Highlights
-
•
Rare data collected from marching locusts during an upsurge highlights importance of field studies.
-
•
Protein hunger does not drive collective movement of locusts, but carbohydrate limitation is common and likely dampens migration.
-
•
Migrating insects are not protein-limited and seek plants low in protein and high in carbohydrate to supply their energy needs.
-
•
Relative plant protein to carbohydrate contents can predict nutritional limitations of locust outbreaks.
Keywords: Collective movement, Nutrition, Migration, Movement ecology, Locust phase change, Swarming, Grasshopper, Orthoptera
Abstract
Locusts are grasshoppers that migrate en masse and devastate food security, yet little is known about the nutritional needs of marching bands in nature. While it has been hypothesized that protein limitation promotes locust marching behavior, migration is fueled by dietary carbohydrates. We studied South American Locust (Schistocerca cancellata) bands at eight sites across Argentina, Bolivia, and Paraguay. Bands ate most frequently from dishes containing carbohydrate artificial diets and minimally from balanced, protein, or control (vitamins and salts) dishes—indicating carbohydrate hunger. This hunger for carbohydrates is likely explained by the observation that local vegetation was generally protein-biased relative to locusts’ preferred protein to carbohydrate ratio. This study highlights the importance of studying the nutritional ecology of animals in their environment and suggests that carbohydrate limitation may be a common pattern for migrating insect herbivores.
Introduction
Locusts are a spectacular example of collective mass migration in herbivores. As juveniles, gregarious locusts form marching bands that can contain millions of individuals and span kilometers (Clark 1949; Ellis and Ashall 1957; Uvarov 1977; Lecoq et al., 1999; Hunter et al., 2008); both marching juveniles and swarming adults can devastate food security (Zhang et al., 2019). While herbivores are often thought to be protein limited, principles of exercise physiology predict that highly active herbivores, including marching juvenile locust bands and adult swarms should be generally carbohydrate hungry due to the high energetic costs of migration (Le Gall et al. 2019, 2021; Talal et al., 2020). However, some Mormon cricket marching bands have been shown to be protein hungry (Simpson et al., 2006; Srygley 2016), suggesting there might be variation in field populations. In addition, the evolution and proximate mechanisms of mass collective movements of locusts have been hypothesized to be influenced by cannibalism and its associated nutritional benefits, in particular meeting protein requirements (Bazazi et al., 2011; Hansen et al., 2011; Guttal et al., 2012), (but see Ariel and Ayali 2015; Maeno et al., 2021; Piou et al., 2021). However, despite the importance for food security and herbivore ecology, no experiments have tested the nutritional status of marching field populations of locusts in multiple environments.
Marching bands of locusts form due to their unique behavioral plasticity. Locusts are grasshoppers (Acrididae) that exhibit extreme phenotypic plasticity, shifting from solitarious forms (shy, low activity) at low population density to gregarious forms (attracted to conspecifics, high activity) at high density (Pener and Simpson 2009). In addition to behavior, other traits can differ depending on the locust species, including color, morphology, and physiology (Cullen et al., 2017). Of the roughly 7000 identified species in the family Acrididae, only about 20 are considered locusts (Song 2011; Cullen et al., 2017). These 20 species are not all closely related, suggesting that this plasticity evolved independently. Indeed, locust species can differ in the types of traits that shift in response to population density, but the core traits that exemplify all locusts are increased aggregation and activity, and, at some point, the formation of migrating groups either as marching bands or flying swarms (Pener and Simpson 2009; Song 2011; Cullen et al., 2017). Species that readily shift to gregarious phenotypes and form large groups that travel especially long distances, such as the Desert Locust (Schistocerca gregaria) are readily classified as locusts. Species that do not shift as readily to gregarious phenotypes or form smaller groups that may not travel as far have been termed non-model locusts (Song 2011). For this paper, we refer collectively to all locusts and non-model locusts as locusts, and we define migration as it relates to individual organisms: “(1) a type of locomotory activity that is notably persistent, undistracted, and straightened out; (2) a relocation of the animal that is on a much greater scale, and involves movement of much longer duration, than those arising in its normal daily activities” (Dingle and Drake 2007).
Is locust marching limited by energy? There is increasing evidence that field populations of locusts are generally carbohydrate limited, and that the availability of carbohydrate-biased plants is important for growth, survival, and buildup of populations, leading to high density and subsequent migration (reviewed in Le Gall et al. 2019). For example, lab populations of Desert Locust (Schistocerca gregaria) nymphs fed carbohydrate-biased diets moved faster than those fed protein-biased diets (Bazazi et al., 2011). While neither the energy cost or fuel usage has been measured for marching locusts, in other insects, sustained terrestrial locomotion increases metabolic rates by 2–12-fold above resting (Herreid and Full 1984). Gregarious locusts rely on carbohydrate and lipid stores for flight and, likely, also to fuel marching as juveniles (Jutsum and Goldsworthy 1976; Gokuldas et al., 1988; Talal et al., 2020, 2021).
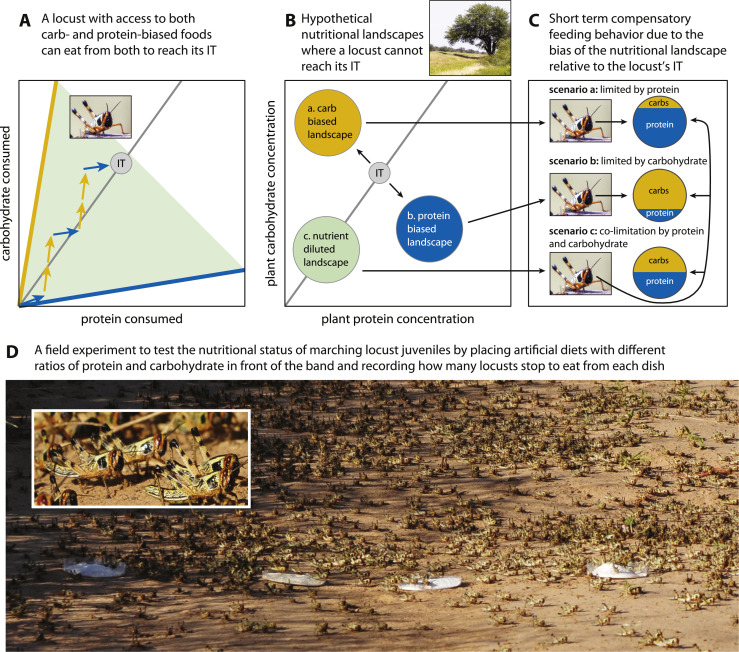
How do locusts acquire sufficient energy in their diet? Animal foraging behavior is highly attuned to balancing protein and carbohydrates (and/or lipids) since these make up the largest majority of nutrients in their diet (Simpson and Raubenheimer 2012). Available food sources have both, so consumers can rarely eat one nutrient without also eating some amount of the other, and balancing these macronutrients has substantial impacts on life history traits. When given a choice between protein- and carbohydrate-biased diets over several days, locusts regulate to a specific balance, or intake target (“IT”, Fig. 1A) (reviewed in Simpson and Raubenheimer 2000). If locusts cannot access foods that allow them to reach their IT (Fig. 1B, landscapes a and b), they become nutritionally imbalanced and will more readily consume food biased in the opposite direction to redress the imbalance (Fig. 1C) (Simpson and Raubenheimer 2000, 2012; Le Gall et al. 2019). This leads to conditional predictions for behavioral responses of marching locusts that are dependent on the type of nutrient limitation experienced. If resources are generally carbohydrate-biased relative to the population IT (Fig. 1B, landscape a), then locusts should eat more from protein-biased food sources (Fig. 1C, locust a). If resources are protein-biased relative to the IT (Fig. 1B, landscape b), locusts should eat more from carbohydrate-biased food sources (Fig. 1C, locust b). If both macronutrients are limiting (i.e. nutrient dilution or total food limitation; Fig. 1B, landscape c), locusts should eat equally from all food resources (Fig. 1C, locust c).
Fig. 1.
Panel A: Feeding behavior by a locust balancing carbohydrate and protein to reach its intake target (IT), RPO and L. Steger locust photo credit. Panel B: Hypothetical nutritional landscapes and predicted nutrient limitation for locusts. Panel C: Predicted compensatory feeding behavior by locusts in the nutrient limiting environments shown in panel B. Panel D: Marching South American Locust (Schistocerca cancellata) nymphs during a field trial in Paraguay, April 2019, ST photo credit. All panels are based on the Geometric Framework for Nutrition (Simpson and Raubenheimer 2012). See text for a detailed description.
We tested these predicted conditional responses (Fig. 1C) using outbreaking South American Locusts (Schistocerca cancellata) (Fig. 1D). This species is related to and exhibits similar gregarious characteristics as Desert Locusts (Pocco et al., 2019; Trumper et al., 2022). South American Locusts are highly polyphagous and have been recorded eating myriad plants ranging from mesquite, soybeans, and peanuts to pasture grasses, maize, and sorghum—it even attacks citrus (Centre for Overseas Pest Research 1982; Le Gall et al. 2019). It was among the most destructive regional agricultural pests from the 1800s to 1954, with its maximum invasion zone covering 4000,000 km2 and six countries (Centre for Overseas Pest Research 1982). However, it typically only persists at pest levels within a central permanent breeding zone in northeast Argentina (Köhler 1962). Between 2015 and 2020, the largest upsurge transpired since 1954 (Medina et al., 2017; Trumper et al., 2022), massive swarms darkened skies, and Argentina, Bolivia, and Paraguay all declared states of emergency (Medina pers. obs.; see also Video S1). The outbreak likely originated from the central permanent breeding zone and expanded across Argentina, Bolivia, and Paraguay (Medina et al., 2017; Trumper et al., 2022). In three different years, we traveled to a total of eight sites across Argentina, Bolivia, and Paraguay to test the nutritional status of marching bands of juveniles across multiple environments by placing dishes containing artificial diets with different amounts of protein and carbohydrate in front of oncoming bands (e.g., Simpson et al., 2006). We also collected host plants from these sites for subsequent nutrient analyses and determined the self-selected intake targets (ITs) of locusts collected from Argentina and Bolivia. We predicted that locusts would have carbohydrate-biased ITs, similar to other final instar locusts (reviewed in Le Gall et al. 2019), and that marching bands would eat predominantly from dishes containing the nutrient (protein or carbohydrate) that was predicted to be limiting based on comparing their IT to the p:c of plants available in their environment (Fig. 1).
Methods
Insects
Field populations
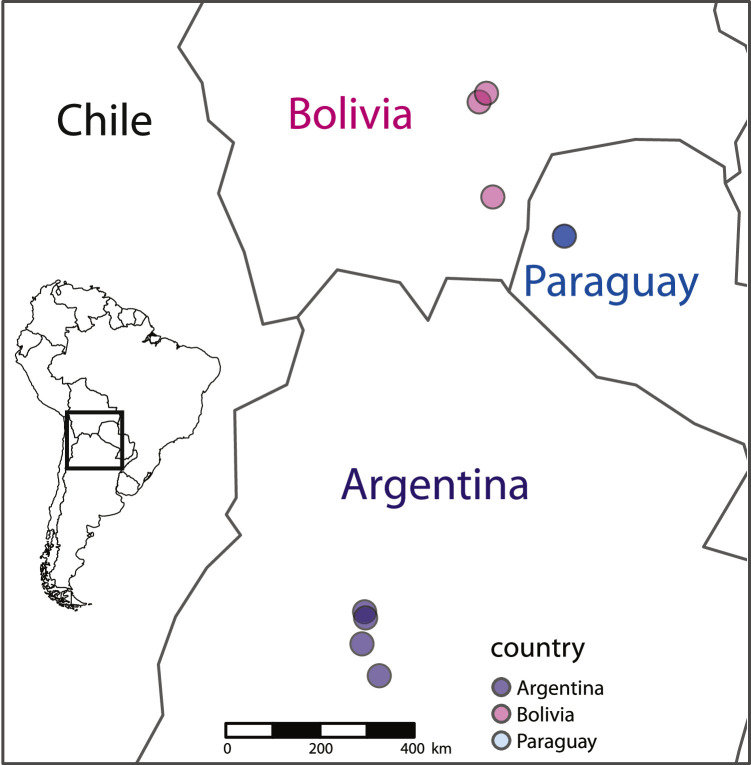
The dramatic emergence of the South American Locust after 60 years of recession provided a unique opportunity to investigate the nutritional ecology of S. cancellata during an outbreak event. We collected data from four sites in Argentina in 2016, three sites in Bolivia in 2017, and one site in Paraguay in 2019 (Paraguay data adapted from Talal et al., 2020) (Fig. 2).
Fig. 2.
Map of experiment sites.
The nutritional status experiments in Argentina took place in April and May of 2016 at four sites in Catamarca and La Rioja provinces, within the S. cancellata permanent breeding zone. In Bolivia, the nutritional status experiments took place in March and April 2017 in the department of Santa Cruz near the communities of Mora and Cabezas in the east and the community of Boyuibe in the southeast. This area in Santa Cruz is near the historical northeastern upper limit of the S. cancellata outbreak range (Trumper et al., 2022). In Paraguay, the nutritional status experiments took place in April 2019 (Talal et al., 2020). All field sites were within the Gran Chaco, a biodiverse and heterogenous, sub-tropical region of central South America. The area generally experiences wet summers and dry winters with high annual variation in temperature as well as high seasonal and geographic variation in precipitation (300 mm–1200 mm). Dominated by a wooded steppe of Prosopis, Schinopsis, Larrea, Bulnesia, and Stesonia (Morales et al., 2019), the Gran Chaco is more recently the stage for increasing deforestation and land-use change primarily for cattle ranching and soy farming (Baumann et al., 2017). We located experiment sites with assistance from SENASA (Servicio Nacional de Sanidad y Calidad Agroalimentaria) field officer Ing Agr Carlos Maldonado and crew in Argentina, SENASAG (Servicio Nacional de Sanidad Agropecuaria e Inocuidad Alimentaria) field officers in Bolivia, and SENAVE (Departamento de Campañas Fitosanitarias, Dirección de Protección Vegetal) field officers in Paraguay. All selected sites had experienced outbreaks, including multiple bands of marching nymphs ranging from third to final instars. During these outbreaks, we observed bands marching past ample vegetation and water puddles (Fig. 3), suggesting they were not generally food or water limited.
Fig. 3.
Panel A: South American Locust nymphs aggregating to roost in the late afternoon near Cabezas, Bolivia, April 2017; inset of roosting nymphs from Catamarca, Argentina February 2020, RPO and L. Steger photo credit. Panel B-C: Field trial location near Boyuibe (Panel B) and outside Rio Seco (Panel C), Bolivia, April 2017, AJC photo credit. Marching bands were often found in disturbed areas, particularly along roadsides.
Lab populations
On the final day of our field seasons in both Argentina and Bolivia, we collected >800 nymphs and transported them to locust rearing facilities at Arizona State University under USDA permit P526P-19–03892. Locusts were kept in standard laboratory conditions for five generations (Argentina) or one generation (Bolivia). Colonies were kept in walk-in environmental growth chambers regulated at 30 °C:25 °C and 14 h:10 h L:D cycle, with 60 Watt incandescent bulbs during the day positioned above cages to allow thermoregulation. Locusts were fed a daily mixture of wheat seedlings, romaine lettuce, and wheat bran, ad libitum, and given cups of mixed sand and vermiculite (3:1 ratio) for oviposition.
Nutrition experiments
Field nutritional status experiment
We evaluated the nutritional status of high density bands of S. cancellata nymphs during the upsurge by presenting four different artificial diet choices at the leading edge of marching bands such that locusts marched roughly perpendicularly across a transect of diets (similar to Simpson et al. (2006)). Three artificial diets varied in protein (p):carbohydrate (c) ratio (0p:42c, 21p:21c, 42p:0c); the fourth diet (0p:0c) contained no macronutrients and only cellulose with the other diet constituents. For the trials, artificial diet dishes were placed 4 cm apart with a random order for each trial. For each trial, a set of diets was monitored for 15–30 min by trained observers. At 30 second intervals, observers recorded the count of individuals arrested at each dish, with at least their head over the contents of the dish (Fig. 1D). For each trial, these 30-second records were summed and normalized by dividing the total for each dish by the sum across all four dishes. The proportion of locusts stopping on the diet treatments was compared using a Kruskal-Wallis one-way analysis of variance test and a Dunn's post-hoc test using the R statistical environment (R Core Team 2018) and the package dunn.test (Dinno 2017).
Lab self-selected macronutrient ratios experiment
We determined the self-selected intake targets (protein to carbohydrate ratio) of locust populations collected from Argentina and Bolivia to assess their nutritional demands using an approach developed by the Geometric Framework for Nutrition (Simpson and Raubenheimer 2012), similar to (Cease et al., 2012; Le Gall et al. 2020b). On day one of the final nymphal instar, we collected locusts from colony populations and placed them into one of two treatments, each containing a water tube, perch, and two food dishes: (1) 7p:35c and 35p:7c or (2) 7p:35c and 28p:14c. Each treatment started with 12 males and 12 females, summing to 48 locusts tested from each population. Individuals who died before day eight were excluded from the analyses (16 from Argentina and 6 from Bolivia). Data for one additional individual from Argentina was removed from the analysis due to its food dishes being spilled. We measured consumption over eight days; no locusts molted to adults during this time period. Dishes plus food were weighed prior to adding them to the cages. Food remaining in the dishes was dried at 60 °C for 48 h, then weighed. We calculated the mass of protein and carbohydrate eaten by multiplying the dry food consumed by the proportion of that constituent in a given diet and summing the two dishes for each locust. We ran Exact Wilcoxon-Mann-Whitney Tests implemented with version 1.4–2 of the coin package (Hothorn et al., 2008) in R to determine if locusts were eating non-randomly. We ran MANCOVAs to test the effect of diet pair and sex on protein and carbohydrate eaten by each population.
Artificial diets
We made artificial dried powdered diets following (Dadd 1961) and modified according to (Simpson and Abisgold 1985). All diets for the lab experiment and three diets from the field experiment included a uniform 42 % digestible macronutrients, protein (p) and carbohydrates (c), by dry mass, with the ratio of p:c varying based on experiment, plus indigestible cellulose, vitamins, linoleic acid, cholesterol, and Wesson's salt mixture. For the field nutritional status experiment, we made one additional diet where the macronutrients were replaced with cellulose, but the amounts of the remaining ingredients were kept constant.
Plant collection and nutrient assays
At each outbreak site we visited, we walked systematically through a roughly 100 m2 area and selected the 3–9 most abundant plant species. We then collected leaf samples from multiple individuals of these abundant species (up to three samples per species per site) throughout the site. This resulted in approximately 80 plant samples for each country. Because the locusts were at such high density, out of necessity, they likely had to feed on most of these species. Indeed, S. cancellata is notorious for eating a wide breadth of grasses, shrubs, trees, and crops (Centre for Overseas Pest Research 1982; Trumper et al., 2022) and we found evidence of locusts feeding on the species we collected. All sites either had active outbreaks (i.e., the sites where we measured nutritional status of marching locusts) or had been inundated with locusts within the prior few days (as evidenced by field reports and substantial vegetation damage). Plant leaves were dried at 60 °C for 72 h, then ground using a ball mill (Retsch MM 400) at 200 rpm for 30 s. Protein was measured using a Bradford assay (Bradford 1976) (for Argentina and Bolivia samples) or estimated from the nitrogen content (elemental analysis) and multiplying by a nitrogen to protein conversion factor of 6.25 (Paraguay samples; Talal et al., 2020). Carbohydrates were measured with a modified phenol-sulfuric acid assay (Dubois et al., 1956; Clissold et al., 2006).
Statistical analyses
Prior to performing statistical analyses, all data sets were tested for assumptions of the statistical analyses used. If data did not meet the assumptions for parametric tests, we transformed the data or used non-parametric tests. For details on specific statistical analyses, see experiment subsections.
Results
Field nutritional status experiment
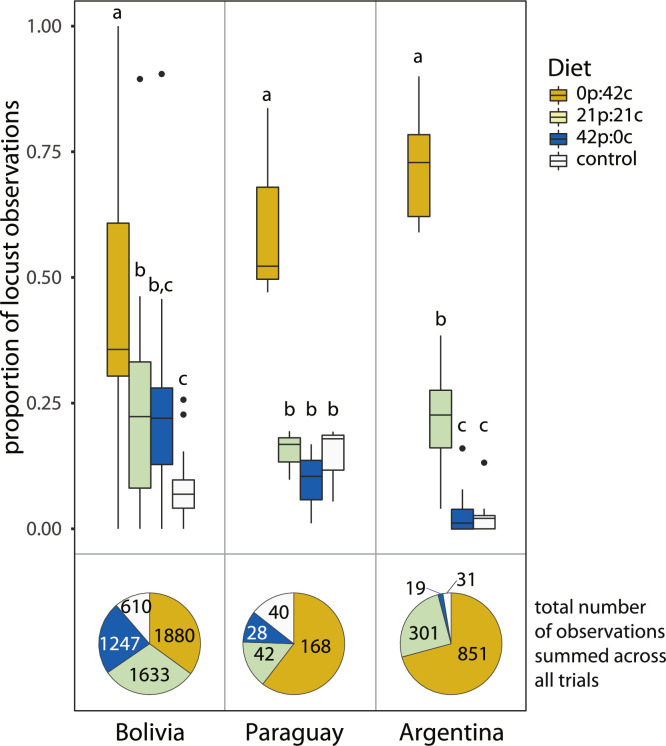
For all populations, more locusts stopped on the carbohydrate-biased diets than any other diet Kruskal-Wallis tests: Argentina (χ2 = 38.4, df = 3, P<0.001), Bolivia (χ2 = 21.6, df = 3, P<0.001) (Fig. 4). Summing all locust observations across all countries and years, the ratio of locusts stopping on the different diet dishes was 4.2 to 2.9 to 1.9 to 1 for the following diets, respectively: 0p:42c, 21p:21c, 42p:0c, control. For direct comparison with prior studies that used chi-squared tests on each individual trial (Talal et al., 2020), we ran independent analyses on all trials. For all trials in Argentina, and in 16 out of 17 trials in Bolivia, locust marching bands stopped on diet dishes in numbers different than what would be expected by chance (i.e., chance would predict equal numbers stopping on all dishes) when considering all four diet dishes or when considering just the three diet dishes that contained macronutrients (0p:42c, 21p:21c, 42p:0c) (see data supplement).
Fig. 4.
Nutritional status of marching nymphs, as indicated by the proportion of locusts stopping at each diet across 12 trials in Argentina, 17 trials in Bolivia, and 3 trials in Paraguay. Boxplots are medians with interquartile ranges. Letters indicate significant differences from Dunn's multiple-comparison tests, following Kruskal-Wallis tests. Paraguay data were adapted from Talal et al., 2020 and analyzed using multiple chi-squared tests with Bonferroni corrections. Pie charts represent the total number of observations summed across all trials for each country.
Lab self-selected macronutrient ratios experiment
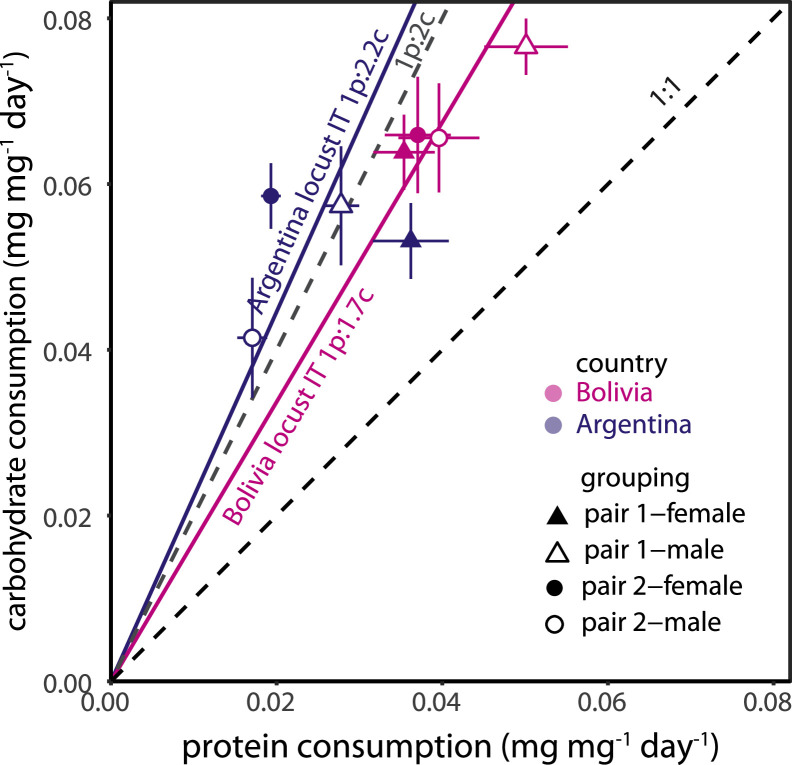
Locusts ate non-randomly when given a choice between diet pairs in all cases, selecting a carbohydrate-biased IT (Fig. 5). From Exact Wilcoxon-Mann-Whitney Tests: Argentina pair 1: Z = 3.87, P<0.001, pair 2: Z = 5.27, P<0.001; Bolivia pair 1 Z = 4.85, P<0.001, pair 2 Z = 3.14, P = 0.001. For Argentina, there was a significant interactive effect of diet and sex (MANCOVA F(2, 25) = 7.25, P = 0.003). These results suggest that, although locusts from the Argentina population were non-randomly selecting an IT of about 1p:2c, they were not tightly regulating around these ratios (e.g., Le Gall et al. 2022). For Bolivia, there were no significant main or interactive effects of diet and sex (MANCOVA F(2, 36)=0.58, P = 0.57), indicating they were tightly regulating for an IT of about 1p:1.7c.
Fig. 5.
Intake targets for Argentina and Bolivia populations. Points represent means and SEMs of mass specific protein and carbohydrate consumption of males (open symbols) and females (closed symbols), eating diet pair 1 (triangle, 7p:35c and 35p:7c) or diet pair 2 (circle, 7p:35c and 28p:14c), from Argentina (purple) or Bolivia (pink) populations.
Plant collection and nutrient assays
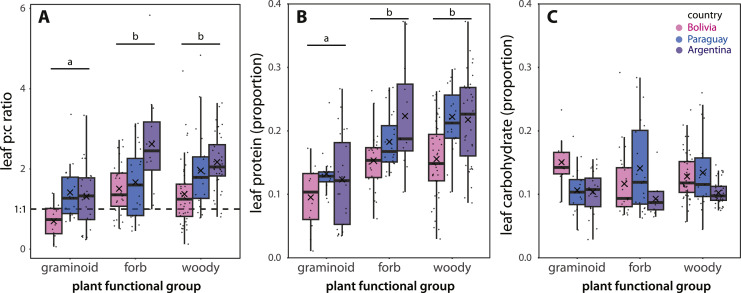
Plant leaves ranged from 1 to 38 % protein and 3–29 % carbohydrate (Fig. 6). Graminoids had a lower p:c ratio on average (1.2 ± 0.1SE) relative to forbs (1.8 ± 0.1SE) and woody vegetation (1.8 ± 0.1SE); all were in the range of plant leaf protein and carbohydrate contents found by other studies (Table S1). There were significant main effects of plant functional group (ANOVA F(2, 213) = 14.8, P<0.001) and country (ANOVA F(2, 213) = 16.5, P<0.001) on plant p:c, but no interactive effect (ANOVA F(4, 213) = 1.9, P = 0.11) (Fig. 7A). These plant p:c differences were mainly driven by plant protein content, which showed the same pattern with significant main effects of plant functional group (ANOVA F(2, 213) = 30.3, P<0.001) and country (ANOVA F(2, 213) = 13.7, P<0.001) on plant protein content, but no interactive effect (ANOVA F(4, 213) = 1.4, P = 0.24) (Fig. 7B). Tukey post-hoc analyses showed that graminoids had lower p:c ratios and protein contents than forbs and woody vegetation, and that Bolivia plants had lower p:c ratios and protein contents than Argentina and Paraguay plants. The pattern for carbohydrate contents was more varied, with a significant interactive effect of country and plant functional group (ANOVA F(4, 213) =3 .0, P = 0.02) and no main effect of plant functional group (ANOVA F(2, 213) = 0.2, P = 0.81) (Fig. 7C). Carbohydrate contents were similar, on average, in Paraguay and Bolivia, but lower in Argentina (ANOVA F(2, 213) = 9.9, P<0.001; followed by a Tukey post-hoc analysis).
Fig. 6.
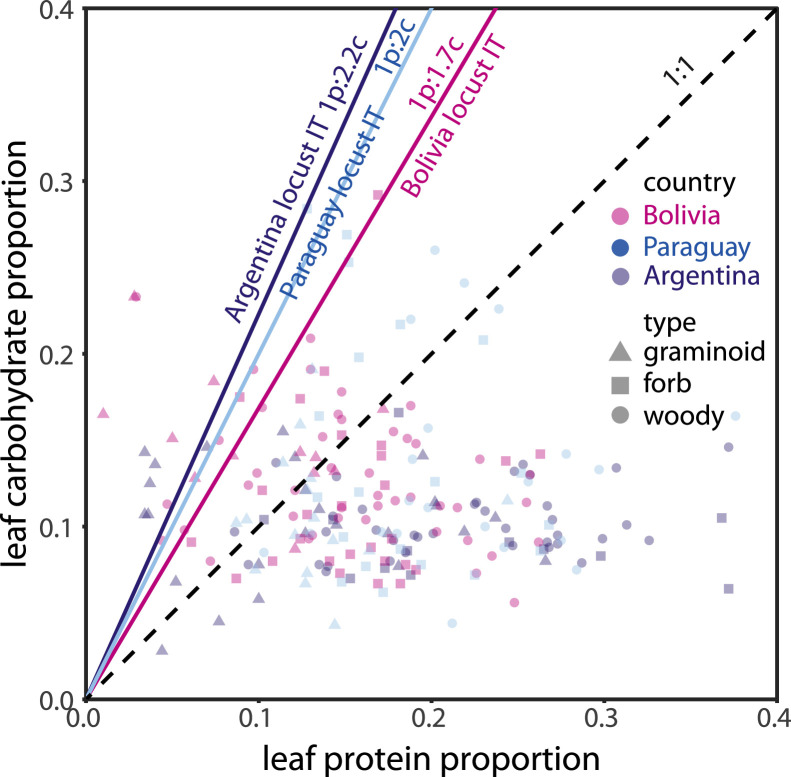
Self-selected protein:carbohydrate intake targets (ITs) of locust populations from Bolivia (pink), Paraguay (blue), and Argentina (purple). Symbols indicate nutrient contents of available host plants at the outbreak sites for graminoids (triangles), forbs (squares), and woody vegetation (circles). The dashed line is the 1p:1c line, for reference. Most available plants fell to the right of the ITs, suggesting that locusts would have a difficult time obtaining sufficient carbohydrate without overeating protein (Fig. 1).
Fig. 7.
Plant p/c ratios (A), protein contents (B), and carbohydrate contents (C) collected from field sites in Bolivia (pink), Paraguay (blue), and Argentina (purple). Boxplots are interquartile ranges with the median shown as a bold line and mean as an X. The small dots are the raw data points. In panel A, values above the 1:1 line are protein-biased (more protein than carbohydrate). There were significant main effects of plant type and country on plant p:c ratios and protein. Graminoids had lower p:c and protein values than forbs or woody vegetation, as represented by the bars and lower-case letters in panel A and B. Bolivia plants had lower p:c and protein values than Paraguay and Argentina (Tukey posthoc analysis not shown). For more statistical details, see the section Plant collection and nutrient assays.
Plants were collected from the following families (genera or species in parentheses):
Argentina: Amaranthaceae (Atriplex sp.), Anacardiaceae (Schinopsis balansae), Asteraceae (Pascalia glauca, Asteraceae sp.), Commelinaceae (Tradescantia sp.), Cyperaceae (Cyperus sp.), Euphorbiaceae (Jatropha macrocarpa), Fabaceae (Acacia spp., Medicago sativa, Mimozyganthus carinatus, Parkinsonia praecox, Prosopis sp., Senna spp.), Malpighiaceae (Tricomaria usillo), Poaceae (Cenchrus spp., Chloris spp., Cynodon dactylon, Panicum spp., Setaria sp., Sorghum sp., Stipa sp.), Malvaceae (Malva spp., Sida rhombifolia), Zygophyllaceae (Larrea divaricata, Larrea cuneifolia).
Bolivia: Amaranthaceae (Amaranthus spp., Gomphrena martiana), Acanthaceae (Anisacanthus boliviensis, Justicia spp.), Asteraceae sp., Capparaceae (Capparicordis tweediana, Sarcotoxicum salicifolium, Cynophalla retusa), Bignoniaceae (Tabebuia nodosa, Tanaecium dichotomum), Cannabaceae (Celtis iguanaea), Euphorbiaceae (Croton spp.), Fabaceae (Acacia sp., Anadenanthera colubrina, Parkinsonia praecox, Senegalia praecox, Vachellia aroma), Poaceae (Digitaria sp., Panicum maximum, Paspalum sp.), Portulacaceae (Portulaca sp.), Polygonaceae (Ruprechtia triflora), Solanaceae (Solanum).
Paraguay: (see Talal et al., 2020).
Comparing plant nutrients with marching band carbohydrate hunger
All but 15 of the 214 leaf samples (90 %) were protein-biased relative to the 1p:2c line, which was about the average IT for the three populations (Fig. 6). This distribution of plants predominantly to the right of the 1p:2c line is far greater than what might be expected by chance if plants fell evenly on both sides (chi-squared test P<0.001).
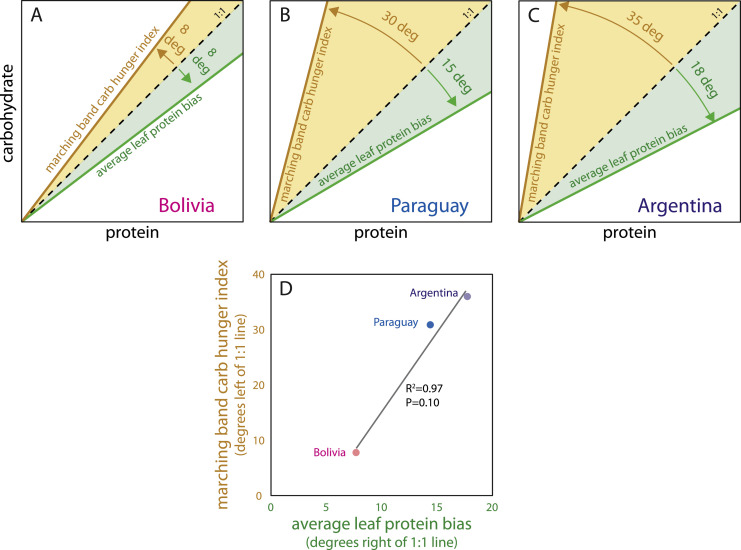
The marching bands in the different nutritional landscapes responded in a pattern predicted by nutritional geometry (Fig. 1) (Simpson and Raubenheimer 2012). To compare the degree to which the marching locusts in Argentina, Paraguay, and Bolivia were hungry for carbohydrate vs protein, we calculated a carbohydrate hunger index for each country. First, we calculated the marching band p:c slope as follows, [(#observations on p dish * 42)+(#observations on p:c dish * 21)] / [(#observations on carb dish * 42)+(#observations on p:c dish * 21)]. We then plotted lines for each country using the marching band p:c slopes on a Cartesian plane and calculated the carbohydrate hunger index by determining how many degrees the p:c line was to the left of the 1:1 line. Lastly, we plotted the average leaf p:c ratio for each country as lines and calculated the average leaf protein bias by determining the number of degrees the plant line was to the right of the 1:1 line (Fig. 8A–C). The magnitude of carbohydrate hunger by locusts marching through the landscape increased as plant leaf protein bias increased (R2=0.97, P = 0.10) (Fig. 8D).
Fig. 8.
Locusts were more hungry for carbohydrate than protein in all landscapes (all lines fell to the left of the 1:1 line), but the carbohydrate hunger increased in magnitude from Bolivia (A), to Paraguay (B), and to the highest degree in Argentina (C). Bands followed a pattern predicted by nutritional geometry in that the more protein biased the plant leaf nutritional landscape that they were marching through was on average, the greater carbohydrate hunger they expressed (D). For more details see the section Comparing plant nutrients with marching band carbohydrate hunger and the discussion.
The availability of a diversity of plants may give locusts the opportunity to select plants that are closer to their IT. The Bolivia field work occurred in March and April when the diversity of green vegetation is high, which may explain, in part, why the number of locusts seeking the carbohydrate-only diet dish was not as extreme as it was for the Paraguay and Argentina populations. However, the variation in plant p:c and protein were similar for all three countries (standard errors for p:c were 0.10, 0.10, and 0.12, and 0.008, 0.008, and 0.009 for protein), suggesting locusts in the three regions had access to similar diversities of plant nutrients.
Discussion
Our experimental evidence collected across three years, three countries, and eight unique outbreak sites containing marching bands of the South American Locust (Schistocerca cancellata) found that marching nymphs ate predominantly from dishes containing carbohydrates and largely ignored dishes containing protein, including diets containing 21p:21c (Fig. 4). Final instar nymphs collected from the field selected carbohydrate-biased intake targets ranging from 1p:1.7c to 1p:2.2c (Figs. 5 and 6), consistent with other locust species (Le Gall et al. 2019). Most available host plants in their environment (93 %) had a p:c ratio >1p:2c, more protein-biased than the average S. cancellata IT (Fig. 6). Therefore, locusts were predicted to be carbohydrate limited (Fig. 1) and our results match the prediction that they would preferentially stop to eat from carbohydrate dishes at a greater frequency than dishes containing protein and carbohydrate or only protein (Fig. 4).
The nutritional status of an individual strongly affects the likelihood they will accept or refuse a meal based on its nutrient content (Simpson and Raubenheimer 2000). For example, if individuals are carbohydrate deprived and protein replete, they will seek out carbohydrate-biased meals and avoid protein-biased meals to redress the imbalance. Across taxa, herbivores and omnivores are more reluctant to overeat protein (Cheng et al., 2008; Simpson and Raubenheimer 2012), potentially due to energetic costs of managing nitrogenous waste products or increased production of oxygen radicals (Harrison et al., 2012). While locusts may not extract the same relative amounts of protein and carbohydrate from plants as measured by lab assays, protein is more readily extracted (Clissold et al., 2006), suggesting that these food plants may be even more protein-biased relative to locust demands than predicted by Fig. 6.
The concept of protein hunger promoting collective movement in locust bands stemmed from marching Mormon crickets (Simpson et al., 2006). This study showed that the band of marching katydids (Tettigoniidae) ate primarily from protein and salt dishes and, when fed high protein diets, individuals from the marching band decreased movement and cannibalism. Subsequent modeling approaches showed how, at high population density, behaviors emerging from nutrient limitation could promote the collective marching behavior seen in locusts (Bazazi et al., 2011; Guttal et al., 2012). However, additional Mormon cricket field data revealed that some bands were protein hungry and others carbohydrate hungry (Srygley 2016), indicating that protein and salt deprivation were not necessary to produce collective movement. In contrast to the varied responses of Mormon cricket bands, marching South American Locusts were consistently carbohydrate hungry across multiple years and populations (Fig. 4). Locusts begin accumulating lipids that can be used for adult flight during their late juvenile stages, largely from de novo synthesis of ingested carbohydrates (Talal et al., 2020, 2021). Thus, whereas Mormon crickets are flightless, the consistent carbohydrate hunger of locusts while marching as juveniles is potentially driven in part to prepare for the energetic demands of adult flight. These patterns are also consistent with their dietary life history—Mormon crickets are omnivorous, but locusts are generally herbivorous. Nevertheless, our results, in combination with prior lab studies (Bazazi et al., 2011) indicate that locusts do not need to be in a specific nutritional state to march, and that satiating protein hunger is not a primary driver of collective movement in locusts. Although the form of marching and collective behavior can be shaped by nutritional state to the extent that this changes local interaction rules and locomotory patterns (Bazazi et al., 2011; Buhl et al., 2012). This is consistent with some of the earliest studies on locust behavior (Uvarov 1928), along with more recent studies (Dkhili et al., 2019), which suggest hunger (of any nutrient) is not necessary for collective movement for locusts.
Nutritional geometry theory predicts that the degree to which a population exhibits carbohydrate hunger should be proportional to the degree to which their nutritional landscape is protein-biased (Simpson and Raubenheimer 2012). The p:c and protein contents of plant leaves were different in the different countries with Bolivia having the lowest p:c ratios and protein contents, on average, and Argentina having the highest (Fig. 7). While it is unclear whether these differences were due to inter-annual, -seasonal, or -regional differences, this variation provided an opportunity to test this prediction. Indeed, locusts marching through the most protein-biased landscape (Argentina) showed the strongest carbohydrate hunger (Fig. 8C) and locusts marching through the least protein-biased landscape (Bolivia) showed the lowest degree of carbohydrate hunger (Fig. 8A). Paraguay is in between the two, creating a positive relationship between plant average protein bias and the marching band carbohydrate hunger index (Fig. 8D). This relationship between plant leaf nutritional landscape and locust carbohydrate and protein hunger suggests that locusts are mainly reliant on plant leaves to meet their protein and non-protein energy demands, and that it is unlikely that marching bands redress nutritional imbalances in nature to a meaningful degree through cannibalism or other food sources. These results are consistent with other studies showing that Migratory Locusts (Locusta migratoria) have evolved a chemical defense to deter cannibalism (Chang et al., 2023) and that cannibalism is fleetingly rare in nature for Desert Locusts (Schistocerca gregaria) (Maeno et al., 2023).
An important remaining question is whether these findings apply more generally. How do South American Locust ITs compare to other locust species and is it an outlier at about 1p:2c? Behmer (2009) reported grasshopper ITs that had been measured up to that point (including two locust species); they spanned from protein-biased (1p:0.6c) to carbohydrate-biased (1p:1.4c). In the subsequent decade, the ITs of additional locust species were measured, including lab and field populations. Le Gall et al. (2019) reported ITs for locust species and showed that all species studied to date (S. gregaria, L. migratoria, C. terminifera, O. asiaticus, O. senegalensis) have carbohydrate-biased ITs (1p:1.2c to 1p:2.2c). Interestingly, while long-term lab grasshopper and locust colonies tend to tightly regulate to a given IT (Behmer 2009), some populations more recently collected from the field will non-randomly select for a given IT, but not as tightly (e.g., Le Gall et al. 2022; Fig. 5). The driver of this variation is not well understood, but likely due to a combination of environmental factors. Nevertheless, S. cancellata has an IT consistent with other locusts and is not an outlier. Indeed, studies including field surveys, host plant choice tests, field cage studies, and lab studies, and from across regions in China (Cease et al., 2012, 2017), Australia (Lawton et al., 2020, 2021), and West Africa (Word et al., 2019; Le Gall et al. 2020b, 2020a, 2021) differing greatly in ecology, have shown that outbreaking late-instar field populations of multiple locust species prefer and perform best on low protein, high carbohydrate diets (reviewed in Le Gall et al. 2019).
Another aspect in exploring the generality of our results is comparing plant nutrient contents. Are South American Locusts likely to encounter similar nutritional landscapes in different years? Are other locust species likely to encounter plants with a similar range of nutrient contents, or are plants in the Chaco region unusually protein-biased? While scientists conducting ecological research often measure leaf nitrogen, which can be used to calculate crude protein, fewer studies report leaf digestible or water-soluble carbohydrate content. Nevertheless, the range of values we found for leaf protein content for woody vegetation and graminoids were similar to other studies in the Chaco region collected in different years (Tecco et al., 2013; Baldassini et al., 2018; Assunção et al., 2020) (Table S1). A review of similar studies suggests that plant leaves in the Chaco are in the range of nutrient contents reported for leaves in many parts of the world. Some studies report more protein-biased results (Scott Brown et al. 2002) or more carbohydrate-biased results (Lenhart et al., 2015; Zembrzuski et al., 2021), but most generally encompass the same nutrient content ranges (Table S1).
In conclusion, while direct tests of the nutritional status of more marching locust species are needed, data to date suggest that carbohydrate hunger is likely the most common nutritional state for marching locusts. Importantly, due to increased energetic demands, carbohydrate-biased foods are likely to support outbreaks and promote migratory behavior in locusts and transboundary migratory pests more generally. Of the 15 plants that had a p:c ratio less than 1p:2c, most (73 %) were graminoids (Fig. 6) and graminoids had a lower p:c on average than forbs or woody vegetation (Fig. 7; Table S1), suggesting that grasses are important for fueling locust migration. Corroborating this idea, deforestation, particularly conversion of forest to pastures, has been linked to outbreaks of other locusts: Locusta migratoria (Farrow 1979; Lecoq and Sukirno 1999), Schistocerca piceifrons (Poot-Pech 2017), and Chortoicetes terminifera (Deveson 2012). In contrast, practices that increase soil fertility, thereby increasing plant p:c ratios, likely dampen large-scale outbreaks and migrations (Cease et al., 2015, 2017; Word et al., 2019). For the South American Locust, it is possible that roadways harboring invasive low p:c grasses (Talal et al., 2020) create a network of refueling stations for migrating locusts (Fig. 2B and C).
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research. One or more authors of this paper self-identifies as an underrepresented ethnic minority in science, a gender minority in their field of research, and/or a member of the LGBTQ+ community. We recognize the strength in team diversity and are proud to represent eight institutions from four countries spanning universities, government organizations, plant protection directorates, and conservation projects.
CRediT authorship contribution statement
Arianne J. Cease: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. Eduardo V. Trumper: Conceptualization, Resources, Writing – original draft, Writing – review & editing, Investigation, Methodology. Héctor Medina: Investigation, Methodology, Resources, Writing – review & editing. Fernando Copa Bazán: Investigation, Methodology, Resources, Writing – review & editing. Jorge Frana: Investigation, Methodology, Resources, Writing – review & editing. Jon Harrison: Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. Nelson Joaquin: Investigation, Methodology, Resources, Writing – review & editing. Jennifer Learned: Conceptualization, Investigation, Methodology, Writing – review & editing. Mónica Roca: Investigation, Methodology, Resources, Writing – review & editing. Julio E. Rojas: Investigation, Methodology, Resources, Writing – review & editing. Stav Talal: Funding acquisition, Investigation, Methodology, Writing – review & editing. Rick P. Overson: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Thanks to Carlos Maldonado, Luis Sanchez Shimura, Geordana Zeballos Céspedes, Alberto Gutiérrez, Wilda Ramírez, Marcelino López Rendón, and Jacob P. Youngblood, as well as SENASA and INTA (Argentina), SENASAG, INIAF, and CIAT (Bolivia), and SENAVE (Paraguay) for generous field support; Marion Le Gall, Natalia Thompson, Ruth Farington, and Douglas Lawton for assistance with the lab studies; and Mira Ries, Greg Sword, Stephen Simpson, and several anonymous reviewers for helpful comments on the manuscript. We thank the communities in the provinces of Catamarca and La Rioja in Argentina, the departments of Santa Cruz and Chuquisaca in Bolivia, and the departments of Presidente Hayes and Boquerón in Paraguay for welcoming researchers into their communities and sharing details of their locust experiences. In the US, the authors recognize that the ASU campus community has and continues to benefit from land that was taken from Indigenous communities, including the Akimel O'odham (Pima) and Pee Posh (Maricopa) Indian Communities, whose stewardship of these lands allows us to be here today. This work was funded by the National Science Foundation, United States [RAPID-1826848, DBI-2021795, and IOS-1942054], and US-Israel Binational Agricultural Research and Development fund [BARD FI-575-2018]. This work was a project by the Global Locust Initiative Laboratory (Arizona State University) and the NSF Behavioral Plasticity Research Institute.
Footnotes
Supplementary material associated with this article includes Table S1, Video S1, and the data and can be found, in the online version, at doi:10.1016/j.cris.2023.100069.
Appendix. Supplementary materials
Video S1. Footage during the S. cancellata upsurge. Scene 1: Nymphs aggregating on the roadside near Boyuibe, Bolivia, April 2017, filmed by RPO. Scene 2: Final instar nymphs marching across the road near Cabezas, Bolivia, April 2017, filmed by RPO. Remaining scenes are high density or swarming adult S. cancellata in northern Argentina, 2017–2018, filmed by SENASA field officers. This video is located in the online supplemental material available on the journal's website.
Data availability
Data presented in this paper are located in the online supplemental material at doi:10.1016/j.cris.2023.100069.
References
- Ariel G., Ayali A. Locust Collective Motion and Its Modeling. PLOS Comput. Biol. 2015;11 doi: 10.1371/journal.pcbi.1004522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assunção V.A., Silva D.M.da, Dalponti G., Sartori Â.L.B., Casagrande J.C., Mansano V.de F. Environmental filters structure plant communities in the Brazilian Chaco. Acta Bot. Bras. 2020;34:746–754. [Google Scholar]
- Baldassini P., Despósito C., Piñeiro G., Paruelo J.M. Silvopastoral systems of the Chaco forests: effects of trees on grass growth. J. Arid Environ. 2018;156:87–95. [Google Scholar]
- Baumann M., Israel C., Piquer-Rodríguez M., Gavier-Pizarro G., Volante J.N., Kuemmerle T. Deforestation and cattle expansion in the Paraguayan Chaco 1987–2012. Reg. Environ. Change. 2017;17:1179–1191. [Google Scholar]
- Bazazi S., Romanczuk P., Thomas S., Schimansky-Geier L., Hale J.J., Miller G.A., et al. Nutritional state and collective motion: from individuals to mass migration. Proc. R. Soc. B Biol. Sci. 2011;278:356–363. doi: 10.1098/rspb.2010.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behmer S.T. Insect herbivore nutrient regulation. Annu. Rev. Entomol. 2009;54:165–187. doi: 10.1146/annurev.ento.54.110807.090537. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buhl J., Sword G.A., Simpson S.J. Using field data to test locust migratory band collective movement models. Interface Focus. 2012;2:757–763. doi: 10.1098/rsfs.2012.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cease A.J., Elser J.J., Fenichel E.P., Hadrich J.C., Harrison J.F., Robinson B.E. Living with locusts: connecting soil nitrogen, locust outbreaks, livelihoods, and livestock markets. Bioscience. 2015;65:551–558. [Google Scholar]
- Cease A.J., Elser J.J., Ford C.F., Hao S., Kang L., Harrison J.F. Heavy livestock grazing promotes locust outbreaks by lowering plant nitrogen content. Science. 2012;335:467–469. doi: 10.1126/science.1214433. [DOI] [PubMed] [Google Scholar]
- Cease A.J., Harrison J.F., Hao S., Niren D.C., Zhang G., Kang L., et al. Nutritional imbalance suppresses migratory phenotypes of the Mongolian locust (Oedaleus asiaticus) R. Soc. Open Sci. 2017;4:1–11. doi: 10.1098/rsos.161039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centre for Overseas Pest Research . Natural Resources Institute; London, UK: 1982. The Locust and Grasshopper Agricultural Manual. [Google Scholar]
- Chang H., Cassau S., Krieger J., Guo X., Knaden M., Kang L., et al. A chemical defense deters cannibalism in migratory locusts. Science. 2023;380:537–543. doi: 10.1126/science.ade6155. [DOI] [PubMed] [Google Scholar]
- Cheng K., Simpson S.J., Raubenheimer D. A geometry of regulatory scaling. Am. Nat. 2008;172:681–693. doi: 10.1086/591686. [DOI] [PubMed] [Google Scholar]
- Clark L.R. Behaviour of swarm hoppers of the Australian plague locust (Chortoicetes terminifera Walk.) CSIRO Bull. 1949;245:1–27. [Google Scholar]
- Clissold F.J., Sanson G.D., Read J. The paradoxical effects of nutrient ratios and supply rates on an outbreaking insect herbivore, the Australian plague locust: supply rate alters ratio of nutrients gained. J. Anim. Ecol. 2006;75:1000–1013. doi: 10.1111/j.1365-2656.2006.01122.x. [DOI] [PubMed] [Google Scholar]
- Cullen D.A., Cease A.J., Latchininsky A.V., Ayali A., Berry K., Buhl J., et al. Advances in Insect Physiology. Elsevier; 2017. From molecules to management: mechanisms and consequences of locust phase polyphenism; pp. 167–285. [Google Scholar]
- Dadd R.H. The nutritional requirements of locusts—IV. Requirements for vitamins of the B complex. J. Insect Physiol. 1961;6:1–12. [Google Scholar]
- Deveson E.D. Naturae Amator and the grasshopper infestations of South Australia's early years. Trans. R. Soc. S. Aust. 2012;136:1–15. [Google Scholar]
- Dingle H., Drake V.A. What is migration? Bioscience. 2007;57:113–121. [Google Scholar]
- Dinno, A. (2017). dunn.test: dunn's Test of Multiple Comparisons Using Rank Sums. R package version 1.3.5.
- Dkhili J., Maeno K.O., Idrissi Hassani L.M., Ghaout S., Piou C. Effects of starvation and Vegetation Distribution on Locust Collective Motion. J. Insect Behav. 2019;32:207–217. [Google Scholar]
- Dubois M., Gilles K.A., Hamilton J.K., Rebers P.t, Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. [Google Scholar]
- Ellis P.E., Ashall C. Field studies on diurnal behaviour, movement and aggregation in the Desert Locust (Schistocerca gregaria Forsk.) AntiLocust Bull. 1957;25:1–103. [Google Scholar]
- Farrow R. Population dynamics of the Australian plague locust, Chortoicetes terminifera (Walker), in Central Western New South Wales. I. Reproduction and migration in relation to weather. Aust. J. Zool. 1979;27:717. [Google Scholar]
- Gokuldas M., Hunt P.A., Candy D.J. The inhibition of lipid synthesis in vitro in the locust, Schistocerca gregaria, by factors from the corpora cardiaca. Physiol. Entomol. 1988;13:43–48. [Google Scholar]
- Guttal V., Romanczuk P., Simpson S.J., Sword G.A., Couzin I.D. Cannibalism can drive the evolution of behavioural phase polyphenism in locusts. Ecol. Lett. 2012;15:1158–1166. doi: 10.1111/j.1461-0248.2012.01840.x. [DOI] [PubMed] [Google Scholar]
- Hansen M.J., Buhl J., Bazazi S., Simpson S.J., Sword G.A. Cannibalism in the lifeboat — Collective movement in Australian plague locusts. Behav. Ecol. Sociobiol. 2011;65:1715–1720. [Google Scholar]
- Harrison J.F., Woods H.A., Roberts S.P. Oxford University Press; Oxford New York: 2012. Ecological and Environmental Physiology of Insects. [Google Scholar]
- Herreid C.F., Full R.J. Cockroaches on a treadmill: aerobic running. J. Insect Physiol. 1984;30:395–403. [Google Scholar]
- Hothorn T., Hornik K., van de Wiel M.A., Zeileis A. Implementing a class of permutation tests: the coin package. J. Stat. Softw. 2008;28:1–23. [Google Scholar]
- Hunter D.M., McCulloch L., Spurgin P.A. Aerial detection of nymphal bands of the Australian plague locust (Chortoicetes terminifera (Walker)) (Orthoptera: acrididae) Crop Prot. 2008;27:118–123. [Google Scholar]
- Jutsum A.R., Goldsworthy G.J. Fuels for flight in Locusta. J. Insect Physiol. 1976;22:243–249. [Google Scholar]
- Köhler P. Ecología de la zona central y de gregarización de la langosta en la República Argentina. Idia. 1962;7:108. [Google Scholar]
- Lawton D., Le Gall M., Waters C., Cease A.J. Mismatched diets: defining the nutritional landscape of grasshopper communities in a variable environment. Ecosphere. 2021;12:1–16. [Google Scholar]
- Lawton D., Waters C., Le Gall M., Cease A. Woody vegetation remnants within pastures influence locust distribution: testing bottom-up and top-down control. Agric. Ecosyst. Environ. 2020;296:1–10. [Google Scholar]
- Le Gall M., Beye A., Diallo M., Cease A.J. Generational variation in nutrient regulation for an outbreaking herbivore. Oikos. 2022;2022:1–15. [Google Scholar]
- Le Gall M., Overson R., Cease A. A global review on locusts (Orthoptera: acrididae) and their interactions with livestock grazing practices. Front. Ecol. Evol. 2019;7:1–24. [Google Scholar]
- Le Gall M., Word M.L., Beye A., Cease A.J. Physiological status is a stronger predictor of nutrient selection than ambient plant nutrient content for a wild herbivore. Curr. Res. Insect Sci. 2021;1 doi: 10.1016/j.cris.2020.100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall M., Word M.L., Thompson N., Beye A., Cease A.J. Nitrogen fertilizer decreases survival and reproduction of female locusts by increasing plant protein to carbohydrate ratio. J. Anim. Ecol. 2020:1–8. doi: 10.1111/1365-2656.13288. [DOI] [PubMed] [Google Scholar]
- Le Gall M., Word M.L., Thompson N., Manneh B., Beye A., Cease A.J. Linking land use and the nutritional ecology of herbivores: a case study with the Senegalese locust. Funct. Ecol. 2020;34:167–181. [Google Scholar]
- Lecoq M., Foucart A., Balança G. Behaviour of Rhammatocerus schistocercoides (Rehn, 1906) hopper bands in Mato Grosso, Brazil (Orthoptera: Acrididae: Gomphocerinae) Ann. Société Entomol. Fr. 1999;35:217–228. [Google Scholar]
- Lecoq M., Sukirno Drought and an exceptional outbreak of the Oriental migratory locust, Locusta migratoria manilensis (Meyen 1835) in Indonesia (Orthoptera: acrididae) J. Orthoptera Res. 1999;8:153–161. [Google Scholar]
- Lenhart P.A., Eubanks M.D., Behmer S.T. Water stress in grasslands: dynamic responses of plants and insect herbivores. Oikos. 2015;124:381–390. [Google Scholar]
- Maeno K.O., Piou C., Kearney M.R., Ely S.O., Mohamed S.O., Jaavar M.E.H., et al. A general model of the thermal constraints on the world's most destructive locust, Schistocerca gregaria. Ecol. Appl. 2021;31:e02310. doi: 10.1002/eap.2310. [DOI] [PubMed] [Google Scholar]
- Maeno K.O., Piou C., Whitman D.W., Ould Ely S., Ould Mohamed S., Jaavar M.E.H., et al. How molting locusts avoid cannibalism. Behav. Ecol. 2023:arad025. [Google Scholar]
- Medina H., Cease A., Trumper E. The resurgence of the South American locust (Schistocerca cancellata) Metaleptea. 2017;37:17–21. [Google Scholar]
- Morales M., Oakley L., Sartori A.L.B., Mogni V.Y., Atahuachi M., Vanni R.O., et al. Diversity and conservation of legumes in the Gran Chaco and biogeograpical inferences. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0220151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pener M.P., Simpson S.J. Advances in Insect Physiology, Advances in Insect Physiology. Academic Press, Elsevier; 2009. Locust Phase Polyphenism: an Update; pp. 1–272. [Google Scholar]
- Piou C., Zagaglia G., Medina H.E., Trumper E., Rojo Brizuela X., Ould Maeno K. Band movement and thermoregulation in Schistocerca cancellata. J. Insect Physiol. 2021 doi: 10.1016/j.jinsphys.2021.104328. [DOI] [PubMed] [Google Scholar]
- Pocco M.E., Cigliano M.M., Foquet B., Lange C.E., Nieves E.L., Song H. Density-dependent phenotypic plasticity in the South American Locust, Schistocerca cancellata (Orthoptera: Acrididae) Ann. Entomol. Soc. Am. 2019;112:458–472. [Google Scholar]
- Poot-Pech M.A. Meeting on the locust situation in South America and the OIRSA region. Metaleptea. 2017;37:2–4. [Google Scholar]
- R Core Team . Foundation for Statistical Computing; Vienna, Austria: 2018. R: A language and Environment For Statistical Computing. [Google Scholar]
- Scott Brown A.S., Simmonds M.S.J., Blaney W.M. Relationship between nutritional composition of plant species and infestation levels of thrips. J. Chem. Ecol. 2002;11 doi: 10.1023/a:1021471732625. [DOI] [PubMed] [Google Scholar]
- Simpson S.J., Abisgold J.D. Compensation by locusts for changes in dietary nutrients: behavioural mechanisms. Physiol. Entomol. 1985;10:443–452. [Google Scholar]
- Simpson S.J., Raubenheimer D. Advances in the Study of Behavior, Vol. 29. Advances in the Study of Behavior; 2000. The hungry locust; pp. 1–44. [Google Scholar]
- Simpson S.J., Raubenheimer D. Princeton University Press; Princeton; Oxford: 2012. The Nature of nutrition: a Unifying Framework from Animal Adaptation to Human Obesity. [Google Scholar]
- Simpson S.J., Sword G.A., Lorch P.D., Couzin I.D. Cannibal crickets on a forced march for protein and salt. Proc. Natl. Acad. Sci. 2006;103:4152–4156. doi: 10.1073/pnas.0508915103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H. Density-dependent phase polyphenism in non-model locusts. Psyche J. Entomol. 2011;2011:1–16. [Google Scholar]
- Srygley R.B. Diet drives the collective migrations and affects the immunity of mormon crickets and locusts: a comparison of these potential superspreaders of disease. Integr. Comp. Biol. 2016;56:268–277. doi: 10.1093/icb/icw035. [DOI] [PubMed] [Google Scholar]
- Talal S., Cease A., Farington R., Medina H.E., Rojas J., Harrison J. High carbohydrate diet ingestion increases post-meal lipid synthesis and drives respiratory exchange ratios above 1. J. Exp. Biol. 2021;224:1–6. doi: 10.1242/jeb.240010. [DOI] [PubMed] [Google Scholar]
- Talal S., Cease A.J., Youngblood J.P., Farington R., Trumper E.V., Medina H.E., et al. Plant carbohydrate content limits performance and lipid accumulation of an outbreaking herbivore. Proc. R. Soc. B Biol. Sci. 2020;287:2–9. doi: 10.1098/rspb.2020.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecco P.A., Urcelay C., Díaz S., Cabido M., Pérez-Harguindeguy N. Contrasting functional trait syndromes underlay woody alien success in the same ecosystem. Austral Ecol. 2013;38:443–451. [Google Scholar]
- Trumper E.V., Cease A.J., Cigliano M.M., Bazán F.C., Lange C.E., Medina H.E., et al. A review of the biology, ecology, and management of the South American Locust, Schistocerca cancellata (Serville, 1838), and future prospects. Agronomy. 2022;12:1–20. [Google Scholar]
- Uvarov B. Centre for Overseas Pest Research; London, UK: 1977. Grasshoppers and Locusts. A Handbook of General acridology. Vol. 2. Behaviour, Ecology, Biogeography, Population Dynamics. [Google Scholar]
- Uvarov B.P. Imperial Bureau of Entomology; London: 1928. Locusts and Grasshoppers. A Handbook for their Study and Control. [Google Scholar]
- Word M.L., Hall S.J., Robinson B.E., Manneh B., Beye A., Cease A.J. Soil-targeted interventions could alleviate locust and grasshopper pest pressure in West Africa. Sci. Total Environ. 2019;663:632–643. doi: 10.1016/j.scitotenv.2019.01.313. [DOI] [PubMed] [Google Scholar]
- Zembrzuski D., Woller D.A., Jech L., Black L.R., Reuter K.C., Overson R., et al. Establishing the nutritional landscape and macronutrient preferences of a major United States rangeland pest, Melanoplus sanguinipes, in field and lab populations. J. Orthoptera Res. 2021;30:163–172. [Google Scholar]
- Zhang L., Lecoq M., Latchininsky A., Hunter D. Locust and grasshopper management. Annu. Rev. Entomol. 2019;64:15–34. doi: 10.1146/annurev-ento-011118-112500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Footage during the S. cancellata upsurge. Scene 1: Nymphs aggregating on the roadside near Boyuibe, Bolivia, April 2017, filmed by RPO. Scene 2: Final instar nymphs marching across the road near Cabezas, Bolivia, April 2017, filmed by RPO. Remaining scenes are high density or swarming adult S. cancellata in northern Argentina, 2017–2018, filmed by SENASA field officers. This video is located in the online supplemental material available on the journal's website.
Data Availability Statement
Data presented in this paper are located in the online supplemental material at doi:10.1016/j.cris.2023.100069.