Abstract
Drug delivery to the brain is crucial in the treatment for central nervous system disorders. While significant progress has been made in recent years, there are still major challenges in achieving controllable drug delivery to the brain. Unmet clinical needs arise from various factors, including controlled drug transport, handling large drug doses, methods for crossing biological barriers, the use of imaging guidance, and effective models for analyzing drug delivery. Recent advances in micro/nanosystems have shown promise in addressing some of these challenges. These include the utilization of microfluidic platforms to test and validate the drug delivery process in a controlled and biomimetic setting, the development of novel micro/nanocarriers for large drug loads across the blood-brain barrier, and the implementation of micro-intervention systems for delivering drugs through intraparenchymal or peripheral routes. In this article, we present a review of the latest developments in micro/nanosystems for controllable drug delivery to the brain. We also delve into the relevant diseases, biological barriers, and conventional methods. In addition, we discuss future prospects and the development of emerging robotic micro/nanosystems equipped with directed transportation, real-time image guidance, and closed-loop control.
Graphical abstract
Public summary
-
•
Micro/nanosystems show their potential to address the challenges of precise drug delivery to the brain.
-
•
Microfluidic platforms enable the creation of biomimetic in vitro brain models.
-
•
Micro/nano materials is emerging as a key player in controllable brain drug delivery.
-
•
The minimally invasive fiberbot microsystem reduces the procedure’s invasiveness.
-
•
Image tracking of micro/nanosystems allows for controlled therapeutic interventions.
Introduction
The global burden of brain diseases is on the rise, making them a significant cause of disability and mortality worldwide.1 As the world is transitioning into an aging population, the prevalence of neurodegenerative disorders including Alzheimer’s disease (AD), Parkinson’s disease (PD), and cerebrovascular diseases like stroke, are posing increasing threats to the society. In addition, mental illnesses have recently gained recognition as substantial contributors to the overall global disease burden.2 Furthermore, primary brain tumors and brain metastases persist as conditions universally associated with high fatality rates.3 Accidents are also a common cause of brain trauma and spinal cord injuries. Moreover, rare diseases, including those affecting the brain such as amyotrophic lateral sclerosis (ALS), have recently garnered substantial attention. Consequently, the imperative of developing drugs with enhanced transport and targeting efficiency for brain-related conditions has come to the forefront. Unfortunately, the commercial development of drugs for brain diseases faces significant obstacles, characterized by protracted research and development timelines and elevated rates of failure.4 This predicament primarily emanates from challenges inherent in delivering drugs to the brain, notably biological barrier limitations.5 Micro/nanosystems offer promising alternatives for facilitating drug delivery to the brain due to their small size and precise manipulability. Moreover, the deployment of in vitro human brain models fabricated utilizing micro/nanosystems, furnishes robust tools for drug screening, personalized treatment strategies, and pathological studies.6
This review seeks to offer a comprehensive overview of the challenges associated with drug delivery for brain diseases and the emerging solutions that capitalize on the advancements in micro/nanosystems. First, we focus on the utilization of micro/nanosystems, with a particular emphasis on microfluidic systems, for the fabrication of in vitro brain models, encompassing both artificial and self-forming structures. Subsequently, we systematically explore micro/nano drug delivery systems, covering both non-invasive vascular-brain delivery approaches and invasive methods. In addition, we present the current state-of-the-art in micro/nano drug delivery systems within clinical settings and evaluate the impediments faced in translating these innovations from laboratory settings to practical bedside applications. Finally, we offer insights into the emerging micro/nanorobotic brain drug delivery systems, characterized by targeted transport, real-time image guidance, and closed-loop control mechanisms.
Prevalence of brain diseases
Brain and nervous system cancers constitute approximately 1%–2% of the total global cancer cases reported annually.7 Within this category, malignant brain tumors, notably glioblastoma, stand out for their exceptionally high mortality rates and significant contribution to overall cancer-related fatalities. The 5-year relative survival rate for these tumors hovers at a meager 30%,8 and glioblastoma itself offers a particularly grim prognosis, with a median survival period of less than 2 years.9 Moreover, approximately 20% of cancer patients encounter brain metastases, which represent the primary cause of death in individuals afflicted by advanced cancer.10 Neurodegenerative diseases encompass a cluster of disorders characterized by the progressive degeneration of nerve cells, leading to declining motor function, memory, and cognition. Common neurodegenerative diseases include AD, PD, Huntington’s disease, and ALS. Globally, an estimated 50 million individuals live with dementia, with higher prevalence rates observed in low- and middle-income countries, thus imposing a substantial burden on affected families and society at large.11 Although other neurodegenerative diseases may exhibit lower incidence rates, their impact remains substantial.12,13,14 Stroke, including both ischemic and hemorrhagic strokes, stands as a significant contributor to disability and mortality worldwide, impacting one in four people during their lifetime, yet with limited treatment options.15,16 The cornerstone for the acute management of stroke presently resides in intravenous tissue plasminogen activator for thrombolysis.17 However, this therapy requires high doses, making it susceptible to complications and therefore demands controlled release mechanisms. Traumatic brain injury represents a grave form of brain damage occurring in over 20 million cases worldwide each year.18 All of these brain disorders share a common challenge: the intricacies of drug delivery to the brain, and excellent therapies are desperately needed.
Challenges of drug delivery to the brain
Drug development in the central nervous system (CNS) is well recognized as a challenging endeavor. Historical data indicate that approval rates by US Food and Drug Administration (FDA) for CNS drugs are less than half of those for non-CNS drugs, with prolonged approval timelines.4 These endeavors encounter several limitations including biological barrier penetration, cell-specific targeting, limited drug distribution, drug toxicity, and preclinical models.
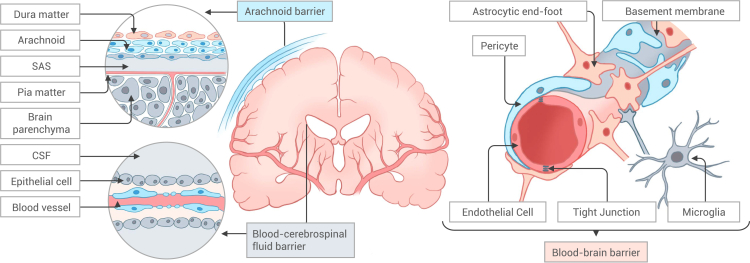
The brain is safeguarded by a complex and intricately regulated biological barrier that serves the dual purpose of preventing the entry of harmful substances into the brain and controlling the delivery of drugs, as illustrated in Figure 1. These barriers primarily consist of the blood-brain barrier (BBB), blood-cerebrospinal fluid (CSF) barrier, and the arachnoid barrier.19 The BBB, in particular, constitutes a highly sophisticated structure that acts as a critical separation between the circulating blood and the interstitial fluid at the brain capillary site, thereby shielding the brain from potentially harmful substances. Positioned at the core of the neurovascular unit (NVU), the BBB is composed of brain microvessel endothelial cells (BMVECs) and the extracellular matrix (ECM). BMVECs are interconnected by tight junctions, resulting in low paracellular permeability, and express transporters and receptors that regulate the transcytosis of essential substance.20 Pericytes are found in the perivascular region, maintaining the integrity and functionality of the BBB. The endfoot of astrocytes encircle the vessels, offering nutrients and facilitating the formation of tight junctions. The abluminal surface of endothelial cells (ECs) is coated by the basement membrane. Together, these components constitute the rest of the NVU.20,21,22,23
Figure 1.
Biological barrier in the brain
Biological barriers in the brain include the arachnoid barrier, the blood-cerebrospinal fluid barrier, and the blood-brain barrier. The blood-brain barrier consists of endothelial cells, pericytes, astrocyte endfeet, and basement membranes, distinguished by tight junctions between endothelial cells.
CSF is a transparent, colorless fluid that envelops the brain and spinal cord and serves to cushion and provide nutrients while facilitating waste product excretion.24 CSF is primarily generated by the choroid plexus in the lateral ventricles and the third and fourth ventricles, and flows via the fourth ventricle into the subarachnoid space of the brain and spinal cord, eventually being absorbed through the arachnoid villi into the superior sagittal sinus of the peripheral blood flow.25 The epithelial cells of the choroid plexus establish the blood-cerebrospinal fluid barrier (BCSFB) that separates the CSF from the blood circulation. Unlike the BBB, the BCSFB exhibits higher permeability. Consequently, it is possible to introduce drugs into the CSF, which can subsequently traverse into the bloodstream and, eventually, access the brain parenchyma via the BBB. However, this approach suffers from a low penetration rate. Alternatively, drugs can diffuse into the brain parenchyma through the CSF, but this typically necessitates high doses and is associated with the risk of inducing neurotoxicity.26 Intrathecal and intracerebroventricular (i.c.v.) injections in nanomedicine hold new promise for CSF delivery, but still require meticulous control.27 The arachnoid barrier, a distinctive type of BCSFB, consists of connective tissue and arachnoid cells that enshroud around the spinal cord and brain. The arachnoid barrier separates the fenestrated vessels of the dura mater from the CSF in the subarachnoid space, regulating the exchange of molecules into and out of the CSF. Recent studies have identified the developmental processes and crucial protective functions of the arachnoid barrier in prenatal CNS,28 revealing how fetal barrier hypoplasia can render susceptibility to meningitis. Subarachnoid injections have been employed for anesthesia and analgesia and hold potential for other drug delivery purposes.29
The scarcity of preclinical models capable of faithfully replicating the intricate structure of the human brain poses constraints on drug development and validation. Conventional in vitro models that emulate the BBB such as parallel artificial membrane permeability assays and transwell assays are structurally rudimentary and lack physiological fidelity.30 In vivo experimental animal models also fall short in faithfully mirroring human biology due to interspecies variability.31 Recent developments in microfluidics have ushered in a novel approach for BBB research, namely BBB-on-chip models (μBBBs). μBBBs facilitate the co-cultivation of diverse human cell types and replicates essential physical processes, including shear stress, thus creating a meticulously controlled microphysiological system.32 The μBBB platform is versatile, accommodating applications in the study of CNS pathology, high-throughput drug screening, and neurotoxicity assessments.33,34,35 When combined with organoids, it offers a more realistic emulation of the human brain and is poised to become the next-generation platform for CNS drug screening.36
Microfluidic systems for in vitro models
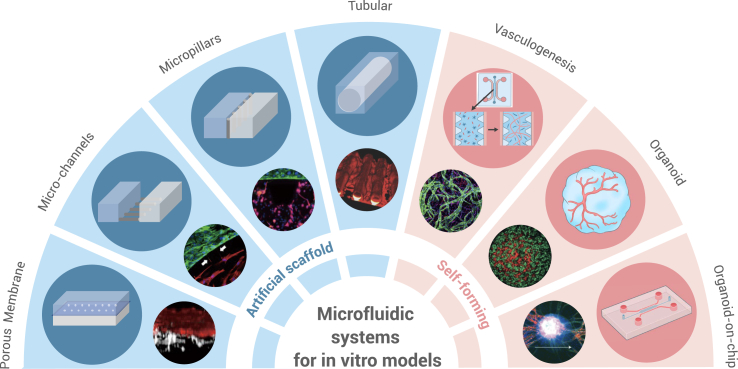
Recent advancements in microfluidics and microfabrication technologies have facilitated the emergence of the organ-on-chip model for in vitro drug screening.37 In the realm of brain research, the introduction of the μBBBs has enabled the emulation of intricate human brain structures in vitro. The μBBB typically comprises three-dimensional (3D) vascular endothelium formed by BMVECs and can be co-cultured with astrocytes and pericytes to more accurately replicate NVU structures.38 The continuous perfusion provides shear stress to the vascular endothelium restoring the mechanical conditions the ECs are subjected to. The μBBBs can be categorized into two main types based on cell distribution and cellular assembly: artificial structures and self-forming structures (Figure 2). In the self-forming model, BMVECs, astrocytes, and pericytes are co-cultured within an ECM gel, allowing them to autonomously develop into a 3D vascular network that closely resembles the native vascular architecture.39 Personalized medicine is made possible through the utilization of patient-derived induced pluripotent stem cell (iPSC)-derived brain microvascular endothelial cells (iBMECs) in constructing self-forming models.40 Nevertheless, this model demands precise control over vascular EC patterning to enhance the efficiency of drug screening.
Figure 2.
Microfluidic systems for in vitro models
Microfluidic systems offer the capability to create both artificial and self-forming in vitro models. Artificial scaffold designs primarily encompass porous membranes, microchannels, micropillars, and tubular configurations. Meanwhile, self-forming in vitro models predominantly include vasculogenesis designs, organoids, and organoids-on-chip. Microscopic images were reproduced from Campisi and co-workers.39,41,42,43,44,45,46 Copyright 2020, Springer Nature; 2019, Wiley Periodicals; 2021, Springer Nature; 2018, Wiley-VCH; 2018, Elsevier; 2013, Springer Nature; 2017, The Royal Society of Chemistry.
Artificial structures
The artificial structure of μBBBs can be categorized into vertical structure, parallel structure, and tubular structure (Figure 3). The vertical design typically comprises two channels, top and bottom, separated by a porous membrane. The top channel represents the blood side and is typically composed of BMECs or iBMECs, while the bottom channel represents the brain side and generally includes astrocytes and pericytes.38 These two channels create a conventional sandwich-like configuration that can be arranged either in an upward or downward orientation.40,41 The vertical design offers a substantial contact area, facilitating the examination of the mechanisms underlying the transport of substances across the BBB and their permeation effects. However, due to the overlapping nature of the upper and lower channels and the limited transparency of the porous membrane, obtaining high-resolution images with an inverted microscope can be challenging, necessitating the utilization of more complex 3D high-resolution imaging techniques.47 Ahn et al. have effectively employed tri-culture vertical μBBB systems to precisely observe the distribution and uptake of nanoparticles (NPs) within various cells.41 In addition, the simple structure of the vertical design allows for batch manufacturing for high-throughput drug screening.34
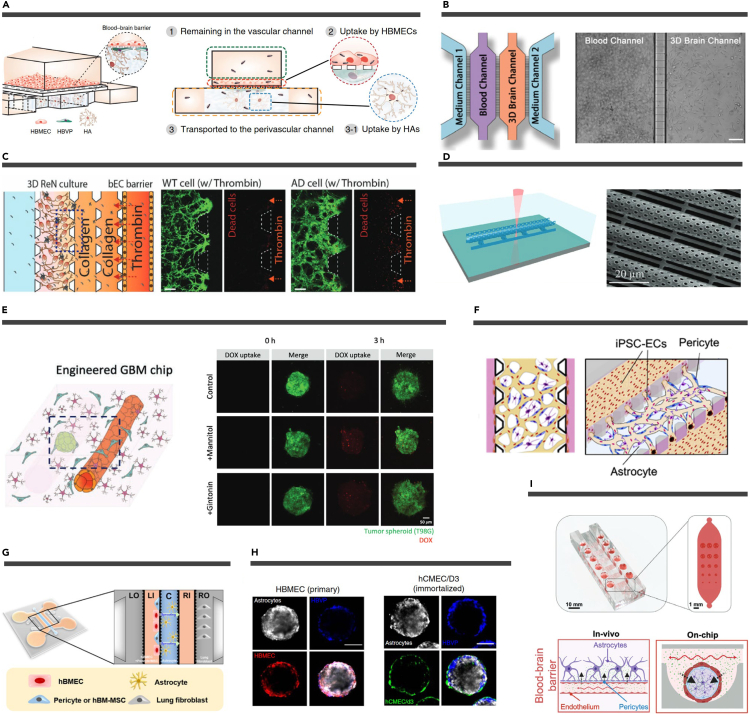
Figure 3.
Artificial structures and self-forming structures of μBBBs
(A) Schematic of a vertically designed μBBBs containing three types of cells. Reproduced from Ahn et al.41 Copyright 2020, Springer Nature.
(B) Schematic diagram (left) and bright-field images (right) of a microchannel-profiled μBBB model. Scale bars, 100 μm. Reproduced from Fan et al.48 Copyright 2023, The Royal Society of Chemistry.
(C) A micropillars-designed μBBB to mimic damage to nerve cells by thrombin influx through a damaged BBB. Dead cells are shown in red. Scale bars, 200 μm. Reproduced from Shin et al.49 Copyright 2019, Wiley-VCH.
(D) Fabrication schematic (left) and SEM image (right) of a TPP printed tubular μBBB design. Reproduced from Marino et al.44 Copyright 2018, Wiley-VCH.
(E) Schematic of a tubular design μBBB + GBM (left). Fluorescent images showing the uptake of DOX by GBM spheres (right). Reproduced from Seo et al.50 Copyright 2022, Wiley-VCH.
(F) Schematic representation of a vasculogenesis BBB microvascular network model that mimics the microvascular structure in the brain environment. Reproduced from Campisi et al.39 Copyright 2018, Elsevier.
(G) Schematic diagram of the angiogenesis microfluidic chip. Reproduced from Kim et al.51 Copyright 2021, Elsevier.
(H) Representative confocal images showing the organization of human astrocytes (white), HBVP (blue), and (left) primary HBMEC (red) or (right) immortalized human cerebral microvascular ECs (hCMEC/D3; green) when co-cultured to form a spheroid. Reproduced from Cho et al.52 Copyright 2017, Springer Nature.
(I) A cutaway rendering of the microfluidic spheroid array (top). Overview of the established 3D BBB model system (bottom). The arrows indicate the transport of the drug. Reproduced from Eilenberger et al.53 Copyright 2021, Wiley-VCH.
The parallel design typically comprises multiple side-by-side channels interconnected by microchannels or micropillars. These narrow channels are capable of housing a single cell, thereby serving as interfaces for cellular communication without permitting cell migration across the channels. Microchannels offer the advantage of observing various processes, such as NP permeation, synaptic connections between neurons, and the infiltration of tumor metastatic cells at the single-cell level.48,54,55,56 Fan et al. developed a microchannels design to investigate the interaction of drug nanocarrier with BBB and revealed that Tf-PEG-AuNP exhibit exceptional BBB penetration with minimal protein absorption.48 Constructing microfluidic models to explore interactions between neuro-glial cells in brain diseases enhances our comprehension of the underlying molecular mechanisms of pathogenesis and facilitates the screening of potential therapeutic approaches.57,58 To more faithfully replicate the human BBB structure, micropillar designs were developed. These designs are often used in combination with matrix gels on the brain side to emulate the basement membrane to which BMVECs adhere.43 These micropillars typically possess a trapezoidal structure, with multiple micropillars arranged to generate surface tension and confine the hydrogel within the brain channel.59 Shin et al. designed a multichannel AD BBB dysfunction in vitro model separated by multiple sets of micropillars to demonstrate the entry of neurotoxin thrombin into the brain through a compromised BBB, as well as to assess the potential mitigating effects of BBB enhancers on AD.49
The above two μBBB structures are typically fabricated by mask-based photolithography resulting in rectangular cross-sectional channels. However, disparities in the morphology of the EC layer emerge between the cuboid geometry and the vessel-like cylindrical structure due to variations in shear stress distribution.30 Consequently, a novel fabrication method has been introduced to produce full-contact tubular μBBB designs, which more faithfully replicate the mechanical properties of in vivo blood flow to ECs. One approach involves the pre-embedding of cylindrical microneedles into polydimethylsiloxane, followed by their removal after curing, to establish microchannels.50,60,61 For example, Seo et al. constructed 3D vascular structures and co-cultured them with glioma spheres to validate combination therapy with BBB-opening and chemotherapeutic agents.50 Furthermore, two-photon polymerization (TPP)-based stereolithography permits the fabrication of intricate microstructures for constructing tubular vascular scaffolds.62 Ciofani and co-workers constructed both two-component and three-component μBBB models by printing side-by-side tubular structures with microporosity in a single molding process using TPP technology.44,63 The demonstrated utility of TPP for microcomponent processing in microfluidic chips carries significant implications for μBBB applications.64 The above three artificial μBBB structures realize regular and controllable cellular arrangements, which have great applications in the fields of CNS drug screening and brain pathological study.
Self-forming structures
The artificial μBBB structure aims to create engineered microvessels within a designated framework. Yet, there remains a gap between these structured vessels and the intricate capillary network of the human body. In recent years, self-forming μBBB structures have emerged, including the vasculogenesis design and vascularized organoids on a chip, which enable the formation of 3D brain microvascular networks (Figure 3).32,36 The vasculogenesis design employs iPSC-derived ECs or primary BMVECs to generate irregular vascular networks and are coupled with primary pericytes and astrocytes to form BBB structures.39,65 Human pluripotent stem cells can also derive pericytes that can be integrated into the models described above.66 Kamm and co-workers used this system to construct a 3D self-organized microvascular model and obtained gene expression profiles and quantitative immunocytochemistry similar to that of natural BBB and with lower permeability than conventional μBBBs.32,39 Moreover, some researchers have developed the angiogenesis design for μBBB models to simulate the angiogenic sprouting during BBB formation in a microfluidic context.67,68 This design is useful for investigating various aspects of BBB generation, such as the role of various perivascular cells,69 the role of interstitial flow,70 and the mechanisms of BBB regeneration.51 Kim et al. demonstrated the potential of stem cell therapy in BBB injury regeneration using an angiogenesis microfluidic chip.51 To achieve a predetermined vascular distribution, ECs can be patterned within hydrogels. Several pre-patterning methods based on geometric constraints,71 3D printing,72 and temporary scaffolds73 have been employed to generate regular vessels. Acoustophoresis, as a non-contact cell manipulation method, also holds potential for controlled vasculogenesis.74
The hydrogel-based self-forming vascular model described above exhibits a limitation in its interaction with NVU cells, thereby diverging from the in vivo context. To address this issue, 3D cellular aggregates, such as spheroids and organoids, composed of multiple organ-specific cells, offer a solution by facilitating optimal intercellular contact.75 The spheroid μBBB model is structured by coating primary or immortalized astrocytes and pericytes on spheroids formed by BMVECs.52 A notable protocol devised by Cho and co-workers successfully generated BBB spheroids comprising ECs, astrocytes, and pericytes. This advancement enabled effective screening of CNS therapeutics through optical and mass spectrometry techniques.52,76 Furthermore, the incorporation of various NVU cell types, including neurons, oligodendrocytes, and microglia, into these spheroids has improved their ability to mimic drug penetration within the body.77,78 Distinct from spheroids, the organoid μBBB model represents a cellular aggregate generated from stem cells.79 Cerebral organoids generated from human embryonic stem cells or human iPSCs have become well established.80 However, vascularization of cerebral organoids has only recently been developed to address the limitations in CNS drug screening.81,82,83 It is worth noting that constructing a BBB organoid typically spans several months and presents a complex internal structure, necessitating additional efforts to translate it into a drug screening model.
Recent techniques have emerged for integrating 3D cellular aggregates into microfluidic chips, creating “organoid-on-chip” systems. Ao et al. developed a microfluidic chip for dynamic perfusion of human brain organoids to study immune cell-driven brain aging.84 Microfluidic chips also offer high-throughput capabilities,85 demonstrated by Eilenberger et al., who created spheroid arrays on these chips to assess BBB permeability.53 Recently, micro-electro-mechanical systems have also been applied to brain organoid-on-chip. Huang et al. fabricated shell microelectrode arrays to record spatiotemporal signals from brain organoids.86 In conclusion, self-forming μBBBs aim to faithfully mimic the human BBB, offering potential for improved CNS therapeutics in preclinical trials.
In the broader context, artificial and self-forming structures exhibit distinctions in their physiological relevance and processing convenience, rendering them suitable for distinct objectives. Artificial structures offer advantages by providing well-defined and stable culture conditions.87 Moreover, they offer a clear optical window enabling high-resolution imaging.30 Consequently, artificial structures find utility in commercial settings for applications like brain drug screening, neurotoxicity testing, and personalized medicine due to their controllability and standardized modeling. Conversely, self-forming structures excel in physiological relevance and microenvironment control, closely resembling the natural microenvironment of organs and tissues.30 While the setup for self-forming structures is increasingly standardized, its technical demands restrict large-scale applications. Self-forming structures are better suited for research-focused objectives such as brain tumors, neuroinflammatory and neurodegenerative diseases, viral infections, and neurodevelopment due to their high fidelity to human physiology.
In the context of in vitro BBB models, both artificial and self-forming structures must prioritize integrity and validity concerning their resemblance to the human BBB. The integrity of μBBBs can be assessed through measurements of transepithelial/transendothelial electrical resistance (TEER) and the permeation of fluorescent dyes.32,88 Currently, μBBBs featuring human iPSC-derived ECs co-cultured with astrocytes can achieve TEER values of 4,000–5,000 Ω cm2,89 closely approximating the in vivo value of 5,900 Ω cm2.90 To further test integrity, permeability experiments utilizing small-molecule fluorescent probes, including fluorescently labeled dextrans of varying molecular weights, are conducted.91 To establish the validity of in vitro BBB models, these models must replicate key attributes of the natural BBB. Expression of tight junction proteins ZO-1 and claudin 5, adherens junction proteins CD31 and VE-cadherin, basement membrane proteins laminin and collagen IV, glycocalyx proteins heparan sulfate proteoglycan 2, and hyaluronic acid should be confirmed in μBBBs by immunofluorescence staining.32 Furthermore, the gene expression profiles related to the BBB should align with in vivo profiles, confirmed through RT-qPCR.39 In vitro BBB models can also be assessed for the secretion of cytokines affecting BBB function, such as C-C motif chemokine ligand 2 (CCL2), IL-8, and IL-6, using ELISA.92 Finally, direct comparisons between in vitro BBB models and in vivo BBB in mice, demonstrating consistent permeability, are now achievable through intravital microscopy.93
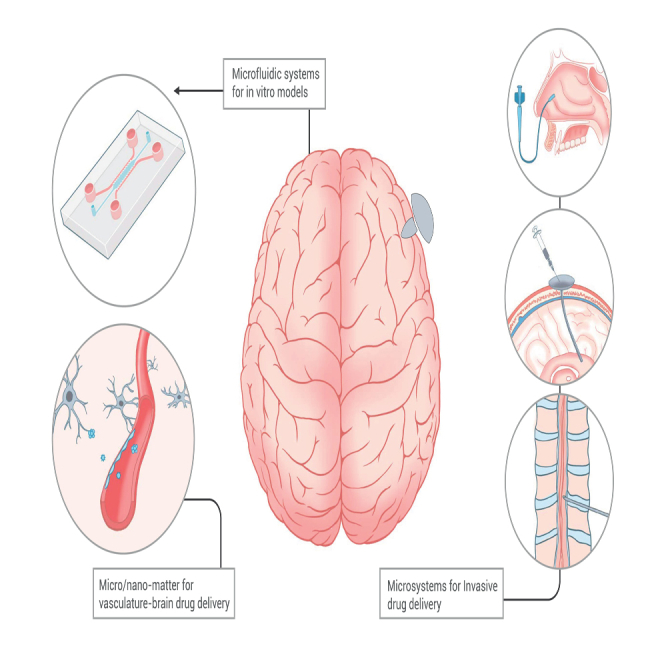
Micro/nanomatters for vasculature-brain drug delivery
The application of micro/nanosystems in brain drug delivery is primarily categorized into two approaches: systemic delivery and interventional delivery.94 Systemic administration primarily involves vasculature-brain drug delivery, typically through intravenous means. BBB blocks over 98% of small-molecule drugs and nearly 100% of large-molecule drugs.95 Micro/nanomatters provide three primary strategies to surmount this challenge: (1) employing carriers of biological origin, (2) engineering carriers with the ability to penetrate the BBB, and (3) regulating the transient opening of the BBB (Figure 4).
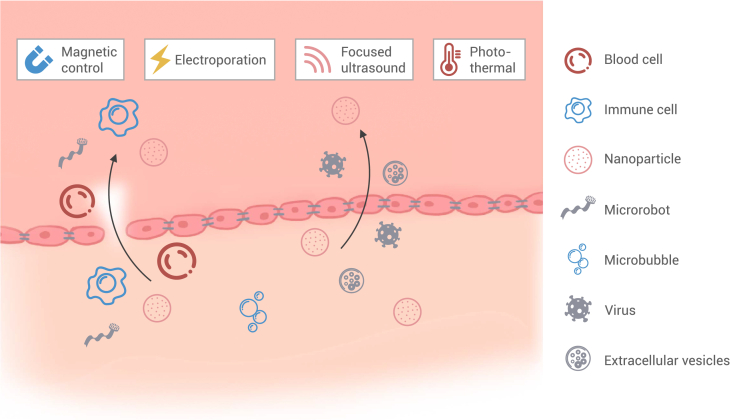
Figure 4.
Micro/nanomatters for vasculature-brain drug delivery
Both biological carriers and artificial micro/nanomatters can cross the BBB through intercellular and intracellular routes. The BBB, along with micro/nano materials, can be controlled using external stimuli, such as magnetic fields, electroporation, focused ultrasound, and photothermal.
Biological carriers
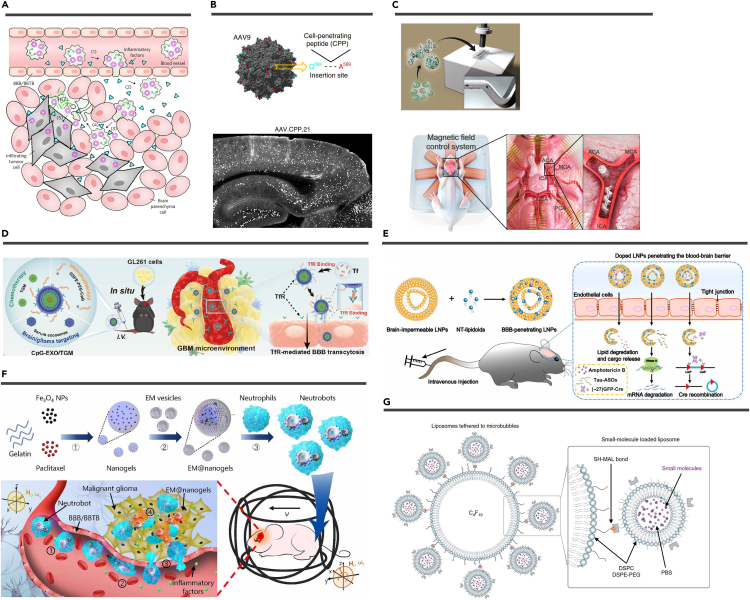
Biological carriers have been engineered to function as innate vehicles for drug delivery due to their intrinsic capacity to target and cross the BBB. Examples of such carriers include blood cells,96 neurotropic viruses,97 and extracellular vesicles (EVs).98,99 In addition, cell-based microrobots have been designed for this purpose.100 The utilization of biological carriers presents a compelling approach to drug delivery (Figure 5).
Figure 5.
Artificial micro/nanomatters
(A) Schematic that shows how engineered neutrophil target glioma after intravenous injection into mice. Reproduced from Xue et al.101 Copyright 2017, Springer Nature.
(B) AAV9 capsid model showing the insertion site for CPPs (top). Representative images of mouse brain regions showing successfully transfected cells by AAV.CPP.21 (white dots) (bottom). Reproduced from Yao et al.102 Copyright 2022, Springer Nature.
(C) Schematic of the microrobots (top). Ex vivo model of a brain blood vessel and the magnetic field control system (bottom). Reproduced from Jeon et al.103 Copyright 2019, American Association for the Advancement of Science.
(D) After CpG-EXO/TGM enters the blood circulation, the TfR on its surface can combine with free Tf in the blood, so that CpG-EXO/TGM is endowed with the ability to target BBB and GBM cells. Reproduced from Cui et al.104 Copyright 2023, American Chemical Society.
(E) Schematic illustration of formulating NT-lipidoid-doped LNPs for cargo delivery into the brain. Reproduced from Ma et al.105 Copyright 2020, American Association for the Advancement of Science.
(F) Schematic of active therapeutics of dual-responsive neutrobots in vivo. Reproduced from Zhang et al.100 Copyright 2021, American Association for the Advancement of Science.
(G) Schematic representation of small-molecule-loaded liposomes tethered to microbubbles. Reproduced from Ozdas et al.106 Copyright 2020, Springer Nature.
Cell-based delivery
The application of biological cells as drug carriers falls within the realm of cell therapy.107 Various immune cell types such as neutrophils,101 monocytes,108 macrophages,109 dendritic cells,110 and Th17 cells111 have been employed for targeted brain drug delivery. These immune cells are recruited to brain tumors, penetrating glioma sites and continuing to be recruited through the release of inflammatory factors post-resection. To illustrate, Xue et al. loaded liposomes containing paclitaxel into neutrophils, effectively inhibiting glioma recurrence following surgery.101 Dendritic cells can also enhance immunosuppression against tumors and deliver chemotherapy drugs.110 Red blood cells, being abundant in the bloodstream, can be modified for brain drug delivery.112 Recent studies have explored using hematopoietic stem cells to treat brain metastases more effectively.113,114 Nevertheless, cell-based drug delivery is still emerging and faces challenges in terms of cell localization within the brain, controlled navigation, and potential systemic toxicity. For example, Zhang et al. engineered "neutrobots" by encapsulating drug-loaded magnetic nanogels in E. coli membranes within neutrophils. These neutrobots demonstrated navigation and enrichment in the vasculature under the influence of an external magnetic field, crossing the BBB using chemotactic movement driven by inflammatory factors, and inhibiting tumor cell proliferation.100 However, further refinement is needed to optimize their therapeutic efficacy and spatiotemporal control before they can become promising drug delivery vectors.
Neurotropic viruses
Neurotropic viruses, represented by adeno-associated viruses (AAVs), have become significant in neurological gene therapy due to their ability to target various brain cells.97,115 In clinical practice, intraparenchymal injection is common but invasive and limited in coverage.116,117 Although subtypes of AAV, particularly AAV9, have shown the ability to transduce neurons and glial cells via intravenous injection, they are hampered by low efficiency and require high doses.118,119 To address this, researchers have developed advanced AAV variants with improved tropism and BBB penetration through rational design and screening of the AAV capsid.120 Gradinaru and co-workers evolved AAV-PHP.B and AAV-PHP.eB, achieving 40-fold higher CNS transduction in mice.121,122 They further created AAV.CAP-B10 with low hepatotoxicity, successfully expressing genes across the brain in marmosets.123 Yao et al. incorporated cell-penetrating peptides into AAV9, leading to AAV.CPP.16 and AAV.CPP.21, with 5-fold higher CNS transduction in cynomolgus macaques.102 These effective AAV toolkits provide promising opportunities for systemic gene therapy for a wide range of CNS diseases.
EVs
EVs, comprising exosomes, microvesicles, and apoptotic bodies, are cell-released membrane-derived vesicles that facilitate intercellular communication and bioregulation.124 EVs have gained widespread recognition for their clinical diagnostic and therapeutic potential, and they are actively under investigation as innovative drug delivery agents. Key intrinsic features of EVs include their size, which ensures stability in circulation, their endogenous composition, allowing immune system evasion, and, notably, the capacity of certain cell-derived EVs to cross the BBB.107 These attributes make EVs efficient drug delivery vehicles for brain diseases.98 EVs can be loaded with various cargoes including exogenous nucleic acids (miRNA, short interfering RNA [siRNA], mRNA) and proteins.125 EVs from specific sources (e.g., macrophages,126 tumor cells,127 neutrophils,128 and choroid plexus129) can cross the BBB or BCSFB without the need for targeted modification. Cui et al. extracted exosomes from serum, loaded them with the chemotherapeutic drug nanomicelles, and anchored the Toll-like receptor 9 agonist CpG oligonucleotide to the exosome membrane creating CpG-EXO/TGM.104 Surface engineering of EVs can enhance their brain-targeting abilities.130 Genetic methods are employed for surface modification, where plasmids expressing a targeting peptide (e.g., rabies virus glycoprotein [RVG]) are transduced into endogenous cells, and EVs with the targeting peptide (e.g., RVG-Lamp2b fusion protein) on their surface are subsequently purified.131 Alternatively, targeted proteins can be directly attached to EV membranes using click chemistry. Peptides like c(RGDyK) or stromal cell-derived factor 1 have been conjugated to EV membranes using click chemistry for the treatment of cerebral ischemia.132,133 However, challenges like low purification and loading efficiency need resolution for EVs to become effective drug carriers.107
Artificial micro/nanomatters
The development of micro/nano technology in the past decade has led to the creation of artificial micro/nanomatters for drug carriers, including nanoparticulate systems, micro- and nanorobots, and microbubbles (MBs) (Figure 5). These artificial micro/nanostructures possess targeting capabilities and can cross the BBB, making systemic administration an effective therapeutic option. They address limitations of poor stability of free drugs, rapid enzymatic degradation, inadequate release, and poor pharmacokinetics.19,134 While relatively few micro/nanomatters are currently approved for treating CNS diseases, much exploration is underway.
Nanoparticulate systems
NPs utilized for systemic brain drug delivery primarily comprise lipid-based NPs, polymeric NPs, and inorganic NPs. Lipid-based NPs, such as liposomes and lipid NPs, are non-toxic carriers with phospholipid mono- or bilayers and have high cargo loading efficiency.135 These NPs are typically constructed from biodegradable materials with hydrophilic heads and hydrophobic tails, including cholesterol, phospholipids, and fatty acids.136 Clinical trials involving liposome-loaded chemotherapeutic agents (e.g., doxorubicin, irinotecan) for glioma treatment are already underway.137,138,139 Liposomes have also emerged as vehicles for gene delivery, most notably in the clinical application of lipid NP-mRNA vaccines against COVID-19, marking a milestone in mRNA therapeutics.140 Lipid-based NPs efficiently transport nucleic acids to the brain for addressing neurodegenerative diseases, brain tumors, and brain inflammation.141,142,143,144 Ma et al. introduced neurotransmitter-derived lipidoids into liposomes for BBB crossing.105 This enabled the successful delivery of antibiotics, antisense oligonucleotides, or Cre recombinase to the mouse brain through systemic intravenous administration, achieving gene silencing or recombination in neuronal cells.105
Polymeric NPs created from homopolymers or copolymers are a promising method for drug delivery to the brain. Common polymeric NPs encompass micelles, nanocapsules, nanospheres, polymersomes, and more.145 These polymeric NPs, derived from biogenic monomers or featuring surface biomodifications, offer outstanding biocompatibility and biodegradability, making them valuable in diverse therapeutic applications for brain diseases. For example, Wu et al. used biocompatible polydopamine and conductive polymer polypyrrole to create NPs loaded with the antiepileptic drug phenytoin, enabling BBB crossing and controlled drug delivery.146 Poly(lactide-co-glycolide) acid, the most successful US FDA-approved biomedical polymer,147 has seen rapid development in polymeric NP-based treatments for neurodegenerative diseases.148 In addition, polymeric NPs have been explored for various therapies in the context of brain tumors. CRISPR-Cas9 nanocapsules, synthesized from crosslinked polymers with disulfide bonds, have been employed for gene therapy in glioblastoma.149 NPs loaded with siRNA, synthesized from polymerized human serum albumin and oligo(ethylene glycol), have been used for a similar purpose.150 Furthermore, nitric oxide-propelled chemotactic nanomotors, synthesized from L-arginine derivatives as monomers, have enhanced immunotherapy in the context of glioblastoma.151
Inorganic NPs, with unique properties like optical, electrical, and magnetic characteristics, are promising for diagnosing and treating brain diseases.152 Gold nanoparticle (AuNP) is widely employed in photothermal therapy (PTT) and theranostics for cancer because of its high photothermal conversion efficiency and good photostability.153 Importantly, AuNPs can cross the BBB via passive diffusion and endocytosis without compromising BBB integrity.154,155 Silica NPs, especially mesoporous silica nanoparticles (MSNs), offer superior chemical stability compared with polymeric NPs.156 MSNs possess a high specific surface area, facilitating effective drug loading.157,158 Tao et al. synthesized MSNs loaded with arsenic trioxide, demonstrating a 2.34-fold increase in pharmacokinetics when targeting brain gliomas compared with a solution.159 Magnetic NPs (MNPs) enable precise manipulation for brain localization, which is discussed in the next section.160 While NP-based brain drug delivery systems hold clinical potential, their BBB penetration and brain targeting require further validation. μBBB models aid in NP infiltration studies,161 and ensuring safety and stability remains a challenge.
Micro- and nanorobots
Micro- and nanorobots are miniature actuators operating at micro- and nanoscales. They have demonstrated considerable potential in biomedicine due to their ability to convert various energy sources into motion and force.162 This rapidly evolving field of robotics research has made notable progress in diverse applications such as drug and cell delivery, microsurgery, cell characterization, pathogen sensing, biopsy, and medical imaging.163,164 Their unique capability to access tight cavities, typically beyond reach by conventional means, enhances treatment efficacy.165 These robots are categorized by actuation methods, encompassing chemical, external stimuli (e.g., optical, ultrasonic, electrical, magnetic), and bio-hybrid actuation.162 Through active propulsion or manipulation via external fields, micro- and nanorobots exhibit precise mobility within brain vessels. Surface modifications enable BBB traversal, such as the development of glucose chemotaxis-driven nanoswimmers functionalized with angiopep-2 for BBB penetration.166 Biocompatible micro- and nanorobots facilitate cell attachment and transport, showing promise in cell therapy and neurological injury repair. Jeon et al. utilized TPP for the fabrication of microrobots with diverse geometries, serving as a structural framework for neuronal cell cultivation. Neural stem cells adhered to these microrobots, subsequently proliferated, and underwent differentiation into astrocytes, oligodendrocytes, and neurons.103 The precise manipulation of these microrobots within mouse brain slices and rat brain vessels was achieved through external magnetic field control.103 The same research group created neuron-loaded microrobots to selectively establish neural network connections for in-depth neural communication studies.167 However, the micro- and nanorobotics field requires continued exploration in materials, fabrication, actuation, and intelligence. In addition, research should focus on swarm manipulation to harness swarm intelligence for more effective treatment.
MBs
Most drug delivery systems targeting the BBB rely on biologically endogenous mechanisms that do not disrupt its integrity. Conventional methods for enhancing BBB penetration using chemical and biological stimuli lack precision and pose a high risk of toxicity.168 In contrast, focused ultrasound (FUS) in conjunction with intravenous MBs can safely and temporarily open the BBB at specific sites through cavitation effects.169 As early as 2001, Hynynen et al. combined MBs with FUS (FUS-MB) and used magnetic resonance imaging (MRI) for real-time monitoring, significantly reducing the required ultrasound power without harming surrounding neurons, resulting in MRI-guided focused ultrasound (MRgFUS).170 US FDA-approved MBs for marketing, such as Lumason, Optison, and Definity, consist of a phospholipid or albumin shell and a perfluorocarbon or sulfur hexafluoride gas core,171 with diameters smaller (1–4 μm) than red blood cells, facilitating passage through brain capillaries.171 Recent research investigates the impact of MB shape on biomedical applications. Non-spherical, anisotropic MBs were found to closely approach blood vessel walls and exhibit longer circulation times in vivo. Consequently, under the same ultrasound stimulation, non-spherical MBs exhibit higher BBB permeability compared with spherical ones.172 MBs can be co-injected with therapeutic agents to enhance drug penetration under FUS. Proteins, AAV, NPs, siRNA, and chemotherapeutic agents have demonstrated improved penetration efficiency in mice when co-injected with MBs.173,174,175,176,177,178 Clinical trials have indicated that MRgFUS enhances the effectiveness of chemotherapeutic agents such as doxorubicin, temozolomide, and carboplatin in brain tumors while ensuring patient safety.179,180 However, due to differing pharmacokinetics and the potential for non-specific toxicity,171 drug-loaded therapeutic and theranostic MBs are also considered options.181 For instance, Liang et al. incorporated indocyanine green onto MB surfaces for real-time NIR-II fluorescence monitoring and PTT of glioblastoma,182 while Fan et al. developed folate-conjugated DNA-loaded MBs for enhanced targeted gene transfection efficiency in tumor cells expressing the folate receptor.183 The temporary opening of the BBB allows blood components to enter the brain, which may lead to CNS toxicity, necessitating further verification of the safety of FUS-MB in humans. MBs can also be used to tether liposomes for precise drug aggregation and uncaging without opening the BBB under precisely modulated FUS, thus avoiding potential neurotoxicity.106
Manipulation of the micro/nanomatters and BBB
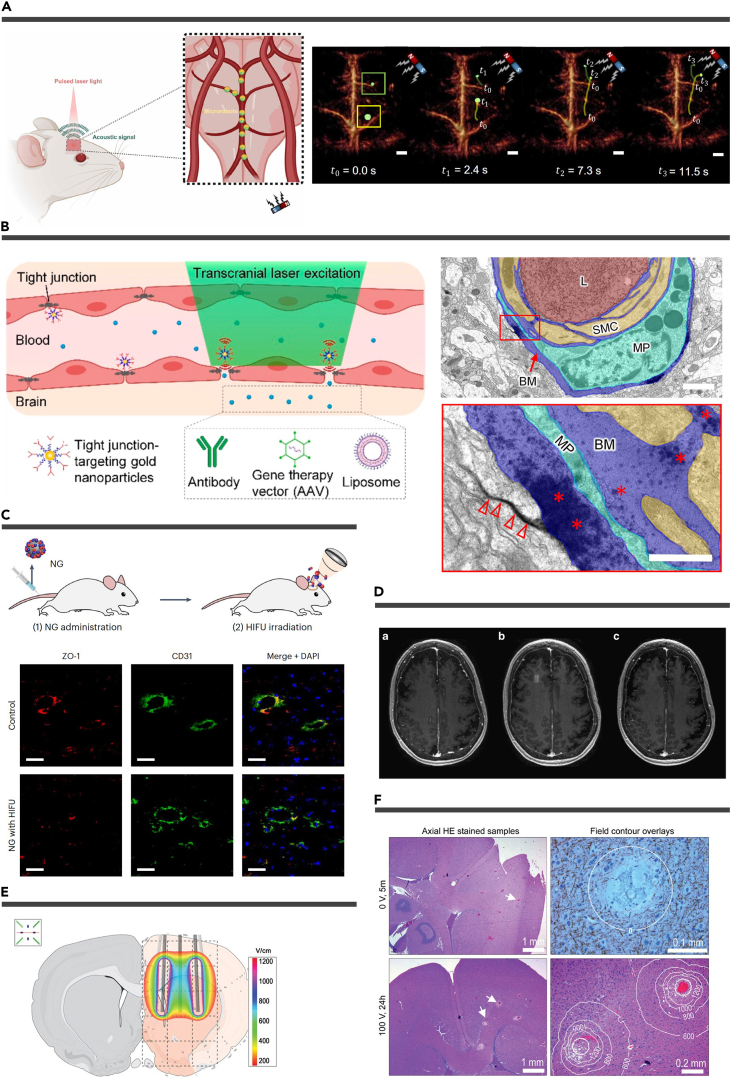
Controlled drug delivery systems for the brain necessitate the precise manipulation of micro/nanomatters. The exact control of micro/nano therapeutic agents within the body primarily relies on the application of magnetic fields, acoustic fields, light fields, or electric fields. Moreover, achieving the reversible and secure opening of the BBB demands meticulous tuning of external stimuli. The principal modalities for BBB manipulation encompass FUS, photothermal effects, and pulsed electric fields (PEFs).19 In this section, we provide a comprehensive overview of several prominent methods for external manipulation (Figure 6).
Figure 6.
Manipulation of the micro/nanomatters and the BBB
(A) Schematic representation of the non-invasive optoacoustic tomography of magnetic microrobots inside the murine brain vasculature. Reproduced from Wrede et al.164 Copyright 2022, American Association for the Advancement of Science.
(B) Schematic representation of reversible modulation of the BBB by laser stimulation of molecularly targeted nanoparticles (left). Electron microscopy imaging of lanthanum diffusion into the basement membrane (∗) and interstitial space (empty arrowheads) (bottom). Reproduced from Li et al154 Copyright 2021, American Chemical Society.
(C) Experimental schemes for nanoparticle administration and HIFU irradiation (top). Representative immunofluorescence images of midbrain sections co-stained for ZO-1 (red), CD31 (green), and nuclei (blue) (bottom). Scale bars, 30 μm. Reproduced from Kim et al.184 Copyright 2023, Springer Nature.
(D) MRI demonstration of BBB opening and closure. Axial T1-weighted gadolinium MRIs of patient 5 at (1) baseline, (2) immediately after stage 2 sonication and BBB opening, and (3) at 24 h after the procedure. Reproduced from Lipsman et al.185 Copyright 2018, Springer Nature.
(E) The electric field overlay (200 V) on the coronal plane corresponds to the center of the electrode device.
(F) Rat brain histology after different voltage treatments. Reproduced from Mahmood et al.186 Copyright 2015, Informa UK Limited.
Magnetic control
Magnetic fields provide a non-invasive, precise means for controlling MNPs and microrobots in drug delivery.19,187,188 Recent advancements in therapy and biosafety have expanded MNP clinical potential. For instance, Li et al. used MNPs in engineered siRNA-loaded exosomes to induce a novel cell death mode, ferroptosis, in glioblastoma cells.189 Zhang et al. successfully manipulated MNP-loaded neutrophils at the cellular level.100 Wang et al. employed intra-tumoral injection of magnetic carbon nanotubes, combined with precise magnetic field control, to induce rotational motion in these nanotubes, resulting in cancer cell death.190 A diverse array of magnetic micro/nanorobots has been designed and applied in biomedicine, demonstrating substantial potential for clinical application.191 For instance, Wrede et al. recently introduced cell-sized spherical Janus magnetic microrobots, enabling real-time optoacoustic imaging and magnetic manipulation within the mouse brain vasculature.164 This group also developed a wireless stent-shaped magnetic soft robot capable of shape-adaptive motion under magnetic field control, targeting the middle cerebral artery for thrombolysis and flow diversion, offering potential solutions for acute ischemic stroke and aneurysm management.192 Currently, there is a growing interest in swarm magnetic control for micro- and nanorobots, as it overcomes limitations associated with individual particles.193 Wang et al. explored the use of microrobot swarm magnetics for active intravascular delivery across various blood flow environments.194 In addition, they incorporated deep learning to enable microrobot swarms to autonomously adjust their movement and distribution in real-time based on their surroundings, enabling traversal through narrow and curved passages.195 Wang et al. employed C-shaped magnetic fields to guide NP swarms for in vivo thrombolysis.196 In another study, Zheng et al. fabricated a starfish-like shape-morphing microrobot capable of autonomously morphing to grasp and release numerous MNPs for swarm motion.197
Ultrasonic control
FUS-mediated BBB opening, as a method to enhance brain drug delivery efficiency, has demonstrated safety and clinical viability based on preclinical trials.198 Recent clinical trials have further confirmed its safety and efficacy in various neurological conditions, including glioma,179,199 brain metastases,200 AD,201,185 PD,202,203 and ALS.204 Ultrasound induces BBB opening through various physical effects within blood vessels, particularly mechanical effects.205 The combination of FUS with MBs results in significant mechanical effects, specifically cavitation effects, such as stable cavitation and inertial cavitation, linked to acoustic pressures.206 High acoustic pressure leads to the rapid collapse of MBs, generating high-speed microjets, shock waves, and sonoporation, which perforate the vascular EC membrane.207 However, strong inertial cavitation can lead to side effects, such as microvascular leakage and neuroinflammation, making it less practical.208 Therefore, using larger-diameter MBs (4–5 and 6–8 μm) at lower pressure amplitudes (0.2–0.3 MPa) is considered a more promising clinical approach.207 At low acoustic pressure, MBs undergo periodic expansion and contraction, resulting in rubbing against the vessel wall and oscillation, generating microstreaming that exerts shear stress on the cell membrane.209 These effects lead to the upregulation of EC carrier proteins and receptor-mediated transcytosis, as well as the downregulation of tight junction proteins, facilitating drug passage through the BBB via trans- and paracellular pathways.210 In addition, ultrasound-induced acoustic streaming modulates the shear stress experienced by ECs, further opening the BBB.211 Low-power FUS (4.6 W) has been employed to instantly open the BBB in Alzheimer’s patients and reversibly close it after 24 h without causing adverse events.185 Low-intensity pulsed ultrasound combined with intravenous MBs has also been tested successfully in clinical trials for drug delivery in recurrent glioblastoma.212 While FUS holds promise as an emerging delivery technology, further clinical trials are needed to validate its safety and optimize acoustic irradiation parameters and MB concentrations for different MBs. Moreover, FUS can be utilized for external stimulation of NPs, as demonstrated by Kim et al., who used FUS to activate piezoelectric NPs for deep brain stimulation and nitric oxide release to disrupt the BBB.184
Other manipulation methods
Apart from the methods mentioned above, photothermal controls and PEFs can also be employed for physical BBB opening. NPs, when stimulated by near-infrared light, generate a thermal effect that can reversibly open the BBB.213,214,215,216,217,218,219 Li et al. synthesized AuNPs designed to target tight junctions, enhancing BBB permeability after transcranial laser stimulation, enabling the entry of antibodies, AAV, and liposomes.154 Electromagnetic fields have demonstrated the ability to induce BBB opening and PEFs showing more promising results.220,221 Mahmood et al. conducted electroporation in the right hemisphere of rats using different voltages (100 and 400 V) and differentiated between transient and permanent membrane permeation using diffusion-weighted MRI.186 However, these methods are less safe than ultrasound and have yet to find widespread clinical application.
The methods for crossing the BBB can be broadly categorized into active targeting and physical disruption. Active targeting involves biological carriers that use biotropism to reach and cross the BBB, as well as micro/nanomatters that use outfield manipulation and surface modification to actively target the BBB. On the other hand, physical disruption involves the reversible disruption of BBB integrity in the target region through external field stimuli. The characteristics of the three methods are summarized in Table 1. Biological carriers, such as exosomes, offer natural selectivity and BBB-crossing abilities, reducing the potential for drug toxicity and enhancing drug stability. However, challenges in production and purification need resolution.130 Clinical trials for gene-based drug delivery using biological carriers are already underway,222 with brain delivery as a promising direction. Artificial micro/nanomatters, such as micro/nanorobots, possess integrated versatility for drug delivery, image guidance, and therapy monitoring. Nevertheless, their biocompatibility and long-term effects require continued attention. Future applications may involve real-time monitoring and navigation of micro/nanomatters using advanced super-resolution medical imaging technology. Physical methods, such as FUS, enhance drug delivery efficiency and reduce drug doses, allowing the use of existing drugs without lengthy approval processes. However, potential side effects and long-term consequences make physical methods challenging for clinical implementation, necessitating careful risk assessment. Among these methods, FUS-MB, representing physical disruption, is the closest to clinical practice.
Table 1.
Comparison of different modes of BBB nanoparticle crossing
| Method | Advantages | Limitations | Potential applications |
|---|---|---|---|
| Active targeting: biological carriers | high selectivity good crossing ability low toxicity and side effects high drug stability |
complicated synthesis difficult to purify difficult to transport |
gene delivery brain tumor treatment neural repair |
| Active targeting: micro/nanomatters | versatility spatiotemporal controllability high degree of modifiability |
unknown side and long-term effects | gene delivery brain embolization therapy |
| Physical destruction | high efficiency of drug delivery region selectivity low drug dosage non-invasive |
possible side effects unknown long-term effects complexity of equipment |
neurodegenerative disease therapy brain tumor treatment |
Microsystems for invasive drug delivery
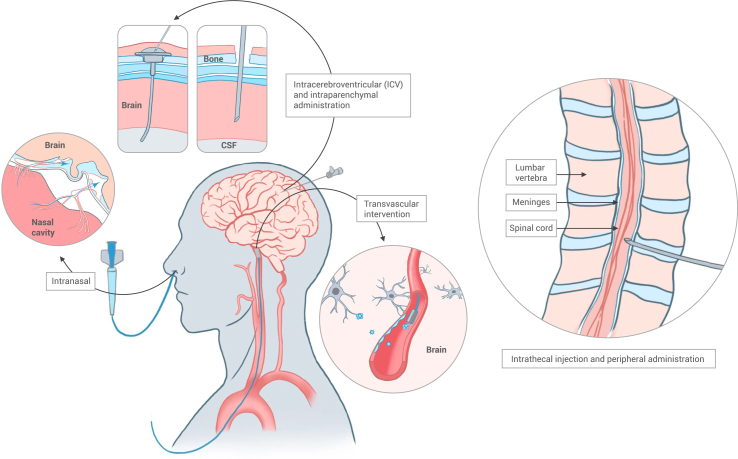
In addition to the previously discussed systemic route for brain drug administration across the BBB, there are alternative effective methods represented by invasive local delivery. These approaches allow for the bypassing of the BBB. The primary modes of administration that facilitate this bypass are intracerebral delivery (e.g., i.c.v. and intraparenchymal) and peripheral delivery (e.g., intrathecal and intranasal) (Figure 7). While these invasive methods are associated with potential damage, they are highly effective in the surgical treatment of conditions like malignant brain tumors, subarachnoid hemorrhage, and PD.94 Utilizing a range of micro/nanosystems, including NPs, implantable scaffolds, hydrogels, and optical fibers, can potentially reduce the invasiveness, lower intervention risks, and enhance treatment outcomes. This section provides examples of how micro/nanosystems are employed in invasive drug delivery, both through intracerebral and peripheral routes (Figure 8).
Figure 7.
Scheme of microsystems for invasive drug delivery
Microsystems can facilitate the invasive delivery of drugs to the brain, including intracerebral administration, intranasal delivery, intrathecal injection, and transvascular intervention. Note: this figure is modified from Furtado et al.19
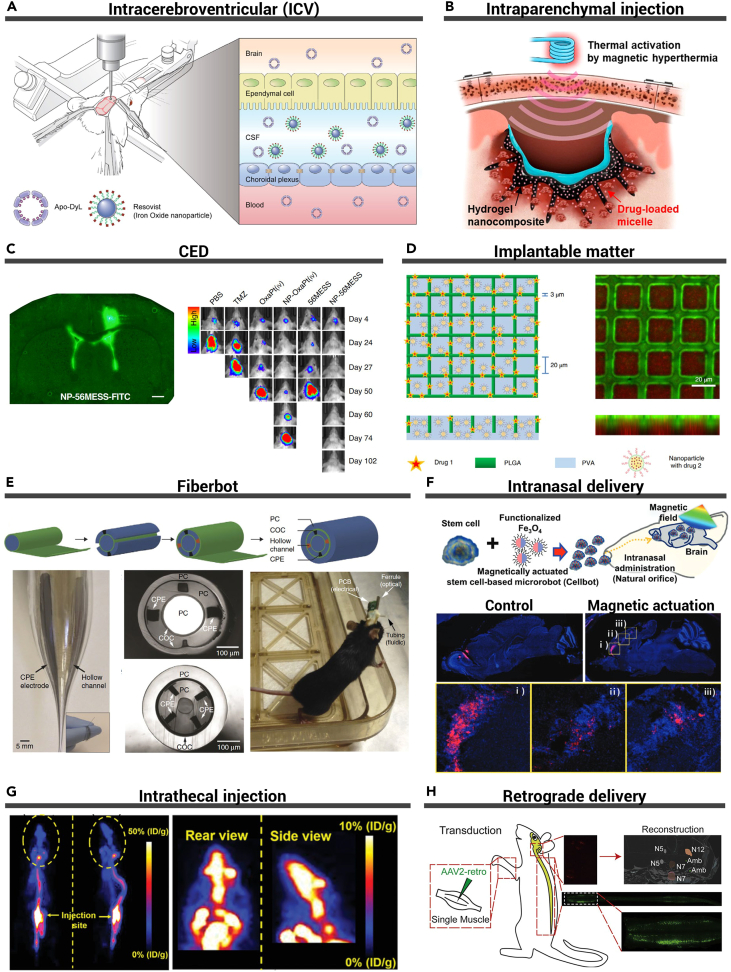
Figure 8.
Microsystems for invasive drug delivery
(A) Schematic illustration of stereotaxic surgery for intracerebroventricular (i.c.v.) injection, CSF-brain barrier, and CSF-blood barrier. Reproduced from Kim et al.223 Copyright 2020, Elsevier.
(B) Schematic illustration of penetrative and sustained drug delivery to deep brain tumors from the intracortical hydrogel nanocomposite by the magnetic activation for mild hyperthermia. Reproduced from Kang et al.224 Copyright 2023, American Chemical Society.
(C) Distribution of nanoparticles in the brain after CED (left). Bioluminescent IVIS images of representative mice (right). Reproduced from Wang et al.225 Copyright 2021, Springer Nature.
(D) Schematic and confocal image of the structure of a micromesh, PLGA strands (green), and the PVA layer carrying RhB nanoparticles (red). Reproduced from Di Mascolo et al.226 Copyright 2021, Springer Nature.
(E) Multimodality fiber probe fabrication and characterization. Reproduced from Canales et al.227 Copyright 2015, Springer Nature.
(F) Schematic of the intranasal administration and magnetic actuation of cellbots (top). Sequential migration and engraftment of the cellbots from the injection site (olfactory bulb) to the cerebral cortex (bottom). Reproduced from Jeon et al.228 Copyright 2021, Wiley-VCH.
(G) Representative PET images of agrin in mice at 30 min after intrathecal injection. Reproduced from Li et al.229 Copyright 2020, Wiley-VCH.
(H) Reverse transport process of rAAV2-Retro to the CNS after intramuscular injection. Reproduced from Chen et al.230 Copyright 2020, Elsevier.
Intracerebral administration
Intracerebral administration can be categorized into two methods: i.c.v. administration and intraparenchymal administration, based on the site of intervention. i.c.v. injection is directed into the CSF-filled lateral ventricle, whereas intraparenchymal administration involves the direct injection of implants or drugs into the brain parenchyma. Both of these approaches bypass the BBB, reducing systemic toxicity and circumventing drug metabolism in the bloodstream. However, they suffer from the drawback of slow drug diffusion in the brain parenchyma.19 Various techniques involving sustained or pressurized release have been developed to enhance drug diffusion,225,224 yet additional methods are still needed to minimize trauma and improve efficacy.
i.c.v. administration
i.c.v. administration stands as a precise and highly efficient technique for the direct delivery of therapeutic agents into the ventricular system. The i.c.v. administration procedure entails surgical exposure of the brain, facilitating the injection of medicinal substances into the lateral ventricles through an outlet catheter connected to an implantable reservoir.19 This method has found application in both preclinical and clinical contexts for diverse therapeutic purposes. Researchers have employed i.c.v. administration to introduce NPs,231 liposomes,232 MBs,233 lentiviruses,234 and siRNAs235 into mouse ventricles. In addition, Kim et al. have developed an i.c.v.-delivered MRI contrast agent, capable of penetrating the brain parenchyma through CSF circulation, enhancing the imaging of brain tumors223 Notably, i.c.v. injection of mesenchymal stem cells (MSCs) has garnered recent attention in the context of AD treatment. A clinical trial using the Ommaya reservoir for continuous injection of MSCs has demonstrated both safety and feasibility.236 Ongoing research endeavors seek to optimize the therapeutic efficacy, such as by incorporating iron oxide NPs to enhance the brain retention of MSCs237 i.c.v. administration ensures consistent, elevated drug concentrations at the target site, thereby minimizing potential side effects associated with systemic drug delivery. Nevertheless, it is imperative to consider strategies for mitigating the inherent risks associated with this invasive procedure.
Intraparenchymal injection
Intraparenchymal administration, akin to i.c.v., involves the targeted delivery of drugs or implants directly into the brain parenchyma. However, it is important to note that drugs introduced through this method exhibit limited diffusion rates and penetration within the brain parenchyma.19 To enhance the efficiency of intraparenchymal administration, micro/nanosystems have been explored, employing sustainably released polymers for drug delivery. Natural or synthetic polymers can be crosslinked to form hydrogels for this purpose. Notably, recent research has investigated the use of injectable hydrogels for postoperative treatment of brain tumors, where the eradication of tumor cells deeply embedded in the brain parenchyma is a primary concern. Kang et al. developed hydrogels incorporating drug-laden micelles and ferrimagnetic iron oxide nanocubes, facilitating drug penetration through magnetic field-induced thermal response.224 Injectable hydrogels also find application in postoperative immunotherapy of brain tumors, as demonstrated by Chen et al., who injected chimeric antigen receptor (CAR) gene-laden nanoporter-hydrogel into the tumor cavity to stimulate macrophages or microglia to produce glioma stem cell-specific CAR.238 In a similar vein, Zhang et al. proposed an injectable hydrogel containing tumor immune regulators for postoperative enhancement of tumor immunity in glioblastoma, thus reducing the recurrence.239 Furthermore, these hydrogels can serve as carriers for CAR-T cells, ensuring their prolonged retention and activation within the solid tumor niche.240 Injectable hydrogels represent a promising alternative strategy for the postoperative treatment of brain tumors.
Convection-enhanced delivery
An alternative strategy for improving the distribution of therapeutic agents within the brain post-intraparenchymal administration involves the application of positive pressure to directly inject substances into the tissue, thus enhancing their diffusion across the target region. This technique, known as convection-enhanced delivery (CED), entails the introduction of therapeutic agents through a surgically implanted catheter, utilizing an external infusion system to generate positive pressure. This pressure aids in the movement of the therapeutic agent from the infusion site into the brain ECs. CED has demonstrated its ability to augment the concentration of NPs and AAV within the brain.241,242,243 For example, Wang et al. employed CED to inject platinum anticancer drug-loaded NPs into glioblastoma xenograft mice, successfully circumventing temozolomide resistance.225 CED is also a valuable option for brain tumor immunotherapy. Parker et al. employed CED to deliver D2C7 immunotoxin and αCD40, resulting in a sustained anti-tumor immune response against malignant gliomas in mice.244 Clinical trials are underway to assess its effectiveness in patients with recurrent malignant glioma (NCT04547777). When implementing CED, it is essential to consider catheter design and material diffusion characteristics to ensure safety and efficacy.245
Implantable matter
Biocompatible brain-implantable matter enables controlled and sustained drug release. Various implantable forms such as extended-release wafers,246 hydrogels,247 conductive polymers,248 and polymer patches249 have been developed. Microfabrication techniques have enabled the creation of fine-structured implants. Di Mascolo et al. designed a biodegradable bilayer micromesh for independent co-loading and combination therapy with two drugs.226 Wang et al. developed microneedle patches for on-demand delivery of separate drugs, reducing glioblastoma volume and enhancing mouse survival.249 Microfabrication techniques also facilitate the creation of neural electrode arrays with microfluidic channels for drug delivery, electrical stimulation, and optical recording.250 However, this emerging field faces challenges related to device biocompatibility, neuroinflammation, long-term stability, and precise control of drug release kinetics. In addition, medical imaging, like photoacoustic imaging (PAI), is crucial for guiding implantation to avoid major blood vessels in the cerebral cortex.
Fiberbot
Optical fibers, renowned for their capacity to transmit optical signals and data, have recently found diverse applications in the biomedical domain.251 Utilizing the thermal drawing process (TDP), nerve electrodes and microfluidic channels can be seamlessly integrated into polymer fibers alongside waveguides, enabling multiple modalities of stimulation including light, electricity, and chemistry.227,252,253 Canales et al. successfully created a multifunctional fiber via TDP for in vivo optogenetic stimulation, neural recording, and drug delivery, establishing a stable brain-machine interface in mice.227 This platform facilitated one-step optogenetics, encompassing neural recording, optical stimulation, and viral vector delivery simultaneously.254 Winding multiple multifunctional fibers into a spiral scaffold results in spatially expandable fiber probes, enabling 3D manipulation of brain activity.255 Moreover, multifunctional fiber optics exhibit promise in tumor treatment, as they can detect tumor impedance while delivering immunotherapeutic agents.256 Advancements in micro and nano 3D printing technology have enabled the creation of minute structures on the tip surface of optical fibers. Barbot et al. used TPP to print microfluidic channels on the fiber tip, allowing for nanoscale droplet transport.257 By incorporating ferromagnetic modifications to the fiber tip, this technology holds potential as a novel tool for minimally invasive endovascular interventions.258 This concept, termed "fiberbot," represents a controlled multifunctional fiber for navigating hard-to-reach sites for multimodal treatment.
Peripheral delivery
An alternative approach to administer substances to the brain, which circumvents BBB, is the peripheral route. This route primarily encompasses intranasal delivery, intrathecal injection, and retrograde delivery. The olfactory region in the nasal cavity provides a pathway for drugs to enter the CNS. Micro/nanomatters are introduced into the CSF via intrathecal injection, enabling an augmentation of the drug dose. When injected into muscle, micro/nanomatters can travel retrogradely along the peripheral nervous system to reach the CNS. These peripheral routes offer an alternative to the risks and limitations associated with invasive intracranial procedures.
Intranasal delivery
Intranasal delivery offers a non-invasive and patient-friendly method to bypass the BBB and reach the CNS. This approach involves the high nasal spray application of therapeutic agents, which are subsequently absorbed by the olfactory and respiratory mucosa. Drugs within the nasal cavity can access the CNS via two pathways: extracellular diffusion through the nasal epithelium and transneuronal transport along olfactory and trigeminal axons.94 Intranasal administration boasts advantages including rapid absorption and onset of action, avoidance of hepatic first-pass effects, and excellent patient compliance. These benefits can be further harnessed by precise targeting and controlled release of micro/nanomatters. Surface-modified NPs, for instance, have demonstrated enhanced targeting efficiency in intranasal delivery.259,260 For example, Zhou et al. successfully delivered neurotrophic factors carried by EVs via intranasal administration to treat ischemic stroke in mice.261 In addition, Jeon et al. devised magnetic stem cell microrobots that can be controllably guided to target tissues following intranasal administration.228 Nonetheless, the clinical application of this route still faces challenges, such as limitations in the quantity of drugs that can be administered and potential adverse reactions, including nasal irritation.
Intrathecal injection
Intrathecal injection is a minimally invasive neurosurgical procedure known for its ability to bypass the BBB and deliver therapeutic agents to the CNS. Typically, a therapeutic agent is injected into the spinal subarachnoid space through lumbar puncture, and it is then transported to the CNS via the CSF.19 Intrathecal injections have been investigated in clinical settings, primarily for conditions like leptomeningeal metastases262,263 and ALS.264,265 Research is ongoing to enhance the distribution of therapeutic agents delivered through intrathecal injections. For example, Li et al. employed positron emission tomography (PET) imaging to examine the spatiotemporal distribution of protein therapeutics following intrathecal injection, offering valuable insights into drug development for conditions such as cerebral ischemia/reperfusion injury.229 Moreover, Castle et al. discovered that the physical localization can influence gene transduction when using intrathecal injections of AAV9 to target the cerebral cortex.266 The use of micro/nano drug carriers holds significant promise for intrathecal drug delivery, although more research is needed to improve controlled manipulation and on-demand release.27 In addition, a deeper understanding of the physiology and anatomy of the subarachnoid space is required to further enhance intrathecal drug delivery.267
Retrograde delivery
Retrograde delivery is a method that leverages the inherent transport mechanisms within neurons to convey therapeutic agents, tracers, or genetic material from peripheral nerves (e.g., muscles) to specific neural circuits or regions within the CNS.268 This approach has been instrumental in neural connectivity mapping, wherein a neural tracer, such as a viral vector, introduced at the injection site is taken up by the axon terminal. It is then transported to the soma and propagated retrogradely to the next neuron.269 Retrograde delivery has also demonstrated utility in targeted gene therapy for neurological disorders in both mice and primates.270,271 Chen et al. intramuscularly injected recombinant AAV, which can undergo reverse transport to reach the lower motor neurons of the brainstem, for gene therapy of motor neuron disease.230 However, this minimally invasive gene delivery method remains in the early stages of research, and further studies focusing on peripheral neurobiology are essential for its development and optimization.
The aforementioned invasive local drug delivery modalities represent viable strategies for surgical brain interventions, each bearing distinct advantages and drawbacks. i.c.v. drug administration facilitates even distribution across multiple brain regions, resulting in elevated drug concentrations within the CSF. Intraparenchymal administration allows for the direct delivery of a highly concentrated drug into specific brain regions, making it well-suited for treating localized lesions. Nonetheless, both of these methodologies entail surgical brain procedures, which present challenges in precise localization, and are susceptible to potential brain tissue damage and surgical risks. Intranasal drug delivery, in contrast, offers the advantages of ease of use and rapid drug onset, but it exhibits reduced drug delivery and absorption efficiency due to limitations related to nasal volume. The selection of the invasive approach should be grounded in a comprehensive evaluation of the patient’s specific disease condition, treatment objectives, and surgical risks.
Imaging and tracking of micro/nanosystems
In the context of their application in clinical medicine, it is imperative that micro/nanomatters not only be capable of manipulation but also exhibit in vivo traceability. Achieving compatibility with established clinical imaging modalities is essential for ensuring the safe and controllable delivery of drugs to the brain. Traditional medical imaging techniques have undergone extensive validation in pre-clinical models.272 One such imaging modality for micro/nanomatters is MRI, which enables in vivo visualization of these entities.273 However, contemporary MRI integration with drug delivery to the brain faces challenges, such as slow imaging rates and real-time tracking limitations. In some instances, the introduction of ferromagnetic materials into micro/nanomatters is necessary to enhance MRI contrast.274 This has led to the exploration of combining micro/nanomatters with another magnetic-based imaging method, magnetic particle imaging, for precise localization of MNP swarms.275 Furthermore, radiological imaging techniques, including X-ray imaging and PET, offer powerful tools for tracking micro/nanomatters.276,277 Nevertheless, the limitation of ionizing radiation-induced damage to the human body is a notable concern. Ultrasound imaging shows promise as a non-invasive and cost-effective method for visualizing micro-objects.278,279 Wang et al. showed in vivo ultrasound Doppler guided magnetic swarms.194 However, its utility for imaging micro/nanomatters may be constrained by position errors and sub-millimeter resolution limitations.
Given that single-mode imaging methods alone cannot fulfill the requirements for in vivo tracking of micro/nanomatters, there is substantial potential in harnessing hybrid medical imaging modalities. Notably, MRgFUS has been employed in clinical settings to accomplish real-time tracking of MB. Another category of hybrid imaging that integrates ultrasound is PAI, which has recently found applications in tracking micro/nanomatters. PAI capitalizes on the contrast advantages of optical absorption and the resolution benefits of ultrasound, achieving resolutions ranging from a few microns to hundreds of microns for multiscale imaging. Various PAI systems, including photoacoustic computed tomography (PACT), optical-resolution photoacoustic microscopy (OR-PAM), and fast-scanning OR-PAM, are utilized for imaging micro/nanomatters.280 Wu et al. notably employed PACT for real-time tracking of microrobots within the intestines.281 In another instance, Wrede et al. achieved real-time 3D tracking of magnetic microrobots within mouse blood vessels using optoacoustic tomography.164 To further enhance resolution, Li et al. employed OR-PAM, achieving micron-level tracking accuracy.282 In the pursuit of in vivo real-time tracking of micro/nanomatters, a synergistic approach employing various imaging methods should be tailored to the specific clinical scenarios at hand.
In invasive brain drug delivery procedures, surgeons are tasked with optimizing drug delivery efficiency while minimizing the risk of postoperative neurological impairment. In this context, advanced preoperative and intraoperative imaging assumes a pivotal role by assisting the surgeon in target area localization, procedural guidance, and ensuring precise drug delivery. Neuronavigation methods are now widely employed in various surgical scenarios, including functional neurosurgery and intracranial tumor interventions, and have become indispensable tools in the realm of minimally invasive neurosurgery.283 These neuronavigation modalities include cortical and subcortical stimulation mapping,284 intraoperative MRI,285 functional neuronavigation,286 navigable intraoperative ultrasonography,287 and fluorescence-guided neuronavigation.288 A burgeoning trend is the adoption of multimodal neuronavigation, which integrates and aligns multiple intraoperative image datasets to mitigate navigation and localization errors arising from brain deformation, thereby significantly enhancing intraoperative navigation precision.289 The ongoing advancement of high-precision neuronavigation technology, coupled with the innovative fiberbot’s neural microprobe drug delivery system, holds promise for introducing novel solutions to the realm of interventional drug delivery within the brain.
Conclusion and future perspectives
The delivery of micro/nanomatters to the brain faces significant challenges due to the tightly regulated BBB. While various approaches have been explored to cross or bypass the BBB, only a limited number of successful preclinical investigations have progressed to human clinical trials, resulting in the approval of only a handful of drugs for market release. Table 2 provides an overview of representative clinical trials in this context. Among the biological carriers, gene vectors, particularly AAV, have demonstrated remarkable progress in clinical trials for the treatment of CNS diseases. In fact, the first marketed drugs within this category are already available.290,291 Numerous ongoing AAV-related clinical trials encompass a spectrum of conditions, including neurodegenerative diseases, neurosensory disorders, and lysosomal storage disorders.292 However, the accumulation of long-term follow-up data and the continuous refinement of carrier structures to mitigate potential risks remain imperative. Conversely, the utilization of exosomes and cellular therapies for CNS diseases is still in its nascent stages within the clinical realm, but it holds substantial promise for the future. Among artificial micro/nanomatters, NPs exhibit the most promise for clinical applications in CNS diseases, primarily due to their demonstrated efficacy in solid tumors.293 However, most ongoing clinical trials use non-targeted delivery, posing a challenge for precise BBB and lesion targeting. MBs, when combined with approved MRgFUS, have produced favorable results in clinical trials for various brain disorders.185,202,204 Clinical trials employing invasive methods for micro/nanomatters brain delivery are underway, but the scarcity of clinical trials relative to successful preclinical experiments highlights translation challenges. Molecular drugs offer established solutions for brain diseases, with comprehensive reviews available for further reference (see Nance and co-workers94,107). This review emphasizes the emerging role of micro/nanosystems.
Table 2.
Represented clinical trials
| Drug name | Material | Conditions | Delivery route | Trial register | Status | Phase | Last update posted |
|---|---|---|---|---|---|---|---|
| EGFR(V)-EDV-Dox | bacterially derived minicell | glioblastoma, astrocytoma | intravenous | NCT02766699 | recruiting | phase 1 | 2019 |
| AVXS-101 | AAV9 | spinal muscular atrophy | intravenous | NCT04851873 | completed | phase 3 | 2023 |
| Skysona | lentiviral | cerebral adrenoleukodystrophy | intravenous | NCT03852498 | active, not recruiting | phase 3 | 2022 |
| DNX-2401 | adenovirus | recurrent high-grade glioma | intraarterial | NCT03896568 | recruiting | phase 1 | 2023 |
| MSCs-Exos | exosome | Alzheimer’s disease | intranasal | NCT04388982 | unknown | phase 1, phase 2 | 2021 |
| LipoCurc | liposome | glioblastoma | intravenous | NCT05768919 | recruiting | phase 1, phase 2 | 2023 |
| AGuIX | polymeric NPs | brain metastases | intravenous | NCT03818386 | recruiting | phase 2 | 2022 |
| NU-0129 | AuNP | gliosarcoma | intravenous | NCT03020017 | completed | early phase 1 | 2022 |
| ExAblate, Definity | microbubbles | Alzheimer’s disease | intravenous | NCT02986932 | completed | not applicable | 2018 |
| Implantable ultrasound device SonoCloud-9 + Abraxane | microbubbles | glioblastoma | intravenous | NCT04528680 | recruiting | phase 1, phase 2 | 2022 |
| MTX110 | NPs | glioma | convection-enhanced delivery | NCT04264143 | recruiting | phase 1 | 2022 |
| Nanoliposomal irinotecan | lipid-based NPs | high-grade glioma | convection-enhanced delivery | NCT02022644 | active, not recruiting | phase 1 | 2022 |
| APH-1105 | NPs | Alzheimer’s disease | intranasal | NCT03806478 | not yet recruiting | phase 2 | 2021 |
| Cytarabine | liposome | central nervous system metastases | intrathecal | NCT00992602 | completed | phase 2 | 2017 |
| GLIADEL | wafer | metastatic brain cancer | intraparenchymal | NCT00525590 | completed | phase 2 | 2020 |
| CD19-CAR T cells | lymphocyte | central nervous system lymphoma | intracerebroventricular | NCT05625594 | recruiting | phase 1 | 2022 |
Brain-targeted micro/nanomatters encounter several clinical translation challenges, including the following.
-
(1)
Optimizing size, surface properties, and functionalization is crucial for improving BBB permeability. Furthermore, achieving precise localization within the brain and preventing off-target effects are imperative. Exploring the integration of multiple surface modifications for sequential targeting (BBB, brain parenchyma, neurons) holds great promise.
-
(2)
Minimizing potential toxic effects, inflammatory responses, and tissue damage caused by these micro/nanomatters or their degradation products is of paramount importance. A comprehensive understanding of the micro/nanomatters' pharmacokinetics, residence time, and clearance routes is essential to optimize drug delivery and reduce systemic distribution. Moreover, thorough evaluation of long-term biocompatibility and the possibility of accumulation in the brain or other organs is necessary.
-
(3)
Precise control over the movement and distribution of these micro/nanomatters in the brain is essential. This necessitates the development of methods for actively actuating and manipulating micro/nanomatters in vivo. The choice of the actuation method should align with the specific application scenario. Utilizing multiphysics field propulsion and manipulation holds promise in this regard.
-
(4)
Real-time imaging guidance is vital for achieving spatiotemporally controlled delivery of micro/nanomatters in the brain. The integration of multimodal imaging techniques with advanced neuroimaging methods, including ultra-high field MRI, fast scanning PAI, and magnetic particle imaging, holds the potential for real-time localization of these micro/nanomatters.
-
(5)
Micro/nanomatters encounter issues related to inefficient transport over extended distances within the brain. The considerable distance between the point of intervention and the target regions in the brain can lead to substantial losses if these particles are transported directly in the bloodstream. In response, the concept of the fiberbot has emerged, aiming to deliver and release the payload locally into the brain’s blood vessels through transvascular intervention.
-
(6)
The ultimate goal is to develop micro/NPs with multifunctional capabilities, encompassing image navigation, motion control, targeted delivery, and outstanding biocompatibility for clinical use. These controllable micro/NPs share traits with robots and have the potential to evolve into micro and nanorobots.294 However, this advancement is accompanied by significant challenges.
Preclinical models, such as animal models, may not faithfully replicate human brain physiology or disease conditions. Consequently, observations of efficacy and safety in preclinical studies might not always translate to human patients. Bridging this gap and substantiating the safety and efficacy of brain-targeted drugs in human trials represents a crucial phase in the clinical translation process. The advent of in vitro BBB microsystem models signifies a breakthrough in brain research. These models, with the ability to mimic the intricate structure and function of the BBB, offer numerous advantages, including enhanced precision in drug screening, a deeper comprehension of disease mechanisms, and potential applications in personalized medicine. Several future prospects for μBBB models include.
-
(1)
Advancements in microfluidic device fabrication techniques, including the integration of 3D printing and organ-on-a-chip technologies, hold the potential to create more realistic and sophisticated BBB models.
-
(2)
The combination of the BBB microfluidic model with other organ models, such as the liver or gut, can facilitate the study of systemic drug effects and enhance the prediction of drug pharmacokinetics295,296
-
(3)
Scaling up the BBB microfluidic model for high-throughput screening and ensuring long-term stability and reproducibility are crucial for its broader utilization.
-
(4)
Manipulating cell distribution within the BBB microfluidic model provides a robust method for investigating the spatial organization and cell-cell interactions of the BBB. Techniques like cellular patterning, control of cell ratios, and their combination with advanced imaging can offer valuable insights into barrier function, neuroinflammatory processes, and disease mechanisms. Existing methods for cell patterning using ultrasound fields may be adapted for use in the μBBB model to achieve vascular patterning74
Brain interventions, like neural stimulation or drug delivery, necessitate tools that are small and precise to minimize harm to delicate neural tissue. The emergence of fiber-based robotic devices, known as fiberbots, offers a promising avenue for attaining this level of precision in brain interventions. The application of fiberbots in brain interventions provides several advantages over conventional methods.
-
(1)
Fiberbots enable precise drug delivery to specific brain regions through minimally invasive surgery, mitigating the risk of complications and side effects.
-
(2)
They facilitate the stimulation or recording of individual neurons or small neural circuits, enhancing our understanding of brain function.
-
(3)
Fiberbots support other minimally invasive procedures like biopsies or tissue sampling without necessitating large incisions or bulky instruments.
Nevertheless, the development of fiberbots presents challenges, including the need for advanced control systems and real-time monitoring of their position and movement. In addition, using biocompatible materials for fiber construction is vital to prevent damage to neural tissue.
Brain drug delivery, an advanced approach for treating CNS disorders, confronts regulatory and ethical challenges during clinical translation. These factors are pivotal in ensuring patient safety and treatment success. Rigorous regulation should be applied throughout the entire process of brain drug development, encompassing safety assessment, drug manufacturing quality, and validation of therapeutic efficacy. Concurrently, government and regulatory authorities should streamline the drug and medical device approval system to foster innovation in brain drug delivery. Given the elevated risk associated with brain drug delivery, clinical trials should give thorough consideration to legal and ethical concerns, including informed patient consent, privacy, long-term safety, and societal impact. Further research is warranted to enhance the design and optimize the utilization of these technologies for safe and efficacious brain interventions.
Acknowledgments
The work is supported by Shanghai Municipal Science and Technology Major Project (grant no. 2021SHZDZX), the Science and Technology Commission of Shanghai Municipality (grant no. 20DZ2220400), and the Shanghai Pilot Program for Basic Research Shanghai Jiao Tong University (grant no. 21TQ1400203). The authors acknowledge support from the Center for Hospital Automation and High Throughput Robotics, Institute of Medical Robotics, School of Biomedical Engineering, Shanghai Jiao Tong University. The authors acknowledge Ziyi Zhang for her help preparing the schematics.
Author contributions
Conceptualization, Z.M. and G.-Z.Y.; writing – original draft, M.T.; writing – review & editing, Z.M. and G.-Z.Y.: supervision, G.-Z.Y.
Declaration of interests
The authors declare no competing interests.
Published Online: November 27, 2023
Contributor Information
Zhichao Ma, Email: zhichaoma@sjtu.edu.cn.
Guang-Zhong Yang, Email: gzyang@sjtu.edu.cn.
Lead contact website
References
- 1.GBD 2016 Neurology Collaborators. Nichols E., Alam T., et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vigo D., Thornicroft G., Atun R. Estimating the true global burden of mental illness. Lancet Psychiatr. 2016;3:171–178. doi: 10.1016/S2215-0366(15)00505-2. [DOI] [PubMed] [Google Scholar]
- 3.Sampson J.H., Gunn M.D., Fecci P.E., Ashley D.M. Brain immunology and immunotherapy in brain tumours. Nat. Rev. Cancer. 2020;20:12–25. doi: 10.1038/s41568-019-0224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gribkoff V.K., Kaczmarek L.K. The need for new approaches in CNS drug discovery: why drugs have failed, and what can be done to improve outcomes. Neuropharmacology. 2017;120:11–19. doi: 10.1016/j.neuropharm.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arvanitis C.D., Ferraro G.B., Jain R.K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer. 2020;20:26–41. doi: 10.1038/s41568-019-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng B., Hao S., Tong Z., et al. Blood–brain barrier (BBB)-on-a-chip: a promising breakthrough in brain disease research. Lab Chip. 2022;22:3579–3602. doi: 10.1039/d2lc00305h. [DOI] [PubMed] [Google Scholar]
- 7.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 8.Miller K.D., Ostrom Q.T., Kruchko C., et al. Brain and other central nervous system tumor statistics, 2021. CA. Cancer J. Clin. 2021;71:381–406. doi: 10.3322/caac.21693. [DOI] [PubMed] [Google Scholar]
- 9.Tan A.C., Ashley D.M., López G.Y., et al. Management of glioblastoma: state of the art and future directions. CA A Cancer J. Clin. 2020;70:299–312. doi: 10.3322/caac.21613. [DOI] [PubMed] [Google Scholar]
- 10.Achrol A.S., Rennert R.C., Anders C., et al. Brain metastases. Nat. Rev. Dis. Prim. 2019;5:5–26. doi: 10.1038/s41572-018-0055-y. [DOI] [PubMed] [Google Scholar]
- 11.Livingston G., Huntley J., Sommerlad A., et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorsey E.R., Sherer T., Okun M.S., et al. The emerging evidence of the Parkinson pandemic. J. Park. Dis. 2018;8:S3–S8. doi: 10.3233/JPD-181474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estevez-Fraga C., Flower M.D., Tabrizi S.J. Therapeutic strategies for Huntington’s disease. Curr. Opin. Neurol. 2020;33:508–518. doi: 10.1097/WCO.0000000000000835. [DOI] [PubMed] [Google Scholar]
- 14.Hardiman O., Al-Chalabi A., Chio A., et al. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Prim. 2017;3 doi: 10.1038/nrdp.2017.85. [DOI] [PubMed] [Google Scholar]
- 15.Tsao C.W., Aday A.W., Almarzooq Z.I., et al. Heart disease and stroke statistics-2023 update: a report from the American heart association. Circulation. 2023;147:e93–e621. doi: 10.1161/CIR.0000000000001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell B.C.V., Khatri P. Stroke. Lancet. 2020;396:129–142. doi: 10.1016/S0140-6736(20)31179-X. [DOI] [PubMed] [Google Scholar]
- 17.Powers W.J., Rabinstein A.A., Ackerson T., et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2018;49:e46–e110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 18.GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:56–87. doi: 10.1016/S1474-4422(18)30415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furtado D., Björnmalm M., Ayton S., et al. Overcoming the blood–brain barrier: the role of nanomaterials in treating neurological diseases. Adv. Mater. 2018;30 doi: 10.1002/adma.201801362. [DOI] [PubMed] [Google Scholar]
- 20.Obermeier B., Daneman R., Ransohoff R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sweeney M.D., Ayyadurai S., Zlokovic B.V. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat. Neurosci. 2016;19:771–783. doi: 10.1038/nn.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armulik A., Genové G., Mäe M., et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 23.Vanlandewijck M., He L., Mäe M.A., et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554:475–480. doi: 10.1038/nature25739. [DOI] [PubMed] [Google Scholar]
- 24.Wichmann T.O., Damkier H.H., Pedersen M. A brief overview of the cerebrospinal fluid system and its implications for brain and spinal cord diseases. Front. Hum. Neurosci. 2021;15:737217. doi: 10.3389/fnhum.2021.737217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pardridge W.M. Drug transport in brain via the cerebrospinal fluid. Fluids Barriers CNS. 2011;8:7. doi: 10.1186/2045-8118-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nutt J.G., Burchiel K.J., Comella C.L., et al. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60:69–73. doi: 10.1212/wnl.60.1.69. [DOI] [PubMed] [Google Scholar]
- 27.Fowler M.J., Cotter J.D., Knight B.E., et al. Intrathecal drug delivery in the era of nanomedicine. Adv. Drug Deliv. Rev. 2020;165–166:77–95. doi: 10.1016/j.addr.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Derk J., Como C.N., Jones H.E., et al. Formation and function of the meningeal arachnoid barrier around the developing mouse brain. Dev. Cell. 2023;58:635–644.e4. doi: 10.1016/j.devcel.2023.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zel J., Hadzic A., Cvetko E., et al. Neurological and histological outcomes after subarachnoid injection of a liposomal bupivacaine suspension in pigs: a pilot study. Br. J. Anaesth. 2019;122:379–387. doi: 10.1016/j.bja.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 30.Oddo A., Peng B., Tong Z., et al. Advances in microfluidic blood–brain barrier (BBB) models. Trends Biotechnol. 2019;37:1295–1314. doi: 10.1016/j.tibtech.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Abbott A. More than a cosmetic change. Nature. 2005;438:144–146. doi: 10.1038/438144a. [DOI] [PubMed] [Google Scholar]
- 32.Hajal C., Offeddu G.S., Shin Y., et al. Engineered human blood–brain barrier microfluidic model for vascular permeability analyses. Nat. Protoc. 2022;17:95–128. doi: 10.1038/s41596-021-00635-w. [DOI] [PubMed] [Google Scholar]
- 33.Osaki T., Shin Y., Sivathanu V., et al. In vitro microfluidic models for neurodegenerative disorders. Adv. Healthcare Mater. 2018;7 doi: 10.1002/adhm.201700489. [DOI] [PubMed] [Google Scholar]
- 34.Xiao R.-R., Jing B., Yan L., et al. Constant-rate perfused array chip for high-throughput screening of drug permeability through brain endothelium. Lab Chip. 2022;22:4481–4492. doi: 10.1039/d2lc00507g. [DOI] [PubMed] [Google Scholar]
- 35.Li Y., Liu Y., Hu C., et al. Study of the neurotoxicity of indoor airborne nanoparticles based on a 3D human blood-brain barrier chip. Environ. Int. 2020;143:105598. doi: 10.1016/j.envint.2020.105598. [DOI] [PubMed] [Google Scholar]
- 36.Zhang S., Wan Z., Kamm R.D. Vascularized organoids on a chip: strategies for engineering organoids with functional vasculature. Lab Chip. 2021;21:473–488. doi: 10.1039/d0lc01186j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esch E.W., Bahinski A., Huh D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov. 2015;14:248–260. doi: 10.1038/nrd4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Achyuta A.K.H., Conway A.J., Crouse R.B., et al. A modular approach to create a neurovascular unit-on-a-chip. Lab Chip. 2013;13:542–553. doi: 10.1039/c2lc41033h. [DOI] [PubMed] [Google Scholar]
- 39.Campisi M., Shin Y., Osaki T., et al. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials. 2018;180:117–129. doi: 10.1016/j.biomaterials.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vatine G.D., Barrile R., Workman M.J., et al. Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell. 2019;24:995–1005.e6. doi: 10.1016/j.stem.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 41.Ahn S.I., Sei Y.J., Park H.-J., et al. Microengineered human blood–brain barrier platform for understanding nanoparticle transport mechanisms. Nat. Commun. 2020;11:175. doi: 10.1038/s41467-019-13896-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown T.D., Nowak M., Bayles A.V., et al. A microfluidic model of human brain (μHuB) for assessment of blood brain barrier. Bioeng. Transl. Med. 2019;4 doi: 10.1002/btm2.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim J., Lee K.-T., Lee J.S., et al. Fungal brain infection modelled in a human-neurovascular-unit-on-a-chip with a functional blood–brain barrier. Nat. Biomed. Eng. 2021;5:830–846. doi: 10.1038/s41551-021-00743-8. [DOI] [PubMed] [Google Scholar]
- 44.Marino A., Tricinci O., Battaglini M., et al. A 3D real-scale, biomimetic, and biohybrid model of the blood-brain barrier fabricated through two-photon lithography. Small. 2018;14 doi: 10.1002/smll.201702959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takebe T., Sekine K., Enomura M., et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 46.Nashimoto Y., Hayashi T., Kunita I., et al. Integrating perfusable vascular networks with a three-dimensional tissue in a microfluidic device. Integr. Biol. 2017;9:506–518. doi: 10.1039/c7ib00024c. [DOI] [PubMed] [Google Scholar]
- 47.Liu D., Zhu M., Lin Y., et al. LY6E protein facilitates adeno-associated virus crossing in a biomimetic chip model of the human blood–brain barrier. Lab Chip. 2022;22:4180–4190. doi: 10.1039/d2lc00698g. [DOI] [PubMed] [Google Scholar]
- 48.Fan Y., Xu C., Deng N., et al. Understanding drug nanocarrier and blood–brain barrier interaction based on a microfluidic microphysiological model. Lab Chip. 2023;23:1935–1944. doi: 10.1039/d2lc01077a. [DOI] [PubMed] [Google Scholar]
- 49.Shin Y., Choi S.H., Kim E., et al. Blood–brain barrier dysfunction in a 3D in vitro model of Alzheimer’s disease. Adv. Sci. 2019;6 doi: 10.1002/advs.201900962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seo S., Nah S.-Y., Lee K., et al. Triculture model of in vitro BBB and its application to study BBB-associated chemosensitivity and drug delivery in glioblastoma. Adv. Funct. Mater. 2022;32 [Google Scholar]
- 51.Kim S., Lee S., Lim J., et al. Human bone marrow-derived mesenchymal stem cells play a role as a vascular pericyte in the reconstruction of human BBB on the angiogenesis microfluidic chip. Biomaterials. 2021;279 doi: 10.1016/j.biomaterials.2021.121210. [DOI] [PubMed] [Google Scholar]
- 52.Cho C.-F., Wolfe J.M., Fadzen C.M., et al. Blood-brain-barrier spheroids as an in vitro screening platform for brain-penetrating agents. Nat. Commun. 2017;8 doi: 10.1038/ncomms15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eilenberger C., Rothbauer M., Selinger F., et al. A microfluidic multisize spheroid array for multiparametric screening of anticancer drugs and blood–brain barrier transport properties. Adv. Sci. 2021;8 doi: 10.1002/advs.202004856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park J., Wetzel I., Marriott I., et al. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat. Neurosci. 2018;21:941–951. doi: 10.1038/s41593-018-0175-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu W., Song J., Du X., et al. AKR1B10 (Aldo-keto reductase family 1 B10) promotes brain metastasis of lung cancer cells in a multi-organ microfluidic chip model. Acta Biomater. 2019;91:195–208. doi: 10.1016/j.actbio.2019.04.053. [DOI] [PubMed] [Google Scholar]
- 56.Freundt E.C., Maynard N., Clancy E.K., et al. Neuron-to-neuron transmission of α-synuclein fibrils through axonal transport. Ann. Neurol. 2012;72:517–524. doi: 10.1002/ana.23747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iannielli A., Ugolini G.S., Cordiglieri C., et al. Reconstitution of the human nigro-striatal pathway on-a-chip reveals OPA1-dependent mitochondrial defects and loss of dopaminergic synapses. Cell Rep. 2019;29:4646–4656.e4. doi: 10.1016/j.celrep.2019.11.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peng B., Tong Z., Tong W.Y., et al. In situ surface modification of microfluidic blood-brain-barriers for improved screening of small molecules and nanoparticles. ACS Appl. Mater. Interfaces. 2020;12:56753–56766. doi: 10.1021/acsami.0c17102. [DOI] [PubMed] [Google Scholar]
- 59.Adriani G., Ma D., Pavesi A., et al. A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood–brain barrier. Lab Chip. 2017;17:448–459. doi: 10.1039/c6lc00638h. [DOI] [PubMed] [Google Scholar]
- 60.Zhao N., Kulkarni S., Zhang S., et al. Modeling angiogenesis in the human brain in a tissue-engineered post-capillary venule. Angiogenesis. 2023;26:203–216. doi: 10.1007/s10456-023-09868-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seo S., Jang M., Kim H., et al. Neuro-glia-vascular-on-a-chip system to assess aggravated neurodegeneration via brain endothelial cells upon exposure to diesel exhaust particles. Adv. Funct. Mater. 2023;33 [Google Scholar]
- 62.Tian M., Ma Z.-C., Han Q., et al. Emerging applications of femtosecond laser fabrication in neurobiological research. Front. Chem. 2022;10 doi: 10.3389/fchem.2022.1051061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tricinci O., De Pasquale D., Marino A., et al. A 3D biohybrid real-scale model of the brain cancer microenvironment for advanced in vitro testing. Adv. Mater. Technol. 2020;5 doi: 10.1002/admt.202000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun R., Song X., Zhou K., et al. Assembly of fillable microrobotic systems by microfluidic loading with dip sealing. Adv. Mater. 2023;35 doi: 10.1002/adma.202207791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qian T., Maguire S.E., Canfield S.G., et al. Directed differentiation of human pluripotent stem cells to blood-brain barrier endothelial cells. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1701679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stebbins M.J., Gastfriend B.D., Canfield S.G., et al. Human pluripotent stem cell-derived brain pericyte-like cells induce blood-brain barrier properties. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aau7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee S., Chung M., Jeon N.L. In: The Blood-Brain Barrier: Methods and Protocols Methods in Molecular Biology. Stone N., editor. Springer US; 2022. BBB-on-a-chip: modeling functional human blood-brain barrier by mimicking 3D brain angiogenesis using microfluidic chip; pp. 251–263. [DOI] [PubMed] [Google Scholar]
- 68.Kim S., Lee H., Chung M., Jeon N.L. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip. 2013;13:1489–1500. doi: 10.1039/c3lc41320a. [DOI] [PubMed] [Google Scholar]
- 69.Lee S., Chung M., Lee S.-R., Jeon N.L. 3D brain angiogenesis model to reconstitute functional human blood–brain barrier in vitro. Biotechnol. Bioeng. 2020;117:748–762. doi: 10.1002/bit.27224. [DOI] [PubMed] [Google Scholar]
- 70.Winkelman M.A., Kim D.Y., Kakarla S., et al. Interstitial flow enhances the formation, connectivity, and function of 3D brain microvascular networks generated within a microfluidic device. Lab Chip. 2021;22:170–192. doi: 10.1039/d1lc00605c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soon K., Li M., Wu R., et al. A human model of arteriovenous malformation (AVM)-on-a-chip reproduces key disease hallmarks and enables drug testing in perfused human vessel networks. Biomaterials. 2022;288 doi: 10.1016/j.biomaterials.2022.121729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yi H.-G., Jeong Y.H., Kim Y., et al. A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy. Nat. Biomed. Eng. 2019;3:509–519. doi: 10.1038/s41551-019-0363-x. [DOI] [PubMed] [Google Scholar]
- 73.Miller J.S., Stevens K.R., Yang M.T., et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012;11:768–774. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma Z., Holle A.W., Melde K., et al. Acoustic holographic cell patterning in a biocompatible hydrogel. Adv. Mater. 2020;32 doi: 10.1002/adma.201904181. [DOI] [PubMed] [Google Scholar]
- 75.Pérez-López A., Torres-Suárez A.I., Martín-Sabroso C., Aparicio-Blanco J. An overview of in vitro 3D models of the blood-brain barrier as a tool to predict the in vivo permeability of nanomedicines. Adv. Drug Deliv. Rev. 2023;196 doi: 10.1016/j.addr.2023.114816. [DOI] [PubMed] [Google Scholar]
- 76.Bergmann S., Lawler S.E., Qu Y., et al. Blood–brain-barrier organoids for investigating the permeability of CNS therapeutics. Nat. Protoc. 2018;13:2827–2843. doi: 10.1038/s41596-018-0066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kumarasamy M., Sosnik A. Heterocellular spheroids of the neurovascular blood-brain barrier as a platform for personalized nanoneuromedicine. iScience. 2021;24 doi: 10.1016/j.isci.2021.102183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nzou G., Wicks R.T., Wicks E.E., et al. Human cortex spheroid with a functional blood brain barrier for high-throughput neurotoxicity screening and disease modeling. Sci. Rep. 2018;8:7413. doi: 10.1038/s41598-018-25603-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lancaster M.A., Renner M., Martin C.-A., et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yin X., Mead B.E., Safaee H., et al. Engineering stem cell organoids. Cell Stem Cell. 2016;18:25–38. doi: 10.1016/j.stem.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cakir B., Xiang Y., Tanaka Y., et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods. 2019;16:1169–1175. doi: 10.1038/s41592-019-0586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ham O., Jin Y.B., Kim J., Lee M.O. Blood vessel formation in cerebral organoids formed from human embryonic stem cells. Biochem. Biophys. Res. Commun. 2020;521:84–90. doi: 10.1016/j.bbrc.2019.10.079. [DOI] [PubMed] [Google Scholar]
- 83.Ahn Y., An J.-H., Yang H.-J., et al. Human blood vessel organoids penetrate human cerebral organoids and form a vessel-like system. Cells. 2021;10:2036. doi: 10.3390/cells10082036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ao Z., Song S., Tian C., et al. Understanding immune-driven brain aging by human brain organoid microphysiological analysis platform. Adv. Sci. 2022;9 doi: 10.1002/advs.202200475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park J., Lee B.K., Jeong G.S., et al. Three-dimensional brain-on-a-chip with an interstitial level of flow and its application as an in vitro model of Alzheimer’s disease. Lab Chip. 2015;15:141–150. doi: 10.1039/c4lc00962b. [DOI] [PubMed] [Google Scholar]
- 86.Huang Q., Tang B., Romero J.C., et al. Shell microelectrode arrays (MEAs) for brain organoids. Sci. Adv. 2022;8 doi: 10.1126/sciadv.abq5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mahto S.K., Charwat V., Ertl P., et al. Microfluidic platforms for advanced risk assessments of nanomaterials. Nanotoxicology. 2015;9:381–395. doi: 10.3109/17435390.2014.940402. [DOI] [PubMed] [Google Scholar]
- 88.Srinivasan B., Kolli A.R., Esch M.B., et al. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015;20:107–126. doi: 10.1177/2211068214561025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Y.I., Abaci H.E., Shuler M.L. Microfluidic blood–brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol. Bioeng. 2017;114:184–194. doi: 10.1002/bit.26045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Butt A.M., Jones H.C., Abbott N.J. Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J. Physiol. 1990;429:47–62. doi: 10.1113/jphysiol.1990.sp018243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Natarajan R., Northrop N., Yamamoto B. Fluorescein isothiocyanate (FITC)-dextran extravasation as a measure of blood-brain barrier permeability. Curr. Protoc. Neurosci. 2017;79:9.58.1–9.58.15. doi: 10.1002/cpns.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hajal C., Shin Y., Li L., et al. The CCL2-CCR2 astrocyte-cancer cell axis in tumor extravasation at the brain. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abg8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Straehla J.P., Hajal C., Safford H.C., et al. A predictive microfluidic model of human glioblastoma to assess trafficking of blood–brain barrier-penetrant nanoparticles. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2118697119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nance E., Pun S.H., Saigal R., et al. Drug delivery to the central nervous system. Nat. Rev. Mater. 2021;7:314–331. doi: 10.1038/s41578-021-00394-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pardridge W.M. Why is the global CNS pharmaceutical market so under-penetrated? Drug Discov. Today. 2002;7:5–7. doi: 10.1016/s1359-6446(01)02082-7. [DOI] [PubMed] [Google Scholar]
- 96.Li Y.-J., Wu J.-Y., Liu J., et al. From blood to brain: blood cell-based biomimetic drug delivery systems. Drug Deliv. 2021;28:1214–1225. doi: 10.1080/10717544.2021.1937384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Challis R.C., Ravindra Kumar S., Chen X., et al. Adeno-associated virus toolkit to target diverse brain cells. Annu. Rev. Neurosci. 2022;45:447–469. doi: 10.1146/annurev-neuro-111020-100834. [DOI] [PubMed] [Google Scholar]
- 98.Rehman F.U., Liu Y., Zheng M., Shi B. Exosomes based strategies for brain drug delivery. Biomaterials. 2023;293 doi: 10.1016/j.biomaterials.2022.121949. [DOI] [PubMed] [Google Scholar]
- 99.Rufino-Ramos D., Albuquerque P.R., Carmona V., et al. Extracellular vesicles: Novel promising delivery systems for therapy of brain diseases. J. Contr. Release. 2017;262:247–258. doi: 10.1016/j.jconrel.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 100.Zhang H., Li Z., Gao C., et al. Dual-responsive biohybrid neutrobots for active target delivery. Sci. Robot. 2021;6 doi: 10.1126/scirobotics.aaz9519. [DOI] [PubMed] [Google Scholar]
- 101.Xue J., Zhao Z., Zhang L., et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat. Nanotechnol. 2017;12:692–700. doi: 10.1038/nnano.2017.54. [DOI] [PubMed] [Google Scholar]
- 102.Yao Y., Wang J., Liu Y., et al. Variants of the adeno-associated virus serotype 9 with enhanced penetration of the blood–brain barrier in rodents and primates. Nat. Biomed. Eng. 2022;6:1257–1271. doi: 10.1038/s41551-022-00938-7. [DOI] [PubMed] [Google Scholar]
- 103.Jeon S., Kim S., Ha S., et al. Magnetically actuated microrobots as a platform for stem cell transplantation. Sci. Robot. 2019;4 doi: 10.1126/scirobotics.aav4317. [DOI] [PubMed] [Google Scholar]
- 104.Cui J., Wang X., Li J., et al. Immune exosomes loading self-assembled nanomicelles traverse the blood–brain barrier for chemo-immunotherapy against glioblastoma. ACS Nano. 2023;17:1464–1484. doi: 10.1021/acsnano.2c10219. [DOI] [PubMed] [Google Scholar]
- 105.Ma F., Yang L., Sun Z., et al. Neurotransmitter-derived lipidoids (NT-lipidoids) for enhanced brain delivery through intravenous injection. Sci. Adv. 2020;6 doi: 10.1126/sciadv.abb4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ozdas M.S., Shah A.S., Johnson P.M., et al. Non-invasive molecularly-specific millimeter-resolution manipulation of brain circuits by ultrasound-mediated aggregation and uncaging of drug carriers. Nat. Commun. 2020;11:4929. doi: 10.1038/s41467-020-18059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Terstappen G.C., Meyer A.H., Bell R.D., Zhang W. Strategies for delivering therapeutics across the blood–brain barrier. Nat. Rev. Drug Discov. 2021;20:362–383. doi: 10.1038/s41573-021-00139-y. [DOI] [PubMed] [Google Scholar]
- 108.Tong H.-I., Kang W., Davy P.M.C., et al. Monocyte trafficking, engraftment, and delivery of nanoparticles and an exogenous gene into the acutely inflamed brain tissue – evaluations on monocyte-based delivery system for the central nervous system. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Klyachko N.L., Polak R., Haney M.J., et al. Macrophages with cellular backpacks for targeted drug delivery to the brain. Biomaterials. 2017;140:79–87. doi: 10.1016/j.biomaterials.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma X., Kuang L., Yin Y., et al. Tumor–antigen activated dendritic cell membrane-coated biomimetic nanoparticles with orchestrating immune responses promote therapeutic efficacy against glioma. ACS Nano. 2023;17:2341–2355. doi: 10.1021/acsnano.2c09033. [DOI] [PubMed] [Google Scholar]
- 111.Shi C., Zhang J., Wang H., et al. Trojan horse nanocapsule enabled in situ modulation of the phenotypic conversion of Th17 cells to Treg cells for the treatment of multiple sclerosis in mice. Adv. Mater. 2023;35 doi: 10.1002/adma.202210262. [DOI] [PubMed] [Google Scholar]
- 112.Brenner J.S., Pan D.C., Myerson J.W., et al. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat. Commun. 2018;9:2684. doi: 10.1038/s41467-018-05079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Andreou T., Rippaus N., Wronski K., et al. Hematopoietic stem cell gene therapy for brain metastases using myeloid cell-specific gene promoters. J. Natl. Cancer Inst. 2020;112:617–627. doi: 10.1093/jnci/djz181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bagci-Onder T., Du W., Figueiredo J.-L., et al. Targeting breast to brain metastatic tumours with death receptor ligand expressing therapeutic stem cells. Brain. 2015;138:1710–1721. doi: 10.1093/brain/awv094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bedbrook C.N., Deverman B.E., Gradinaru V. Viral strategies for targeting the central and peripheral nervous systems. Annu. Rev. Neurosci. 2018;41:323–348. doi: 10.1146/annurev-neuro-080317-062048. [DOI] [PubMed] [Google Scholar]
- 116.Kaplitt M.G., Feigin A., Tang C., et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet Lond. Engl. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 117.LeWitt P.A., Rezai A.R., Leehey M.A., et al. AAV2-GAD gene therapy for advanced Parkinson’s disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011;10:309–319. doi: 10.1016/S1474-4422(11)70039-4. [DOI] [PubMed] [Google Scholar]
- 118.Foust K.D., Nurre E., Montgomery C.L., et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Feldman A.G., Parsons J.A., Dutmer C.M., et al. Subacute liver failure following gene replacement therapy for spinal muscular atrophy type 1. J. Pediatr. 2020;225:252–258.e1. doi: 10.1016/j.jpeds.2020.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen X., Ravindra Kumar S., Adams C.D., et al. Engineered AAVs for non-invasive gene delivery to rodent and non-human primate nervous systems. Neuron. 2022;110:2242–2257.e6. doi: 10.1016/j.neuron.2022.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chan K.Y., Jang M.J., Yoo B.B., et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 2017;20:1172–1179. doi: 10.1038/nn.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Deverman B.E., Pravdo P.L., Simpson B.P., et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 2016;34:204–209. doi: 10.1038/nbt.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Goertsen D., Flytzanis N.C., Goeden N., et al. AAV capsid variants with brain-wide transgene expression and decreased liver targeting after intravenous delivery in mouse and marmoset. Nat. Neurosci. 2022;25:106–115. doi: 10.1038/s41593-021-00969-4. [DOI] [PubMed] [Google Scholar]
- 124.Cheng L., Hill A.F. Therapeutically harnessing extracellular vesicles. Nat. Rev. Drug Discov. 2022;21:379–399. doi: 10.1038/s41573-022-00410-w. [DOI] [PubMed] [Google Scholar]
- 125.EL Andaloussi S., Mäger I., Breakefield X.O., Wood M.J.A. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 126.Yuan D., Zhao Y., Banks W.A., et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 2017;142:1–12. doi: 10.1016/j.biomaterials.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Morad G., Carman C.V., Hagedorn E.J., et al. Tumor-derived extracellular vesicles breach the intact blood–brain barrier via transcytosis. ACS Nano. 2019;13:13853–13865. doi: 10.1021/acsnano.9b04397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang J., Tang W., Yang M., et al. Inflammatory tumor microenvironment responsive neutrophil exosomes-based drug delivery system for targeted glioma therapy. Biomaterials. 2021;273 doi: 10.1016/j.biomaterials.2021.120784. [DOI] [PubMed] [Google Scholar]
- 129.Pauwels M.J., Xie J., Ceroi A., et al. Choroid plexus-derived extracellular vesicles exhibit brain targeting characteristics. Biomaterials. 2022;290 doi: 10.1016/j.biomaterials.2022.121830. [DOI] [PubMed] [Google Scholar]
- 130.Xu M., Feng T., Liu B., et al. Engineered exosomes: desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics. 2021;11:8926–8944. doi: 10.7150/thno.62330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Alvarez-Erviti L., Seow Y., Yin H., et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 132.Tian T., Zhang H.-X., He C.-P., et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–149. doi: 10.1016/j.biomaterials.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 133.Ruan H., Li Y., Zheng D., et al. Engineered extracellular vesicles for ischemic stroke treatment. Innovation. 2023;4:100394. doi: 10.1016/j.xinn.2023.100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xie J., Shen Z., Anraku Y., et al. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials. 2019;224 doi: 10.1016/j.biomaterials.2019.119491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Luo M., Lee L.K.C., Peng B., et al. Delivering the promise of gene therapy with nanomedicines in treating central nervous system diseases. Adv. Sci. 2022;9 doi: 10.1002/advs.202201740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hajj K.A., Whitehead K.A. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2017;2:17056–17117. [Google Scholar]
- 137.Clarke J.L., Molinaro A.M., Cabrera J.R., et al. A phase 1 trial of intravenous liposomal irinotecan in patients with recurrent high-grade glioma. Cancer Chemother. Pharmacol. 2017;79:603–610. doi: 10.1007/s00280-017-3247-3. [DOI] [PubMed] [Google Scholar]
- 138.Gaillard P.J., Kerklaan B.M., Aftimos P., et al. Abstract CT216: Phase I dose escalating study of 2B3-101, glutathione PEGylated liposomal doxorubicin, in patients with solid tumors and brain metastases or recurrent malignant glioma. Cancer Res. 2014;74:CT216. [Google Scholar]
- 139.Chastagner P., Devictor B., Geoerger B., et al. Phase I study of non-pegylated liposomal doxorubicin in children with recurrent/refractory high-grade glioma. Cancer Chemother. Pharmacol. 2015;76:425–432. doi: 10.1007/s00280-015-2781-0. [DOI] [PubMed] [Google Scholar]
- 140.Hou X., Zaks T., Langer R., Dong Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021;6:1078–1094. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu D., Cheng Y., Qiao S., et al. Nano-codelivery of temozolomide and siPD-L1 to reprogram the drug-resistant and immunosuppressive microenvironment in orthotopic glioblastoma. ACS Nano. 2022;16:7409–7427. doi: 10.1021/acsnano.1c09794. [DOI] [PubMed] [Google Scholar]
- 142.Erel-Akbaba G., Carvalho L.A., Tian T., et al. Radiation-induced targeted nanoparticle-based gene delivery for brain tumor therapy. ACS Nano. 2019;13:4028–4040. doi: 10.1021/acsnano.8b08177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lin C.-Y., Lin Y.-C., Huang C.-Y., et al. Ultrasound-responsive neurotrophic factor-loaded microbubble- liposome complex: Preclinical investigation for Parkinson’s disease treatment. J. Contr. Release. 2020;321:519–528. doi: 10.1016/j.jconrel.2020.02.044. [DOI] [PubMed] [Google Scholar]
- 144.Marcos-Contreras O.A., Brenner J.S., Kiseleva R.Y., et al. Combining vascular targeting and the local first pass provides 100-fold higher uptake of ICAM-1-targeted vs untargeted nanocarriers in the inflamed brain. J. Contr. Release. 2019;301:54–61. doi: 10.1016/j.jconrel.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Letchford K., Burt H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007;65:259–269. doi: 10.1016/j.ejpb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 146.Wu D., Fei F., Zhang Q., et al. Nanoengineered on-demand drug delivery system improves efficacy of pharmacotherapy for epilepsy. Sci. Adv. 2022;8 doi: 10.1126/sciadv.abm3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Martins C., Sousa F., Araújo F., Sarmento B. Functionalizing PLGA and PLGA derivatives for drug delivery and tissue regeneration applications. Adv. Healthcare Mater. 2018;7 doi: 10.1002/adhm.201701035. [DOI] [PubMed] [Google Scholar]
- 148.Pinto M., Silva V., Barreiro S., et al. Brain drug delivery and neurodegenerative diseases: Polymeric PLGA-based nanoparticles as a forefront platform. Ageing Res. Rev. 2022;79 doi: 10.1016/j.arr.2022.101658. [DOI] [PubMed] [Google Scholar]
- 149.Zou Y., Sun X., Yang Q., et al. Blood-brain barrier–penetrating single CRISPR-Cas9 nanocapsules for effective and safe glioblastoma gene therapy. Sci. Adv. 2022;8 doi: 10.1126/sciadv.abm8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gregory J.V., Kadiyala P., Doherty R., et al. Systemic brain tumor delivery of synthetic protein nanoparticles for glioblastoma therapy. Nat. Commun. 2020;11:5687. doi: 10.1038/s41467-020-19225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen H., Li T., Liu Z., et al. A nitric-oxide driven chemotactic nanomotor for enhanced immunotherapy of glioblastoma. Nat. Commun. 2023;14:941. doi: 10.1038/s41467-022-35709-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jiao M., Zhang P., Meng J., et al. Recent advancements in biocompatible inorganic nanoparticles towards biomedical applications. Biomater. Sci. 2018;6:726–745. doi: 10.1039/c7bm01020f. [DOI] [PubMed] [Google Scholar]
- 153.Huang X., Neretina S., El-Sayed M.A. Gold nanorods: from synthesis and properties to biological and biomedical applications. Adv. Mater. 2009;21:4880–4910. doi: 10.1002/adma.200802789. [DOI] [PubMed] [Google Scholar]
- 154.Li X., Vemireddy V., Cai Q., et al. Reversibly modulating the blood–brain barrier by laser stimulation of molecular-targeted nanoparticles. Nano Lett. 2021;21:9805–9815. doi: 10.1021/acs.nanolett.1c02996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhou Y., Peng Z., Seven E.S., et al. Crossing the blood-brain barrier with nanoparticles. J. Contr. Release. 2018;270:290–303. doi: 10.1016/j.jconrel.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 156.Martano S., De Matteis V., Cascione M., Rinaldi R. Inorganic nanomaterials versus polymer-based nanoparticles for overcoming neurodegeneration. Nanomaterials. 2022;12:2337. doi: 10.3390/nano12142337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang Y., Wang X., Xie R., et al. Overcoming the blood–brain barrier for gene therapy via systemic administration of GSH-responsive silica nanocapsules. Adv. Mater. 2023;35 doi: 10.1002/adma.202208018. [DOI] [PubMed] [Google Scholar]
- 158.Rosenholm J.M., Sahlgren C., Lindén M. Towards multifunctional, targeted drug delivery systems using mesoporous silica nanoparticles--opportunities & challenges. Nanoscale. 2010;2:1870–1883. doi: 10.1039/c0nr00156b. [DOI] [PubMed] [Google Scholar]
- 159.Tao J., Fei W., Tang H., et al. Angiopep-2-conjugated “Core-Shell” hybrid nanovehicles for targeted and pH-triggered delivery of arsenic trioxide into glioma. Mol. Pharm. 2019;16:786–797. doi: 10.1021/acs.molpharmaceut.8b01056. [DOI] [PubMed] [Google Scholar]
- 160.Chen J., Yuan M., Madison C.A., et al. Blood-brain barrier crossing using magnetic stimulated nanoparticles. J. Contr. Release. 2022;345:557–571. doi: 10.1016/j.jconrel.2022.03.007. [DOI] [PubMed] [Google Scholar]
- 161.Luo M., Li Y., Peng B., et al. A multifunctional porous silicon nanocarrier for glioblastoma treatment. Mol. Pharm. 2023;20:545–560. doi: 10.1021/acs.molpharmaceut.2c00763. [DOI] [PubMed] [Google Scholar]
- 162.Li J., Esteban-Fernández de Ávila B., Gao W., et al. Micro/Nanorobots for biomedicine: delivery, surgery, sensing, and detoxification. Sci. Robot. 2017;2 doi: 10.1126/scirobotics.aam6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang D., Gorochowski T.E., Marucci L., et al. Advanced medical micro-robotics for early diagnosis and therapeutic interventions. Front. Robot. AI. 2022;9 doi: 10.3389/frobt.2022.1086043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wrede P., Degtyaruk O., Kalva S.K., et al. Real-time 3D optoacoustic tracking of cell-sized magnetic microrobots circulating in the mouse brain vasculature. Sci. Adv. 2022;8 doi: 10.1126/sciadv.abm9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yoo J., Tang S., Gao W. Micro- and nanorobots for biomedical applications in the brain. Nat. Rev. Bioeng. 2023;1:308–310. [Google Scholar]
- 166.Joseph A., Contini C., Cecchin D., et al. Chemotactic synthetic vesicles: Design and applications in blood-brain barrier crossing. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1700362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kim E., Jeon S., An H.-K., et al. A magnetically actuated microrobot for targeted neural cell delivery and selective connection of neural networks. Sci. Adv. 2020;6 doi: 10.1126/sciadv.abb5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Poon C., McMahon D., Hynynen K. Noninvasive and targeted delivery of therapeutics to the brain using focused ultrasound. Neuropharmacology. 2017;120:20–37. doi: 10.1016/j.neuropharm.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dauba A., Delalande A., Kamimura H.A.S., et al. Recent advances on ultrasound contrast agents for blood-brain barrier opening with focused ultrasound. Pharmaceutics. 2020;12:1125. doi: 10.3390/pharmaceutics12111125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hynynen K., McDannold N., Vykhodtseva N., Jolesz F.A. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220:640–646. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- 171.Chowdhury S.M., Abou-Elkacem L., Lee T., et al. Ultrasound and microbubble mediated therapeutic delivery: underlying mechanisms and future outlook. J. Contr. Release. 2020;326:75–90. doi: 10.1016/j.jconrel.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 172.Dasgupta A., Sun T., Palomba R., et al. Nonspherical ultrasound microbubbles. Proc. Natl. Acad. Sci. USA. 2023;120 doi: 10.1073/pnas.2218847120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kinoshita M., McDannold N., Jolesz F.A., Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood–brain barrier disruption. Proc. Natl. Acad. Sci. USA. 2006;103:11719–11723. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Treat L.H., McDannold N., Zhang Y., et al. Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound Med. Biol. 2012;38:1716–1725. doi: 10.1016/j.ultrasmedbio.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang S., Karakatsani M.E., Fung C., et al. Direct brain infusion can be enhanced with focused ultrasound and microbubbles. J. Cerebr. Blood Flow Metabol. 2017;37:706–714. doi: 10.1177/0271678X16637881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ogawa K., Kato N., Yoshida M., et al. Focused ultrasound/microbubbles-assisted BBB opening enhances LNP-mediated mRNA delivery to brain. J. Contr. Release. 2022;348:34–41. doi: 10.1016/j.jconrel.2022.05.042. [DOI] [PubMed] [Google Scholar]
- 177.Guo Y., Lee H., Fang Z., et al. Single-cell analysis reveals effective siRNA delivery in brain tumors with microbubble-enhanced ultrasound and cationic nanoparticles. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abf7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wu P., Zhu M., Li Y., et al. Cascade-amplifying synergistic therapy for intracranial glioma via endogenous reactive oxygen species-triggered “all-in-one” nanoplatform. Adv. Funct. Mater. 2021;31 [Google Scholar]
- 179.Idbaih A., Canney M., Belin L., et al. Safety and feasibility of repeated and transient blood-brain barrier disruption by pulsed ultrasound in patients with recurrent glioblastoma. Clin. Cancer Res. 2019;25:3793–3801. doi: 10.1158/1078-0432.CCR-18-3643. [DOI] [PubMed] [Google Scholar]
- 180.Mainprize T., Lipsman N., Huang Y., et al. Blood-brain barrier opening in primary brain tumors with non-invasive MR-guided focused ultrasound: a clinical safety and feasibility study. Sci. Rep. 2019;9:321. doi: 10.1038/s41598-018-36340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang J., Li Z., Pan M., et al. Ultrasound-mediated blood–brain barrier opening: An effective drug delivery system for theranostics of brain diseases. Adv. Drug Deliv. Rev. 2022;190 doi: 10.1016/j.addr.2022.114539. [DOI] [PubMed] [Google Scholar]
- 182.Liang S., Hu D., Li G., et al. NIR-II fluorescence visualization of ultrasound-induced blood–brain barrier opening for enhanced photothermal therapy against glioblastoma using indocyanine green microbubbles. Sci. Bull. 2022;67:2316–2326. doi: 10.1016/j.scib.2022.10.025. [DOI] [PubMed] [Google Scholar]
- 183.Fan C.-H., Chang E.-L., Ting C.-Y., et al. Folate-conjugated gene-carrying microbubbles with focused ultrasound for concurrent blood-brain barrier opening and local gene delivery. Biomaterials. 2016;106:46–57. doi: 10.1016/j.biomaterials.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 184.Kim T., Kim H.J., Choi W., et al. Deep brain stimulation by blood–brain-barrier-crossing piezoelectric nanoparticles generating current and nitric oxide under focused ultrasound. Nat. Biomed. Eng. 2023;7:149–163. doi: 10.1038/s41551-022-00965-4. [DOI] [PubMed] [Google Scholar]
- 185.Lipsman N., Meng Y., Bethune A.J., et al. Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat. Commun. 2018;9:2336. doi: 10.1038/s41467-018-04529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mahmood F., Hansen R.H., Agerholm-Larsen B., et al. Detection of electroporation-induced membrane permeabilization states in the brain using diffusion-weighted MRI. Acta Oncol. 2015;54:289–297. doi: 10.3109/0284186X.2014.991045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Qiao R., Fu C., Forgham H., et al. Magnetic iron oxide nanoparticles for brain imaging and drug delivery. Adv. Drug Deliv. Rev. 2023;197 doi: 10.1016/j.addr.2023.114822. [DOI] [PubMed] [Google Scholar]
- 188.Reiling J. The magnetic field is not a health hazard. JAMA. 2021;326:1752. doi: 10.1001/jama.2021.15756. [DOI] [PubMed] [Google Scholar]
- 189.Li B., Chen X., Qiu W., et al. Synchronous disintegration of ferroptosis defense axis via engineered exosome-conjugated magnetic nanoparticles for glioblastoma therapy. Adv. Sci. 2022;9 doi: 10.1002/advs.202105451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang X., Gong Z., Wang T., et al. Mechanical nanosurgery of chemoresistant glioblastoma using magnetically controlled carbon nanotubes. Sci. Adv. 2023;9 doi: 10.1126/sciadv.ade5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang L., Meng Z., Chen Y., Zheng Y. Engineering magnetic micro/nanorobots for versatile biomedical applications. Adv. Intell. Syst. 2021;3 [Google Scholar]
- 192.Wang T., Ugurlu H., Yan Y., et al. Adaptive wireless millirobotic locomotion into distal vasculature. Nat. Commun. 2022;13:4465. doi: 10.1038/s41467-022-32059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang Q., Zhang L. External power-driven microrobotic swarm: from fundamental understanding to imaging-guided delivery. ACS Nano. 2021;15:149–174. doi: 10.1021/acsnano.0c07753. [DOI] [PubMed] [Google Scholar]
- 194.Wang Q., Chan K.F., Schweizer K., et al. Ultrasound Doppler-guided real-time navigation of a magnetic microswarm for active endovascular delivery. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abe5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yang L., Jiang J., Gao X., et al. Autonomous environment-adaptive microrobot swarm navigation enabled by deep learning-based real-time distribution planning. Nat. Mach. Intell. 2022;4:480–493. [Google Scholar]
- 196.Wang L., Wang J., Hao J., et al. Guiding drug through interrupted bloodstream for potentiated thrombolysis by C-shaped magnetic actuation system in vivo. Adv. Mater. 2021;33 doi: 10.1002/adma.202105351. [DOI] [PubMed] [Google Scholar]
- 197.Zheng Z., Wang H., Dong L., et al. Ionic shape-morphing microrobotic end-effectors for environmentally adaptive targeting, releasing, and sampling. Nat. Commun. 2021;12:411. doi: 10.1038/s41467-020-20697-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gorick C.M., Breza V.R., Nowak K.M., et al. Applications of focused ultrasound-mediated blood-brain barrier opening. Adv. Drug Deliv. Rev. 2022;191 doi: 10.1016/j.addr.2022.114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Carpentier A., Canney M., Vignot A., et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 2016;8:343re2. doi: 10.1126/scitranslmed.aaf6086. [DOI] [PubMed] [Google Scholar]
- 200.Meng Y., Reilly R.M., Pezo R.C., et al. MR-guided focused ultrasound enhances delivery of trastuzumab to Her2-positive brain metastases. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abj4011. [DOI] [PubMed] [Google Scholar]
- 201.Rezai A.R., Ranjan M., D’Haese P.-F., et al. Noninvasive hippocampal blood-brain barrier opening in Alzheimer’s disease with focused ultrasound. Proc. Natl. Acad. Sci. USA. 2020;117:9180–9182. doi: 10.1073/pnas.2002571117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gasca-Salas C., Fernández-Rodríguez B., Pineda-Pardo J.A., et al. Blood-brain barrier opening with focused ultrasound in Parkinson’s disease dementia. Nat. Commun. 2021;12:779. doi: 10.1038/s41467-021-21022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Martínez-Fernández R., Máñez-Miró J.U., Rodríguez-Rojas R., et al. Randomized trial of focused ultrasound subthalamotomy for Parkinson’s disease. N. Engl. J. Med. 2020;383:2501–2513. doi: 10.1056/NEJMoa2016311. [DOI] [PubMed] [Google Scholar]
- 204.Abrahao A., Meng Y., Llinas M., et al. First-in-human trial of blood-brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound. Nat. Commun. 2019;10:4373. doi: 10.1038/s41467-019-12426-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dalecki D. Mechanical bioeffects of ultrasound. Annu. Rev. Biomed. Eng. 2004;6:229–248. doi: 10.1146/annurev.bioeng.6.040803.140126. [DOI] [PubMed] [Google Scholar]
- 206.Dayton P., Klibanov A., Brandenburger G., Ferrara K. Acoustic radiation force in vivo: a mechanism to assist targeting of microbubbles. Ultrasound Med. Biol. 1999;25:1195–1201. doi: 10.1016/s0301-5629(99)00062-9. [DOI] [PubMed] [Google Scholar]
- 207.Wan M., Feng Y. In: ter Haar G., editor. Springer Netherlands; 2015. Cavitation in Biomedicine: Principles and Techniques. [Google Scholar]
- 208.Miller D.L. Overview of experimental studies of biological effects of medical ultrasound caused by gas body activation and inertial cavitation. Prog. Biophys. Mol. Biol. 2007;93:314–330. doi: 10.1016/j.pbiomolbio.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 209.Chen H., Kreider W., Brayman A.A., et al. Blood vessel deformations on microsecond time scales by ultrasonic cavitation. Phys. Rev. Lett. 2011;106 doi: 10.1103/PhysRevLett.106.034301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sheikov N., McDannold N., Vykhodtseva N., et al. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med. Biol. 2004;30:979–989. doi: 10.1016/j.ultrasmedbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 211.Qi H., Zhang S., Liang J., et al. Controllable blood–brain barrier (BBB) regulation based on gigahertz acoustic streaming. Nanotechnol. Precis. Eng. 2022;5 [Google Scholar]
- 212.Sonabend A.M., Gould A., Amidei C., et al. Repeated blood-brain barrier opening with an implantable ultrasound device for delivery of albumin-bound paclitaxel in patients with recurrent glioblastoma: a phase 1 trial. Lancet Oncol. 2023;24:509–522. doi: 10.1016/S1470-2045(23)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Li B., Li N., Chen L., et al. Alleviating neuroinflammation through photothermal conjugated polymer nanoparticles by regulating reactive oxygen species and Ca2+ signaling. ACS Appl. Mater. Interfaces. 2022;14:48416–48425. doi: 10.1021/acsami.2c13322. [DOI] [PubMed] [Google Scholar]
- 214.Gong L., Zhang X., Ge K., et al. Carbon nitride-based nanocaptor: An intelligent nanosystem with metal ions chelating effect for enhanced magnetic targeting phototherapy of Alzheimer’s disease. Biomaterials. 2021;267 doi: 10.1016/j.biomaterials.2020.120483. [DOI] [PubMed] [Google Scholar]
- 215.Zhou H., Gong Y., Liu Y., et al. Intelligently thermoresponsive flower-like hollow nano-ruthenium system for sustained release of nerve growth factor to inhibit hyperphosphorylation of tau and neuronal damage for the treatment of Alzheimer’s disease. Biomaterials. 2020;237 doi: 10.1016/j.biomaterials.2020.119822. [DOI] [PubMed] [Google Scholar]
- 216.Xiong S., Li Z., Liu Y., et al. Brain-targeted delivery shuttled by black phosphorus nanostructure to treat Parkinson’s disease. Biomaterials. 2020;260 doi: 10.1016/j.biomaterials.2020.120339. [DOI] [PubMed] [Google Scholar]
- 217.Cheng G., Li Z., Liu Y., et al. “Swiss Army Knife” black phosphorus-based nanodelivery platform for synergistic antiparkinsonian therapy via remodeling the brain microenvironment. J. Contr. Release. 2023;353:752–766. doi: 10.1016/j.jconrel.2022.12.024. [DOI] [PubMed] [Google Scholar]
- 218.Gao Y., Cheng Y., Chen J., et al. NIR-assisted MgO-based polydopamine nanoparticles for targeted treatment of Parkinson’s disease through the blood–brain barrier. Adv. Healthcare Mater. 2022;11 doi: 10.1002/adhm.202201655. [DOI] [PubMed] [Google Scholar]
- 219.Jin L., Hu P., Wang Y., et al. Fast-acting black-phosphorus-assisted depression therapy with low toxicity. Adv. Mater. 2020;32 doi: 10.1002/adma.201906050. [DOI] [PubMed] [Google Scholar]
- 220.Kuo Y.-C., Kuo C.-Y. Electromagnetic interference in the permeability of saquinavir across the blood-brain barrier using nanoparticulate carriers. Int. J. Pharm. 2008;351:271–281. doi: 10.1016/j.ijpharm.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 221.Salford L.G., Brun A., Sturesson K., et al. Permeability of the blood-brain barrier induced by 915 MHz electromagnetic radiation, continuous wave and modulated at 8, 16, 50, and 200 Hz. Microsc. Res. Tech. 1994;27:535–542. doi: 10.1002/jemt.1070270608. [DOI] [PubMed] [Google Scholar]
- 222.Cully M. Exosome-based candidates move into the clinic. Nat. Rev. Drug Discov. 2021;20:6–7. doi: 10.1038/d41573-020-00220-y. [DOI] [PubMed] [Google Scholar]
- 223.Kim H.-K., Baek A.R., Choi G., et al. Highly brain-permeable apoferritin nanocage with high dysprosium loading capacity as a new T2 contrast agent for ultra-high field magnetic resonance imaging. Biomaterials. 2020;243 doi: 10.1016/j.biomaterials.2020.119939. [DOI] [PubMed] [Google Scholar]
- 224.Kang T., Cha G.D., Park O.K., et al. Penetrative and sustained drug delivery using injectable hydrogel nanocomposites for postsurgical brain tumor treatment. ACS Nano. 2023;17:5435–5447. doi: 10.1021/acsnano.2c10094. [DOI] [PubMed] [Google Scholar]
- 225.Wang Y., Jiang Y., Wei D., et al. Nanoparticle-mediated convection-enhanced delivery of a DNA intercalator to gliomas circumvents temozolomide resistance. Nat. Biomed. Eng. 2021;5:1048–1058. doi: 10.1038/s41551-021-00728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Di Mascolo D., Palange A.L., Primavera R., et al. Conformable hierarchically engineered polymeric micromeshes enabling combinatorial therapies in brain tumours. Nat. Nanotechnol. 2021;16:820–829. doi: 10.1038/s41565-021-00879-3. [DOI] [PubMed] [Google Scholar]
- 227.Canales A., Jia X., Froriep U.P., et al. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat. Biotechnol. 2015;33:277–284. doi: 10.1038/nbt.3093. [DOI] [PubMed] [Google Scholar]
- 228.Jeon S., Park S.H., Kim E., et al. A magnetically powered stem cell-based microrobot for minimally invasive stem cell delivery via the intranasal pathway in a mouse brain. Adv. Healthcare Mater. 2021;10 doi: 10.1002/adhm.202100801. [DOI] [PubMed] [Google Scholar]
- 229.Li S., Wang Y., Jiang D., et al. Spatiotemporal distribution of agrin after intrathecal injection and its protective role in cerebral ischemia/reperfusion injury. Adv. Sci. 2020;7 doi: 10.1002/advs.201902600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Chen Z., Fan G., Li A., et al. rAAV2-retro enables extensive and high-efficient transduction of lower motor neurons following intramuscular injection. Mol. Ther. Methods Clin. Dev. 2020;17:21–33. doi: 10.1016/j.omtm.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Peviani M., Capasso Palmiero U., Cecere F., et al. Biodegradable polymeric nanoparticles administered in the cerebrospinal fluid: Brain biodistribution, preferential internalization in microglia and implications for cell-selective drug release. Biomaterials. 2019;209:25–40. doi: 10.1016/j.biomaterials.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 232.Bhattacherjee A., Daskhan G.C., Bains A., et al. Increasing phagocytosis of microglia by targeting CD33 with liposomes displaying glycan ligands. J. Contr. Release. 2021;338:680–693. doi: 10.1016/j.jconrel.2021.09.010. [DOI] [PubMed] [Google Scholar]
- 233.Mee-Inta O., Hsieh C.-F., Chen D.-Q., et al. High-frequency ultrasound imaging for monitoring the function of meningeal lymphatic system in mice. Ultrasonics. 2023;131 doi: 10.1016/j.ultras.2023.106949. [DOI] [PubMed] [Google Scholar]
- 234.Regev L., Ezrielev E., Gershon E., et al. Genetic approach for intracerebroventricular delivery. Proc. Natl. Acad. Sci. USA. 2010;107:4424–4429. doi: 10.1073/pnas.0907059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Helmschrodt C., Höbel S., Schöniger S., et al. Polyethylenimine nanoparticle-mediated siRNA delivery to reduce α-Synuclein expression in a model of Parkinson’s disease. Mol. Ther. Nucleic Acids. 2017;9:57–68. doi: 10.1016/j.omtn.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kim H.J., Cho K.R., Jang H., et al. Intracerebroventricular injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: a phase I clinical trial. Alzheimer's Res. Ther. 2021;13:154. doi: 10.1186/s13195-021-00897-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Jung M., Kim H., Hwang J.W., et al. Iron oxide nanoparticle-incorporated mesenchymal stem cells for Alzheimer’s disease treatment. Nano Lett. 2023;23:476–490. doi: 10.1021/acs.nanolett.2c03682. [DOI] [PubMed] [Google Scholar]
- 238.Chen C., Jing W., Chen Y., et al. Intracavity generation of glioma stem cell-specific CAR macrophages primes locoregional immunity for postoperative glioblastoma therapy. Sci. Transl. Med. 2022;14 doi: 10.1126/scitranslmed.abn1128. [DOI] [PubMed] [Google Scholar]
- 239.Zhang J., Chen C., Li A., et al. Immunostimulant hydrogel for the inhibition of malignant glioma relapse post-resection. Nat. Nanotechnol. 2021;16:538–548. doi: 10.1038/s41565-020-00843-7. [DOI] [PubMed] [Google Scholar]
- 240.Grosskopf A.K., Labanieh L., Klysz D.D., et al. Delivery of CAR-T cells in a transient injectable stimulatory hydrogel niche improves treatment of solid tumors. Sci. Adv. 2022;8 doi: 10.1126/sciadv.abn8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Saucier-Sawyer J.K., Seo Y.-E., Gaudin A., et al. Distribution of polymer nanoparticles by convection-enhanced delivery to brain tumors. J. Contr. Release. 2016;232:103–112. doi: 10.1016/j.jconrel.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zhang C., Nance E.A., Mastorakos P., et al. Convection enhanced delivery of cisplatin-loaded brain penetrating nanoparticles cures malignant glioma in rats. J. Contr. Release. 2017;263:112–119. doi: 10.1016/j.jconrel.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lonser R.R., Akhter A.S., Zabek M., et al. Direct convective delivery of adeno-associated virus gene therapy for treatment of neurological disorders. J. Neurosurg. 2020;134:1751–1763. doi: 10.3171/2020.4.JNS20701. [DOI] [PubMed] [Google Scholar]
- 244.Parker S., McDowall C., Sanchez-Perez L., et al. Immunotoxin-αCD40 therapy activates innate and adaptive immunity and generates a durable antitumor response in glioblastoma models. Sci. Transl. Med. 2023;15 doi: 10.1126/scitranslmed.abn5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Allard E., Passirani C., Benoit J.-P. Convection-enhanced delivery of nanocarriers for the treatment of brain tumors. Biomaterials. 2009;30:2302–2318. doi: 10.1016/j.biomaterials.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 246.Grossman S.A., Reinhard C., Colvin O.M., et al. The intracerebral distribution of BCNU delivered by surgically implanted biodegradable polymers. J. Neurosurg. 1992;76:640–647. doi: 10.3171/jns.1992.76.4.0640. [DOI] [PubMed] [Google Scholar]
- 247.Ranganath S.H., Fu Y., Arifin D.Y., et al. The use of submicron/nanoscale PLGA implants to deliver paclitaxel with enhanced pharmacokinetics and therapeutic efficacy in intracranial glioblastoma in mice. Biomaterials. 2010;31:5199–5207. doi: 10.1016/j.biomaterials.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 248.George P.M., Bliss T.M., Hua T., et al. Electrical preconditioning of stem cells with a conductive polymer scaffold enhances stroke recovery. Biomaterials. 2017;142:31–40. doi: 10.1016/j.biomaterials.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wang Z., Yang Z., Jiang J., et al. Silk microneedle patch capable of on-demand multidrug delivery to the brain for glioblastoma treatment. Adv. Mater. 2022;34 doi: 10.1002/adma.202106606. [DOI] [PubMed] [Google Scholar]
- 250.Wan J., Zhou S., Mea H.J., et al. Emerging roles of microfluidics in brain research: from cerebral fluids manipulation to brain-on-a-chip and neuroelectronic devices engineering. Chem. Rev. 2022;122:7142–7181. doi: 10.1021/acs.chemrev.1c00480. [DOI] [PubMed] [Google Scholar]
- 251.Rezapour Sarabi M., Jiang N., Ozturk E., et al. Biomedical optical fibers. Lab Chip. 2021;21:627–640. doi: 10.1039/d0lc01155j. [DOI] [PubMed] [Google Scholar]
- 252.Frank J.A., Antonini M.-J., Chiang P.-H., et al. In vivo photopharmacology enabled by multifunctional fibers. ACS Chem. Neurosci. 2020;11:3802–3813. doi: 10.1021/acschemneuro.0c00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Du M., Huang L., Zheng J., et al. Flexible fiber probe for efficient neural stimulation and detection. Adv. Sci. 2020;7 doi: 10.1002/advs.202001410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Park S., Guo Y., Jia X., et al. One-step optogenetics with multifunctional flexible polymer fibers. Nat. Neurosci. 2017;20:612–619. doi: 10.1038/nn.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Jiang S., Patel D.C., Kim J., et al. Spatially expandable fiber-based probes as a multifunctional deep brain interface. Nat. Commun. 2020;11:6115. doi: 10.1038/s41467-020-19946-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Chin A.L., Jiang S., Jang E., et al. Implantable optical fibers for immunotherapeutics delivery and tumor impedance measurement. Nat. Commun. 2021;12:5138. doi: 10.1038/s41467-021-25391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Barbot A., Wales D., Yeatman E., Yang G.Z. Microfluidics at fiber tip for nanoliter delivery and sampling. Adv. Sci. 2021;8 doi: 10.1002/advs.202004643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Leber A., Dong C., Laperrousaz S., et al. Highly integrated multi-material fibers for soft robotics. Adv. Sci. 2023;10 doi: 10.1002/advs.202204016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Goel H., Kalra V., Verma S.K., et al. Convolutions in the rendition of nose to brain therapeutics from bench to bedside: Feats & fallacies. J. Contr. Release. 2022;341:782–811. doi: 10.1016/j.jconrel.2021.12.009. [DOI] [PubMed] [Google Scholar]
- 260.Sonvico F., Clementino A., Buttini F., et al. Surface-modified nanocarriers for nose-to-brain delivery: from bioadhesion to targeting. Pharmaceutics. 2018;10:34. doi: 10.3390/pharmaceutics10010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Zhou X., Deng X., Liu M., et al. Intranasal delivery of BDNF-loaded small extracellular vesicles for cerebral ischemia therapy. J. Contr. Release. 2023;357:1–19. doi: 10.1016/j.jconrel.2023.03.033. [DOI] [PubMed] [Google Scholar]
- 262.Fan C., Zhao Q., Li L., et al. Efficacy and safety of intrathecal Pemetrexed combined with Dexamethasone for treating tyrosine kinase inhibitor-failed leptomeningeal metastases from EGFR-mutant NSCLC-a prospective, open-label, single-arm phase 1/2 clinical trial (unique identifier: ChiCTR1800016615) J. Thorac. Oncol. 2021;16:1359–1368. doi: 10.1016/j.jtho.2021.04.018. [DOI] [PubMed] [Google Scholar]
- 263.Pan Z., Yang G., He H., et al. Concurrent radiotherapy and intrathecal methotrexate for treating leptomeningeal metastasis from solid tumors with adverse prognostic factors: A prospective and single-arm study. Int. J. Cancer. 2016;139:1864–1872. doi: 10.1002/ijc.30214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Miller T.M., Pestronk A., David W., et al. An antisense oligonucleotide against SOD1 delivered intrathecally for patients with SOD1 familial amyotrophic lateral sclerosis: a phase 1, randomised, first-in-man study. Lancet Neurol. 2013;12:435–442. doi: 10.1016/S1474-4422(13)70061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Gotkine M., Caraco Y., Lerner Y., et al. Safety and efficacy of first-in-man intrathecal injection of human astrocytes (AstroRx®) in ALS patients: phase I/IIa clinical trial results. J. Transl. Med. 2023;21:122. doi: 10.1186/s12967-023-03903-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Castle M.J., Cheng Y., Asokan A., Tuszynski M.H. Physical positioning markedly enhances brain transduction after intrathecal AAV9 infusion. Sci. Adv. 2018;4 doi: 10.1126/sciadv.aau9859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Møllgård K., Beinlich F.R.M., Kusk P., et al. A mesothelium divides the subarachnoid space into functional compartments. Science. 2023;379:84–88. doi: 10.1126/science.adc8810. [DOI] [PubMed] [Google Scholar]
- 268.Tosolini A.P., Sleigh J.N. Intramuscular delivery of gene therapy for targeting the nervous system. Front. Mol. Neurosci. 2020;13:129. doi: 10.3389/fnmol.2020.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Xu X., Holmes T.C., Luo M.-H., et al. Viral vectors for neural circuit mapping and recent advances in trans-synaptic anterograde tracers. Neuron. 2020;107:1029–1047. doi: 10.1016/j.neuron.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Tosolini A.P., Morris R. Targeting motor end plates for delivery of adenoviruses: an approach to maximize uptake and transduction of spinal cord motor neurons. Sci. Rep. 2016;6 doi: 10.1038/srep33058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Towne C., Schneider B.L., Kieran D., et al. Efficient transduction of non-human primate motor neurons after intramuscular delivery of recombinant AAV serotype 6. Gene Ther. 2010;17:141–146. doi: 10.1038/gt.2009.119. [DOI] [PubMed] [Google Scholar]
- 272.Aziz A., Pane S., Iacovacci V., et al. Medical imaging of microrobots: toward in vivo applications. ACS Nano. 2020;14:10865–10893. doi: 10.1021/acsnano.0c05530. [DOI] [PubMed] [Google Scholar]
- 273.Martel S. Magnetic navigation control of microagents in the vascular network: challenges and strategies for endovascular magnetic navigation control of microscale drug delivery carriers. IEEE Control Syst. Mag. 2013;33:119–134. [Google Scholar]
- 274.Yan X., Zhou Q., Vincent M., et al. Multifunctional biohybrid magnetite microrobots for imaging-guided therapy. Sci. Robot. 2017;2 doi: 10.1126/scirobotics.aaq1155. [DOI] [PubMed] [Google Scholar]
- 275.Griese F., Knopp T., Gruettner C., et al. Simultaneous magnetic particle imaging and navigation of large superparamagnetic nanoparticles in bifurcation flow experiments. J. Magn. Magn Mater. 2020;498 [Google Scholar]
- 276.Vilela D., Cossío U., Parmar J., et al. Medical imaging for the tracking of micromotors. ACS Nano. 2018;12:1220–1227. doi: 10.1021/acsnano.7b07220. [DOI] [PubMed] [Google Scholar]
- 277.Ceylan H., Yasa I.C., Kilic U., et al. Translational prospects of untethered medical microrobots. Prog. Biomed. Eng. 2019;1 [Google Scholar]
- 278.Yu J., Jin D., Chan K.-F., et al. Active generation and magnetic actuation of microrobotic swarms in bio-fluids. Nat. Commun. 2019;10:5631. doi: 10.1038/s41467-019-13576-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Liberman A., Wang J., Lu N., et al. Mechanically tunable hollow silica ultrathin nanoshells for ultrasound contrast agents. Adv. Funct. Mater. 2015;25:4049–4057. doi: 10.1002/adfm.201500610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Li D., Zhang Y., Liu C., et al. Review of photoacoustic imaging for microrobots tracking in vivo [Invited] Chin. Opt Lett. 2021;19 [Google Scholar]
- 281.Wu Z., Li L., Yang Y., et al. A microrobotic system guided by photoacoustic computed tomography for targeted navigation in intestines in vivo. Sci. Robot. 2019;4 doi: 10.1126/scirobotics.aax0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Li D., Liu C., Yang Y., et al. Micro-rocket robot with all-optic actuating and tracking in blood. Light Sci. Appl. 2020;9:84. doi: 10.1038/s41377-020-0323-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.D’Amico R.S., Englander Z.K., Canoll P., Bruce J.N. Extent of resection in glioma–a review of the cutting edge. World Neurosurg. 2017;103:538–549. doi: 10.1016/j.wneu.2017.04.041. [DOI] [PubMed] [Google Scholar]
- 284.Eisner W., Burtscher J., Bale R., et al. Use of neuronavigation and electrophysiology in surgery of subcortically located lesions in the sensorimotor strip. J. Neurol. Neurosurg. Psychiatry. 2002;72:378–381. doi: 10.1136/jnnp.72.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Senft C., Bink A., Franz K., et al. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol. 2011;12:997–1003. doi: 10.1016/S1470-2045(11)70196-6. [DOI] [PubMed] [Google Scholar]
- 286.Berntsen E.M., Gulati S., Solheim O., et al. Functional magnetic resonance imaging and diffusion tensor tractography incorporated into an intraoperative 3-dimensional ultrasound-based neuronavigation system: Impact on therapeutic strategies, extent of resection, and clinical outcome. Neurosurgery. 2010;67:251–264. doi: 10.1227/01.NEU.0000371731.20246.AC. [DOI] [PubMed] [Google Scholar]
- 287.Moiyadi A.V., Shetty P.M., Mahajan A., et al. Usefulness of three-dimensional navigable intraoperative ultrasound in resection of brain tumors with a special emphasis on malignant gliomas. Acta Neurochir. 2013;155:2217–2225. doi: 10.1007/s00701-013-1881-z. [DOI] [PubMed] [Google Scholar]
- 288.Stummer W., Pichlmeier U., Meinel T., et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 289.Garcia-Navarrete R., Contreras-Vázquez C., León-Alvárez E., et al. Central Nervous System Tumors. IntechOpen; 2021. Multimodal neuronavigation for brain tumor surgery. [Google Scholar]
- 290.Russell S., Bennett J., Wellman J.A., et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–860. doi: 10.1016/S0140-6736(17)31868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Mendell J.R., Al-Zaidy S., Shell R., et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 292.Hudry E., Vandenberghe L.H. Therapeutic AAV gene transfer to the nervous system: a clinical reality. Neuron. 2019;101:839–862. doi: 10.1016/j.neuron.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Wang S., Cheng K., Chen K., et al. Nanoparticle-based medicines in clinical cancer therapy. Nano Today. 2022;45 [Google Scholar]
- 294.Yang G.-Z., Bellingham J., Dupont P.E., et al. The grand challenges of Science Robotics. Sci. Robot. 2018;3:eaar7650. doi: 10.1126/scirobotics.aar7650. [DOI] [PubMed] [Google Scholar]
- 295.Trapecar M., Wogram E., Svoboda D., et al. Human physiomimetic model integrating microphysiological systems of the gut, liver, and brain for studies of neurodegenerative diseases. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abd1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Novak R., Ingram M., Marquez S., et al. Robotic fluidic coupling and interrogation of multiple vascularized organ chips. Nat. Biomed. Eng. 2020;4:407–420. doi: 10.1038/s41551-019-0497-x. [DOI] [PMC free article] [PubMed] [Google Scholar]