Summary
Craniofacial anomalies (CFAs) are a diverse group of disorders affecting the shapes of the face and the head. Malformation of the cranial base in humans leads CFAs, such as midfacial hypoplasia and craniosynostosis. These patients have significant burdens associated with breathing, speaking, and chewing. Invasive surgical intervention is the current primary option to correct these structural deficiencies. Understanding molecular cellular mechanism for craniofacial development would provide novel therapeutic options for CFAs. In this study, we found that enhanced bone morphogenetic protein (BMP) signaling in cranial neural crest cells (NCCs) (P0-Cre;caBmpr1a mice) causes premature fusion of intersphenoid synchondrosis (ISS) resulting in leading to short snouts and hypertelorism. Histological analyses revealed reduction of proliferation and higher cell death in ISS at postnatal day 3. We demonstrated to prevent the premature fusion of ISS in P0-Cre;caBmpr1a mice by injecting a p53 inhibitor Pifithrin-α to the pregnant mother from E15.5 to E18.5, resulting in rescue from short snouts and hypertelorism. We further demonstrated to prevent premature fusion of cranial sutures in P0-Cre;caBmpr1a mice by injecting Pifithrin-α through E8.5 to E18.5. These results suggested that enhanced BMP-p53-induced cell death in cranial NCCs causes premature fusion of ISS and sutures in time-dependent manner.
Keywords: BMPs, cell death, craniofacial anomalies, neural crest cells, synchondrosis development
1 ∣. INTRODUCTION
Craniofacial anomalies (CFAs) are a diverse group of deformities due to abnormal growth of the head and the face. The incidence is approximately 1 in 100 births (Kitami, Kitami, Kaku, Wang, & Komatsu, 2018; Sakai & Trainor, 2016; Twigg & Wilkie, 2015; Wei, Hu, Mishina, & Liu, 2016). Surgery is the most used clinical treatment; however, invasive surgery at neonatal stages decreases their quality of life and concerning the financial burdens for the patients. Understanding molecular cellular mechanisms for CFAs could provide us new therapeutic options. It has been reported that the abnormal cranial base development in patients with CFAs, such as Apert syndrome (1:160,000 to 1:200,000 births), Crouzon syndrome (1:60,000 births), Pfeiffer syndrome (1:100,000 births), and Down syndrome (13:10000 births) (Canfield et al., 2006; Cohen Jr. & Kreiborg, 1992; Kaplan, 1991; Vogels & Fryns, 2006). Clarifying molecular cellular mechanisms for the cranial base development will provide novel clinical treatments options for the CFAs caused by premature fusion of synchondroses.
The cranial base is a bottom part of the skull, which supports and separates the brain from other oral structures (Hallett, Ono, Franceschi, & Ono, 2022; Lieberman, Pearson, & Mowbray, 2000; Martínez-Abadías et al., 2012; Zhang et al., 2022). The cranial base consists of basioccipital bone (BO), the basisphenoid bone (BS), and the presphenoid (PS) bones, and cartilaginous tissues connecting the bones, which are the sphenoid-occipital synchondrosis (SOS) between BO and BS, and the intersphenoidal synchondrosis (ISS) between BS and PS, respectively (Funato, 2020; Zhang et al., 2022). Different with majority skull bones are formed via intermembranous ossification, in which mesenchymal cells differentiate into osteoblasts to form bones (Nakashima & de Crombrugghe, 2003), bones in the cranial base are developed by endochondral ossification, which cartilage are replaced into bones, similar with bones in the trunk and extremities (Rengasamy Venugopalan & Van Otterloo, 2021; Young et al., 2006). Unlike endochondral ossification in the long bones, synchondroseal growth is bidirectional (Bailleul & Horner, 2016; Hallett et al., 2022). We recently reported that a feedback loop between Indian hedgehog and parathyroid hormone-related protein (PTHrP) plays critical roles in the posterior-to-anterior ossification of cartilage primordia in the cranial base (Zhang et al., 2022). Interestingly, however, PTHrP-expressing cells, which contribute to formation of columnar chondrocytes in long bones, do not contribute to the formation of columnar structures in synchondrosis (Hallett, Zhou, et al., 2022). These facts suggest that there are different molecular cellular mechanisms of endochondral ossification between the cranial base and the long bones. Therefore, it is necessary to identify common and unique mechanisms of formation and maintenance of synchondroses in the cranial base.
Cranial neural crest cells (NCCs) are the origin of the face and the anterior part of the head. Cranial NCCs are multipotent cells giving rise to bones, cartilage, adipo-tissues, and other tissues and organs (Hall, 2018; Ishii et al., 2012; Mishina & Snider, 2014; Santagati & Rijli, 2003; Schneider, 2018). Defects in their behaviors, such as migration, proliferation, cell death, differentiation, and cell fate specification have been often reported to cause CFAs (He & Soriano, 2017; Teng, Mundell, Frist, Wang, & Labosky, 2008). Many studies have revealed that signaling pathways regulate behaviors of cranial NCCs during craniofacial development (Murillo-Rincón & Kaucka, 2020; Neben & Merrill, 2015). Among them, bone morphogenetic proteins (BMPs) signaling is largely known to play important roles in craniofacial development including chondrogenesis (Salazar, Gamer, & Rosen, 2016; Shu et al., 2011). We previously developed a transgenic mouse model with augmentation of BMP signaling in NCCs (P0-Cre;caBmpr1a mice). P0-Cre;caBmpr1a mice exhibit premature fusion of cranial sutures along with round face, short snouts, and hypertelorism by postnatal day 17 (Komatsu et al., 2013). However, we observed the abnormal head morphology of P0-Cre;caBmpr1a mice at newborn stage (Hayano, Komatsu, Pan, & Mishina, 2015), earlier than the stage P0-Cre;caBmpr1a mice develop cranial suture fusions. These data suggest that premature fusion of cranial suture is not a sole reason for the CFAs in P0-Cre;caBmpr1a mice. It has been reported that higher BMP ligand expression and lower BMP antagonist expression, such as Noggin are found in the prematurely fused synchondrosis caused by a gain of function mutation of fibroblast growth factor receptor 3 (Matsushita et al., 2009). It is also reported that absence of Id2, a downstream target of BMP signaling, suppresses chondrogenic differentiation and proliferation in synchondrosis (Sakata-Goto et al., 2012). Despite the facts that whether BMP signaling plays critical roles in synchondrosis development remains unclear, these reports suggest a mechanism that augmented BMP signaling in P0-Cre;caBmpr1a mice alters synchondrosis development and contributes to the CFAs observed in this model.
In this study, we found that augmentation of BMP signaling in cranial NCCs mediated by BMPR1A, a type I receptor for BMPs, develops premature fusion of ISS along with reduced cell proliferation and higher cell death. Moreover, suppressing p53 activity significantly rescues the premature fusion of ISS restoring CFAs such as short snouts and hypertelorism. We identified a novel mechanism that BMP signaling plays critical roles in synchondrosis development via regulating p53 activity. Our findings also provide a potential preventive strategy for premature fusion of synchondrosis during development.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Mouse breeding
Transgenic mouse line carrying the Cre-inducible constitutively activated Bmpr1a (caBmpr1a) was described previously (Komatsu et al., 2013). We then crossed the caBmpr1a mice with P0-Cre mice (C57BL/6JTg(P0-Cre)94Imeg (ID 148) provided by CARD, Kumamoto University, Japan) (Yamauchi et al., 1999) to generate mice with neural crest specific enhanced BMP signaling (P0-Cre;caBmpr1a). All mice used in this study were kept in a mixed background of C57BL6/J and 129S6. All mice were housed in cages in a 20°C room with a 12 hours (h) light/dark cycle. All animal experiments were performed in accordance with the policy and federal law of judicious use of vertebrate animals as approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Michigan (Protocol # 00009613).
2.2 ∣. Micro-computed tomography
Mice were euthanized at postnatal day 17 (P17). After cleaning of extra tissues, mouse heads were fixed with 10% formalin overnight. All mouse heads were embedded in 1% agarose gel, placed in a 19 mm diameter tube, and scanned over their whole length by using a micro-CT system (μCT40 Scanco Medical). Bone surface images were generated as described, respectively (Komatsu et al., 2013; Pan et al., 2017).
2.3 ∣. Histology, immunohistochemistry, and TUNEL staining
P0-Cre mice (Control mice) and P0-Cre;caBmpr1a mice at postnatal day 3 (P3) and P6 were euthanized and mouse heads were dissected and fixed with 4% paraformaldehyde (PFA) at 4°C for overnight. Then, mouse heads were transferred into 30% sucrose in phosphate-buffered saline (PBS) at 4°C until the heads sank down. The heads were embedded in OCT-compound (ThermoFisher Scientific, Waltham, MA, USA) and 10-μm cryo-sections were prepared by a cryostat (Leica CM1950). Hematoxylin and eosin (HE) staining are performed according to a standard protocol. Immunohistochemistry was performed as previously described (Hayano et al., 2015; Komatsu et al., 2013). The following primary antibodies were used: rabbit antibody against human SOX9 (1:500, Cat# AB5535, Millipore, Burlington, MA, USA), rabbit antibody against Ki67 (1:200, Cat# 9129 S, Cell signaling technology, Danvers, MA, USA). Sections were washed with PBS for 5 min, three times. Goat antibody against rabbit IgG conjugated with AlexaFluor 568 (1:200, Invitrogen, Carlsbad, CA, USA) was used as secondary antibody. Finally, sections were mounted with ProLong gold antifade mountant with DAPI (Thermo Fisher Scientific, Waltham, MA, USA). Cell death was detected with TdT-mediated dUTP nick-end labeling (TUNEL) staining by using Click-iT Plus TUNEL assay (Cat# C10619, Thermo Fisher Scientific, Waltham, MA, USA) according to a standard protocol. After TUNEL staining, sections were mounted with ProLong gold mountant with DAPI. Fluorescence images were taken by a confocal microscopy.
2.4 ∣. Pifithrin-α treatment and whole-mount skeletal staining
Pifithrin-α (PFT) was diluted with 50% DMSO in PBS to prepare PFT stock solution (1.68 mg/ml) (Hayano et al., 2015). This solution was further diluted 10 times with PBS prior to inject to pregnant mice. The day when vaginal plugs were found was designed as embryonic day 0.5 (E0.5). PFT (2.2 mg/kg body weight) was intraperitoneally injected into pregnant mice from E15.5 to E18.5 or from E8.5 to E18.5, respectively (Hayano et al., 2015; Jones et al., 2008). Pups were euthanized at P10 and P17, then skeletal preparation by alizarin red and alcian blue were performed as previously described (Komatsu et al., 2013).
2.5 ∣. Statistic analyses
Statistical analyses were performed by using GraphPad Prism 9.0 software. Student's t-test was used for comparison of two groups, and One-way ANOVA with Tukey's test was used for multiple comparison were performed. p-Value is indicated with asterisks; *p < .05, and **p < .01.
3 ∣. RESULTS
3.1 ∣. Augmentation of BMP signaling in cranial NCCs causes premature fusion of synchondrosis (ISS) in the neonatal skull
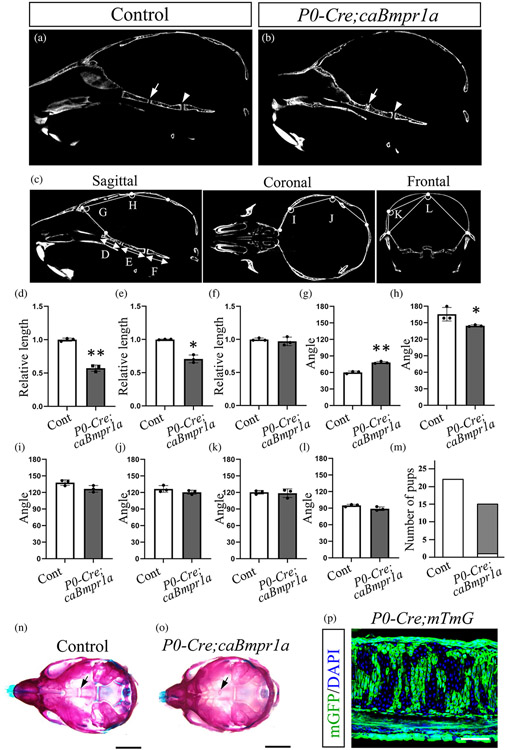
Our previous results showed that transgenic mice with enhanced BMP signaling in cranial NCCs developed round face, short snouts, and hypertelorism (Komatsu et al., 2013). To investigate the function of BMP signaling in synchondrosis of the cranial base leading CFAs, we crossed caBmpr1a with P0-Cre mice (hereafter, P0-Cre;caBmpr1a mice). We evaluated the synchondrosis development of both P0-Cre mice (control mice) (Figure 1a) and P0-Cre;caBmpr1a mutant mice (Figure 1b) by micro computed tomography (μCT) at postnatal day 17 (P17). Intersphenoidal synchondrosis (ISS) of P0-Cre;caBmpr1a mice was prematurely fused at P17 while ISS of control mice kept the patency (Figure 1a, b, arrows). The sphenoid-occipital synchondrosis (SOS), of which cellular origins are NCCs and mesenchymal cells (Kulkarni et al., 2018), was not fused in both control and P0-Cre;caBmpr1a mice at P17 (Figure 1a, b, arrowheads). Assessments for liner and angular dimensions showed shorter cranial base and wider anterior part of the skull in P0-Cre;caBmpr1a mice than control (Figure 1c-l). Whole-mount skeletal staining by alizarin red and alcian blue staining revealed that ISS in P0-Cre;caBmpr1a mice prematurely fused at P10 (14 pups out of 15 pups), but not in control mice (Figure 1m-o, black arrows), while SOS were still patent in both genotypes. P0-Cre;mTmG showed that chondrocytes and perichondrial cells in the ISS are NC-derived cells (Figure 1p).
FIGURE 1.
Augmentation of BMP signaling in cranial NCCs develops premature fusion of intersphenoidal synchondrosis (ISS). Mid-sagittal planes were generated by using micro-CT scans of Control (a) (n = 3) and P0-Cre;caBmpr1a mice (b) (n = 3). (c–l) Angular and length between control and P0-Cre;caBmpr1a mice were then measured. Each point of sagittal view, coronal view, and frontal view were indicated in c. (m–o) Skeletal preparations for control (n) and P0-Cre;caBmpr1a mice (o) at postnatal day 10 were stained with alizarin Red and alcian blue. Arrows in a, b, m, and n indicated ISS. The number of pups with patent ISS (white bars) and premature fused ISS (gray bars) at P10 are shown in m. (p) P0-Cre;mTmG mice showed that cells in ISS are neural crest-derived cells (mGFP; green). Scale bar; 5 mm (m, n) and 100 μm (p). Student's t-test was used for statistical analyses. **p < .01, *p < .05
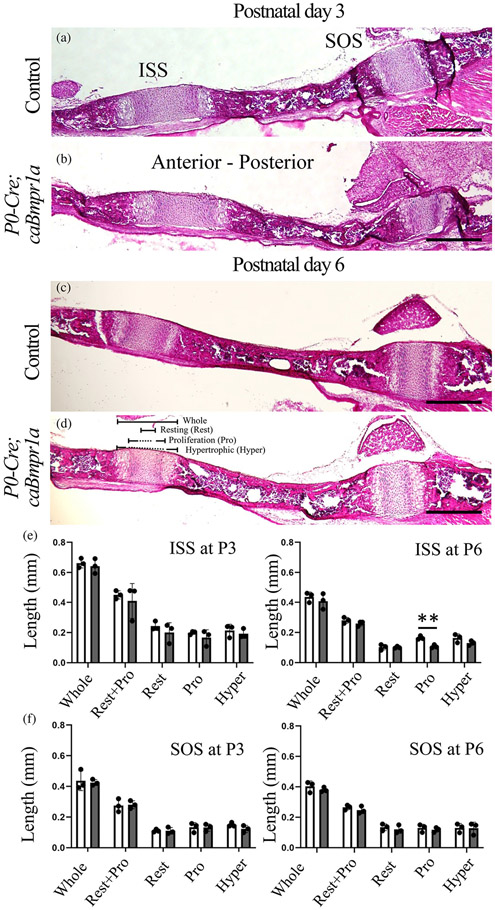
Next, we evaluated structures of ISS and SOS at P3 and P6 (Figure 2a-d), stages earlier than that of premature fusion of ISS in the mutant mice. Histological assessments by H&E staining showed proliferation zone of ISS in P0-Cre;caBmpr1a mice at P6 significantly reduced compared with stage-matched controls (Figure 2c, d). Sizes of other zones were comparable between two genotypes at P6. We did not see overt changes in the ISS, including proliferation zone, at P3 (Figure 2e). For SOS, no significant changes were detected at both stages between two genotypes (Figure 2f).
FIGURE 2.
Histological observations of intersphenoidal synchondrosis (ISS) and sphenooccipital synchondrosis (SOS) for control and P0-Cre;caBmpr1a mice at neonatal stage. (a–d) Histological sections of the ISS and SOS at postnatal day 3 (P3) and P6 were stained with hematoxylin and eosin staining. (e, f) Length between control (white bars) and P0-Cre;caBmpr1a (gray bars) at P3 and P6 were measured (n = 3, two replicate experiments were performed independently). Whole; whole synchondrosis, Resting; cells in resting zone, Pro; proliferating cells, Hyper, hypertrophic cells. Scale bar; 200 μm. Student's t-test was used for statistical analyses. **p < .01
3.2 ∣. Upregulated p53 activities as a downstream event of enhanced BMP signaling which causes premature fusion of ISS
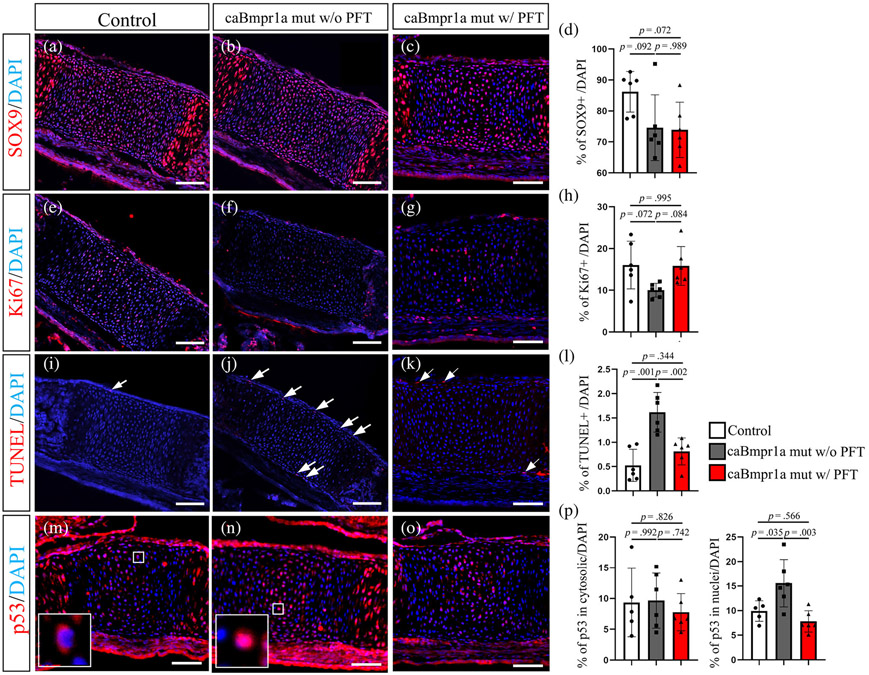
We have reported that augmentation of BMP signaling in cranial NCCs induced more cell death associated with increased p53 activity (Hayano et al., 2015). We then hypothesized that the reduced proliferating zone of ISS in mutants are due to higher cell death and lower cell proliferation caused by elevated p53 activity. Thus, we next quantified the number of SOX9-positive cells as chondrogenic stem/progenitor cells, Ki67-positive cells to mark proliferating cells, and TUNEL-positive cells for cell death. The number of SOX9- and Ki67-positive cells in ISS of P0-Cre;caBmpr1a mice tended to be lower than that in control mice (Figure 3a, b, d, e, f, h). On the other hand, the number of TUNEL-positive cells in ISS of P0-Cre;caBmpr1a was significantly higher than that in ISS of control mice (Figure 3i, j, l, arrows). Immunostaining for p53 demonstrates the presence of p53 in the cytosol (Figure 3m, boxed area) and p53 in nuclear (Figure 3n, boxed area) in ISS. The number of cells with p53 in nuclear in ISS of P0-Cre;caBmpr1a mice was significantly higher than in ISS of control mice (Figure 3p, right). It is well known that p53 translocates into nuclei and then regulates cell cycle and apoptosis. These results suggest that augmentation of BMP signaling in P0-Cre;caBmpr1a mice reduces cell proliferation and increases cell death and p53 activity.
FIGURE 3.
Staining of undifferentiating cells, proliferating cells, and cell death in intersphenoidal synchondrosis (ISS). Immunohistochemistry for SOX9 (a–c) and Ki67 (e–g), TdT-mediated dUTP nick-end labeling (TUNEL) staining (i–k) and p53 (m–o) are shown (n = 6). Nuclei were stained with DAPI (Blue). Control pups (control) and pups of P0-Cre;caBmpr1a mice (caBmpr1a mut w/o PFT) were harvested at P3. The pregnant mice were injected Pifithrin-a from E15.5 to E18.5 and the pups were harvested at P3 (caBmpr1a mut w/ PFT). Number of cells positive for respective markers were quantified (d, h, l, j). Scale bar; 100 μm. Statistical analyses for respective markers were evaluated by one-way ANOVA with Tukey's test
Next, we examined whether elevated p53 activity is a reason for the premature fusion of the ISS. Since Bmp2 is expressed in hypertrophic chondrocytes and perichondrial cells in synchondrosis at embryonic day 16.5 (E16.5) (Kettunen, Nie, Kvinnsland, & Luukko, 2006), we hypothesized that BMP signaling in ISS at E16.5 prompts hypertrophy of chondrocytes and thus augmented BMP signaling develops premature fusion of ISS at or later than E16.5. We injected Pifithrin-a (PFT), an inhibitor of p53, into the pregnant mothers from E15.5, which is 1 day before when Bmp2 is expressed in hypertrophic chondrocytes, to E18.5 to evaluate impacts of p53 suppression on ISS development. We firstly confirmed that PFT administration from E15.5 to E18.5 reduced p53 activity in ISS, as the number of cells with p53 in nuclei in ISS of PFT-treated P0-Cre;caBmpr1a mice was significantly lower than that in ISS of P0-Cre;caBmpr1a mice (Figure 3p). We then determined whether PFT treatment rescued cell proliferation and apoptosis in ISS of mutants. The immunostaining and quantification results showed that although the number of SOX9-positive cells in ISS of PFT-treated P0-Cre;caBmpr1a mice was still lower than that in ISS of controls (Figure 3c,d), the number of Ki67-positive cells (Figure 3g, h) and TUNEL-positive cells (Figure 3k, l) in ISS of PFT-treated P0-Cre;caBmpr1a mice was comparable with that in ISS of control mice. Of note, the number of cells positive for p53 in nuclear in the ISS of PFT-treated P0-Cre;caBmpr1a mice was significantly lower than that in ISS of P0-Cre;caBmpr1a mice (Figure 3p). These results indicated that augmentation of BMP signaling in ISS causes lower cell proliferation and elevated cell death through prompting p53 activities.
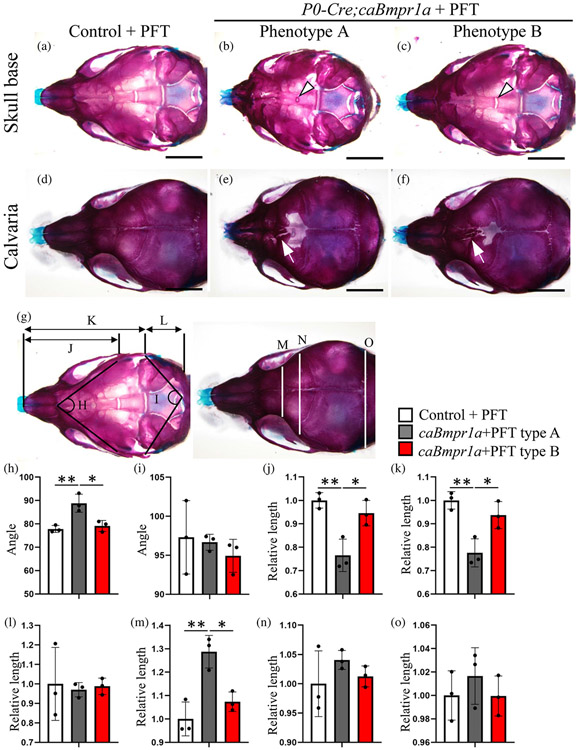
By the same schedule of PFT treatment, about half of the P0-Cre;caBmpr1a mice treated with PFT showed rescued ISS morphology (6 out of 11, Phenotype B, Figure 4c), while the other half still showed premature fusion of ISS (5 out of 11, Phenotype A, Figure 4b). All PFT-treated mutant mice showed premature fusion of the anterior frontal suture and foramina in the posterior portion of the frontal bone (Figure 4d-f, arrows), similar to the P0-Cre;caBmpr1a mice without PFT treatment (Komatsu et al., 2013). These results suggested that PFT treatment during mid-late embryonic stage does not affect the formation of abnormal structures in the calvaria of P0-Cre;caBmpr1a mice. Assessments for the liner and angular dimensions of the skull demonstrated that the abnormal skull shape such as short snout and hypertelorism was rescued in Phenotype B mice (Figure 4h-o).
FIGURE 4.
Inhibition of p53 during late embryonic stage partially rescued the premature fusion of ISS in P0-Cre;caBmpr1a mice. Pifithrin-α (PFT), an inhibitor for p53, was injected to the pregnant mice from embryonic day 15.5 (E15.5) to E18.5, then the skulls of control and P0-Cre;caBmpr1a mice were stained with alizarin red and alcian blue (skeletal preparation) following the pups were harvested at postnatal day 17. Two phenotypes of P0-Cre;caBmpr1a mice were found by PFT injection; phenotype A (4 out of 7 pups) and phenotype B (3 out of 7 pups). Skeletal preparation of the cranial base (a–c) and calvaria (d–f) are shown. (g) Schematic measurement points of angular and length are shown. (h–o) Measurements with significant differences between control (white bar), P0-Cre;caBmpr1a phenotype A (gray bar) and P0-Cre;caBmpr1a phenotype B (red bar) were shown. Scale bar; 2 mm. One-way ANOVA with Turkey's test was used for statistical analyses. **p < .01, *p < .05.
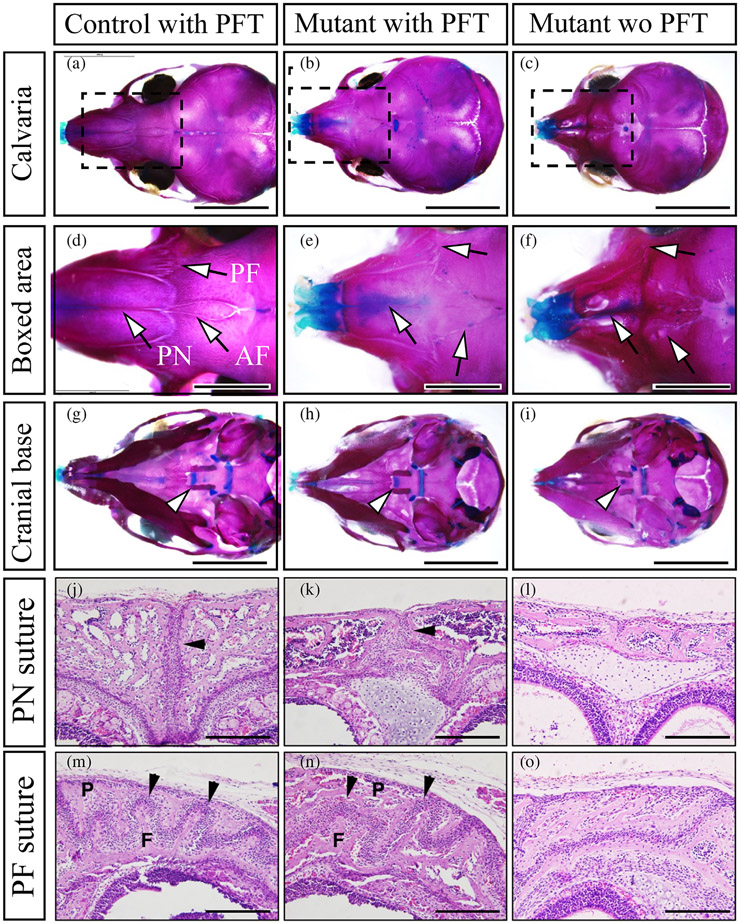
We next determine whether p53 suppression during early embryonic stages can rescue premature fusion of the cranial sutures in P0-Cre;caBmpr1a mice by injecting PFT from E8.5 to E18.5. Skeletal preparation and histological observation demonstrated that premature fusion of the anterior frontal suture (AF), the premaxilla-frontal suture (PF suture), and the posterior nasal suture (PN) are partially rescued by PFT injection during early-late embryonic stage (3 out of 9 pups, Figure 5a-f, j-o, black arrowheads), in addition to rescue the premature fusion of ISS (Figure 5g-i, arrowheads). These results suggest that augmentation of BMP signaling in NCCs at mid-late embryonic stage develops premature fusion of ISS while augmentation of BMP signaling in NCCs at early-mid embryonic stage develops premature fusion of cranial sutures. Taken together, our results suggest that enhanced BMP-p53 axis in cranial NCCs will cause premature fusion of ISS resulting in CFAss such as short snouts and hypertelorism.
FIGURE 5.
Inhibition of p53 during early to late embryonic stage partially rescued the premature fusion of cranial sutures in P0-Cre;caBmpr1a mice. Pifithrin-α (PFT), an inhibitor for p53, was injected to the pregnant mice from embryonic day 8.5 (E8.5) to E18.5, then the skulls of control and P0-Cre;caBmpr1a mice were stained with alizarin red and alcian blue (Skeletal preparation) following the pups were harvested at postnatal day 10. (a–c) Cranial sutures in control mice with PFT (control with PFT), P0-Cre;caBmpr1a mice with PFT (mutant with PFT), and P0-Cre;caBmpr1a mice without PFT (Mutant wo PFT) were shown. (d–f) Boxed area in a–c were enlarged. Arrows indicated the anterior frontal suture (AF), premaxilla-frontal suture (PF), and posterior nasal suture (PN), respectively. (d–f) Synchondroses in control with PFT, Mutant with PFT, and Mutant without PFT were shown. Arrowheads indicated intersphenoidal synchondrosis (ISS). (j–o) Histological observations for the PN suture (j–l) and the PF suture (m–o) by hematoxylin and eosin staining were done. Black arrows indicated patent sutures (2 out of 5 pups showed the partial restoration). F and P in the PF suture mean frontal bones and posterior bones. Scale; 5 mm (a–c, g–i), 2 mm (d–f), 200 μm (j–o).
4 ∣. DISCUSSION
Dysregulations of cranial NCCs have been investigated as causative reasons for CFAs. Despite much studies, molecular mechanisms underlying pathological synchondrosis development leading to CFAs are still unclear. In this study, we found that augmentation of BMP signaling in cranial NCCs causes premature fusion of ISS leading to CFAs such as short snouts and hypertelorism. We also found that inhibition of p53 activity during mid-late embryonic stage rescued premature fusion of ISS along with restoration of the CFAs.
4.1 ∣. Augmentation of BMP-SMAD signaling induces apoptosis of chondrocytes through accumulating the SMAD-p53 interaction
In this study, we found reduction of proliferation and more cell death in ISS of P0-Cre;caBmpr1a mice (Figure 3). We previously reported that augmentation of BMP signaling in NCCs results in more cell death due to accumulation of phosphorylated p53 through a more robust interaction between phosphorylated SMAD and MDM2 in nuclei (Hayano et al., 2015). Thus, we hypothesized that augmentation of BMP signaling also upregulates p53 activity in synchondroses. It has been demonstrated that loss of function mutation of Polycystin-1 (Pkd1) in NCCs (Wnt1-Cre; Pkd1) develops premature fusion of synchondrosis associated with reduction of proliferation and higher levels of apoptosis (Kolpakova-Hart et al., 2008). Notably, Pkd1 expression is suppressed by p53 (Galluzzi et al., 2018; Van Bodegom et al., 2006). It has been investigated that p53 induces apoptosis in articular and the Meckel's cartilage (Okazaki et al., 2003; Trichilis & Wroblewski, 1997). During endochondral ossification, chondrocytes terminally differentiate into hypertrophic chondrocytes, subsequently, hypertrophic chondrocytes undergo apoptosis (Hallett, Ono, & Ono, 2021). Apoptotic chondrocytes are known to promote calcium accumulation and matrix calcification (Chen et al., 2021; Gibson, 1998). These facts suggest that apoptosis induced by BMP-p53 interaction in NCCs promotes premature fusion of synchondrosis. It is still unclear whether hypertrophic chondrocytes in synchondroses undergo apoptosis to promote calcium accumulation and matrix calcification as well as long bones. Gain of function mutation of fibroblast growth factor receptor 3 (Fgfr3G374R), a causative reason for craniosynostosis, promotes premature fusion of synchondrosis in mice (Ko, 2016); (Matsushita et al., 2009). The Fgfr3G374R mice show lower cell proliferation but do not show higher cell death. It is an important effort to determine whether cell death of hypertrophic chondrocytes in synchondrosis promotes calcium accumulation.
4.2 ∣. Defects of BMP signaling in cranial NCCs develops CFAs
In mice, we and others have reported that loss of function or gain of function mutation of BMP signaling mediated by BMP type 1 receptor develops CFAs such as midfacial defects, cleft palate, and craniosynostosis (Dudas, Sridurongrit, Nagy, Okazaki, & Kaartinen, 2004; Hayano et al., 2015; Komatsu et al., 2013; Li et al., 2013; Maruyama et al., 2021; Yang et al., 2021). In addition to many studies about BMP-related CFAs by using transgenic animals, there are a few case reports of BMP-related CFAs in human subjects. Homozygous missense of BMPR1A (results in amino acid substitution of BMPR1AR406L) develops brachycephaly with unilateral coronal craniosynostosis in humans (Russell et al., 2019). Duplication of human chromosome 10 q22.3q23.2, including BMPR1A, develops hypertelorism (van Bon et al., 2011). Genome-wide association studies (GWAS) of patients with nonsyndromic craniosynostosis demonstrated the single nucleotide polymorphisms (SNPs) near the location of BMP2, which may upregulate BMP2 expression in the patients (Justice et al., 2012). SMAD6 is an inhibitory SMAD that negatively regulates BMP signaling (Hata, Lagna, Massagué, & Hemmati-Brivanlou, 1998). Exome sequencing of patients with nonsyndromic craniosynostosis demonstrated de novo SMAD6 mutation, inferred loss of function mutation of SMAD6 (Komatsu & Mishina, 2016; Timberlake et al., 2016). Notably, all patients carrying both the SMAD6 mutation and the SNP near BMP2 develops craniosynostosis (Komatsu & Mishina, 2016; Timberlake et al., 2016). Many of the global knockouts of BMP ligands, receptors, and Smads and overexpressions of BMP signaling components in mice result in embryonic lethality (Mishina, Crombie, Bradley, & Behringer, 1999; Mishina, Suzuki, Ueno, & Behringer, 1995; Winnier, Blessing, Labosky, & Hogan, 1995; Zhang & Bradley, 1996). It is reasonable to speculate that a significant change in BMP signaling activity may also cause lethality in human subjects. Mutations identified in humans with CFAs are likely those which result in slight up- or down-regulation of BMP signaling. Indeed, chondrocytes with homozygous BMPR1AR406L show slight elevation of the pSMAD 1/5/9 level (Russell et al., 2019). Taken together, this finding suggests that mutations in regulatory sequences, which result in a slight up- or down-regulation of components of the BMP-SMAD pathway, may lead to CFAs even if no mutation is found in their coding sequences.
In summary, we propose that BMP-induced apoptosis and reduction of proliferation develop premature fusion of synchondrosis leading to CFAs. We hope these findings will contribute to further define the molecular mechanisms of CFAs, ultimately provide better treatment options for pathologic conditions including midfacial hypoplasia.
ACKNOWLEDGEMENT
We thank Drs. Kenichi Yamamura for providing P0-Cre mice, W. Benton Swanson for critical reading, and Michelle Lynch for microCT operation.
FUNDING INFORMATION
This work was supported by grants from the NIH (R01DE020843 and R01DE027662 to Yuji Mishina). The micro-CT core at the University of Michigan School of Dentistry is funded in part by NIH/NCRR S10RR026475-01.
Funding information
National Institute of Dental and Craniofacial Research, Grant/Award Numbers: R01DE020843, R01DE027662, S10RR026475
Footnotes
CONFLICT OF INTEREST
All authors state that they have no conflict of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Bailleul AM, & Horner JR (2016). Comparative histology of some craniofacial sutures and skull-base synchondroses in non-avian dinosaurs and their extant phylogenetic bracket. Journal of Anatomy, 229(2), 252–285. 10.1111/joa.12471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield MA, Honein MA, Yuskiv N, Xing J, Mai CT, Collins JS, … Kirby RS (2006). National estimates and race/ethnic-specific variation of selected birth defects in the United States, 1999-2001. Birth Defects Research. Part A, Clinical and Molecular Teratology, 76(11), 747–756. 10.1002/bdra.20294 [DOI] [PubMed] [Google Scholar]
- Chen H, Tan XN, Hu S, Liu RQ, Peng LH, Li YM, & Wu P (2021). Molecular mechanisms of chondrocyte proliferation and differentiation. Frontiers in Cell and Development Biology, 9, 664168. 10.3389/fcell.2021.664168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MM Jr., & Kreiborg S (1992). Birth prevalence studies of the Crouzon syndrome: Comparison of direct and indirect methods. Clinical Genetics, 41(1), 12–15. 10.1111/j.1399-0004.1992.tb03620.x [DOI] [PubMed] [Google Scholar]
- Dudas M, Sridurongrit S, Nagy A, Okazaki K, & Kaartinen V (2004). Craniofacial defects in mice lacking BMP type I receptor Alk2 in neural crest cells. Mechanisms of Development, 121(2), 173–182. 10.1016/j.mod.2003.12.003 [DOI] [PubMed] [Google Scholar]
- Funato N. (2020). New insights into cranial synchondrosis development: A mini review. Frontiers in Cell and Development Biology, 8, 706. 10.3389/fcell.2020.00706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, … Kroemer G (2018). Molecular mechanisms of cell death: Recommendations of the nomenclature committee on cell death 2018. Cell Death and Differentiation, 25(3), 486–541. 10.1038/s41418-017-0012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. (1998). Active role of chondrocyte apoptosis in endochondral ossification. Microscopy Research and Technique, 43(2), 191–204. [DOI] [PubMed] [Google Scholar]
- Hall BK (2018). Germ layers, the neural crest and emergent organization in development and evolution. Genesis, 56(6–7), e23103. 10.1002/dvg.23103 [DOI] [PubMed] [Google Scholar]
- Hallett SA, Ono W, Franceschi RT, & Ono N (2022). Cranial Base synchondrosis: Chondrocytes at the hub. International Journal of Molecular Sciences, 23(14), 7817. 10.3390/ijms23147817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett SA, Ono W, & Ono N (2021). The hypertrophic chondrocyte: To be or not to be. Histology Histopathology, 36(10), 1021–1036. 10.14670/hh-18-355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett SA, Zhou A, Herzog C, Arbiv A, Ono W, & Ono N (2022). Cranial base synchondrosis lacks PTHrP-expressing column-forming chondrocytes. International Journal of Molecular Sciences, 23(14), 7873. 10.3390/ijms23147873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A, Lagna G, Massagué J, & Hemmati-Brivanlou A (1998). Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes & Development, 12(2), 186–197. 10.1101/gad.12.2.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano S, Komatsu Y, Pan H, & Mishina Y (2015). Augmented BMP signaling in the neural crest inhibits nasal cartilage morphogenesis by inducing p53-mediated apoptosis. Development, 142(7), 1357–1367. 10.1242/dev.118802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, & Soriano P (2017). Dysregulated PDGFRα signaling alters coronal suture morphogenesis and leads to craniosynostosis through endochondral ossification. Development, 144(21), 4026–4036. 10.1242/dev.151068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii M, Arias AC, Liu L, Chen YB, Bronner ME, & Maxson RE (2012). A stable cranial neural crest cell line from mouse. Stem Cells and Development, 21(17), 3069–3080. 10.1089/scd.2012.0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NC, Lynn ML, Gaudenz K, Sakai D, Aoto K, Rey JP, … Trainor PA (2008). Prevention of the neurocristopathy treacher collins syndrome through inhibition of p53 function. Nature Medicine, 14(2), 125–133. 10.1038/nm1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice CM, Yagnik G, Kim Y, Peter I, Jabs EW, Erazo M, … Boyadjiev SA (2012). A genome-wide association study identifies susceptibility loci for nonsyndromic sagittal craniosynostosis near BMP2 and within BBS9. Nature Genetics, 44(12), 1360–1364. 10.1038/ng.2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan LC (1991). Clinical assessment and multispecialty management of Apert syndrome. Clinics in Plastic Surgery, 18(2), 217–225. [PubMed] [Google Scholar]
- Kettunen P, Nie X, Kvinnsland IH, & Luukko K (2006). Histological development and dynamic expression of Bmp2-6 mRNAs in the embryonic and postnatal mouse cranial base. The Anatomical Record. Part A, Discoveries in Molecular, Cellular, and Evolutionary Biology, 288(12), 1250–1258. 10.1002/ar.a.20402 [DOI] [PubMed] [Google Scholar]
- Kitami K, Kitami M, Kaku M, Wang B, & Komatsu Y (2018). BRCA1 and BRCA2 tumor suppressors in neural crest cells are essential for craniofacial bone development. PLoS Genetics, 14(5), e1007340. 10.1371/journal.pgen.1007340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JM (2016). Genetic syndromes associated with craniosynostosis. Journal of Korean Neurosurgical Association, 59(3), 187–191. 10.3340/jkns.2016.59.3.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolpakova-Hart E, McBratney-Owen B, Hou B, Fukai N, Nicolae C, Zhou J, & Olsen BR (2008). Growth of cranial synchondroses and sutures requires polycystin-1. Developmental Biology, 321(2), 407–419. 10.1016/j.ydbio.2008.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu Y, & Mishina Y (2016). An epistatic explanation. eLife, 5. 10.7554/eLife.21162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu Y, Yu PB, Kamiya N, Pan H, Fukuda T, Scott GJ, … Mishina Y (2013). Augmentation of Smad-dependent BMP signaling in neural crest cells causes craniosynostosis in mice. Journal of Bone and Mineral Research, 28(6), 1422–1433. 10.1002/jbmr.1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni AK, Louie KW, Yatabe M, Ruellas ACO, Mochida Y, Cevidanes LHS, … Zhang H (2018). A ciliary protein EVC2/LIMBIN plays a critical role in the skull base for mid-facial development. Frontiers in Physiology, 9, 1484. 10.3389/fphys.2018.01484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wang Y, Lin M, Yuan G, Yang G, Zheng Y, & Chen Y (2013). Augmented BMPRIA-mediated BMP signaling in cranial neural crest lineage leads to cleft palate formation and delayed tooth differentiation. PLoS One, 8(6), e66107. 10.1371/journal.pone.0066107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman DE, Pearson OM, & Mowbray KM (2000). Basicranial influence on overall cranial shape. Journal of Human Evolution, 38(2), 291–315. 10.1006/jhev.1999.0335 [DOI] [PubMed] [Google Scholar]
- Martínez-Abadías N, Esparza M, Sjøvold T, González-José R, Santos M, Hernández M, & Klingenberg CP (2012). Pervasive genetic integration directs the evolution of human skull shape. Evolution, 66(4), 1010–1023. 10.1111/j.1558-5646.2011.01496.x [DOI] [PubMed] [Google Scholar]
- Maruyama T, Stevens R, Boka A, DiRienzo L, Chang C, Yu HI, … Hsu W (2021). BMPR1A maintains skeletal stem cell properties in craniofacial development and craniosynostosis. Science Translational Medicine, 13(583). 10.1126/scitranslmed.abb4416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita T, Wilcox WR, Chan YY, Kawanami A, Bükülmez H, Balmes G, … Murakami S (2009). FGFR3 promotes synchondrosis closure and fusion of ossification centers through the MAPK pathway. Human Molecular Genetics, 18(2), 227–240. 10.1093/hmg/ddn339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina Y, Crombie R, Bradley A, & Behringer RR (1999). Multiple roles for activin-like kinase-2 signaling during mouse embryogenesis. Developmental Biology, 213(2), 314–326. 10.1006/dbio.1999.9378 [DOI] [PubMed] [Google Scholar]
- Mishina Y, & Snider TN (2014). Neural crest cell signaling pathways critical to cranial bone development and pathology. Experimental Cell Research, 325(2), 138–147. 10.1016/j.yexcr.2014.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina Y, Suzuki A, Ueno N, & Behringer RR (1995). Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes & Development, 9(24), 3027–3037. 10.1101/gad.9.24.3027 [DOI] [PubMed] [Google Scholar]
- Murillo-Rincón AP, & Kaucka M (2020). Insights into the complexity of craniofacial development from a cellular perspective. Frontiers in Cell and Development Biology, 8, 620735. 10.3389/fcell.2020.620735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, & de Crombrugghe B (2003). Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends in Genetics, 19(8), 458–466. 10.1016/s0168-9525(03)00176-8 [DOI] [PubMed] [Google Scholar]
- Neben CL, & Merrill AE (2015). Signaling pathways in craniofacial development: Insights from rare skeletal disorders. Current Topics in Developmental Biology, 115, 493–542. 10.1016/bs.ctdb.2015.09.005 [DOI] [PubMed] [Google Scholar]
- Okazaki R, Sakai A, Ootsuyama A, Sakata T, Nakamura T, & Norimura T (2003). Apoptosis and p53 expression in chondrocytes relate to degeneration in articular cartilage of immobilized knee joints. The Journal of Rheumatology, 30(3), 559–566. [PubMed] [Google Scholar]
- Pan H, Zhang H, Abraham P, Komatsu Y, Lyons K, Kaartinen V, & Mishina Y (2017). BmpR1A is a major type 1 BMP receptor for BMP-Smad signaling during skull development. Developmental Biology, 429(1), 260–270. 10.1016/j.ydbio.2017.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengasamy Venugopalan S, & Van Otterloo E (2021). The Skull's girder: A brief review of the Cranial Base. Journal of Developmental Biology, 9(1). 10.3390/jdb9010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell BE, Rigueur D, Weaver KN, Sund K, Basil JS, Hufnagel RB, … Dauber A (2019). Homozygous missense variant in BMPR1A resulting in BMPR signaling disruption and syndromic features. Molecular Genetics & Genomic Medicine, 7(11), e969. 10.1002/mgg3.969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai D, & Trainor PA (2016). Face off against ROS: Tcof1/treacle safeguards neuroepithelial cells and progenitor neural crest cells from oxidative stress during craniofacial development. Development, Growth & Differentiation, 58(7), 577–585. 10.1111/dgd.12305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata-Goto T, Takahashi K, Kiso H, Huang B, Tsukamoto H, Takemoto M, … Bessho K (2012). Id2 controls chondrogenesis acting downstream of BMP signaling during maxillary morphogenesis. Bone, 50(1), 69–78. 10.1016/j.bone.2011.09.049 [DOI] [PubMed] [Google Scholar]
- Salazar VS, Gamer LW, & Rosen V (2016). BMP signalling in skeletal development, disease and repair. Nature Reviews. Endocrinology, 12(4), 203–221. 10.1038/nrendo.2016.12 [DOI] [PubMed] [Google Scholar]
- Santagati F, & Rijli FM (2003). Cranial neural crest and the building of the vertebrate head. Nature Reviews. Neuroscience, 4(10), 806–818. 10.1038/nrn1221 [DOI] [PubMed] [Google Scholar]
- Schneider RA (2018). Neural crest and the origin of species-specific pattern. Genesis, 56(6–7), e23219. 10.1002/dvg.23219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu B, Zhang M, Xie R, Wang M, Jin H, Hou W, … Chen D (2011). BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development. Journal of Cell Science, 124(Pt 20), 3428–3440. 10.1242/jcs.083659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng L, Mundell NA, Frist AY, Wang Q, & Labosky PA (2008). Requirement for Foxd3 in the maintenance of neural crest progenitors. Development, 135(9), 1615–1624. 10.1242/dev.012179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timberlake AT, Choi J, Zaidi S, Lu Q, Nelson-Williams C, Brooks ED, … Lifton RP (2016). Two locus inheritance of nonsyndromic midline craniosynostosis via rare SMAD6 and common BMP2 alleles. eLife, 5. 10.7554/eLife.20125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trichilis A, & Wroblewski J (1997). Expression of p53 and hsp70 in relation to apoptosis during Meckel's cartilage development in the mouse. Anatomy and Embryology (Berl), 196(2), 107–113. 10.1007/s004290050083 [DOI] [PubMed] [Google Scholar]
- Twigg SR, & Wilkie AO (2015). New insights into craniofacial malformations. Human Molecular Genetics, 24(R1), R50–R59. 10.1093/hmg/ddv228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bodegom D, Saifudeen Z, Dipp S, Puri S, Magenheimer BS, Calvet JP, & El-Dahr SS (2006). The polycystic kidney disease-1 gene is a target for p53-mediated transcriptional repression. The Journal of Biological Chemistry, 281(42), 31234–31244. 10.1074/jbc.M606510200 [DOI] [PubMed] [Google Scholar]
- van Bon BW, Balciuniene J, Fruhman G, Nagamani SC, Broome DL, Cameron E, … de Vries BB (2011). The phenotype of recurrent 10q22q23 deletions and duplications. European Journal of Human Genetics, 19(4), 400–408. 10.1038/ejhg.2010.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels A, & Fryns JP (2006). Pfeiffer syndrome. Orphanet Journal of Rare Diseases, 1, 19. 10.1186/1750-1172-1-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Hu M, Mishina Y, & Liu F (2016). Developmental regulation of the growth plate and cranial synchondrosis. Journal of Dental Research, 95(11), 1221–1229. 10.1177/0022034516651823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, & Hogan BL (1995). Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes & Development, 9(17), 2105–2116. 10.1101/gad.9.17.2105 [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, Abe K, Mantani A, Hitoshi Y, Suzuki M, Osuzu F, … Yamamura K (1999). A novel transgenic technique that allows specific marking of the neural crest cell lineage in mice. Developmental Biology, 212(1), 191–203. 10.1006/dbio.1999.9323 [DOI] [PubMed] [Google Scholar]
- Yang J, Kitami M, Pan H, Nakamura MT, Zhang H, Liu F, … Mishina Y (2021). Augmented BMP signaling commits cranial neural crest cells to a chondrogenic fate by suppressing autophagic β-catenin degradation. Science Signaling, 14(665). 10.1126/scisignal.aaz9368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B, Minugh-Purvis N, Shimo T, St-Jacques B, Iwamoto M, Enomoto-Iwamoto M, … Pacifici M (2006). Indian and sonic hedgehogs regulate synchondrosis growth plate and cranial base development and function. Developmental Biology, 299(1), 272–282. 10.1016/j.ydbio.2006.07.028 [DOI] [PubMed] [Google Scholar]
- Zhang H, & Bradley A (1996). Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development, 122(10), 2977–2986. 10.1242/dev.122.10.2977 [DOI] [PubMed] [Google Scholar]
- Zhang H, Louie KW, Kulkarni AK, Zapien-Guerra K, Yang J, & Mishina Y (2022). The posterior part influences the anterior part of the mouse Cranial Base development. JBMR Plus, 6(2), e10589. 10.1002/jbm4.10589 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.