inoculation with Saccharomyces: Rhizopus: Aspergillus niger in a ratio of 2:3:2 with cis-linalool oxide (12.38 %), linalool oxide2 (9.47 %), and phenylethyl alcohol (9.15 %) having higher relative contents and were primarily characterized by floral and fruity aromas (Table 1). Clustering enabled division of the five fermentation methods of Pu'er tea into two groups. These included natural fermentation and Rhizopus fermentation as one category, and inoculated Saccharomyces: Rhizopus: Aspergillus niger in ratios of 2:1:2, 2:2:2, and 2:3:2 as another category. After subdivision, natural fermentation and Rhizopus fermentation could be separated, as well as inoculated Saccharomyces: Rhizopus: Aspergillus niger in a ratio of 2:1:2 and inoculated Saccharomyces: Rhizopus: Aspergillus niger in ratios of 2:2:2 and 2:3:2. Therefore, the fermentation method determined the relative content and type of aroma compounds in Pu'er tea; the microbial composition of Pu'er tea also influenced the formation of Pu'er tea aroma compounds (Hu, Shi, & Ma, 2022).
Table 1.
ROAV of the aroma components in Pu'er tea fermented by microorganisms.
| Serial number | Aroma compounds | RI | Flavor type | The threshold value (μg/kg) | ROAV |
||||
|---|---|---|---|---|---|---|---|---|---|
| ZR | GM | HJ1 | HJ2 | HJ3 | |||||
| 1 | cis-linalool oxide | 12.20 | Strong scent of flowers and trees | 6.00 | 5.78 | 0.78 | 97.99 | 0.00 | 100.00 |
| 2 | Linalool | 13.16 | Bouquet of lilacs, lily of the valley and rose, wood and fruit | 3.80 | 4.10 | 1.57 | 60.03 | 59.47 | 50.06 |
| 3 | Linalool oxide1 | 15.62 | Strong woody, floral and fragrant scent | 320.00 | 0.04 | 0.01 | 0.28 | 0.39 | 0.35 |
| 4 | Linalool oxide2 | 15.80 | Strong woody, floral and fragrant scent | 320.00 | 0.10 | 0.01 | 0.95 | 1.75 | 1.43 |
| 6 | 2,6-dimethylcyclohexane alcohol | 13.45 | – | 11.00 | 0.00 | 0.00 | 0.00 | 5.58 | 4.66 |
| 10 | Phenylethyl alcohol | 13.61 | Rose aroma | 1100.00 | 0.01 | 0.00 | 0.38 | 0.53 | 0.40 |
| 11 | Isoamyl alcohol | 2.97 | Apple brandy aroma and spicy notes | 250.00 | 0.00 | 0.00 | 0.46 | 0.54 | 0.43 |
| 12 | 1-octen-3-ol | 8.95 | Aroma of mushroom, lavender, rose and hay | 1.00 | 0.00 | 0.00 | 88.76 | 0.00 | 0.00 |
| 13 | 1,2-dimethoxybenzene | 16.38 | Aroma of aged fragrance | 259.00 | 0.19 | 0.06 | 0.00 | 0.00 | 0.00 |
| 14 | 3,4,5-trimethoxytoluene | 16.05 | Aroma of aged fragrance | 100.00 | 0.31 | 0.03 | 0.00 | 0.00 | 0.00 |
| 15 | 1,2,3-trimethoxybenzene | 20.59 | Aroma of aged fragrance | 500.00 | 0.12 | 0.05 | 0.12 | 0.17 | 0.20 |
| 16 | 4-ethyl-1,2-dimethoxybenzene | 19.84 | Aroma of aged fragrance | 3.40 | 5.62 | 2.34 | 0.00 | 0.00 | 0.00 |
| 17 | 1,2,4-trimethoxybenzene | 22.48 | Aroma of aged fragrance | 100.00 | 0.21 | 0.06 | 0.28 | 0.51 | 0.34 |
| 21 | β-Ionone | 15.62 | Bouquet of violets and fruit wood | 0.09 | 100.00 | 100.00 | 0.00 | 0.00 | 0.00 |
| 22 | α-Ionone | 15.79 | Flower and wood fragrance | 0.40 | 0.00 | 10.73 | 0.00 | 0.00 | 0.00 |
| 23 | Methyl salicylate | 19.02 | Holly flavor | 40.00 | 0.18 | 0.19 | 0.00 | 0.00 | 0.00 |
| 25 | Dihydroactinidiolide | 27.37 | Coumarin-like aroma and a musky odor | 43.00 | 0.00 | 0.00 | 0.40 | 0.43 | 0.37 |
| 26 | Hexanal | 4.02 | Smell of grass and apple | 4.50 | 0.00 | 0.00 | 20.73 | 22.89 | 24.53 |
| 27 | Benzaldehyde | 8.33 | Bitter almond smell | 350.00 | 0.09 | 0.01 | 0.07 | 0.06 | 0.00 |
| 28 | Nonanal | 13.33 | Strong grease smell | 1.00 | 0.00 | 0.00 | 100.00 | 100.00 | 85.57 |
| 30 | 2-hexenal | 5.24 | Special green leaf aroma | 17.00 | 0.00 | 0.00 | 0.00 | 0.50 | 0.86 |
| 31 | (E,E)-2,4-heptadienal | 8.78 | Clear aroma, aldehyde aroma, chicken aroma | 56.00 | 0.33 | 0.15 | 0.00 | 0.00 | 0.00 |
| 32 | Benzeneacetaldehyde | 9.03 | Rich aroma of hosta flowers | 4.00 | 10.21 | 1.84 | 0.00 | 0.00 | 0.00 |
| 36 | Limonene | 10.62 | Fresh orange and lemon-like aroma | 10.00 | 0.00 | 0.00 | 9.02 | 7.14 | 7.89 |
Note: confirmation by authentic standards. ZR: Traditional natural fermentation. GM: Rhizopus inoculated for fermentation. HJ1: Synergistic fermentation by inoculated Saccharomyces: Rhizopus: Aspergillus niger in a ratio of 2:1:2. HJ2: Synergistic fermentation by inoculated Saccharomyces: Rhizopus: Aspergillus niger in a ratio of 2:2:2. HJ3: Synergistic fermentation by inoculated Saccharomyces: Rhizopus: Aspergillus niger in a ratio of 2:3:2.
A PLS-DA was performed to visually discern the relative content of key aroma components in five different fermented Pu'er tea; the five fermentation samples could be effectively divided into the two groups. The discrimination results were consistent with the cluster analysis (Fig. 3B). Simultaneously, the contribution of variable aroma component content to the sample classification difference between groups was measured based on PLS-DA results using VIP analysis (VIP > 1 indicated that the aroma component was an “important” variable, and larger values indicated a higher level of importance). Eight signature aroma components were screened (Fig. 3C), including linalool oxide2 (4), benzeneacetaldehyde (32), 3,4,5-trimethoxytoluene (14), benzaldehyde (27), 1,2-dimethoxy aldehyde (13), β-ionone (21), methyl salicylate (23), and furfuryl alcohol (5). These eight flavor components significantly influenced the aroma and flavor differences of Pu'er tea fermented using the five methods.
3.3. ROAV analysis of the aroma components of Pu'er tea fermented by different microorganisms
The overall flavor of Pu’er tea depends on the concentration of different aroma substances and is closely related to the threshold value of substances. Pu’er tea that has been fermented in different manners has a unique flavor. Owing to the different characteristics and threshold values of different aroma substances, an ROAV was used to analyze the degree of contribution of the key aroma compounds in fermented Pu’er tea samples to the overall aroma of Pu’er tea. The threshold values used were obtained from the Compilation of Aroma Threshold of Compounds (Second Edition) (Vanheimer, 2018) and related studies (Wei et al., 2020).
The ROAV values were determined using the aroma threshold and relative content of some aromatic compounds (Table 1). A more detailed analysis of the main aromatic compounds was performed in five fermented Pu'er tea samples. Six key aroma components (ROAV ≥ 1) from naturally fermented Pu'er tea were identified as the following compounds: cis-linalool oxide (1), linalool (2), 4-ethyl-1, 2-dimethoxybenzene (16), β-ionone (21), and benzeneacetaldehyde (32). Seven major aroma components (ROAV ≥ 1) identified from the Pu'er tea fermented by Rhizopus, including linalool (2), 4-ethyl-1,2-dimethoxybenzene (16), β-ionone (21), α-ionone (22) and benzeneacetaldehyde (32). β-ionone and 4-ethyl-1, 2-dimethoxybenzene displayed aged and wood fragrance characteristics (Xu, Mo, Yan, & Zhu, 2007). Pang et al. (Pang, Yu, Cao, Yuan, Qiu, Kong, & Wu, 2019) demonstrated that β-ionone has a low threshold and plays a crucial role in the fragrance development of ripened Pu'er tea (Pang et al., 2019). The seven key aroma components (ROAV ≥ 1) identified in the synergistic fermentation of Pu'er tea by Saccharomyces: Rhizopus: Aspergillus niger in a ratio of 2:1:2 were cis-linalool oxide (1), linalool (2), 1-octen-3-ol (12), hexanal (26), nonanal (28), and limonene (36). The six key aroma components (ROAV > 1) identified in the synergistic fermentation of Pu'er tea by Saccharomyces: Rhizopus: Aspergillus niger in a ratio of 2:2:2 include linalool (2), linalool oxide2 (4), 2,6-dimethylcyclohexane alcohol (6), hexanal (26), nonanal (28), and limonene (36). The seven key aroma components (ROAV ≥ 1) discovered in the synergistic fermentation of Pu'er tea by Saccharomyces: Rhizopus: Aspergillus niger in a ratio of 2:3:2 included cis-linalool oxide (1), linalool (2), linalool oxide 2 (4), 2, 6-dimethylcyclohexane alcohol (6), hexanal (26), nonanal (28), and limonene (36). Nonanal, linalool, cis-linalool oxide, hexanal, and limonene have floral and fruity aromas (Liu et al., 2021). Linalool and its oxide, and limonene were discovered to be the primary compounds for the formation of floral aromas (Yue et al., 2022).
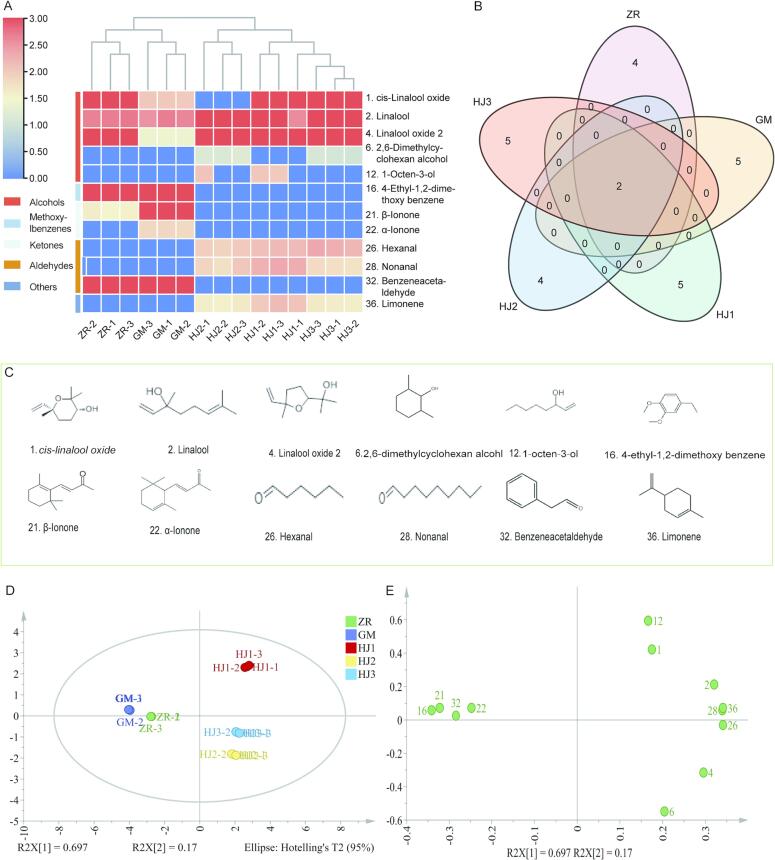
Natural fermented Pu'er tea was screened for 6 and Rhizoma inoculum fermented Pu'er tea was identified for 7 kinds of key aroma components. Inoculation of Saccharomyces: Rhizopus: Aspergillus niger in a ratio of (2:1:2) synergistic fermentation of Pu'er tea was identified 7 kinds of key aroma components, inoculation of Saccharomyces: Rhizopus: Aspergillus niger in a ratio of (2:2:2) synergistic fermentation of Pu'er tea was identified 6 kinds of key aroma components, and inoculation of Saccharomyces: Rhizopus: Aspergillus niger in a ratio of (2:3:2) synergistic fermentation of Pu'er tea was identified 6 kinds of key aroma constituents (Fig. 4A, B). Among them, the structural formula of the key components is shown in Fig. 4C. An analysis of the above-mentioned ROAV values revealed that compounds with aged and woody aromas in natural fermentation and Rhizopus fermentation of Pu'er tea had higher ROAV values, while those with floral and fruity aromas in microbial synergistic fermentation of Pu'er tea had higher ROAV values. Simultaneously, the aromatic components of microbial synergistic fermentation of Pu'er tea were more abundant than those of natural and Rhizopus fermentation. These results imply that microbial synergistic fermentation increases the richness of Pu'er tea aroma and reduces the inferior fermentation flavor of Pu'er tea, and produces more high-quality floral and fruit aromas.
Fig. 4.
The key aroma components of Pu'er tea fermented by different microorganisms. A: Clustering heat map of key aroma components, B: Venn diagram of key aroma components. C: Chemical structure formula of key aroma components. D: PCA analysis of key aroma components. E: Load plot of PCA analysis. ZR: Traditional natural fermentation. GM: Inoculation with Rhizopus for fermentation. HJ1: Synergistic fermentation following inoculation with Saccharomyces: Rhizopus: Aspergillus niger in a ratio of 2:1:2. HJ2: Synergistic fermentation following inoculation with Saccharomyces: Rhizopus: Aspergillus niger in a ratio of 2:2:2. HJ3: Synergistic fermentation following inoculation with Saccharomyces: Rhizopus: Aspergillus niger in a ratio of 2:3:2.
3.4. PCA analysis of aroma components in different microbial synergistic fermentation of Pu'er tea
The key aroma substances in five fermented Pu’er tea samples were determined in more detail. A total of 24 aromatic components (Table 1) that contributed to the volatile flavor of Pu’er tea (ROV ≥ 1) were found in relevant studies for the PCA. The score and load charts are shown in Fig. 4.
The variance contribution rate of principal component 1 (PC1) was 69.7 %; the variance contribution rate of principal component 2 (PC2) was 17.0 %, and the cumulative variance contribution rate was 86.7 %, which potentially explains the primary characteristics of the beneficial microbial fermentation of Pu'er tea aroma substances (Fig. 4D, E). Among them, the aroma component with the highest positive load in PC1 was limonene (36), and the aroma component with the highest negative load was 4-ethyl-1,2-dimethoxybenzene (16). The aroma component with the highest positive load in PC2 was α-ionone (22), and the aroma component with the highest negative load was 2,6-dimethylcyclohexane alcohol (6). The results described above show that limonene (36), 4-ethyl-1,2-dimethoxybenzene (16), α-ionone (22), and 2,6-dimethylcyclohexane alcohol (6) are the aroma components with the most obvious change in content in Pu’er tea that was fermented using different methods. Thus, the compounds described above can be provided to distinguish Pu’er tea that was produced using different fermentation methods.
The aroma components of natural fermentation and Pu'er tea that had been synergistic fermented following inoculation with Rhizopus and Saccharomyces: Rhizopus: Aspergillus niger in ratios of 2:1:2, 2:2:2, and 2:3:2 had no overlap among the five fermentation sample regions of Pu'er tea, which could be distinguished from each other. This finding suggests that the relative contents of aroma components can identify various fermentation methods. The contents and types of major flavor substances differed significantly between the five fermentation methods. The PCA results agreed with the previous clustering and PLS-DA results (Fig. 3), demonstrating that the five fermentation methods potentially influence the aroma components of Pu'er tea.
3.5. Aroma sensory and aroma components PLSR analysis of different microbial synergistic fermented Pu'er tea
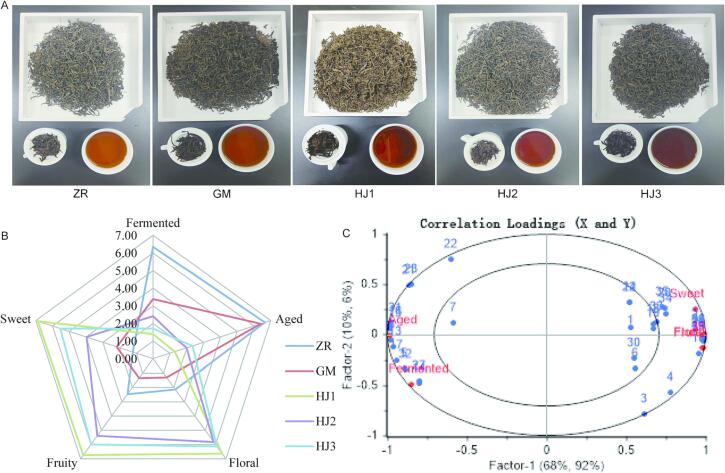
The aroma sensory quantitative description analytical results of the varying types of synergistic fermentation of Pu’er tea by different microorganisms were profiled (Fig. 5A, B). The “aged” and “fermented” aromas of naturally fermented Pu’er tea were the strongest. Synergistic fermented Pu’er tea following inoculation with Saccharomyces: Rhizopus: Aspergillus niger in ratios of 2:1:2, 2:2:2, and 2:3:2 had stronger “sweet,” “floral,” and “fruity” aromas. The fermentation with the ratio of 2:1:2 was the strongest, while that fermented with the ratio 2:2:2 was the second strongest.
Fig. 5.
Partial least square method (PLSR) analysis of the aroma components of Pu'er tea fermented by different microorganisms. A: Tea samples fermented by different microorganisms. B: Aroma sensory of tea samples fermented by different microorganisms. C: PLSR model load diagram of the correlation between aroma components and sensory indices. ZR: Traditional natural fermentation. GM: Inoculation with Rhizopus for fermentation. HJ1: Synergistic fermentation following inoculation with Saccharomyces: Rhizopus: Aspergillus niger in a the ratio 2:1:2. HJ2: Synergistic fermentation following inoculation with Saccharomyces: Rhizopus: Aspergillus niger in a ratio 2:2:2. HJ3: Synergistic fermentation following inoculation with Saccharomyces: Rhizopus: Aspergillus niger in a ratio 2:3:2.
The “aged” and “fermented” aromas of naturally fermented Pu'er tea were the strongest. Synergistically fermented Pu'er tea following inoculation with Saccharomyces: Rhizopus: Aspergillus niger in ratios of 2:1:2, 2:2:2, and 2:3:2 had stronger “sweet,” “floral,” and “fruity” aromas. Fermentation with a ratio of 2:1:2 was the strongest, while that fermented with the ratio 2:2:2 was the second strongest.
A regression analysis was performed with the 36 aroma compounds following the microbial fermentation of Pu'er tea as the X variable and five aroma sensory attributes (“fermented,” “aged,” “floral,” “fruity,” and “sweet”) as the Y variable. The aroma components and aroma sensory attributes of Pu'er tea samples were analyzed using multi-independent and multi-dependent variables (PLS2). The two major components retrieved from the established PLSR model explained 78 % of the variance of X (aroma compounds) and 98 % of the variance of Y (sensory attributes), indicating that the model could accurately reflect the overall information of the sample (Fig. 5C).
The PLSR is a multivariable approach utilized in sensory and aroma compound datasets to determine the relationship between sensory attributes and aroma compounds (Zhang, Ji, Liu, & Gao, 2020a). Five sensory indicators and 28 aroma compounds were found to be distributed between two ovals (R2 = 0.5 and R2 = 1.0). The values represent 50 % and 100 % of the explanatory variance, respectively. The following 28 volatile compounds determined the aroma characteristics of microbial synergistic fermentation of Pu'er tea samples: linalool (2), linalool oxide1 (3), linalool oxide2 (4), furfuryl alcohol (5), geranyl alcohol (8), benzyl alcohol (9), phenylethyl alcohol (10), isoamyl alcohol (11), 1, 2-dimethoxybenzene (13), 3,4,5-trimethoxytoluene (14), 1,2,3-trimethoxy benzene (15), 4-ethyl-1,2-dimethoxybenzene (16), 1,2,4-trimethoxybenzene (17), 2-methylcyclopentanone (20), β-ionone (21), α-ionone (22), methyl salicylate (23), dihydroactinidiolide (25), hexanal (26), benzaldehyde (27), nonanal (28), (E,E)-2, 4-heptadienal (31), benzeneacetaldehyde (32), phenol (33), β-pinene (34), longifolene (35), and limonene (36).
The findings demonstrated that aroma sensory analysis depends not only on the content of aroma components and the odor threshold, but also on aroma components that potentially interact with each other, further influencing the sensory evaluation of individual aroma components (Guichard, 2002). “Fermented” correlated positively with furfuryl alcohol (5), 1,2-dimethoxybenzene (13), 3,4,5-trimethoxytoluene (14), 1,2,4-trimethoxy benzene (17), benzaldehyde (27), and benzeneacetaldehyde (32), with 1,2-dimethoxybenzene (13), 3,4,5-trimethoxytoluene (14), benzaldehyde (27), and benzeneacetaldehyde (32) being the primary contributors to “fermented.” “Aged” positively correlated with geranyl alcohol (8), 1,2,3-trimethoxybenzene (15), 4-ethyl-1,2-dimethoxybenzene (16), β-ionone (21), α-ionone (22), methyl salicylate (23), and (E,E)-2,4-heptadienal (31), with 1,2,3-trimethoxybenzene (15) being the primary contributor to “aged.” Methoxy compounds are considered the primary contributors to the aroma of aged fragrances; their increased content resulted in the synthesis of the aged aroma of Pu'er tea (Liu et al., 2022).
“Floral” and “fruity” were positively correlated with linalool oxide1 (3), linalool oxide2 (4), benzyl alcohol (9), phenylethyl alcohol (10), isoamyl alcohol (11), dihydroactinidiolide (25), hexanal (26), and nonanal (28), with phenylethyl alcohol (10) being the primary contributor to “floral” and “fruity.” “Sweet” was positively correlated with linalool (2), 2-methylcyclopentanone (20), β-pinene (34), longifolene (35), and limonene (36), with linalool (2) being the primary contributor to “sweet.” Enzymatic hydrolysis of glycosides can generate alcohol compounds, particularly linalool and its oxides, increasing their relative content; this explains why Pu'er tea has a distinct floral and fruity scent (Xu et al., 2007).
4. Conclusions
The goal of this study was to investigate the key aroma components that influence the quality of Pu'er tea that had been inoculated with different proportions of Rhizopus, Saccharomyces cerevisiae, and Aspergillus niger. SDE and GC–MS were used to detect and analyze the aroma components of Pu’er tea by traditional natural fermentation, which included the inoculation of Rhizopus and Saccharomyces: Rhizopus: Aspergillus niger in ratios of 2:1:2, 2:2:2, and 2:3:2. A total of 36 aroma components were extracted. The results showed that the five fermentation methods of Pu'er tea were clearly differentiated, and ROAV showed that low-threshold β-ionone (21) was the key aroma component of naturally fermented and rhizobium-inoculated Pu'er tea. Floral and fruity aroma compounds such as linalool and its oxides were the key aroma components of synergistically fermented Pu'er teas. PLSR analyses showed that the fermentation method and microbial composition had a great influence on the sensory characteristics of Pu'er tea. The results of this study can provide a theoretical basis for optimising the fermentation technology and improving the flavour quality of Pu'er tea. In addition, the contribution of aroma components from different fermentation methods to the overall aroma of Pu'er tea should be determined by reconstitution and omission experiments.
CRediT authorship contribution statement
Xuehang Yan: Writing – review & editing, Writing – original draft, Visualization, Investigation, Formal analysis, Data curation, Conceptualization. Yang Tian: Validation, Project administration. Feng Zhao: Investigation, Formal analysis. Ruifang Wang: Investigation, Formal analysis. Hongjie Zhou: Resources. Naiming Zhang: Resources. Yuefei Wang: Supervision. Zhiguo Shan: Writing – review & editing, Funding acquisition, Data curation, Conceptualization. Chunhua Zhang: Writing – review & editing, Methodology, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.101048.
Contributor Information
Zhiguo Shan, Email: 353879230@qq.com.
Chunhua Zhang, Email: 190888358@qq.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- General Administration of Quality Supervision, I.a.Q.o.t.P.s.R.o.C., Administration, C.N.S., 2018. Method for sensory evaluation of tea. General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China; China National Standardization Administration, p. 28.
- Barra A., Baldovini N., Loiseau A.M., Albino L., Lesecq C., Lizzani Cuvelier L. Chemical analysis of French beans (Phaseolus vulgaris L.) by headspace solid phase microextraction (HS-SPME) and simultaneous distillation/extraction (SDE) Food Chemistry. 2007;101:1279–1284. [Google Scholar]
- Deng X., Huang G., Tu Q., Zhou H., Li Y., Shi H.…Xu W. Evolution analysis of flavor-active compounds during artificial fermentation of Pu-erh tea. Food Chemistry. 2021;357 doi: 10.1016/j.foodchem.2021.129783. [DOI] [PubMed] [Google Scholar]
- Guichard E. Interactions between flavor compounds and food ingredients and their influence on flavor perception. Food Reviews International. 2002;18:49–70. [Google Scholar]
- Hu T., Shi S., Ma Q. Modulation effects of microorganisms on tea in fermentation. Frontiers in Nutrition. 2022;9 doi: 10.3389/fnut.2022.931790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji A., Gong W., Peng W., Liu C., Zeng Y., Yan L. Modern Agricultural Science and Technology; 2016. Research progress in microorganisms in Pu'er tea; pp. 253–255. [Google Scholar]
- Li Y., Hao J., Zhou J., He C., Yu Z., Chen S.…Ni D. Pile-fermentation of dark tea: Conditions optimization and quality formation mechanism. LWT. 2022;166 [Google Scholar]
- Li J., Wu J., Xu N., Yu Y., Brake J., Xu R., Wu X. Dynamic evolution and correlation between microorganisms and metabolites during manufacturing process and storage of Pu-erh tea. LWT. 2022;158 doi: 10.3390/metabo11100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Xu Y., Wu J., Wen J., Yu Y., An K., Zou B. GC-IMS and olfactometry analysis on the tea aroma of Yingde black teas harvested in different seasons. Food Research International. 2021;150 doi: 10.1016/j.foodres.2021.110784. [DOI] [PubMed] [Google Scholar]
- Liu S., Yang L., Zhou Y., He S., Li J., Sun H.…Xu S. Effect of mixed moulds starters on volatile flavor compounds in rice wine. LWT. 2019;112 [Google Scholar]
- Liu P., Zheng P., Feng L., Gong Z., Zheng L., Gao S.…Liu Z. Dynamic changes in the aroma profile of Qingzhuan tea during its manufacture. Food Chemistry. 2022;375 doi: 10.1016/j.foodchem.2021.131847. [DOI] [PubMed] [Google Scholar]
- Lv S., Wu Y., Li C., Xu Y., Liu L., Meng Q. Comparative analysis of Pu-erh and Fuzhuan teas by fully automatic headspace solid-phase microextraction coupled with gas chromatography-mass spectrometry and chemometric methods. Journal of Agricultural and Food Chemistry. 2014;62:1810–1818. doi: 10.1021/jf405237u. [DOI] [PubMed] [Google Scholar]
- Lv H.-P., Zhong Q.-S., Lin Z., Wang L., Tan J.-F., Guo L. Aroma characterisation of Pu-erh tea using headspace-solid phase microextraction combined with GC/MS and GC–olfactometry. Food Chemistry. 2012;130:1074–1081. [Google Scholar]
- Nian B., Jiao W., He M., Liu Q., Zhou L., Jiang B.…Zhao M. Comparison of biochemical components and aroma substances between flower and fruit scented and aged Pu'er tea. Modern Food Science and Technology. 2020;36:241–248. [Google Scholar]
- Pan X., Zhang S., Xu X., Lao F., Wu J. Volatile and non-volatile profiles in jujube pulp co-fermented with lactic acid bacteria. LWT. 2022;154 [Google Scholar]
- Pang, X., Yu, W., Cao, C., Yuan, X., Qiu, J., Kong, F., & Wu, J., 2019. Comparison of Potent Odorants in Raw and Ripened Pu-Erh Tea Infusions Based on Odor Activity Value Calculation and Multivariate Analysis: Understanding the Role of Pile Fermentation. Journal of Agricultural and Food Chemistry 2019. [DOI] [PubMed]
- Piao M., Zhang Y., Chen T. Effects of different de-enzyming methods on microbial composition and volatile compounds of raw Pu’ er tea based on microbiome and metabolomics. Food Bioscience. 2022;48 [Google Scholar]
- Shang A., Li J., Zhou D.-D., Gan R.-Y., Li H.-B. Molecular mechanisms underlying health benefits of tea compounds. Free Radical Biology and Medicine. 2021;172:181–200. doi: 10.1016/j.freeradbiomed.2021.06.006. [DOI] [PubMed] [Google Scholar]
- Vanheimer L. (2nd Edition)(Chinese Edition). 2018. Compound Olfactory Threshold Compilation. [Google Scholar]
- Wang C., Li J., Wu X., Zhang Y., He Z., Zhang Y.…Liu Z. Pu-erh tea unique aroma: Volatile components, evaluation methods and metabolic mechanism of key odor-active compounds. Trends in Food Science & Technology. 2022;124:25–37. [Google Scholar]
- Wang S., Qiu Y., Gan R.-Y., Zhu F. Chemical constituents and biological properties of Pu-erh tea. Food Research International. 2022;154 doi: 10.1016/j.foodres.2021.110899. [DOI] [PubMed] [Google Scholar]
- Wang R., Sun J., Lassabliere B., Yu B., Liu S.Q. Biotransformation of green tea (Camellia sinensis) by wine yeast Saccharomyces cerevisiae. Journal of Food Science. 2020;85:306–315. doi: 10.1111/1750-3841.15026. [DOI] [PubMed] [Google Scholar]
- Wang R., Sun J., Lassabliere B., Yu B., Liu S.Q. UPLC-Q-TOF-MS based metabolomics and chemometric analyses for green tea fermented with Saccharomyces boulardii CNCM I-745 and Lactiplantibacillus plantarum 299V. Current Research in Food Science. 2022;5:471–478. doi: 10.1016/j.crfs.2022.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Zhang Y., Wang Y., Ju H., Niu C., Song Z.…Yue T. Assessment of chemical composition and sensorial properties of ciders fermented with different non-Saccharomyces yeasts in pure and mixed fermentations. International Journal of Food Microbiology. 2020;318 doi: 10.1016/j.ijfoodmicro.2019.108471. [DOI] [PubMed] [Google Scholar]
- Xu X., Mo H., Yan M., Zhu Y. Analysis of characteristic aroma of fungal fermented Fuzhuan brick-tea by gas chromatography/mass spectrophotometry. Journal of the Science of Food and Agriculture. 2007;87:1502–1504. [Google Scholar]
- Xu A., Xiao Y., He Z., Liu J., Wang Y., Gao B.…du Z. Use of non-saccharomyces yeast co-fermentation with saccharomyces cerevisiae to improve the polyphenol and volatile aroma compound contents in nanfeng tangerine wines. Journal of Fungi. 2022;8:128. doi: 10.3390/jof8020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X. Identification of volatile and odor-active compounds in Hunan black tea by SPME/GC-MS and multivariate analysis. LWT. 2022;164 [Google Scholar]
- Yue Y., Shi T., Liu J., Tian Q., Yang X., Wang L. Genomic, metabonomic and transcriptomic analyses of sweet osmanthus varieties provide insights into floral aroma formation. Scientia Horticulturae. 2022;306 [Google Scholar]
- Zhang H., Huang D., Pu D., Zhang Y., Chen H., Sun B., Ren F. Multivariate relationships among sensory attributes and volatile components in commercial dry porcini mushrooms (Boletus edulis) Food Research International. 2020;133 doi: 10.1016/j.foodres.2020.109112. [DOI] [PubMed] [Google Scholar]
- Zhang D., Ji H., Liu S., Gao J. Similarity of aroma attributes in hot-air-dried shrimp (Penaeus vannamei) and its different parts using sensory analysis and GC–MS. Food Research International. 2020;137 doi: 10.1016/j.foodres.2020.109517. [DOI] [PubMed] [Google Scholar]
- Zhang C., Shan Z., Yuan W., Li Y., Sun T., Qin T.…Zhou H. Study on the effect of solid state fermentation with different beneficial bacteria on the aroma components of Pu'er Tea. Journal of Tea Science. 2010;30:251–258. [Google Scholar]
- Zhang M., Zhong T., Heygi F., Wang Z., Du M. Effects of inoculation protocols on aroma profiles and quality of plum wine in mixed culture fermentation of Metschnikowia pulcherrima with Saccharomyces cerevisiae. LWT. 2022;161 [Google Scholar]
- Zheng, Y., Zhang, C., Ren, D., Bai, R., Li, W., Wang, J., Shan, Z., Dong, W., & Yi, L., 2023. Headspace solid-phase microextraction coupled with gas chromatography-mass spectrometry (HS-SPME-GC-MS) and odor activity value (OAV) to reveal the flavor characteristics of ripened Pu-erh tea by co-fermentation. Frontiers in Nutrition 10. [DOI] [PMC free article] [PubMed]
- Zheng X.Q., Li Q.S., Xiang L.P., Liang Y.R. Recent advances in volatiles of teas. Molecules (Basel, Switzerland) 2016;21:338. doi: 10.3390/molecules21030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.