Abstract
The murine cytomegalovirus (MCMV) proteins encoded by US22 genes M139, M140, and M141 function, at least in part, to regulate replication of this virus in macrophages. Mutant MCMV having one or more of these genes deleted replicates poorly in macrophages in culture and in the macrophage-dense environment of the spleen. In this report, we demonstrate the existence of stable complexes formed by the products of all three of these US22 genes, as well as a complex composed of the products of M140 and M141. These complexes form in the absence of other viral proteins; however, the pM140/pM141 complex serves as a requisite binding partner for the M139 gene products. Products from all three genes colocalize to a perinuclear region of the cell juxtaposed to or within the cis-Golgi region but excluded from the trans-Golgi region. Interestingly, expression of pM141 redirects pM140 from its predominantly nuclear residence to the perinuclear, cytoplasmic locale where these US22 proteins apparently exist in complex. Thus, complexing of these nonessential, early MCMV proteins likely confers a function(s) independent of each individual protein and important for optimal replication of MCMV in its natural host.
The US22 gene family, first identified in the human cytomegalovirus (HCMV) genome, is conserved among all betaherpesviruses and thus likely provides important functions for survival of this subfamily within the host population. The US22 family of genes is defined by the presence of up to four motifs (I to IV) consisting of stretches of hydrophobic residues interspersed with charged amino acids (5, 7, 13, 16, 17, 19). In spite of their conservation, little is known about the function of US22 gene products. US22 genes expressed at immediate-early times include HCMV UL36 and IRS1/TRS1 and murine CMV (MCMV) m128, m142, and m143. The latter two genes provide an essential (but unknown) function for replication of MCMV (14), while m128 and the HCMV genes function, at least in vitro, as transcriptional transactivators (3, 6, 12, 24). Recent studies indicate that HCMV UL36 and MCMV M36 gene products confer antiapoptotic functions, with M36 conferring this function selectively in macrophages (14, 22). Another MCMV US22 gene, M43, regulates growth of the virus in some cell types in vitro (14) and in the salivary glands of mice in vivo (25). Recently, HCMV UL26 was also identified as a US22 family gene whose product is a virion tegument protein that transactivates the HCMV major immediate-early enhancer-promoter (23). The other HCMV US22 gene products identified as tegument proteins are IRS1/TRS1 (20) and UL23, UL24, UL43, and US22 (1).
Products of MCMV US22 genes M139, M140 and M141 function by an unknown mechanism to regulate, directly or indirectly, the replication of MCMV in macrophages, a cell type central to CMV pathogenesis due to the ability of this cell to support productive and latent infections (11, 15, 18). Deletion or mutation in these genes renders the virus highly attenuated for growth in cultured, activated macrophages, in spleen tissue, and in severe combined immunodeficient (SCID) mice (4, 9, 10, 14). The M139, M140, and M141 protein products (p75M139, p61M139, pM140, and pM141) are expressed abundantly and coordinately from 3′-coterminal transcripts at early and late times in the nucleus and cytoplasm of infected fibroblasts and macrophages (8, 9). Furthermore, there is evidence of functional cooperation among these US22 gene products. One or more of the proteins convey stability to neighboring gene products, and the comparative growth phenotypes of individual gene deletion mutants imply cooperative functions (9, 14). Thus, it appears that these US22 gene products function in an interactive and/or cooperative manner to optimize MCMV replication in its natural host.
The above findings led us to explore the possibility that the products of these US22 genes form a stable complex in MCMV-infected cells. Binding of some of these partners has been reported for pM140 and pM141 (Z. Karabekian, L. K. Hanson, J. S. Slater, and A. E. Campbell, Abstr. 25th Int. Herpesvirus Workshop, abstr. 9.26, 2000) and for p75M139, p61M139, and pM141 (14). However, studies to specifically identify and characterize the complex or complexes formed among these gene products in vitro or in vivo have not been conducted. Here we demonstrate for the first time that, under stringent conditions, at least three independent complexes are formed. Formation of at least two of these complexes is not dependent upon other viral proteins. Interestingly, while pM140 was expressed predominantly in the nucleus in the absence of pM141, and pM141 was expressed diffusely throughout the cytoplasm in the absence of pM140, coexpression of the two proteins within the same cell resulted in colocalization of both proteins to a perinuclear region juxtaposed to or within the cis-Golgi region, where the M139 products also reside. Thus, it is likely that the products of these three US22 genes function as partners to directly or indirectly confer replication competency in selective cell types. While deciphering the molecular mechanisms of how these viral proteins confer this function is the ultimate goal, defining the structural entities in which these proteins exist is a valuable component of understanding how these proteins perform their functions.
MATERIALS AND METHODS
Cells and viruses.
NIH 3T3 fibroblasts (ATCC CRL-1658; American Type Culture Collection, Manassas, Va.) and IC-21 macrophages (ATCC TIB 186) were propagated as described previously (9, 10). Wild-type (WT) virus was Smith strain MCMV (ATCC VR 194). Construction and characterization of insertion or deletion mutants of MCMV disrupting expression of M139, M140, or M141 were previously described (9), with the exception of mutant virus RV12 with M139 and M140 deleted. This virus was constructed by excising a HindIII-PvuII fragment (bases 195847 to 199657) from MCMV HindIII-I and inserting it into pGEM7Z. This clone was digested with NaeI and religated, removing bases 196050 to 197355. The subclone was digested with BamHI, and a BglII-BamHI fragment (bases 192588 to 194337) from MCMV HindIII-J was inserted into the BamHI site. An e1-β-glucuronidase cassette (4) was then inserted into the BglII site at position 197627 between open reading frames M140 and M141. β-Glucuronidase-expressing progeny virus resulting from homologous recombination between this construct and the MCMV genome resulted in mutant MCMV having M139 (bases 195847 to 194337) and M140 (bases 197355 to 196050) deleted.
Immunoprecipitation of viral proteins from infected or transfected cells.
Mock- or MCMV-infected cell lysates (1 PFU/cell) from NIH 3T3 fibroblasts or IC-21 macrophages at late times postinfection were used in standard protocols for immunoprecipitation under stringent conditions to detect stable complexes. In some experiments, proteins were immunoprecipitated from lysates of cells transiently expressing epitope-tagged or untagged M139, M140, or M141 gene products 24 h after transfection. The procedure was essentially as described previously (9), with the exception that cells were radiolabeled with 100 μCi of [35S]methionine and -cysteine (DuPont NEN, Wilmington, Del.)/ml. Precipitating antibodies were rabbit polyclonal antiserum to M139, M140, or M141 (described in reference 9) or rabbit polyclonal H-15 anti-His antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.). Preimmune rabbit sera were used as controls.
In those experiments, using denatured cell lysates prior to immunoprecipitation, a 10% sodium dodecyl sulfate (SDS) stock solution was added to the cell lysates to attain a final concentration of 1% SDS. The SDS-containing samples were boiled for 10 min, and lysates were diluted 10-fold with NET-GEL buffer (150 mM NaCl, 5 mM EDTA, 50 mM Tris [pH 7.5], 0.05% NP-40, and 0.25% gelatin).
In vitro transcription-translation reactions.
Vectors were designed to express full-length M139 and M141 proteins, and His/Xpress-tagged full-length pM140, in in vitro transcription-translation reactions. The M139 expression vector was generated by cloning a 2.4-kb HincII fragment (MCMV bases 193670 to 196059) into the EcoRV site of pcDNA3.1(+) (Invitrogen, Carlsbad, Calif.). The M141 expression vector was generated by cloning the 1.6-kb AvrII-to-BglII fragment (bases 197629 to 199283) into the XbaI and BamHI sites of pcDNA3.1Zeo(−).
To construct the plasmid expressing His/Xpress-tagged pM140, the 1.7-kb XbaI-to-HindIII fragment (bases 195850 to 197549) containing the entire M140 open reading frame was cloned into XbaI/HindIII-digested pcDNA3.1Zeo(−) (Invitrogen). Then, a fragment of the M140 gene in this vector was PCR amplified, generating a BamHI site 12 bp upstream of the M140 start codon. Primers used for this amplification were M1405′BAMf (5′-CCA TTA TCA CCA TCA TCG GAT CCG GCG AGA TGG-3′) and M1403′Ecor (5′-ATG ACG AGC AGG TGG TGG ATG CTG AAG AC-3′). The PCR product was cloned into an intermediate vector, pCR 4-TOPO (Invitrogen). Concurrently, a SmaI-to-NotI fragment (bases 197153 to 195452) from pJI (10) was inserted into EcoRV/NotI-digested pcDNA3.1/His-C from Invitrogen. Finally, the intermediate vector was digested with BamHI and EcoRV, generating a 683-bp fragment that was inserted into BamHI/EcoRV-digested pHis140T. This vector expressed a full-length, N-terminally tagged M140 protein.
In vitro transcription-translation reactions were conducted using the TnT coupled reticulocyte lysate system (Promega, Madison, Wis.), with 30 μCi of [35S]methionine (1,000 Ci/mmol at 15 mCi/ml; Amersham Biosciences, Piscataway, N.J.) and using 0.4 μg of HisM140 expression vector DNA and 0.8 μg each of M139 and M141 expression vector DNA.
To immunoprecipitate products of in vitro translation, 40 μl of sample was diluted sixfold in 10S buffer (50 mM HEPES [pH 7.2], 250 mM NaCl, 0.3% NP-40, 0.1% Triton X-100, 0.005% SDS, 10 mM NaPO4 [pH 7.0], 1 mM NaF, 0.1 mM phenylmethylsulfonyl fluoride, 0.5 mM dithiothreitol) and incubated with 2 μl of antibody to the HisG epitope (Invitrogen) for 2 h at 4°C with a constant rotation of 360°. Then the sample was incubated with 30 μl of protein A/G agarose (Santa Cruz Biotechnology) overnight with constant rotation. Immune complexes were washed and processed as described previously (9).
Construction of vectors expressing epitope-tagged viral proteins in transient transfections.
To generate a plasmid expressing FLAG-tagged M140, a BglII-to-PstI fragment from pHindIII-I (bases 197627 to 196074 within the MCMV genome) was inserted into BamHI/PstI-digested pA3 M (the generous gift of Nancy Rabb-Traub, University of North Carolina, Chapel Hill) as an intermediate vector. A SalI-to-XhoI fragment from this construct (MCMV bases 196906 to 196074) was inserted into the SalI site of p3xFLAG-CMV-7 (Sigma-Aldrich, St. Louis, Mo.). Expression from this plasmid resulted in a FLAG-tagged M140 having 81 N-terminal amino acids deleted. FLAG-tagged bacterial alkaline phosphatase (FBAP; Sigma-Aldrich) was used as a control vector for expression of all FLAG-tagged products.
For the generation of FLAG-tagged M141, an AvrII-to-XbaI fragment from pHindIII-I (MCMV bases 199281 to 197547) was inserted into the XbaI site of p3xFLAG-CMV-7, resulting in expression of a full-length M141 product with an N-terminal tag. For the generation of Myc-tagged M141, the expression vector FLAG-M141 was digested with HindIII and HincII, and the fragment was inserted into pA3 M digested with HindIII and EcoRV (MCMV bases 199281 to 197826). This vector expressed a C-terminally tagged M141 protein truncated by 39 amino acids at the carboxy terminus.
For generation of His/Xpress-tagged M139, the EcoRI fragment from HindIII-J containing MCMV bases 195847 to 193529 was inserted into the EcoRI site of pcDNA3.1/His/Xpress-B. This resulted in a tagged M139 with an N-terminal truncation of 55 amino acids.
For generation of His/Xpress-tagged M140, a SmaI-to-NotI fragment (MCMV bases 197153 to 195452) from pJI (10) was inserted into EcoRV- and NotI-digested pcDNA3.1/His/Xpress-C, yielding a tagged M140 product with an N-terminal deletion of 121 amino acids. For generation of pGFP-M140, a BamHI-to-HindIII fragment from the His/Xpress-tagged M140 was inserted into vector pGFP-C1 (Clontech) with BglII and HindIII digestions. For all of the above constructs, expression of the tagged proteins of the expected sizes was confirmed by transient transfections and Western blotting with antitag antibodies, as described below.
Transfections.
NIH 3T3 or IC-21 cells (106) were transfected with one or more of the above plasmids and either empty vector pcDNA3.1 or a control plasmid expressing (from the same promoter) His/XpressLacZ (pcDNA3.1/His/XpressB/LacZ) (Invitrogen) in lieu of the viral products (total of 6 μg of DNA) with the Cytofectene transfection reagent according to the manufacturer's instructions (Bio-Rad Laboratories, Hercules, Calif.). Twenty-four hours later, cells were harvested for immunoprecipitations, Western blotting, or confocal microscopy.
Western blotting.
Western blotting was performed as described previously (9) using the following antibodies: polyclonal rabbit antiserum to M139, M140, or M141 (9); mouse monoclonal M2 anti-FLAG (Sigma-Aldrich); rabbit polyclonal A-14 anti-Myc (Santa Cruz Biotechnology); or rabbit polyclonal H-15 anti-His (Santa Cruz Biotechnology). Goat anti-rabbit or goat anti-mouse antibodies conjugated to horseradish peroxidase (HRP) (Sigma-Aldrich) were used as secondary antibodies for detection with the Bio-Rad Immun Star HRP chemiluminescence kit.
Sucrose gradient analysis of infected-cell lysates.
The formation of viral protein complexes in the infected-cell lysates was assessed by sedimentation velocity centrifugation on sucrose gradients. A 400-μl aliquot of lysates from WT- or RVΔ140-infected NIH 3T3 cells was layered onto an 11-ml 5 to 25% (wt/vol) sucrose gradient in Tris buffer (50 mM Tris [pH 8], 5 mM EDTA, 150 mM NaCl). The cellular lysates were sedimented at 274,000 × g (40,000 rpm) for 8 h at 17°C in a Beckman SW41 Ti rotor, and the gradient was then fractionated on an ISCO gradient fractionator at 0.75 ml/min and 0.5 min/fraction.
The M139, M140, or M141 protein products present in each fraction were analyzed by Western blotting. Each fraction (80- to 100-μl samples) was applied to a dot blot array by using the American Bionetics VacuDot-VS apparatus (Hayward, Calif.) and vacuum blotted onto a supported nitrocellulose membrane (Osmonics Inc., Minnetonka, Minn.). Wells were then washed three times with phosphate-buffered saline. After the final wash the apparatus was disassembled and the membrane was allowed to air dry.
The membrane was blocked overnight at 4°C in Tris-buffered saline containing 5% powdered milk and 0.3% Tween 20. The membrane was then incubated in primary antibody (1/500 rabbit polyclonal anti-M139, anti-M140, or anti-M141) in blocking solution at room temperature for 45 min. to 1 h. Blots were then washed three times for 10 min each with Tris-buffered saline-0.3% Tween 20. Goat anti-rabbit antibody-HRP (Sigma) secondary antibody was diluted in blocking buffer and incubated for 45 min at room temperature. Blots were then vigorously washed five times, and antibody reactivity was detected using the chemiluminescence kit as described above. The density of each spot was quantitated using UN-SCAN-IT software (Silk Scientific, Inc., Orem, Utah).
To discern the location within the gradient of each gene product expressed as individual proteins, M139, M140, and M141 were transcribed and translated in vitro as separate gene products in the presence of [35S]methionine. A 144-μl sample of each product was applied to the sucrose gradient, fractions were collected, and approximately 100 μl of each fraction was run on a 12.5% polyacrylamide gel. Bands were visualized by autoradiography, and their densities were quantitated using UN-SCAN-IT software.
Confocal microscopy.
Fibroblasts were cotransfected with plasmids expressing one or more epitope-tagged viral proteins or the control plasmid pcDNA3.1/His/XpressB/LacZ. Twenty-four hours later, the cells were harvested and seeded into chambered slides. After 24 h of incubation, the cells were fixed in 2% paraformaldehyde, permeabilized with 0.5% Triton X-100 in phosphate-buffered saline, and blocked with 5% normal goat serum in phosphate-buffered saline. The cells were stained with the antitag antibodies described above and either goat anti-rabbit-fluorescein isothiocyanate (FITC) or goat anti-mouse-tetramethyl rhodamine isothiocyanate (TRITC) (Sigma-Aldrich). In some experiments, cells were stained with the Golgi marker wheat germ agglutinin-Alexa Fluor 488 conjugate (Molecular Probes, Eugene, Oreg.), anti-GS15, or anti-GS28 (mouse monoclonal antibodies; BD Biosciences, Palo Alto, Calif.) and TRITC-conjugated goat anti-mouse secondary antibody. Fluorescence was visualized with a Zeiss LSM510 laser scanning confocal microscope equipped with MetaMorph image analysis and deconvolution software.
For analysis of live cells expressing green fluorescent protein (GFP)-pM140, cells transfected with pGFP-M140 (or control pGFP-C1) were seeded 24 h posttransfection onto Lab-Tek chambered slides (Fisher Scientific, Pittsburgh, Pa.). The cells were then infected (5 PFU/cell) 12 h later and viewed by confocal microscopy at 18 h postinfection.
RESULTS
Previous reports implicated functional and/or physical interactions among some of the M139, M140, and/or M141 gene products (9, 14). To assess the nature of the physical interactions, we devised five approaches. Initially, fibroblasts or macrophages infected with WT virus or mutant MCMV having M139, M140, or M141 deleted were subjected to a series of immunoprecipitation experiments to detect coprecipitating proteins during virus infection. Secondly, coimmunoprecipitation of the viral proteins was examined in cells transiently expressing epitope-tagged M139, M140, and M141, in the absence of other viral proteins. Thirdly, M139, M140, and M141 were cotranscribed and translated in vitro to assess association of the proteins in the absence of cellular proteins. Fourthly, the subcellular location where the viral proteins reside, both individually and together, was identified by confocal microscopy. Finally, sedimentation gradient analysis was used to assess the number of complexes that exist as physical entities in the context of virus infection.
Identification of proteins coprecipitating with M139, M140, or M141 gene products.
We began by identifying protein products that coprecipitated with the M139, M140, and M141 gene products within infected cells. Immunoprecipitation assays were performed using rabbit polyclonal antisera to detect potential coprecipitation of the two M139 products (p75M139 and p61M139), pM140 (56 kDa), and pM141 (52 kDa) during the course of MCMV infection in either fibroblasts or macrophages. Note that there is negligible cross-reactivity among the three antisera for each of the protein products, as demonstrated by Western blotting (9) and immunoprecipitation of radiolabeled proteins (as seen in Fig. 2).
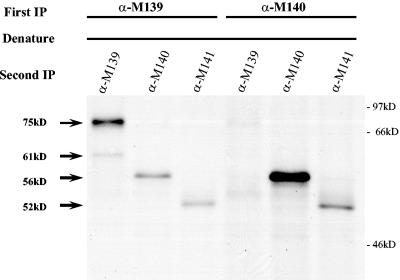
FIG. 2.
Sequential immunoprecipitations identify binding partners in the complex. Radiolabeled proteins were immunoprecipitated from lysates of NIH 3T3 cells infected with WT MCMV (1 PFU/cell) with rabbit polyclonal antisera to M139 or M140 gene products (First IP). The immune complexes were then denatured, separated from the protein A agarose beads, diluted 10-fold, divided into equal aliquots, and then reimmunoprecipitated with the indicated antibodies (Second IP).
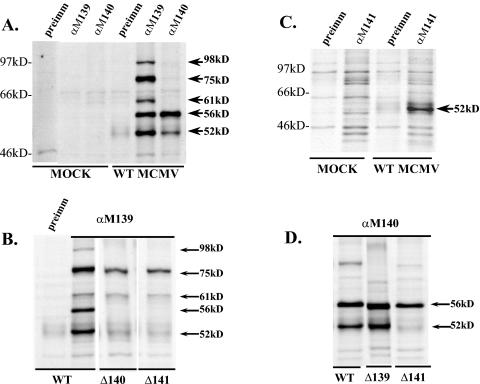
Immunoprecipitation of the proteins from infected cells revealed several interesting patterns of coprecipitation. As shown in Fig. 1A, the anti-M139 antisera precipitated the expected M139 gene products of 75 and 61 kDa, in addition to proteins of 98, 56, and 52 kDa (9). The identity of the 98-kDa protein is currently unknown; however, this protein coprecipitates with anti-M139 antisera only in the context of WT virus infection. The smaller two proteins were identified as products of M140 and M141, respectively (9). These identifications were based on molecular weights and on the fact that these protein bands were not coprecipitated with anti-M139 antisera from cells infected with mutant MCMV having M140 or M141 deleted (Fig. 1B).
FIG. 1.
Coimmunoprecipitation reveals complex formation among M139, M140, and M141 gene products. The figure shows autoradiographs of radiolabeled proteins immunoprecipitated from lysates of NIH 3T3 cells either mock infected, infected with WT MCMV, or infected with mutant MCMV having gene M139 (Δ139), M140 (Δ140), or M141 (Δ141) deleted. Cells were infected using 1 PFU/cell, radiolabeled from 20 to 24 h postinfection, and harvested in immunoprecipitation lysis buffer. The rabbit polyclonal antibodies used for each immunoprecipitation are denoted above each lane. “Preimm” signifies use of preimmune rabbit serum.
Anti-M141 antiserum consistently precipitated the 52-kDa pM141, as shown in Fig. 1C. In some experiments, coprecipitation of the 56-kDa pM140 with this antiserum was also evident.
Figure 1A also shows that anti-M140 antisera precipitated the expected product of 56 kDa in addition to the 52-kDa M141 product. The identity of the 52-kDa protein as the M141 product was confirmed by genetic analyses, which demonstrated that the 52-kDa product was not coprecipitated with anti-M140 antiserum when cells were infected with MCMV having M141 deleted (Fig. 1D).
These genetic analyses also demonstrated that pM140 and pM141 are able to complex in the absence of M139 gene products but that M139 products do not bind pM140 or pM141 expressed as individual proteins. Anti-M140 antiserum coprecipitated pM141 from cells infected with mutant MCMV having M139 deleted (Fig. 1D). However, in the absence of pM140, pM141 did not coprecipitate with anti-M139 antiserum, and likewise, in the absence of M141, pM140 did not complex with the M139 proteins (Fig. 1B). Our previously published data indicate that, in the absence of pM140, the half-life of pM141 is reduced from 2 to 1 h, as determined by pulse-chase experiments (9). However, those experiments also revealed that, in the absence of a chase, pM141 is clearly detected by immunoprecipitation with anti-M141 antiserum in cells infected with RVΔ140 (9). Therefore, newly synthesized pM141 is available as a potential binding partner for M139 gene products in the absence of pM140. Collectively, the data in Fig. 1B and D indicate that (i) pM140 and pM141 form a stable complex independent of the M139 proteins and (ii) M139 proteins stably interact with pM140 and pM141 only when these binding partners are in complex.
To confirm the identity of complexes formed in the context of MCMV infection, proteins immunoprecipitated from infected cells were denatured and subsequently reimmunoprecipitated with each specific antibody. From these experiments, it was evident that anti-M139 precipitates a complex composed of M139, M140, and M141 products, while anti-M140 precipitates a complex of pM140 and pM141 that does not contain M139 proteins (Fig. 2). This consistent pattern, with anti-M139 serum coprecipitating pM140 and pM141 and anti-M140 serum coprecipitating pM141, was independent of virus preparation or source of specific antisera and was evident in infected macrophages as well (data not shown). In addition, coprecipitation was not evident when proteins were denatured prior to immunoprecipitation (data not shown). Note that the relative intensities of each of the bands likely reflect immunoprecipitation of both complexed and free forms of the proteins. These data confirmed that at least two stable complexes coexist within MCMV-infected cells: a pM140/pM141 complex and one composed of products from all three genes.
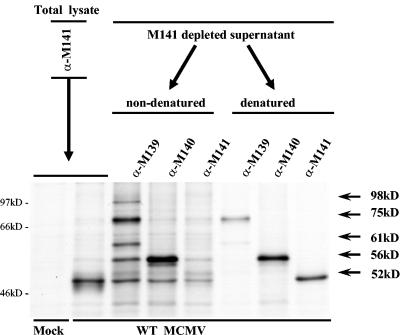
Although coprecipitation of pM141 by anti-M140 serum was highly consistent, the ability to detect coprecipitation of pM140 with anti-M141 serum was variable. Thus, we considered the possibility that anti-M141 preferentially recognizes a free form of pM141, compared to the complexed form, or that the majority of pM141 exists independently of the complex. To address these possibilities, we performed a two-step immunoprecipitation assay in which infected-cell lysates were first subjected to immunoprecipitation with anti-pM141, and the supernatants from the pelleted immune complexes were then subjected to a second round of immunoprecipitations with anti-M140 or -M141 sera. The data in Fig. 3 demonstrated coprecipitation of pM140 (56 kDa) by anti-M141 antisera from nondenatured supernatants partially depleted of pM141. Furthermore, a significant amount of pM141 was detectable in the second round of immunoprecipitations when complexed proteins remaining after the initial anti-M141 immunoprecipitation were first denatured. These data suggested that the antibody preferentially recognizes a free form of pM141. In addition, these data demonstrated that both anti-M140 and anti-M141 sera are capable of coprecipitating both pM140 and pM141 as a complex. Collectively, these data further supported the contention that pM140 and pM141 exist as a stable complex, independently from the larger pM139-associated complex, during MCMV infection.
FIG. 3.
The M141 protein exists in a free and complexed form. Radiolabeled proteins from lysates of NIH 3T3 cells, either mock infected or infected with WT MCMV (1 PFU/cell), were immunoprecipitated with anti-M141 serum (first immunoprecipitation). The immune complexes from this sample (total lysate) were set aside, and the pM141-depleted supernatant was divided into aliquots. Half of the aliquots was immediately reimmunoprecipitated with each of the three indicated antibodies (nondenatured). The other half was denatured and then reimmunoprecipitated (denatured). All immune complexes, obtained from the first and second immunoprecipitations, were processed identically and run on a 12.5% polyacrylamide gel.
Complex formation in the absence of virus infection.
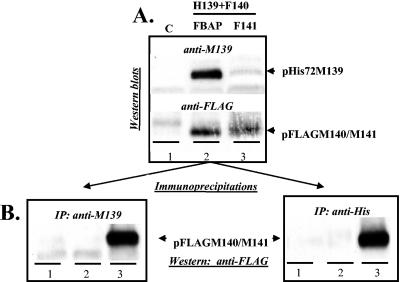
In order to assess whether these viral proteins formed stable complexes in the absence of other viral proteins (or cellular proteins induced by virus infection), we repeated the immunoprecipitation experiments by using lysates from fibroblasts transiently expressing M139, M140, and M141. For these experiments we constructed plasmids expressing epitope-tagged versions of the viral proteins to completely avoid any antibody cross-reactivity and to facilitate detection of the viral proteins by Western blotting. Initially, NIH 3T3 cells were cotransfected with vectors expressing His/Xpress-tagged M139, FLAG-tagged M140, and either FLAG-tagged M141 or, as a control, FBAP. The anti-His antibody detects only the 75-kDa form of the His/Xpress-tagged M139 protein, in contrast to the polyclonal anti-M139 serum, which detects both the 75- and 61-kDa forms. In addition, in these initial experiments, the FLAG-tagged M140 (truncated by 81 amino acids) and FLAG-tagged M141 proteins were of similar sizes, thus preventing their distinction based on molecular weight but nonetheless providing epitope-tagged binding partners for tagged p75M139.
The data in Fig. 4A confirmed that the viral gene products were expressed as expected in the transfected fibroblasts. The p75M139 protein was expressed abundantly in samples cotransfected with His/Xpress-tagged-p75M139 and FLAG-tagged M140 (lane 2). This product was detected at much lower, but detectable, levels in cells transfected with all three US22 genes (lane 3). The FLAG-tagged viral proteins were detected in cells transfected with FLAG-tagged M140- and M141-expressing vectors (lanes 2 and 3). After immunoprecipitation of the transfected-cell lysates with either anti-M139 or anti-His serum, coprecipitating FLAG-tagged M140 and M141 were identified by Western blotting (Fig. 4B). Importantly, FLAG-tagged M140 did not coprecipitate with His/Xpress-M139 in the absence of M141 (lane 2). Only when all three viral genes were expressed did FLAG-M140 and FLAG-M141 coprecipitate with His/Xpress-M139 (lane 3). Identical results were seen when using anti-M139 or anti-His serum as the precipitating antibody. These data substantiate our hypothesis that the 75-kDa M139-derived protein binds to a pM140/pM141 complex in a conformation-dependent manner but not to the individual proteins. The results corroborate the data in Fig. 1B, which showed that pM140 or pM141 did not coprecipitate with anti-M139 serum from lysates of cells infected with MCMV having M141 or M140 deleted, respectively.
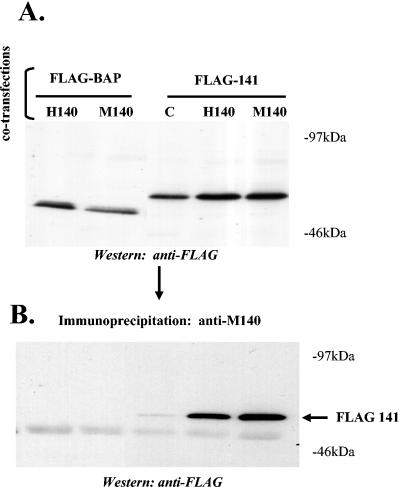
FIG. 4.
Products of M139, M140, and M141 form a complex when the genes are transiently expressed in the absence of other viral proteins. NIH 3T3 cells were cotransfected with vectors expressing His/Xpress-tagged M139 (H139), FLAG-tagged M140 (F140), and either FBAP or FLAG-tagged M141 (F141). (A) To verify expression of each protein, cell lysates were subjected to Western blotting with anti-M139 or anti-FLAG antiserum. Lane 1 (C) depicts nontransfected cells. (B) Lysates were immunoprecipitated with either anti-M139 or anti-His antibody, and immunoprecipitated proteins were subjected to Western blotting to detect coprecipitation of FLAG-tagged M140 or M141 (pFLAGM140/M141).
A similar approach was used to further assess complexing of pM140 and pM141 in transiently transfected cells. In these experiments, vectors expressing either WT or His/Xpress-tagged-M140 were cotransfected with a vector expressing FLAG-tagged M141 or the FBAP control. The data in Fig. 5A indicate that the FLAG-tagged proteins were expressed to comparable levels in the transfected cells. Importantly, when the cell lysates were immunoprecipitated with anti-M140 antiserum, coprecipitation of FLAG-tagged M141 with either the WT or His/Xpress-tagged M140 was evident by Western blotting (Fig. 5B). Thus, these two proteins form a stable complex in the absence of other viral proteins or cellular proteins induced by viral infection.
FIG. 5.
Products of M140 and M141 form a complex, independent of M139, when the two genes are transiently expressed in the absence of other viral proteins. NIH 3T3 cells were transiently transfected with vectors expressing FLAG-tagged M141 (F141) and either His/Xpress-tagged M140 (H140) or untagged M140 (M140). As a control, cells were alternatively cotransfected with FBAP and either tagged or untagged M140. C denotes co-transfection with empty vector. (A) Western blotting was performed to verify that the FLAG-tagged products were expressed in all samples. (B) Cell lysates were immunoprecipitated with rabbit polyclonal anti-M140 serum, and the immune complexes were subjected to Western blotting with anti-FLAG antibody.
Complex formation in vitro.
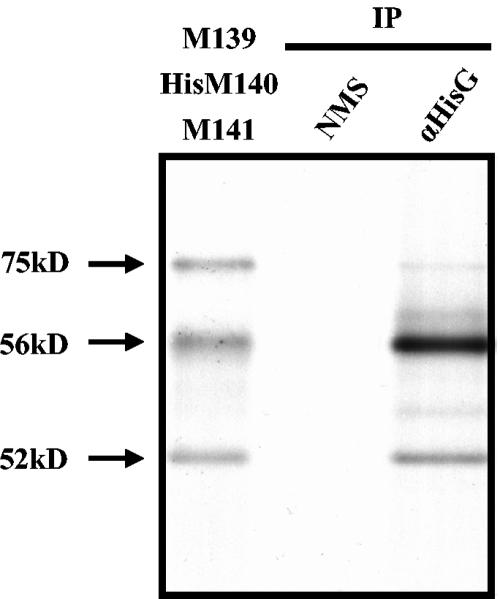
In order to determine if cellular proteins are required to form or stabilize the complex of M139, M140, and M141 proteins, we assessed the ability of the three proteins to coimmunoprecipitate as products of in vitro transcription-translation reactions. When full-length M139 and M141 were cotranscribed and translated along with His/Xpress-tagged full-length M140 in a single reaction, all three products were expressed to nearly comparable levels (Fig. 6, first lane). The exception was the 61-kDa product of M139, which was not expressed to detectable levels. Immunoprecipitation with anti-His antibody yielded trace amounts of p75M139 and more abundant levels of pM140 and pM141 (Fig. 6, third lane). The differences in the intensities of p75M139 compared to the other two proteins may reflect detection by anti-His of a monomeric form of pM140 or a pM140/pM141 complex in addition to a complex composed of all three proteins; thus “pulling down” more pM140 and pM141 than p75M139. Importantly, these data demonstrate that complexes composed of M139, M140, and M141 gene products can form in the absence of cellular proteins. We cannot rule out the possibility, however, that the stoichiometry and/or stability of the complex may be influenced by cellular proteins not present in the translation reaction.
FIG. 6.
Proteins p75M139, pM140, and pM141 form a complex when expressed as products of in vitro transcription and translation. Genes M139, His/Xpress-tagged M140, and M141 were cotranscribed and translated in vitro in the presence of [35S]methionine as described in Materials and Methods. A 2-μl sample of the products was loaded directly into the first lane of a 12.5% polyacrylamide gel. Samples of 40 μl were immunoprecipitated with normal mouse serum (NMS) or mouse monoclonal anti-HisG antibody. Immunoprecipitated proteins were visualized by autoradiography.
Sedimentation gradient analyses to reveal the identity of the two complexes.
The experiments demonstrating coimmunoprecipitation of products of M139, M140, and M141 from infected cells, transfected cells, or in vitro-transcribed genes provided strong evidence that these proteins complex and that at least two separate complexes exist under steady-state conditions. To further prove the physical identity of these complexes, lysates of cells infected with WT virus or RVΔ140 were subjected to sedimentation sucrose gradient analyses, and the various fractions were analyzed for the presence of M139, M140, and M141 proteins. Although the data with fibroblasts are presented for consistency, results were identical using IC-21 macrophages as hosts for infection.
Initial experiments were designed to determine where the monomeric forms of these proteins, expressed in the absence of other binding partners, sedimented within the gradient. Products of each single gene transcribed and translated in vitro were applied to the gradient, and the fractions in which they sedimented were detected by autoradiography. As shown in Fig. 7A, three distinct peaks were evident, representing each of the indicated proteins. The peak fraction for the M139 gene product represents p75M139, as p61M139 was not evident from the autoradiograph, similar to previous results of in vitro transcription-translation reactions (Fig. 6). It is interesting that, in this analysis, the M141 product appeared to be of a greater size and/or weight than the product of M140. In spite of the faster migration of pM141 than of pM140 in SDS-polyacrylamide gels, a higher molecular weight for pM141 is actually predicted based on the amino acid sequence. The relative position of each of the proteins expressed as single genes was used to determine the identity of the “monomeric” forms of these proteins, although the presence of homodimers cannot be ruled out. However, upon consideration of the molecular weight of each protein on SDS-polyacrylamide gels, the relative positions of the three proteins within the gradient are suggestive of monomeric forms.
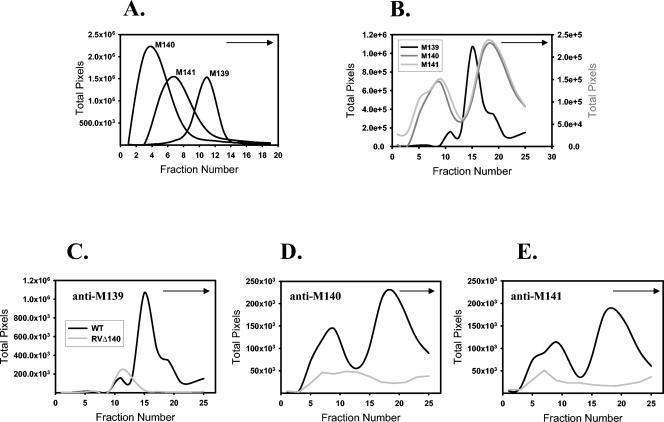
FIG. 7.
Sucrose gradient fractionation of infected-cell lysates reveals three distinguishable complexes. (A) Fractionation of monomeric forms of each protein. Genes M139, M140, and M141 were individually transcribed and translated in vitro in the presence of [35S]methionine, and each product was subjected to sucrose gradient fractionation. Each fraction was applied to an SDS-polyacrylamide gel, and the location within the gradient where each protein sedimented was determined by autoradiography. The films were scanned, and bands were subjected to densitometric quantitation with UN-SCAN-IT software. (B) A composite graph of plots from WT-infected cell lysates in panels C to E to show the relative sedimentation within the gradient of M139, M140, and M141 gene products. (C to E) Fractions containing the M139, M140, and M141 proteins from cells infected with WT MCMV (black line) or mutant virus having M140 deleted (gray line) were determined by Western dot blotting and quantitated as described above. Arrows in each graph indicate the direction of sedimentation, from top (smaller proteins) to bottom (larger proteins).
Sedimentation of the three proteins in the context of virus infection was determined by subjecting lysates from infected fibroblasts or macrophages to gradient analyses and identifying the presence or absence of pM139 (p75M139 with or without p61M139), pM140, or pM141 in each fraction by Western dot blotting. As with conventional Western blotting, the polyclonal antibodies used to detect the viral proteins were not cross-reactive in these dot blot assays. A composite showing the relative sedimentation of each of the gene products from WT virus-infected cells is shown in Fig. 7B.
The M139 protein(s) was identified in three separate fractions (Fig. 7C). Based on the data in Fig. 7A, the peak of anti-M139 reactivity at fraction 11 corresponds to a monomeric form of this protein. As predicted from the immunoprecipitation assays, this monomeric form was also present in RVΔ140-infected cells. A second peak at fraction 15 was unpredicted but may correspond to a complex of p75M139 and/or p61M139 and an unidentified cellular or viral protein. Importantly, this peak was not present in cells infected with RVΔ140, in which pM140 is absent and pM141 is unstable under steady-state conditions (9, 14). A third anti-M139 reactive peak, detected in fraction 18 of WT virus-infected cells but not RVΔ140-infected cells, corresponds to the peak of pM140 and pM141 reactivity (Fig. 7B), thus confirming the existence of a complex composed of all three gene products.
In Fig. 7D, a distinct monomeric form of pM140 was not evident; however, the broad left shoulder of the first anti-M140 reactive peak may mask a monomeric form at fraction 4. Two distinct pM140-containing peaks were evident in WT virus-infected cells, at fractions 9 and 18. Importantly, these same peaks correspond to pM141-containing fractions (Fig. 7B and E). The first peak represents the pM140/pM141 complex. The second corresponds to the complex of all three gene products. Whether the unknown 98-kDa protein identified in the initial coimmunoprecipitation experiments (Fig. 1) is a component of one of these fractions has yet to be determined.
Finally, a monomeric form of pM141 was evident in fractions 6 to 7 (Fig. 7E), in addition to the two peaks mentioned above containing both pM140 and pM141 (Fig. 7B and E). In fractions of RVΔ140-infected cells, a small peak that may represent a monomeric form of pM141 was seen; however, considering the instability of this protein in the absence of pM140 under steady-state conditions, the true identify of this “peak” is in question.
Collectively, these data revealed the identify of three separate complexes composed of two or more of the M139, M140, and M141 gene products. As expected, one complex is composed of all three protein products (fraction 18) and a second (fraction 9) is composed of pM140 and pM141. A third complex (fraction 15) contains p75M139 and/or p61M139 and one or more as-yet-unidentified protein(s) either encoded by M140 or regulated by the product of this gene. Significantly, these experiments revealed structural identities to which function can eventually be ascribed.
Colocalization of M139, M140, and M141 proteins to a perinuclear region of the cell.
The above evidence demonstrates the presence of at least three complexes composed of the M139, M140, and M141 proteins in various combinations. Previous studies indicated that each gene product was detected, via cell fractionation and Western blotting, in both the nucleus and cytoplasm of MCMV-infected cells (9). To identify the cellular region where the M139, M140, and M141 gene products converge and, hence, where the complexes might function within infected cells, we performed confocal microscopy analyses. Here we made use of cells transiently expressing epitope-tagged products.
The confocal images in Fig. 8A indicate that, when coexpressed, products from all three genes colocalize predominantly to a perinuclear region of the cell. Based on the Western blot data above, we assume that the majority of the FITC staining of His/Xpress-tagged M139 is due to expression of p75M139. This product was expressed predominately in the perinuclear region of the cytoplasm, although some nuclear staining, in discrete regions, was observed as expected. FLAG-tagged pM140 and pM141 also localized to the perinuclear region of the cytoplasm, with faint nuclear staining. Merged images revealed perinuclear regions where the M139, M140, and M141 products converged. This area of colocalization likely reflects the site where the M139-M140-M141 complex resides.
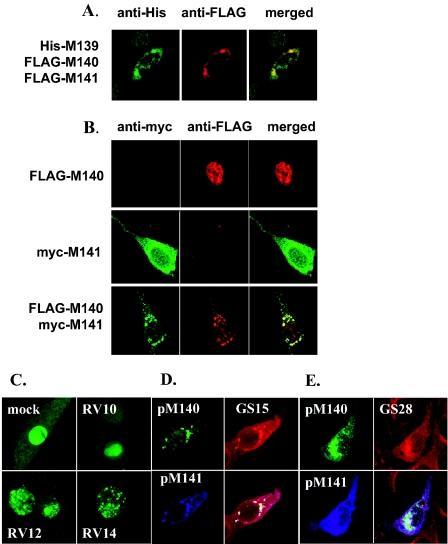
FIG. 8.
Products of M139, M140, and M141 colocalize to a perinuclear region of the cell juxtaposed to or within the cis-Golgi region. (A) NIH 3T3 cells were cotransfected with His/Xpress-tagged M139 (His-M139), FLAG-tagged M140 (FLAG-M140), and FLAG-tagged M141 (FLAG-M141). After 24 h, cells were seeded onto chamber slides, fixed, and stained with the indicated primary antibodies and secondary antibodies conjugated to FITC (green) or to TRITC (red). Original magnification, ×40. (B) As in panel A above, except that cells were transfected with a single vector expressing FLAG-M140 or Myc-tagged M141 (myc-M141) or were cotransfected with both vectors. Original magnification, ×40. (C) NIH 3T3 cells were transfected with GFP-M140, seeded onto chambered slides, and either mock infected or infected (5 PFU/cell) with mutant MCMV having M139, M140, and M141 deleted (RV10); having M139 and M140 deleted (RV12); or having M140 alone deleted (RV14). Cells were viewed 18 h postinfection. Original magnification, ×40. (D) NIH 3T3 cells were cotransfected with GFP-M140 and Myc-M141. Fixed cells were stained with rabbit anti-Myc antibody and Cy5-conjugated goat anti-rabbit antibody or with mouse anti-GS15 antibody and Texas red-conjugated goat anti-mouse serum. Convergence of all three labels appears white. Original magnification, ×40. (E) NIH 3T3 cells were transfected, fixed, and stained with primary and secondary antibodies as described above, with the exception that mouse anti-GS28 antibody was used.
As expression and localization of FLAG-tagged M140 and FLAG-tagged M141 could not be distinguished in Fig. 8A, we performed additional experiments to determine the specific localization of each of these two proteins. In these experiments, FLAG-tagged M140 expressed in the absence of pM141 resided predominantly within the nucleus, with faint cytoplasmic staining (Fig. 8B). This finding was somewhat surprising, as the sequence of M140 does not reveal a consensus nuclear localization signal. In contrast, Myc-tagged pM141 expressed alone displayed a diffuse cytoplasmic staining, with some nuclear localization. However, when pM140 and pM141 were coexpressed, they colocalized to the perinuclear region in a punctate pattern of staining, with some pM140 remaining within the nucleus. These data suggest that coexpression of pM140 and pM141, under conditions where the two proteins complex, alters the predominant subcellular localization of each protein. Presumably, the physical interaction of pM140 with pM141 either prevents pM140 targeting to the nucleus or retrieves pM140 from the nucleus. Furthermore, these data indicate that the free forms of these proteins reside in cellular regions different from the pM140/pM141 complex or the pM139/pM140/pM141 complex.
We next performed experiments to determine if the transition of pM140 from a nuclear to a perinuclear region upon expression of pM141 was evident within the environment of an MCMV-infected cell. Therefore, fibroblasts transfected with GFP-tagged M140 were superinfected with MCMV mutants having M140 deleted and either expressing M141 or having M141 deleted. The images in Fig. 8C indicate that, in the absence of pM141, when cells were either mock infected or infected with mutant MCMV having M139, M140, and M141 deleted (RV10), pM140 remained largely nuclear. However, when the GFP-M140-expressing cells were superinfected with MCMV expressing M141 but having either M139 and M140 deleted (RV12) or M140 alone deleted (RV14), pM140 was expressed predominantly in the cytoplasm in discrete, punctate patterns. Although precise subcellular localization of the punctate staining is more difficult to interpret in live cells with virus-induced cytopathic effects, the differences in the staining pattern of pM140 in the absence versus the presence of pM141 confirmed that pM141 influences the localization of pM140 within the context of MCMV infection. Results with RV12- compared to RV14-infected cells indicated that, as expected from results with transiently transfected cells, the influence of pM141 on pM140 localization was independent of M139 expression.
The particular perinuclear staining pattern of the M139, M140, and M141 products suggested that these proteins may converge in a Golgi-related compartment. Therefore, fibroblasts cotransfected with FLAG-tagged M140 and His-tagged M141 were stained with antibody to GS15 or GS28, components of the SNARE complex that mediates transport from the endoplasmic reticulum to the cis-Golgi region and intra-Golgi transport (26), or with FITC-labeled wheat germ agglutinin, which binds to sialic acid moieties in the lumen of the trans-Golgi region. The data in Fig. 8D and E indicate that pM140 and pM141 colocalize with GS15 and GS28, suggesting a cis- or medial Golgi site of convergence. Cross-reactivity of anti-GS15 or -GS28 antibodies with MCMV proteins was ruled out (W. Salb, unpublished data). The complete absence of colocalization with wheat germ agglutinin provided evidence that pM140 and pM141 do not colocalize to the lumen of the trans-Golgi region and apparently do not reside even proximal to this site, such as the cytoplasmic side of the trans-Golgi region (data not shown). Thus, the data are consistent with localization of at least pM140 and pM141 to a region either closely juxtaposed to or within the cis-Golgi region.
The majority of these experiments were performed using transfected fibroblasts, as the transfection efficiency of these cells is quite high. Selective experiments were repeated using IC-21 macrophages, which have a transfection efficiency of approximately 5%. The same results in colocalization of both the viral proteins and Golgi markers were seen in the macrophages (data not shown).
DISCUSSION
Conservation of US22 genes among all betaherpesviruses signifies their potential importance in the pathogenesis of these viruses within their host populations. For MCMV US22 genes M139, M140, and M141, their coordinated kinetics of expression and abundance within infected cells further suggests the importance of this gene cluster in functioning cooperatively to optimize virus replication in selective cell types. Indeed, functional cooperation has been reported for pM140 and pM141, with pM140 stabilizing pM141 (9). Accordingly, an M140 deletion mutant of MCMV that fails to express pM140 and therefore has substantially reduced levels of pM141 as well has a more profound replication defect than does an M141 mutant, both in vitro and in vivo (9). Similarly, deletions of or mutations within M139, M140, or M141 significantly reduce the steady-state levels of products of the remaining two genes (14). In this report, we demonstrate an additional functional interaction between pM140 and pM141 in dictating cellular localization and demonstrate the coexistence of monomeric and at least three complexed forms of the M139, M140, and M141 gene products within infected cells.
The findings from this study indicate that the functional interactions occur as a consequence of the physical complexing among these US22 proteins. Here we reveal the presence of at least three stable complexes: one composed of the products of all three genes (M139, M140, and M141), one composed of pM140 and pM141, and a third comprised of p75M139 and/or p61M139 complexed to unidentified proteins, the formation of which directly or indirectly involves pM140. Complex formation was confirmed structurally or functionally by using a combination of approaches: coimmunoprecipitations, sedimentation gradient analyses, and confocal microscopy employing cells expressing all three genes or selective combinations of genes expressed from the virus or plasmids. The genetic analyses with mutant viruses in coimmunoprecipitation experiments demonstrated that the pM140/pM141 complex is a prerequisite for optimal binding of the M139 gene products.
This finding is in contrast to results reported by Menard et al., showing that anti-M141 peptide antibodies coprecipitate the M139 gene products from MCMV-infected cells, although with no apparent binding of pM140 (14). It is possible that, under less stringent conditions than used here, other complexes comprised of various combinations of M139, M140, and M141 gene products exist either as stable complexes in steady state or as intermediates of the larger complex defined here as p75M139, p61M139, pM140, pM141, and the as-yet-unidentified 98-kDa protein. Data from the sedimentation gradient experiments employed here confirm the existence of an additional complex comprised of p75M139 and/or p61M139 and an undefined binding partner(s). Differences in the immunoprecipitating antibodies may also explain the divergent results. Menard at el. used an antibody to an N-terminal peptide of M141, while our polyclonal antiserum was generated to a pM141 protein missing the first 24 N-terminal amino acids. Thus, if the more C-terminal peptides of pM141 were masked as a result of complex formation, our anti-M141 antibody may not detect a pM139-pM141 complex. Importantly, however, the work of Menard et al. indicates that coprecipitation of the M139 proteins by anti-M141 serum was not evident in the absence of M140, a finding that supports our hypothesis that the pM140/pM141 complexing favors binding of M139 products.
Other than the 98-kDa protein, additional radiolabeled viral or cellular proteins were not consistently coprecipitated with the M139, M140, and M141 gene products; however, we cannot rule out the possibility that other proteins are transient or low-affinity components of one or more of the complexes. Future goals include defining, via mass spectrometry, the binding partners of each complex. In addition, it will be important to identify the stoichiometry of the complexes. From the gradient analyses, it appeared that monomeric forms of the viral proteins exist in steady-state conditions with the complexes, and various combinations of multimers may also exist. Defining the stoichiometry will be important in relating the various physical forms of these proteins to their functions in the replication of MCMV and its pathogenesis.
A functional impact of complex formation on at least cellular distribution is evident from the confocal microscopy data. When expressed alone, pM140 resides predominantly in the nucleus. When coexpressed with M141, pM141 either redirects a substantial portion of pM140 from the nucleus to the cytoplasm or prevents nuclear localization of pM140. Presumably, this redistribution of pM140 from the nucleus to the cytoplasm is the result of pM140/pM141 complex formation. The complex likely conveys a specific function independent from the monomeric proteins. Colocalization of the M139 products at the same site suggests that the larger complex may have a function similar to, or complementing, that of the pM140/pM141 complex.
The association of these US22 proteins with the cis- or medial-Golgi region is intriguing and worthy of further pursuit. The exact nature of this association needs to be defined to determine, for example, whether the M139, M140, and M141 products are tethered to the Golgi matrix or actually reside within the Golgi region. Additional preliminary data indicate that there is partial colocalization of pM140 and pM141 with GM130, a golgin that binds to the cytoplasmic side of the cis-Golgi region (2). Of the three gene products, only pM139 is predicted to have a transmembrane domain but minimal potential glycosylation sites. None of the three genes is predicted to have an N-terminal myristoylation site, nor sites of palmitoylation that would link these proteins to membranes, a process that may require another cellular or viral protein not yet identified. An association of these US22 proteins with Golgi compartments might implicate their involvement in some aspect of viral tegumentation or envelopment (21). Future studies will be directed toward deciphering the functions of the M139, M140, and M141 products and how these functions relate to the various structural entities and their subcellular localization.
Acknowledgments
We acknowledge the input of Nag Hedge from David Johnson's laboratory, Oregon Health Sciences University, Portland, in preliminary discussions leading to this work. We are appreciative of the assistance of Jeff Duprees in obtaining the confocal images. We gratefully acknowledge the advice from Jay Brown, University of Virginia, on analyses of data from the sedimentation gradients.
This work was supported by The Thomas F. Jeffress and Kate Miller Jeffress Memorial Trust and NIH grant R01-CA41451 to A.E.C.
REFERENCES
- 1.Adair, R., E. R. Douglas, J. B. Maclean, S. Y. Graham, J. D. Aitken, F. E. Jamieson, and D. J. Dargan. 2002. The products of human cytomegalovirus genes UL23, UL24, UL43 and US22 are tegument components. J. Gen. Virol. 83:1315-1324. [DOI] [PubMed] [Google Scholar]
- 2.Barr, F. A., and B. Short. 2003. Golgins in the structure and dynamics of the Golgi apparatus. Curr. Opin. Cell Biol. 15:405-413. [DOI] [PubMed] [Google Scholar]
- 3.Cardin, R. D., G. B. Abenes, C. A. Stoddart, and E. S. Mocarski. 1995. Murine cytomegalovirus IE2, an activator of gene expression, is dispensable for growth and latency in mice. Virology 209:236-241. [DOI] [PubMed] [Google Scholar]
- 4.Cavanaugh, V. J., R. M. Stenberg, T. L. Staley, H. W. Virgin IV, M. R. MacDonald, S. Paetzold, H. E. Farrell, W. D. Rawlinson, and A. E. Campbell. 1996. Murine cytomegalovirus with a deletion of genes spanning HindIII-J and -I displays altered cell and tissue tropism. J. Virol. 70:1365-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chee, M. S., A. T. Bankier, S. Beck, R. Bohni, C. M. Brown, R. Cerny, T. Horsnell, C. A. Hutchison III, T. Kouzarides, J. A. Martignetti, et al. 1990. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr. Top. Microbiol. Immunol. 154:125-169. [DOI] [PubMed] [Google Scholar]
- 6.Colberg-Poley, A. M., L. D. Santomenna, P. P. Harlow, P. A. Benfield, and D. J. Tenney. 1992. Human cytomegalovirus US3 and UL36-38 immediate-early proteins regulate gene expression. J. Virol. 66:95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Efstathiou, S., G. L. Lawrence, C. M. Brown, and B. G. Barrell. 1992. Identification of homologues to the human cytomegalovirus US22 gene family in human herpesvirus 6. J. Gen. Virol. 73:1661-1671. [DOI] [PubMed] [Google Scholar]
- 8.Hanson, L. K., B. L. Dalton, Z. Karabekian, H. E. Farrell, W. D. Rawlinson, R. M. Stenberg, and A. E. Campbell. 1999. Transcriptional analysis of the murine cytomegalovirus HindIII-I region: identification of a novel immediate-early gene region. Virology 260:156-164. [DOI] [PubMed] [Google Scholar]
- 9.Hanson, L. K., J. S. Slater, Z. Karabekian, G. Ciocco-Schmitt, and A. E. Campbell. 2001. Products of US22 genes M140 and M141 confer efficient replication of murine cytomegalovirus in macrophages and spleen. J. Virol. 75:6292-6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanson, L. K., J. S. Slater, Z. Karabekian, H. W. Virgin IV, C. A. Biron, M. C. Ruzek, N. van Rooijen, R. P. Ciavarra, R. M. Stenberg, and A. E. Campbell. 1999. Replication of murine cytomegalovirus in differentiated macrophages as a determinant of viral pathogenesis. J. Virol. 73:5970-5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry, S. C., K. Schmader, T. T. Brown, S. E. Miller, D. N. Howell, G. G. Daley, and J. D. Hamilton. 2000. Enhanced green fluorescent protein as a marker for localizing murine cytomegalovirus in acute and latent infection. J. Virol. Methods 89:61-73. [DOI] [PubMed] [Google Scholar]
- 12.Iskenderian, A. C., L. Huang, A. Reilly, R. M. Stenberg, and D. G. Anders. 1996. Four of eleven loci required for transient complementation of human cytomegalovirus DNA replication cooperate to activate expression of replication genes. J. Virol. 70:383-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kouzarides, T., A. T. Bankier, S. C. Satchwell, E. Preddy, and B. G. Barrell. 1988. An immediate early gene of human cytomegalovirus encodes a potential membrane glycoprotein. Virology 165:151-164. [DOI] [PubMed] [Google Scholar]
- 14.Menard, C., M. Wagner, Z. Ruzsics, K. Holak, W. Brune, A. E. Campbell, and U. H. Koszinowski. 2003. Role of murine cytomegalovirus US22 gene family members in replication in macrophages. J. Virol. 77:5557-5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michelson, S. 1997. Interaction of human cytomegalovirus with monocytes/macrophages: a love-hate relationship. Pathol. Biol. (Paris) 45:146-158. [PubMed] [Google Scholar]
- 16.Nicholas, J. 1996. Determination and analysis of the complete nucleotide sequence of human herpesvirus. J. Virol. 70:5975-5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholas, J., and M. E. Martin. 1994. Nucleotide sequence analysis of a 38.5-kilobase-pair region of the genome of human herpesvirus 6 encoding human cytomegalovirus immediate-early gene homologs and transactivating functions. J. Virol. 68:597-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollock, J. L., R. M. Presti, S. Paetzold, and H. W. Virgin IV. 1997. Latent murine cytomegalovirus infection in macrophages. Virology 227:168-179. [DOI] [PubMed] [Google Scholar]
- 19.Rawlinson, W. D., H. E. Farrell, and B. G. Barrell. 1996. Analysis of the complete DNA sequence of murine cytomegalovirus. J. Virol. 70:8833-8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romanowski, M. J., E. Garrido-Guerrero, and T. Shenk. 1997. pIRS1 and pTRS1 are present in human cytomegalovirus virions. J. Virol. 71:5703-5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez, V., K. D. Greis, E. Sztul, and W. J. Britt. 2000. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J. Virol. 74:975-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skaletskaya, A., L. M. Bartle, T. Chittenden, A. L. McCormick, E. S. Mocarski, and V. S. Goldmacher. 2001. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc. Natl. Acad. Sci. USA 98:7829-7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stamminger, T., M. Gstaiger, K. Weinzierl, K. Lorz, M. Winkler, and W. Schaffner. 2002. Open reading frame UL26 of human cytomegalovirus encodes a novel tegument protein that contains a strong transcriptional activation domain. J. Virol. 76:4836-4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stasiak, P. C., and E. S. Mocarski. 1992. Transactivation of the cytomegalovirus ICP36 gene promoter requires the α gene product TRS1 in addition to IE1 and IE2. J. Virol. 66:1050-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao, J., T. Tong, X. Zhan, E. Haghjoo, and F. Liu. 2000. In vitro and in vivo characterization of a murine cytomegalovirus with a transposon insertional mutation at open reading frame M43. J. Virol. 74:9488-9497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu, Y., S. Martin, D. E. James, and W. Hong. 2002. GS15 forms a SNARE complex with syntaxin 5, GS28, and Ykt6 and is implicated in traffic in the early cisternae of the Golgi apparatus. Mol. Biol. Cell 13:3493-3507. [DOI] [PMC free article] [PubMed] [Google Scholar]