Abstract
Lambda interferon (IFN-λ) induces an intracellular IFN-α/β-like antiviral response through a receptor complex distinct from the IFN-α/β receptor. We therefore determined the ability of IFN-λ to inhibit hepatitis B virus (HBV) and hepatitis C virus (HCV) replication. IFN-λ inhibits HBV replication in a differentiated murine hepatocyte cell line with kinetics and efficiency similar to IFN-α/β and does not require the expression of IFN-α/β or IFN-γ. Furthermore, IFN-λ blocked the replication of a subgenomic and a full-length genomic HCV replicon in human hepatocyte Huh7 cells. These results suggest the possibility that IFN-λ may be therapeutically useful in the treatment of chronic HBV or HCV infection.
Hepatitis B virus (HBV) replication is noncytopathically inhibited by alpha/beta interferon (IFN-α/β) and IFN-γ (9). Transgenic mouse models of HBV gene expression and replication have been used to demonstrate that multiple events contribute to this process, including an early step in which viral DNA replication intermediates are cleared from the liver with no change in the level of viral mRNA and a later step where HBV mRNA levels are reduced by both transcriptional and posttranscriptional mechanisms (18, 19). While it has been shown that HBV replication is inhibited by a reduction in the assembly or stability of viral pregenomic RNA-containing capsids through a mechanism that requires proteasome activity (15, 20), the precise molecular events that mediate this inhibition have not yet been defined.
Recently, a new family of cytokines (IFN-λ1, 2, 3, or interleukin-29 [IL-29], IL-28A, or IL-28B, respectively) has been characterized (11, 16). Although displaying low sequence homology to IFN-α/β, the IFN-λs share a number of common biological characteristics with IFN-α/β. IFN-λ expression is induced by stimuli that induce IFN-α/β, such as Toll-like receptor triggering (with poly(I) · poly(C), lipopolysaccharide, or CpG DNA) or virus (influenza or encephalomyocarditis virus [EMCV]) infection (3, 11, 16). Also like IFN-α/β, IFN-λ activates IFN-stimulated regulatory factor 3 and induces the expression of genes containing IFN-stimulated response elements (11, 16). Furthermore, IFN-λ was shown to inhibit the replication of a number of viruses, including vesicular stomatitis virus and EMCV (11, 16). However, IFN-λ does not bind to the IFN-α receptor complex but, rather, induces its cellular activity through a receptor consisting of the IL-10 receptor 2 subunit and a unique subunit known as IFN-λ receptor 1 (4, 11, 16).
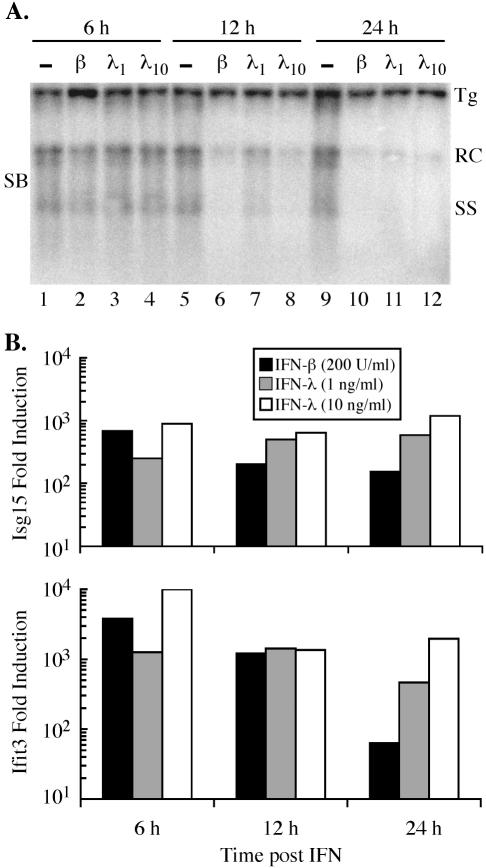
We therefore examined the ability of IFN-λ to inhibit the replication of HBV. For these studies, we used an immortalized murine hepatocyte cell line (HBV-Met) that replicates HBV in an IFN-α/β- and IFN-γ-sensitive manner (13). After HBV-Met cells were differentiated in 2% dimethyl sulfoxide for 12 days, HBV gene expression and replication were induced from a 1.3 HBV genome-length integrated transgene (13). Treatment of differentiated HBV-Met cells with 1 to 10 ng of murine IFN-λ2 (Peprotech, Rocky Hill, N.J.) per ml inhibited HBV replication >90% at 24 h after the IFN addition, as determined by Southern blot analysis of HBV DNA replication intermediates (Fig. 1A). The magnitude and kinetics of this effect were similar to the inhibition observed after treating the cells with 200 U (approximately 20 ng) of murine IFN-β (provided by K. Harada, Toray Industries, Chiba, Japan) per ml for the same time period (Fig. 1A). Under these conditions, neither IFN-β nor IFN-λ altered the level of the HBV 3.5- and 2.1-kb mRNAs (data not shown). Thus, like IFN-α/β and IFN-γ, IFN-λ induces an antiviral response in hepatocytes that inhibits HBV replication.
FIG. 1.
IFN-λ inhibits HBV replication and induces expression of IFN-stimulated genes. (A) Differentiated HBV-Met cells were untreated (−; lanes 1, 5, and 9) or treated with 200 U of murine IFN-β (β; lanes 2, 6, and 10) per ml, 1 ng of murine IFN-λ2 (λ1; lanes 3, 7, and 11) per ml, or 10 ng of murine IFN-λ2 (λ10; lanes 4, 8, and 12) per ml and harvested at the indicated time points. HBV replication was monitored by Southern blot (SB) analysis of the HBV relaxed circle (RC) and single-stranded (SS) DNA replication forms. Tg, integrated transgene. (B) Quantitative RT-PCR analysis of two representative IFN-induced genes, Isg15 and Ifit3. Data are expressed as increases in induction (n-fold) relative to untreated cells and are normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) expression levels.
We also examined the kinetics of IFN-λ-mediated, IFN-stimulated response element-dependent gene expression by quantitative reverse transcription PCR (RT-PCR) in the HBV-Met cells. For this analysis, we selected two representative IFN-induced genes, Isg15 and Ifit3 (5, 17). The expression of both Isg15 and Ifit3 was induced by IFN-λ to comparable levels and with similar kinetics relative to those of IFN-β (Fig. 1B). Induction of these genes by IFN-λ, however, appears to be prolonged relative to their induction by IFN-β, which peaked at 6 h and declined thereafter (Fig. 1B). Surprisingly, we were unable to induce intrahepatic IFN-stimulated gene expression (greater than twofold induction of Isg15 or Ifit3) or HBV inhibition (>50% reduction) in 1.3.32 HBV transgenic mice 24 h after intravenous injection with 100 μg of murine IFN-λ2, possibly due to either a short in vivo half-life of IFN-λ or a higher receptor density on extrahepatic tissues (data not shown).
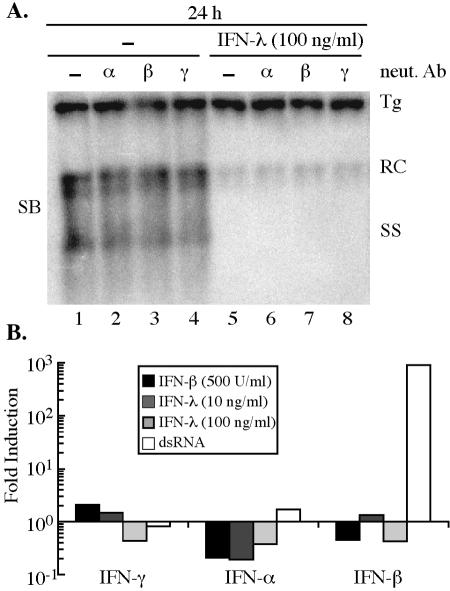
Although the kinetics of gene expression and the antiviral effect were consistent with a mechanism by which IFN-λ-induced genes directly inhibit HBV replication, the possibility existed that the inhibition of HBV occurs through an indirect mechanism in which IFN-λ induces the expression of IFN-α/β and/or IFN-γ. However, the antiviral effect of IFN-λ (100 ng/ml) was not blocked by coincubation with neutralizing antibodies (1,000 neutralization units/ml; Calbiochem, San Diego, Calif.) to IFN-α, IFN-β, or IFN-γ (Fig. 2A). Furthermore, we were not able to detect an increase in IFN-γ, IFN-α2, or IFN-β mRNA expression levels upon treatment with 10 to 100 ng of IFN-λ per ml by quantitative RT-PCR (Fig. 2B), providing further evidence that the antiviral effect of IFN-λ is through the direct induction of genes with antiviral activity.
FIG. 2.
Inhibition of HBV replication by IFN-λ does not require IFN-α/β or IFN-γ. (A) Differentiated HBV-Met cells were untreated (lanes 1 to 4) or treated with 100 ng of murine IFN-λ2 (lanes 5 to 8) per ml in the presence of 1,000 neutralization units of antibody to murine IFN-α (α; lanes 2 and 6), IFN-β (β; lanes 3 and 7), or IFN-γ (γ; lanes 4 and 8) per ml and harvested 24 h later. HBV replication was monitored by Southern blot (SB) analysis of the HBV relaxed circle (RC) and single-stranded (SS) DNA replication forms. Tg, integrated transgene; neut Ab, neutralizing antibody. (B) Quantitative RT-PCR analysis of changes in IFN-γ, IFN-α2, and IFN-β expression levels induced by IFN-β (500 U/ml), IFN-λ (10 and 100 ng/ml), or transfection with an irrelevant double-stranded RNA (dsRNA). Data are expressed as the change in induction (n-fold) relative to untreated cells and are normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) expression levels.
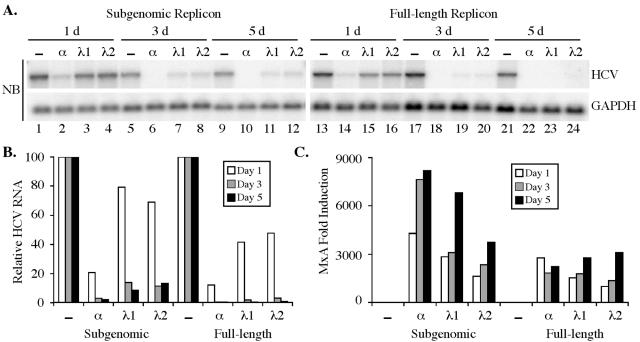
Finally, we examined the ability of IFN-λ to inhibit hepatitis C virus (HCV) replication. Like HBV replication, HCV replication is sensitive to the antiviral effects of both IFN-α/β and IFN-γ (2, 6, 7, 10, 12). Human Huh7 hepatoma cells containing subgenomic (1) or full-length genomic (14) HCV replicons (genotype 1b) were treated for 1 to 5 days with 100 ng of human IFN-λ1 or IFN-λ2 (Peprotech) per ml. As with IFN-α, IFN-λ reduced the level of HCV plus-strand RNA 3 to 5 days after IFN addition, as determined by Northern blotting (Fig. 3A) and quantitative RT-PCR (Fig. 3B) analysis. The reduction in HCV replicon RNA was approximately 90% in the subgenomic and >99% in the full-length genomic replicon-containing cells by day 5 posttreatment. However, this reduction was less than that achieved with 500 U (approximately 5 ng) of IFN-α per ml, especially at the earlier time points (Fig. 3A and B). IFN-λ also induced the expression of the representative IFN-stimulated gene MxA to levels similar to those induced by IFN-α in replicon-containing Huh7 cells (Fig. 3C). Thus, like HBV replication, HCV replication is sensitive to the antiviral effects of IFN-λ.
FIG. 3.
IFN-λ inhibits HCV replication. (A) Huh7 cells containing HCV subgenomic (lanes 1 to 12) or full-length genomic (lanes 13 to 24) replicons were untreated (−; lanes 1, 5, 9, 13, 17, and 21) or treated with 500 U of human IFN-α(A/D) (α; lanes 2, 6, 10, 14, 18, and 22) per ml, 100 ng of human IFN-λ1 (λ1; lanes 3, 7, 11, 15, 19, and 23) per ml, or 100 ng of human IFN-λ2 (λ2; lanes 4, 8, 12, 16, 20, and 24) per ml. Cells were harvested 1, 3, or 5 days after IFN treatment, and Northern blot (NB) analysis (on 5 μg of total RNA from subgenomic and 8 μg of total RNA from genomic HCV replicons) was performed for HCV plus-strand RNA and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels. (B) Quantitative RT-PCR analysis of HCV plus-strand RNA from the same experiment. Data are expressed as HCV RNA levels relative to untreated controls and are normalized to GAPDH levels. (C) Quantitative RT-PCR analysis of MxA expression. Data are expressed as the increase in induction (n-fold) relative to untreated controls and are normalized to GAPDH levels.
Although displaying little sequence homology to IFN-α/β and exerting its biological activities through a distinct receptor, IFN-λ induces the expression of IFN-stimulated genes with antiviral activity. IFN-λ1 has been previously shown to inhibit vesicular stomatitis virus and EMCV replication with a specific activity similar to that of IFN-α, although the activity of IFN-λ2 against EMCV may be lower than that of IFN-λ1 (11, 16). We have demonstrated here that replication of HBV and HCV is sensitive to the antiviral activities of IFN-λ. However, the precise potency of IFN-λ with respect to HBV and HCV inhibition remains to be determined, as does the question of whether IFN-λ activates alternate antiviral pathways and would, therefore, act synergistically with IFN-α. Finally, the apparent low potency of IFN-λ in vivo warrants further characterization, as to our knowledge this has not been previously examined.
IFN-α is utilized therapeutically in the treatment of chronic HBV and HCV infections and is presumably clinically beneficial through both its immunomodulatory and antiviral properties (8). IFN-α therapy has a number of disadvantages, however, including significant side effects. The results of the present study raise the possibility that IFN-λ may also be clinically useful in the treatment of chronic HBV and HCV infections, either alone or in combination with IFN-α. Further studies will be needed to determine if these therapeutic strategies will be feasible.
Acknowledgments
This work was supported by grant CA40489 (F.V.C.) and National Research Service Award AI49665 (M.D.R.) from the National Institutes of Health.
We thank Toray Industries for the gift of murine IFN-β; Charles Rice for providing the HCV subgenomic replicon; Ralf Bartenschlager for providing the HCV full-length genomic replicon; and Stefan Wieland, Sharookh Kapadia, and Jin Zhong for helpful assistance and discussions.
Footnotes
Paper no. 16793-MEM from The Scripps Research Institute.
REFERENCES
- 1.Blight, K. J., A. A. Kolykhalov, and C. M. Rice. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972-1974. [DOI] [PubMed] [Google Scholar]
- 2.Cheney, I. W., V. C. Lai, W. Zhong, T. Brodhag, S. Dempsey, C. Lim, Z. Hong, J. Y. Lau, and R. C. Tam. 2002. Comparative analysis of anti-hepatitis C virus activity and gene expression mediated by alpha, beta, and gamma interferons. J. Virol. 76:11148-11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coccia, E. M., M. Severa, E. Giacomini, D. Monneron, M. E. Remoli, I. Julkunen, M. Cella, R. Lande, and G. Uze. 2004. Viral infection and Toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur. J. Immunol. 34:796-805. [DOI] [PubMed] [Google Scholar]
- 4.Donnelly, R. P., F. Sheikh, S. V. Kotenko, and H. Dickensheets. 2004. The expanded family of class II cytokines that share the IL-10 receptor-2 (IL-10R2) chain. J. Leukoc. Biol. 76:314-321. [DOI] [PubMed] [Google Scholar]
- 5.Farrell, P. J., R. J. Broeze, and P. Lengyel. 1979. Accumulation of an mRNA and protein in interferon-treated Ehrlich ascites tumour cells. Nature 279:523-525. [DOI] [PubMed] [Google Scholar]
- 6.Frese, M., T. Pietschmann, D. Moradpour, O. Haller, and R. Bartenschlager. 2001. Interferon-alpha inhibits hepatitis C virus subgenomic RNA replication by an MxA-independent pathway. J. Gen. Virol. 82:723-733. [DOI] [PubMed] [Google Scholar]
- 7.Frese, M., V. Schwarzle, K. Barth, N. Krieger, V. Lohmann, S. Mihm, O. Haller, and R. Bartenschlager. 2002. Interferon-gamma inhibits replication of subgenomic and genomic hepatitis C virus RNAs. Hepatology 35:694-703. [DOI] [PubMed] [Google Scholar]
- 8.Ganem, D., and A. M. Prince. 2004. Hepatitis B virus infection-natural history and clinical consequences. N. Engl. J. Med. 350:1118-1129. [DOI] [PubMed] [Google Scholar]
- 9.Guidotti, L. G., and F. V. Chisari. 1999. Cytokine-induced viral purging—role in viral pathogenesis. Curr. Opin. Microbiol. 2:388-391. [DOI] [PubMed] [Google Scholar]
- 10.Guo, J. T., V. V. Bichko, and C. Seeger. 2001. Effect of alpha interferon on the hepatitis C virus replicon. J. Virol. 75:8516-8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotenko, S. V., G. Gallagher, V. V. Baurin, A. Lewis-Antes, M. Shen, N. K. Shah, J. A. Langer, F. Sheikh, H. Dickensheets, and R. P. Donnelly. 2003. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 4:69-77. [DOI] [PubMed] [Google Scholar]
- 12.Lanford, R. E., B. Guerra, H. Lee, D. R. Averett, B. Pfeiffer, D. Chavez, L. Notvall, and C. Bigger. 2003. Antiviral effect and virus-host interactions in response to alpha interferon, gamma interferon, poly(I)-poly(C), tumor necrosis factor alpha, and ribavirin in hepatitis C virus subgenomic replicons. J. Virol. 77:1092-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasquetto, V., S. F. Wieland, S. L. Uprichard, M. Tripodi, and F. V. Chisari. 2002. Cytokine-sensitive replication of hepatitis B virus in immortalized mouse hepatocyte culture. J. Virol. 76:5646-5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pietschmann, T., V. Lohmann, A. Kaul, N. Krieger, G. Rinck, G. Rutter, D. Strand, and R. Bartenschlager. 2002. Persistent and transient replication of full-length hepatitis C virus genomes in cell culture. J. Virol. 76:4008-4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robek, M. D., S. F. Wieland, and F. V. Chisari. 2002. Inhibition of hepatitis B virus replication by interferon requires proteasome activity. J. Virol. 76:3570-3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheppard, P., W. Kindsvogel, W. Xu, K. Henderson, S. Schlutsmeyer, T. E. Whitmore, R. Kuestner, U. Garrigues, C. Birks, J. Roraback, C. Ostrander, D. Dong, J. Shin, S. Presnell, B. Fox, B. Haldeman, E. Cooper, D. Taft, T. Gilbert, F. J. Grant, M. Tackett, W. Krivan, G. McKnight, C. Clegg, D. Foster, and K. M. Klucher. 2003. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat. Immunol. 4:63-68. [DOI] [PubMed] [Google Scholar]
- 17.Smith, J. B., and H. R. Herschman. 1996. The glucocorticoid attenuated response genes GARG-16, GARG-39, and GARG-49/IRG2 encode inducible proteins containing multiple tetratricopeptide repeat domains. Arch. Biochem. Biophys. 330:290-300. [DOI] [PubMed] [Google Scholar]
- 18.Tsui, L. V., L. G. Guidotti, T. Ishikawa, and F. V. Chisari. 1995. Posttranscriptional clearance of hepatitis B virus RNA by cytotoxic T lymphocyte-activated hepatocytes. Proc. Natl. Acad. Sci. USA 92:12398-12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uprichard, S. L., S. F. Wieland, A. Althage, and F. V. Chisari. 2003. Transcriptional and posttranscriptional control of hepatitis B virus gene expression. Proc. Natl. Acad. Sci. USA 100:1310-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wieland, S. F., L. G. Guidotti, and F. V. Chisari. 2000. Intrahepatic induction of alpha/beta interferon eliminates viral RNA-containing capsids in hepatitis B virus transgenic mice. J. Virol. 74:4165-4173. [DOI] [PMC free article] [PubMed] [Google Scholar]