Abstract
Background:
Care gaps remain in modern health care despite the availability of robust, evidence-based medications. Although sodium-glucose cotransporter-2 (SGLT2) inhibitors have demonstrated profound benefits in improving both cardiovascular and kidney outcomes in patients, the uptake of these medications remain suboptimal, and the causes have not been systematically explored.
Objective:
The purpose of this study was to use the Consolidated Framework for Implementation Research (CFIR) to describe the barriers and facilitators faced by clinicians in British Columbia, Canada, when prescribing an SGLT2 inhibitor. To achieve this, we conducted semistructured interviews using the CFIR with practicing family physicians, nephrologists, endocrinologists, and cardiologists in British Columbia.
Design:
Semistructured interviews.
Setting:
British Columbia, Canada.
Participants:
Actively practicing family physicians, nephrologists, endocrinologists, and cardiologists in British Columbia.
Methods:
Twenty-one clinicians were interviewed using questions derived from the CFIR. The audio recordings were transcribed verbatim, and each transcription was individually analyzed in duplicate using thematic analysis. The analysis focused on identifying barriers and facilitators to using SGLT2 inhibitors in clinical practice and coded using the CFIR constructs. Once the transcriptions were coded, overarching themes were created.
Results:
Five overarching themes were identified to the barriers and facilitators to using SGLT2 inhibitors: current perceptions and beliefs, clinician factors, patient factors, medication factors, and health care system factors. The current perceptions and beliefs were that SGLT2 inhibitors are efficacious and have distinct advantages over other agents but are underutilized in British Columbia. Clinician factors included varying levels of knowledge of and comfort in prescribing SGLT2 inhibitors, and patient factors included intolerable adverse events and additional pill burden, but many were enthusiastic about potential benefits. Multiple SGLT2 inhibitor related adverse events like mycotic infections and euglycemic diabetic ketoacidosis and the difficulty in obtaining reimbursement for these medications were also identified as a barrier to prescribing these medications. Facilitators for the use of SGLT2 inhibitors included consensus among colleagues, influential leaders, and peers in support of their use, and endorsement by national guidelines.
Limitations:
The experience from the clinicians regarding costs and the reimbursement process is limited to British Columbia as each province has its own procedures. There may be responder bias as clinicians were approached through purposive sampling.
Conclusion:
This study highlights different themes to the barriers and facilitators of using SGLT2 inhibitors in British Columbia. The identification of these barriers provides a specific target for improvement, and the facilitators can be leveraged for the increased use of SGLT2 inhibitors. Efforts to address and optimize these barriers and facilitators in a systematic approach may lead to an increase in the use of these efficacious medications.
Keywords: SGLT2 inhibitors, barriers, facilitators, qualitative research, clinician perspectives, CFIR, evidence-based care, interviews
Abrégé
Contexte:
Des lacunes subsistent dans les soins de santé modernes, malgré l’existence de médicaments éprouvés et fondés sur des données probantes. Les inhibiteurs du cotransporteur de sodium-glucose de type 2 (SGLT2) ont démontré d’importants effets dans l’amélioration des résultats cardiovasculaires et rénaux des patients, mais l’utilization de ces médicaments demeure sous-optimale et les raisons qui expliquent cette situation n’ont pas été systématiquement explorées.
Objectif:
Utiliser le Consolidated Framework for Implementation Research (CFIR) pour décrire les obstacles et les éléments facilitateurs rencontrés par les cliniciens de la Colombie-Britannique (Canada) lorsqu’ils prescrivent un inhibiteur du SGLT2. Pour ce faire, nous avons mené des entretiens semi-structurés au moyen du CFIR auprès de médecins de famille, de néphrologues, de cardiologues et d’endocrinologues exerçant en Colombie-Britannique.
Conception:
Entretiens semi-structurés.
Cadre:
Colombie-Britannique (Canada).
Participants:
Médecins de famille, cardiologues, endocrinologues et néphrologues exerçant en Colombie-Britannique.
Méthodologie:
Les questions dérivées du CFIR ont été posées à vingt-et-un cliniciens. Les enregistrements audio ont été transcrits verbatim et chaque transcription a été analysée individuellement en double en utilisant l’analyze thématique. L’analyze s’est concentrée sur l’identification des obstacles et des facilitateurs à l’utilization des inhibiteurs du SGLT2 dans la pratique clinique et sur le codage selon les concepts du CFIR. Une fois les transcriptions codées, des thèmes généraux ont été créés.
Résultats:
Cinq thèmes généraux ont été identifiés pour les obstacles et les facilitateurs à l’utilization des inhibiteurs du SGLT2: les perceptions et les croyances actuelles, les facteurs liés aux cliniciens, les facteurs liés aux patients, les facteurs liés aux médicaments et les facteurs liés au système de santé. Les perceptions et croyances actuelles étaient que les inhibiteurs du SGLT2 sont efficaces, qu’ils présentent des avantages distincts des autres agents, mais qu’ils sont sous-utilisés en Colombie-Britannique. Les facteurs liés aux cliniciens incluaient des niveaux variables de connaissance et de confort vis-à-vis la prescription d’inhibiteurs du SGLT2. Les facteurs liés aux patients incluaient les événements indésirables intolérables et la charge médicamenteuse supplémentaire, mais plusieurs répondants voyaient positivement les bienfaits potentiels. Les nombreux événements indésirables liés aux inhibiteurs du SGLT2, notamment les infections mycosiques et l’acidocétose diabétique euglycémique, et la difficulté à obtenir le remboursement de ces médicaments ont également été cités comme raisons limitant la prescription de ces médicaments. Le consensus parmi les collègues, les leaders influents et les pairs en faveur des inhibiteurs du SGLT2 et l’inclusion de ces médicaments dans les lignes directrices nationales figuraient parmi les facilitateurs.
Limites:
Les expériences rapportées par les cliniciens en ce qui concerne les coûts et le processus de remboursement se limitent à la Colombie-Britannique, car chaque province a ses propres procédures. L’étude comporte un possible biais de réponse puisque les cliniciens ont été approchés par échantillonnage dirigé.
Conclusion:
Cette étude met en évidence différents thèmes concernant les obstacles et les facilitateurs à l’utilization des inhibiteurs du SGLT2 en Colombie-Britannique. L’identification de ces obstacles fournit une cible précise pour l’amélioration, alors que les facilitateurs peuvent être mis à profit pour accroître l’utilization des inhibiteurs de SGLT2. Les efforts déployés pour aborder et optimiser ces obstacles et ces facilitateurs dans le cadre d’une approche systématique pourraient mener à une augmentation de l’utilization de ces médicaments efficaces.
Introduction
Persistent care gaps remain in modern health care despite the availability of robust evidence supporting the use of guideline-based medications. Studies have demonstrated suboptimal prescription and poor uptake of evidence-based therapies for the management of common conditions such as diabetes (T2DM) and cardiovascular disease (CVD).1,2 Sodium-glucose cotransporter-2 (SGLT2) inhibitors are a novel drug class with profound potential to improve important kidney and cardiovascular outcomes for people with chronic kidney disease (CKD), T2DM, and CVD.3 -7 SGLT2 inhibitors have demonstrated significant benefits in reduction of the composite risk of kidney failure, progression of CKD, and kidney and cardiovascular death by 37% in those with or without T2DM. 8 SGLT2 inhibitors reduced the composite outcome of cardiovascular death or hospitalization for heart failure by 23%. 8 Additional benefits for blood pressure, blood sugar control, and body weight have also been demonstrated.3,9 With such significant disease-modifying effects, it is essential that SGLT2 inhibitors enter widespread clinical practice and so reach all for whom they are indicated.
Unfortunately, in Canada, the uptake of SGLT2 inhibitors remains suboptimal. Using a retrospective linked administrative data set of more than 340 000 people with T2DM who met the indication criteria for SGLT2 inhibitors between 2014 and 2019, including concurrent CVD among 31% of the cohort, only 14% filled a prescription for an SGLT2 inhibitor. 10 In a cohort of more than 446 000 patients with diabetes, less than 8% were prescribed an SGLT2 inhibitor, and among the 76 630 individuals with both CKD and diabetes, only 7% received this treatment. 11 Various obstacles from a clinician, patient, and health system perspective may hinder the appropriate usage of SGLT2 inhibitors and so contribute to preventable morbidity and mortality. A qualitative study with primary-care physicians in Hong Kong identified physician barriers such as lack of awareness of cardio-renal benefits and hesitancy to initiate SGLT2 inhibitors in patients with CKD; patient barriers such as fear of adverse effects; and health system barriers such as prohibitive cost of SGLT2 inhibitors. 12 Retrospective studies of administrative databases demonstrated older patients, despite being eligible, were prescribed SGLT2 inhibitors less than their younger counterparts, and up-titration or initiation of other heart failure therapies were prioritized.11,13,14 No studies have systematically described the implementation facilitators and barriers of SGLT2 inhibitors using an established framework.
Various implementation frameworks have been developed to address these knowledge and care gaps. The Consolidated Framework for Implementation Research (CFIR) is a widely recognized and used framework developed to guide systematic assessment of multilevel implementation contexts to identify barriers and facilitators of an intervention, according to published implementation theories.15 -17 An advantage of the framework is that it can be used at any phase of implementation and hence guide the framework for the evaluation of planned intervention programs arising from stakeholder engagement initiatives. The CFIR was chosen over other frameworks for this study because it explicitly outlines relevant factors associated with SGLT2 inhibitors, such as relative advantage over alternatives and the product cost. 18 The ultimate goal with the CFIR is to close the substantial lag between research-generated evidence and incorporation into routine clinical medicine, currently estimated to take up to 17 years.15,19 The purpose of this study was to use the CFIR to describe the barriers and facilitators faced by clinicians in British Columbia, Canada, when prescribing an SGLT2 inhibitor. To achieve this, we conducted semistructured interviews using the CFIR with practicing family physicians, nephrologists, endocrinologists, and cardiologists in British Columbia.
Methods
The reporting of the study methods below is consistent with the Consolidated Criteria for Reporting Qualitative Research (COREQ), and the COREQ Checklist is included in the Supplementary Material S1. 20
Participants
The initial participants were selected through purposive sampling of practicing clinicians (family physicians, nephrologists, endocrinologists, and cardiologists) in British Columbia, Canada, aiming to include key opinion leaders in their respective fields, with varying years of clinical experience, a balance of genders, and including regional participants. To obtain a broader range of opinions, a snowball sampling approach was subsequently used, as participating clinicians recommended other colleagues with experience or views on the topic, with attention again given to inclusion of different disciplines and levels of clinical experience. The participants were initially approached by email. Emails were sent to 24 clinicians, and 21 responded and agreed to participate. Clinicians were eligible for the study if they were actively practicing physicians in British Columbia. The only exclusion criterion was a lack of English language proficiency.
Interview Guide Development
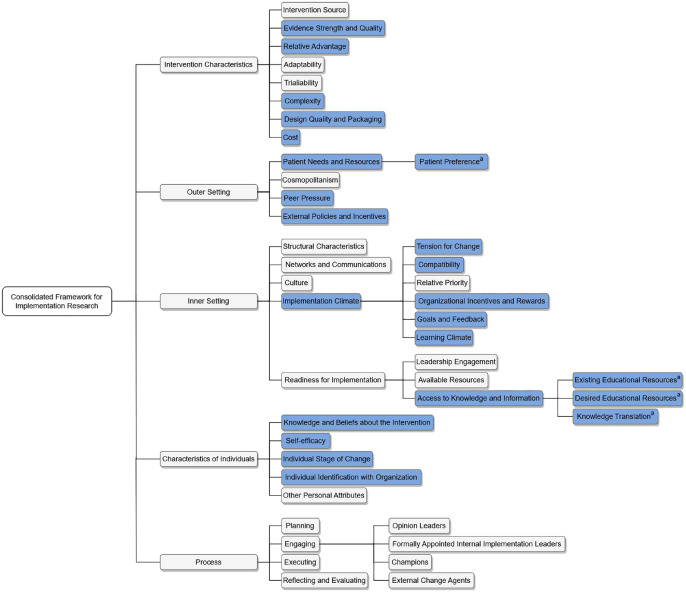
The interview guide was iteratively developed by the research team, which includes TWY and AL, who are practicing nephrologists in Canada; DVO, BS, and MJ who are practicing nephrologists in Australia; and RLM who is a health economist and experienced qualitative researcher. Initial interview topics were developed according to the researchers’ expectation of the broad questions that would most likely identify barriers and facilitators of SGLT2 inhibitor implementation, before being re-evaluated through consideration of the CFIR organizational framework. Examination of the CFIR domains and constructs led to the generation of initial interview questions which were later refined and mapped to corresponding constructs. Additional questions were designed to cover all relevant remaining constructs. Some CFIR domains were considered not relevant and were excluded, such as the “process” domain which applies to later stage of the implementation cycle once an intervention has been applied. The interview questions were developed using 4 of the 5 CFIR domains: Intervention Characteristics, Outer Setting, Inner Setting, and Individual Characteristics. 15 Figure 1 demonstrates the constructs, and the blue bars indicate relevant constructs used to inform the interview questions. The interview guide underwent 2 iterative reviews internally with the study team and was piloted with a test clinician interview (not included in the Results) including transcription, data coding, and analysis, with review of the data by the senior investigator (RLM). Minor adjustments to the interview questions were made after the initial few interviews. The final interview guide is attached in the Supplementary Material S2.
Figure 1.
Consolidated Framework for Implementation Research constructs.
Note. Blue bars highlight constructs used to inform interview questions.
aSubconstructs created during the analysis process.
Data Collection
The semistructured interviews were conducted via Zoom (San Jose, California) by TWY, and participants were aware of the aims and rationale of the study. The interviews were conducted individually so that clinicians could speak unencumbered and to accommodate busy schedules. Semistructured interviews were conducted as they allowed for more in-depth discussion using open-ended questions and an interview guide, supplemented by follow-up prompts and comments.21,22 Informed consent for audio recording of interviews was obtained before the interviews. Interviews lasted between 30 and 45 minutes. Interviews were recorded, and the audio recordings were transcribed verbatim using a transcription service. Interviews were only conducted once, and transcriptions were not returned to the participants.
Data Analysis
Two members of the research team (TWY and DVO) separately conducted thematic analysis of all transcripts using both an inductive and deductive approach. 23 Data were coded into the relevant CFIR constructs, as well as generating new codes for any themes that did not fit with the existing CFIR constructs. TWY and DVO compared all data coding and reached a consensus to develop a reconciled single database of coded data and themes. Agreement was reached through iterative discussion, with referral to a third reviewer (RLM) for final decision if needed. For clarity in presenting the results, the CFIR domains were then interpreted in terms of the overarching theme and the level of consensus among participating clinicians. Previous research demonstrates data saturation (defined as how much new data repeated what was expressed in previous data) may occur between 12 and 17 interviews.24 -27 Data saturation was considered achieved after 21 interviews as no new data were identified in the final 2 interviews. The study team maintained an audit trail for all analytic procedures.28 -30 Transcripts were analyzed using NVivo software (QSR International, Burlington, Massachusetts).
This study was approved by the Research Ethics Board at the University of British Columbia (H21-00006).
Results
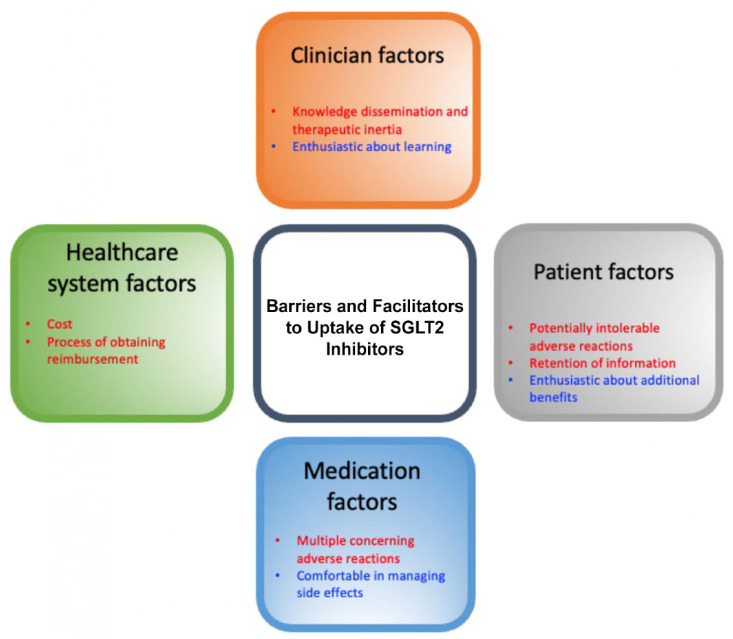
A total of 21 interviews were conducted with family physicians (n = 5), nephrologists (n = 5), endocrinologists (n = 5), and cardiologists (n = 6). Participants have been in practice as follows: 6 less than 5 years, 3 between 5 and 10 years, 3 between 10 and 15 years, 3 between 15 and 20 years, and 6 more than 20 years in clinical practice. The identified barriers were grouped into 5 encompassing themes: current perceptions and beliefs, clinician factors, patient factors, medication factors, and health care system factors. Subthemes were identified in each as described in Figure 2 and mapped to the CFIR Framework in Table 1.
Figure 2.
Most salient barriers and facilitators for the uptake of SGLT2 inhibitors in BC.
Note. Red highlights barriers and blue highlights facilitators. SGLT2 = sodium-glucose cotransporter-2; BC = British Columbia.
Table 1.
Summary of CFIR Constructs Identified in Each Theme.
| Themes identified | CFIR constructs |
|---|---|
| Current perceptions and beliefs regarding the use of SGLT2 inhibitors in BC, Canada. | |
| SGLT2 inhibitors are underutilized | Tension for change, cost, desired sources |
| SGLT2 inhibitors are efficacious | Evidence strength and quality, Knowledge and beliefs about the intervention |
| SGLT2 inhibitors have distinct advantages over other agents | Relative advantage |
| Clinician factors | |
| Clinicians identified knowledge translation is a key barrier | Peer pressure, access to knowledge and information, existing sources, desired sources, and knowledge translation |
| Clinicians were using SGLT2 inhibitors as part of their routine clinical practice | Individual stage of change |
| All clinicians need to play a role in prescribing SGLT2 inhibitors | Tension for change, patient needs and resources, knowledge translation, and individual identification with organization |
| Clinicians varied in their knowledge of and comfort prescribing SGLT2 inhibitors | Implementation climate, knowledge translation, and self-efficacy |
| Clinicians are influenced by their colleagues’ use of SGLT2 inhibitors | Peer pressure |
| Clinicians are interested and enthusiastic about learning | Implementation climate, learning climate |
| There is some caution with new therapies in general | Implementation climate |
| Therapeutic inertia plays a substantial role | Implementation climate, compatibility |
| Patient factors from a clinician’s perspective | |
| SGLT2 inhibitors are sometimes intolerable due to adverse events | Complexity |
| Pill burden is an important issue | Complexity, design quality and packaging |
| Patients were enthusiastic about potential benefits | Complexity, relative advantage |
| Individualized approach to patient selection and use of SGLT2 inhibitors is required | Complexity, external policy and incentives |
| Patient retention of information is an issue | Complexity |
| Some patients prefer not to take any medications | Patient preference |
| Medication factors | |
| Concerning adverse events | Complexity |
| Clinicians were comfortable managing adverse events | Complexity |
| Clinicians prioritized different potential adverse events when discussing them with their patients | Complexity |
| Health care system factors | |
| Cost and reimbursement administration is a barrier | Cost, external policies and incentives |
| Audit of evidence-based care delivery is not routinely performed | External policies and incentives, goals and feedback, organizational incentives and rewards |
Note. CFIR = Consolidated Framework for Implementation Research; SGLT2 = sodium-glucose cotransporter-2; BC = British Columbia.
Theme 1: Current Perceptions and Beliefs Regarding Use of SGLT2 Inhibitors
SGLT2 inhibitors are underutilized
Most interviewees agreed that SGLT2 inhibitors are underutilized in BC. As Endocrinologist 2 stated,
I think that there are probably many patients out there that could be on an SGLT2 inhibitor and that whether it’s just because they don’t have access to it or their care provider might not know as much about it, whether it’s their GP or in some other settings and so I do think that it is underutilized.
There were, however, exceptions in certain clinical settings, such as a multidisciplinary heart failure clinic where all patients were screened systematically for consideration of SGLT2 inhibitor therapy.
SGLT2 inhibitors are efficacious
Most clinicians emphasized that the evidence regarding the efficacy of SGLT2 inhibitors is strong, with very compelling evidence for benefits such as delaying kidney failure and reducing adverse heart failure and cardiac outcomes. No clinicians felt that the evidence for these benefits was inadequate. Cardiologist 1 stated “Absolutely, I think the evidence is very compelling.”
SGLT2 inhibitors have distinct advantages over other agents
In addition to the established cardiac and kidney benefits and glycemic control of SGLT2 inhibitors, clinicians identified further additional benefits to using SGLT2 inhibitors relative to other antihyperglycemic agents. These included lower risks for hypoglycemia, improved weight loss, another treatment option before insulin, and the avoidance of expected adverse events of insulin and an injection. Endocrinologist 5 mentioned that
People have often been quite accepting. I know it’s another oral agent with a bit of weight loss and so most people welcome. And given that often the alternative is an injection drug, because they’re already being on two oral agents, they’re actually pretty accepting on the third oral agent.
Theme 2: Clinician Factors
Clinicians identified knowledge translation is a key barrier
A third of clinicians indicated knowledge translation for the use of SGLT2 inhibitors was a key barrier. SGLT2 inhibitors were initially developed for glucose-lowering in the treatment of diabetes, and this appeared to have set the tone of being a “diabetes” drug rather than a cardiovascular or renal agent. Cardiologist 2 pointed out that “there’s an understanding that these are a good class of agents, I think people are confused because of the nuances and the differences in the clinical trials and there’s no clear guidance document.” Yet, several clinicians also identified that for those working in an academic center, new evidence is ingrained in rounds and reinforced through working with trainees and pharmacists, so that knowledge translation is less of an issue. All clinicians indicated several resources that they have found useful for learning about new evidence, such as clinical guidelines, conferences, rounds, journal club, reputable journals, webinars, talks provided by specialists/experts in the field, medical networks on social media, websites, and continuing education courses (Table 2).
Table 2.
Formats for Presenting Learning Materials Suggested by Clinicians.
| • Applications ○ Phone ○ Electronic medical record prompts ○ Self-practice audit ○ Alerts for new evidence/publications • Websites • Summaries ○ Short 1-page summaries ○ Prescription flowcharts ○ Sick day fact sheets ○ Safety advice ○ Efficacy ○ Benefits of SGLT2 inhibitors versus other medications ○ Action plan for mycotic infections or other common adverse events ○ Guidelines ○ Physiology and mechanism of action • Decision support tools ○ Selection of GLP-1 receptor agonists versus SGLT2 inhibitors • Journals • Talks and webinars from professional societies rather than drug companies • Multidisciplinary clinics • Social media • Video • Initiation protocols ○ Initiation of SGLT2 inhibitors ○ Titration ○ Follow-up blood work |
Note. SGLT2 = sodium-glucose cotransporter-2; GLP1 = glucagon-like peptide-1.
Clinicians were using SGLT2 inhibitors as part of their routine clinical practice
All clinicians reported prescribing SGLT2 inhibitors, although there were significant variations in the scale of this use. Endocrinologists described substantial higher use of SGLT2 inhibitors compared to nephrologists, cardiologists, and family physicians. Endocrinologist 1 mentioned that in their clinical practice,
everybody who is the below the age of 80 with established cardiovascular disease and diabetes I am trying to get them on an SGLT2 inhibitor regardless of glycemic control. I am not looking exclusively at glycemic control however I actually do think that the benefit is mostly with congestive heart failure.
All clinicians need to play a role in prescribing SGLT2 inhibitors
Most clinicians agreed that all prescribers who felt comfortable initiating SGLT2 inhibitors should take the opportunities to do so, while several clinicians deferred initiation to other physicians. Several clinicians emphasized the need for increasing collaboration with specialists to provide treatment early in the disease course so that individuals can derive the most benefit, for example, initiation of an SGLT2 inhibitor at a higher eGFR (estimated glomerular filtration rate) to maximize the prevention of progression of CKD. Some potential reasons as to why there may be hesitancy around prescription included perceived lack of association to nephrology and cardiology, as SGLT2 inhibitors were initially marketed as diabetes medications; and a lack of resources and time to organize frequent follow-ups for initiation and troubleshooting of these medications. Nephrologist 5 nicely summarized the issue
I think the big problem to crack is everybody pointing fingers at somebody else to say it’s their job to do it. And part of that’s going to have to be us as, that’s what I say in my mind it’s us as nephrologists at least taking responsibility for the ones that clearly fall under our, you know, envelope and everybody doing the same. I think that’s the biggest one to crack.
Clinicians varied in their knowledge of and comfort prescribing SGLT2 inhibitors
The level of familiarity and understanding of the evidence surrounding SGLT2 inhibitors ranged significantly, from being “probably not that familiar, like I know a little bit here and there, but probably not the most up to date” (Family Physician 3) and not as comfortable with the evidence base, to “I am completely confident and comfortable” (Endocrinologist 1). Increasing exposure and prescriptions appeared to help with confidence in using these medications, particularly in the context of other concurrent medications such as angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), and mineralocorticoid receptor antagonists (MRAs). Clinicians also identified some nuances in prescribing SGLT2 inhibitors, including the need to start these medications early in the disease course to gain maximal benefits rather than waiting until the kidney function is too low, the need to identify the appropriate patients to prescribe these medications, and the need for further evidence among people with type 1 diabetes or those who have received a kidney transplant.
Clinicians are influenced by their colleague’s use of SGLT2 inhibitors
Around two-thirds of clinicians indicated having a consensus among colleagues and peers encouraged a more supportive environment to prescribe newer medications such as SGLT2 inhibitors. As mentioned by Endocrinologist 2: “I think that kind of collegial discussion and that all of us are doing this together . . . makes it easier for individual practitioners to feel comfortable prescribing.” However, a few physicians indicated that they were comfortable prescribing SGLT2 inhibitors already and that it did not matter what colleagues were doing. Some physicians indicated that due to the practice environment like individual private offices, there may be limited interactions with peers and colleagues. Most of the clinicians identified that expert opinion leaders, influential peers, and endorsement by national guidelines play an important role in changing clinical practice and increasing the uptake of SGLT2 inhibitors.
Clinicians are interested and enthusiastic about learning
Most of the clinicians were interested in engaging with new evidence and updates. As Endocrinologist 4 mentioned
I could say, as a group of endocrinologists we’re really familiar with the evidence, we keep up to date, like you know [SGLT2 inhibitor clinical trials] DAPA-CKD, CREDENCE, like we’re always on top of this, but I don’t know how up to date other specialists are. Kind of like, you know, it’s in our area of expertise so like we are sort of obligated.
Some stated that another reason they are obligated to keep up with new emerging evidence is due to working with trainees. However, several physicians indicated that despite best efforts, it remains a challenge to always stay on top of emerging evidence while maintaining a full clinical practice.
There is some caution with new therapies in general
Around half of clinicians did not express any hesitation in incorporating relatively new therapies into their clinical practice, while others indicated they were not necessarily “early adopters” but liked to wait for an intervention to be well established in clinical care. A clinician observed that their colleagues might recommend to another physician to initiate SGLT2 inhibitors but may not necessarily start it themselves. Family Physician 1, with over 25 years of clinical experience, mentioned clinicians with even more seniority may also be “more cautious about jumping right into things” due to negative experiences with medications that have had unexpected postmarketing complications.
Therapeutic inertia plays a substantial role
The concept of therapeutic inertia was identified by around a third of the clinicians as one of the reasons for the perceived lack of uptake of SGLT2 inhibitors. As discussed by Cardiologist 1:
With any new medication there is always going to be a time period where people need to be coaxed into using guideline-based therapy. That’s just human nature. You’re more likely to use med[ication]s freely if you already know about them as a practitioner.
Physicians indicated that disrupting previously established prescription habits for older medications before SGLT2 inhibitors was very difficult due to concern about causing new issues—“I guess we often have a habit, like if they’re already on their medications, everything’s status quo, why would I switch them to a new medication?” (Family Physician 2).
Theme 3: Patient Factors From a Clinician’s Perspective
SGLT2 inhibitors are sometimes intolerable due to adverse events
Several clinicians indicated that some of their patients were unable to tolerate or even initiate SGLT2 inhibitors due to potential adverse events, particularly genital mycotic infections and urinary tract infections. As described by Endocrinologist 3, patients’ previous experiences limit the use of SGLT2 inhibitors due to particularly
Yeast infections for women. I’ve definitely had women that say ‘I’ve had yeast infections, you know, in the past, I just don’t want to go there’. So they don’t even start.
However, clinicians expressed that many of these adverse events are manageable, and with careful monitoring, many patients can continue to take these medications.
Pill burden is an important issue
Around a third of the clinicians identified they had encountered some resistance from their patients due to the idea of adding another pill to their medication regimen—“I do have some patients who say, “Oh I’m already on so many different medications do I need to be on another one?” (Endocrinologist 2). Although pill burden was a barrier for some, many patients preferred commencing another pill if that meant an injection was potentially avoidable.
Patients were enthusiastic about potential benefits
Several clinicians discussed that explaining the potential benefits of SGLT2 inhibitors could improve patient enthusiasm about their use. Cardiologist 1 explained “Generally it’s a very easy sell to patients . . . it’s one of the few drugs where like they’re like excited to you know, I might lose weight on it, my blood pressure will come down like there’s all these bonus effects” while Nephrologist 4 emphasized that patients “need to be clear what the indication of the medication is.” The potential benefit of weight loss was particularly appealing to patients—“People have often been quite accepting. I know it’s another oral agent with a bit of weight loss and so most people welcome” (Endocrinologist 5).
Individualized approach to patient selection and use of SGLT2 inhibitors is required
More than half of the clinicians indicated that precise patient selection is required for the use of SGLT2 inhibitors due to concerns about their use in patients with borderline T2DM or T1DM, borderline eGFR, and other clinical factors such as advanced age, frailty, or marginalization. Concern was raised about people at higher risk of volume depletion, such as those with an ileostomy and cognitive impairment. Careful patient selection is important as Nephrologist 3 stated “I don’t know, it’s just not everybody is necessarily gonna get the benefit that you want so I think we have to think about what we’re actually trying to achieve.”
Patient retention of information is an issue
Several clinicians indicated that patients often forgot specific advice like medication management during periods of illness. A few strategies included highlighting the most common potential adverse reactions, reinforcing information at subsequent clinics, and providing information in a piecemeal fashion that is easier to absorb, to avoid what Nephrologist 1 said
What would I call it the phenomenon of oversaturation right? You’ve already just loaded a pile of information.
Some patients prefer not to take any medications
Two clinicians indicated that some patients strongly prefer not to take any medications and would prefer adjusting lifestyle measures. From Cardiologist 5’s experience, “You know, there are some folks who don’t want any medical therapy, and that would be more of their personal patient preference. But for the ones that are motivated to take advice given, there is no pushback.”
Theme 4: Medication Factors
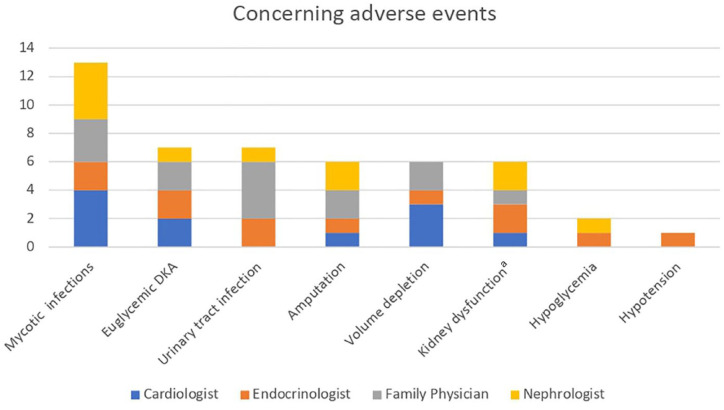
Concerning adverse events
Figure 3 describes the adverse events clinicians identified as being the most concerning.
Figure 3.
Potentially concerning adverse events with using SGLT2 inhibitors identified by clinicians.
Note. SGLT2 = sodium-glucose cotransporter-2; DKA = diabetic ketoacidosis; eGFR = estimated glomerular filtration rate.
aAcute kidney injury or eGFR drop with initiation.
Clinicians were comfortable managing adverse events
Only a few clinicians expressed uncertainty in the management of SGLT2 inhibitors peri-operatively or the treatment of mycotic genital infections. However, most of them were familiar dealing with the various adverse reactions, as Endocrinologist 1 stated
I am completely confident and comfortable with providing that. It’s one of the things about SGLT2’s that is so easy to use you can basically hand them to your patients and most of the things that happen to them it’s pretty easy to figure out whether it’s urinary tract infection or whatever so myself I’m pretty confident.
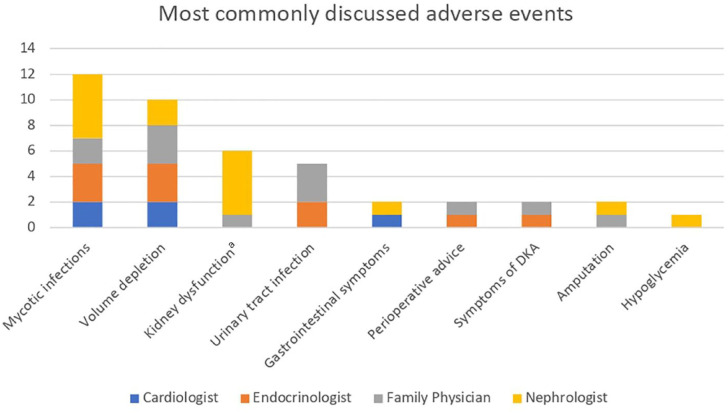
Clinicians prioritized different potential adverse events when discussing them with their patients
Figure 4 describes the adverse events physicians most commonly discuss with their patients when prescribing an SGLT2 inhibitor. Some physicians also mentioned that they do not discuss diabetic ketoacidosis as it is conceptually difficult to explain, as Nephrologist 3 says “For me I feel like it’s hard for me to talk about euglycemic DKA, it’s like something that’s complicated but we usually try to spin it in like the sick day advice like kind of lens” and elect to instead advise the patient to contact their office when feeling ill, for blood work. They also opted to reinforce the importance of sick day medication management.
Figure 4.
Potential adverse events clinicians were most likely to discuss with patients.
Note. DKA = diabetic ketoacidosis.
aAcute kidney injury or eGFR drop with initiation.
Theme 5: Health Care System Factors
Cost and reimbursement administration is a barrier
All clinicians agreed that the out-of-pocket costs associated with the lack of coverage for SGLT2 inhibitors for specific usage (i.e. kidney indications) was a barrier in their use. All clinicians indicated the actual processing of forms and documents to acquire coverage was a huge barrier as it is very disruptive to the flow of clinical practice. As Nephrologist 1 stated, “Well I think if you could remove the need for a special authority form to be filled out for nephrologists, endocrinologists, and cardiologists that would be great and that would be the biggest carrot anyone could offer me.” In Endocrinologist 4’s experience, “I have seen sort of situations where it’s just been started but no special authority form has been filled, so then patients don’t end up filling the medication because of the cost.” Also, the current coverage in BC for SGLT2 inhibitor for diabetes management requires treatment failure on metformin and sulfonylurea, which was identified as a significant barrier and source of delay in initiating optimal treatment. Clinicians also identified that prescribing an SGLT2 inhibitor when a patient is already on a glucagon-like peptide 1 (GLP-1) receptor agonist was an issue as only one of the medications can be covered at a time for an individual patient. Specifically, the delay in the expansion in coverage for renal indications, despite CREDENCE and DAPA-CKD demonstrating significant benefits in 2019 and 2020, is a barrier to the use of SGLT2 inhibitors. A few clinicians indicated that incorporation of the required documents into their electronic medical records, or assistance from a pharmacist, greatly reduced the obstacle of filling out the required forms in their clinics.
Audit of evidence-based care delivery is not routinely performed
Most clinicians expressed that they did not have routine self-review or audit of practice. There was some prompting of these practices from Section 3 of the Royal College of Physicians and Surgeons of Canada Maintenance of Certification Program, as well as through measurement of quality improvement metrics in some local hospitals at a clinic level, as Cardiologist 2 said
We do quality assurance with heart failure, and we have reporting of our outcomes in compliance with guideline medical care. So there’s that but that’s done in a, you know, clinic level rather than a per patient basis.
Most feedback processes required a clinician to initiate their own self-practice audits. The few who had experience with practice audit were in the context of their training program as a resident.
Discussion
This is the first study that assessed the barriers and facilitators to using SGLT2 inhibitors perceived by various clinicians in British Columbia, Canada, using a framework such as the CFIR. We have identified 5 themes that overarch the barriers in uptake of SGLT2 inhibitors in BC: current perceptions and beliefs, clinician factors, patient factors, health care system factors, and medication factors. Figure 2 highlights the most salient facilitators and barriers identified in the interviews.
There is usually a delay between evidence generation and its application into clinical practice, due to the well-established barriers of “therapeutic inertia” and knowledge translation, which were also identified as key barriers by clinicians in this study.19,31 Therapeutic inertia describes the failure to escalate or deescalate treatment, broadly contributed by provider, patient, and health-system-related factors. 32 It is pervasive, as a study demonstrated around 82 000 patients with T2DM taking more than 3 years to escalate from one antihyperglycemic agent to 2 agents despite having an HbA1c > 7%. 32 The impact is significant, as it is estimated that therapeutic inertia may contribute up to 80% of heart attacks and strokes due to poor management of T2DM, hypertension, and dyslipidemia with evidence-based care. 33 Knowledge translation is a broader concept encompassing the process of moving what is learned through research, to its application into the clinical setting, and is composed of 4 elements: synthesis, dissemination, exchange, and ethically sound application of the knowledge.34,35 In this study, clinicians primarily considered knowledge dissemination when discussing knowledge translation, as this theme highlighted that clinicians may not be aware of and exposed to new evidence. Other CFIR constructs revealed further barriers to true knowledge translation into routine care, including lack of time and resources, differences in opinion about who should prescribe, and varying levels of comfort. Each contributing factor may require its own tailored approach. Multifaceted approaches are likely necessary, including engaging key opinion leaders and stakeholders, launching awareness and education campaigns, revising health care policies, and potentially testing new interventions to improve clinician knowledge and confidence.36,37
An advantage of using the CFIR is that this knowledge can be used to guide the choice of implementation strategies to address identified barriers, which can be enhanced by pairing with a structured intervention development framework such as the Expert Recommendations for Implementing Change (ERIC). 38 The CFIR-ERIC Implementation Strategy Match Tool provides implementation suggestions, such as addressing an identified lack of knowledge dissemination with educational meetings, development, and distribution of educational materials, ongoing training, and capturing and sharing local knowledge. 39 Adapting these strategies to the local setting, available resources, and infrastructure, may offer an improved likelihood to resolve these barriers. This can undergo an iterative process to navigate each identified CFIR construct to develop effective implementation strategies. Our research group is conducting ongoing work with BC Renal, a provincial organization responsible for funding, care delivery, and health outcome to help address these gaps.
In this study, clinicians identified that a major barrier in prescribing SGLT2 inhibitors was the potential for adverse reactions patients may experience. Mycotic genital infection was the most common adverse event of concern to clinicians. Although this is consistent with the higher relative risk of 3.57 (95% confidence interval CI: [3.14, 4.06]), the absolute risk increase remains low at around 2.8%. 8 Clinicians also identified euglycemic diabetic ketoacidosis (eDKA) and urinary tract infections (UTIs) as significant concerns which prevented them prescribing SGLT2 inhibitors to their patients. Interestingly, clinicians did not necessarily discuss eDKA with patients. Clinicians explained that this complication is uncommon and is difficult to explain, including nonspecific symptomatology which could cause patient alarm. Literature on the topic suggests advising patients to withhold their SGLT2 inhibitors and seek prompt medical attention if they are not feeling well, which clinicians in this study reported doing. 40 Although those who are on SGLT2 inhibitors are at an increased risk for eDKA relative to those who are not, the absolute rate of incidence is low, at around 0.6 to 2.2 events per 1000 patient-years. 41 With regards to the potential increased risk of UTIs, clinical trials and real-world cohort studies suggests that the risk of UTIs is not increased in those on SGLT2 inhibitors.4,6,42,43 In addition, amputation risk was raised as a concern based upon the results of one study, the CANVAS program, but as further studies have been reassuring regarding this risk, it was often not discussed with patients. 40 Although the clinician concerns regarding overall medication adverse events should not be minimized, the potential adverse events are actually uncommon and treatable, but cited frequently by interviewees. As the effectiveness of SGLT2 inhibitors is relatively well established and understood, perhaps focus for further education should be on addressing these potential adverse reactions because the absolute risks are low. Given the low absolute risks, clinicians should reconsider initiation of SGLT2 inhibitors in the appropriate patient as the benefits of these medications far outweigh the potential harm.
The history of SGLT2 inhibitors as a class of medication initially designed to treat hyperglycemia in T2DM, expanded to cardiovascular and kidney-related indications since 2019, may be a reason why some cardiologists and nephrologists choose to defer the initiation of these medication as it may appear more appropriate for family physicians or endocrinologists to initiate them.4 -7,44,45 Similarly, interviews of general practitioners and endocrinologists in Australia demonstrated under-appreciation of the cardio-renal benefits of SGLT2 inhibitors, and preference for an endocrinologist to initiate therapy by general practitioners. 46 Fortunately, the general consensus from the interviewed clinicians indicate that all, family physicians, nephrologists, endocrinologists, and cardiologists, should take the responsibility in initiating SGLT2 inhibitors for patients.
The facilitators of SGLT2 inhibitor implementation identified in this study provide opportunities to further improve practice change and adoption. Clinicians indicated consensus among colleagues and the endorsement from expert opinion leaders and influential peers were facilitators for the use of SGLT2 inhibitors. Expert opinion leaders and peers can change group practice patterns as they may play an important role to address the practice gap by supporting early adoption of new evidence.47,48 Facilitating local case discussions, with input from expert opinion leaders, may help leverage these facilitators. The clinicians were also keen to continue to engage with the evolving evidence base. Continuing Medical Education (CME) is an important component in medicine that aims to improve not only physician performance but also patient health outcomes. 49 The clinicians generated a comprehensive list of preferred methods of delivery in regards to educational resources, outlined in Table 2. This table highlights previous findings that physicians find CME activities that are more interactive, using various methods, and involving multiple exposures to be more effective and to lead to more positive outcomes. 50 The best method to engage and promote knowledge dissemination, as identified by the clinicians, may be multifactorial in nature, using various methods like guidelines, pocket guides, on-demand webinars, educational slide decks, and online calculators, as demonstrated by the Canadian Cardiology Society. 51
The strength of this study is that it used an established framework to determine the barriers and facilitators to using SGLT2 inhibitors in clinical practice and involved a sufficient number of interviewees to achieve data saturation. 24 This study also included clinicians from 4 key specialties. The clinical encounters regarding adverse events and patient experiences may be generalizable to other clinicians. Limitations of this study may include the specific context of BC, one province in Canada, which impacts issues related to the process of government approval, reimbursement, and costs of SGLT2 inhibitors experienced by clinicians. There may be responder bias due to the sampling technique. We believe further research is required to explore the impact of potential strategies to increase the use of SGLT2 inhibitors in BC. The development of multipronged implementation strategies to address these identified barriers and the subsequent formalized evaluation plan to determine the effectiveness of this intervention is required.
In conclusion, this study highlights different themes to the barriers and facilitators of using SGLT2 inhibitors in BC. The identification of these barriers provides specific targets for improvement, and the facilitators can be leveraged for the increased use of SGLT2 inhibitors. Efforts to address and optimize these barriers and facilitators in a systematic approach may lead to increased usage of these efficacious medications.
Supplemental Material
Supplemental material, sj-docx-1-cjk-10.1177_20543581231217857 for Identifying Barriers and Facilitators for Increasing Uptake of Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors in British Columbia, Canada, using the Consolidated Framework for Implementation Research by Tae Won Yi, Daniel V. O’Hara, Brendan Smyth, Meg J. Jardine, Adeera Levin and Rachael L. Morton in Canadian Journal of Kidney Health and Disease
Footnotes
Ethics Approval and Consent to Participate: This study was approved by the Research Ethics Board at the University of British Columbia (H21-00006).
Consent for Publication: All authors provided their consent for publication.
Availability of Data and Materials: The data underlying this article will be shared on reasonable request to the corresponding author and subject to approval from the Research Ethics Board at the University of British Columbia.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: TWY is supported by Research Scholar Award from BC Renal. BS is supported by a Research Establishment Fellowship from the Royal Australasian College of Physicians. MJJ is supported by an NHMRC Investigator Fellowship. RLM is supported by an NHMRC Investigator Fellowship #1194703. DVO received support through the NHMRC Clinical Trials Centre Postgraduate Research Scholarship and subsequently the Royal Australasian College of Physicians Jacquot Research Entry Scholarship, as well as the Australian Government Research Training Program Scholarship
ORCID iDs: Tae Won Yi  https://orcid.org/0000-0003-3350-1170
https://orcid.org/0000-0003-3350-1170
Brendan Smyth  https://orcid.org/0000-0003-1838-3348
https://orcid.org/0000-0003-1838-3348
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Nelson AJ, Ardissino M, Haynes K, et al. Gaps in Evidence-based therapy use in insured patients in the United States with type 2 diabetes mellitus and atherosclerotic cardiovascular disease. J Am Heart Assoc. 2021;10(2):e016835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chin KL, Skiba M, Tonkin A, et al. The treatment gap in patients with chronic systolic heart failure: a systematic review of evidence-based prescribing in practice. Heart Fail Rev. 2016;21(6):675-697. [DOI] [PubMed] [Google Scholar]
- 3. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436-1446. [DOI] [PubMed] [Google Scholar]
- 4. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306. [DOI] [PubMed] [Google Scholar]
- 5. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. [DOI] [PubMed] [Google Scholar]
- 6. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644-657. [DOI] [PubMed] [Google Scholar]
- 7. The EMPA-KIDNEY Collaborative Group; Herrington WG, Staplin N, Wanner C, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baigent C, Emberson J, Haynes R, et al. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400(10365):1788-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herrington WG, Savarese G, Haynes R, et al. Cardiac, renal, and metabolic effects of sodium-glucose co-transporter 2 inhibitors: a position paper from the European Society of Cardiology ad-hoc task force on sodium-glucose co-transporter 2 inhibitors. Eur J Heart Fail. 2021;23(8):1260-1275. [DOI] [PubMed] [Google Scholar]
- 10. Quinn A, Campbell D, Au F, et al. Describing the uptake and patterns of SGLT2 inhibitor use among adults with type 2 diabetes in Alberta, Canada. Can J Diabetes. 2021;45(suppl 7):S15. [Google Scholar]
- 11. Lau D, Pannu N, Yeung RO, Scott-Douglas N, Klarenbach S. Use of sodium–glucose cotransporter 2 inhibitors in Alberta adults with chronic kidney disease: a cross-sectional study identifying care gaps to inform knowledge translation. CMAJ Open. 2023;11(1):E101-E109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ng NM, Ng YS, Chu TK, Lau P. Factors affecting prescription of sodium-glucose co-transporter 2 inhibitors in patients with type 2 diabetes mellitus with established cardiovascular disease/ chronic kidney disease in Hong Kong: a qualitative study. BMC Primary Care. 2022;23(1):317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jeong SJ, Lee SE, Shin DH, Park IB, Lee HS, Kim K-A. Barriers to initiating SGLT2 inhibitors in diabetic kidney disease: a real-world study. BMC Nephrol. 2021;22(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dhaliwal A, Toma M, Kochan A, Didi A. Identifying barriers to SGLT2 inhibitor use in eligible patients with heart failure: a real-world experience from a single centre. J Card Fail. 2022;28(5):S48-S49. [Google Scholar]
- 15. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kirk MA, Kelley C, Yankey N, Birken SA, Abadie B, Damschroder L. A systematic review of the use of the Consolidated Framework for Implementation Research. Implement Sci. 2016;11:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Birken SA, Powell BJ, Presseau J, et al. Combined use of the Consolidated Framework for Implementation Research (CFIR) and the Theoretical Domains Framework (TDF): a systematic review. Implement Sci. 2017;12(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nilsen P, Birken SA. Handbook on Implementation Science. Cheltenham, England: Edward Elgar Publishing Limited; 2020. [Google Scholar]
- 19. Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104(12):510-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349-357. [DOI] [PubMed] [Google Scholar]
- 21. Jamshed S. Qualitative research method-interviewing and observation. J Basic Clin Pharm. 2014;5(4):87-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DeJonckheere M, Vaughn LM. Semistructured interviewing in primary care research: a balance of relationship and rigour. Fam Med Community Health. 2019;7(2):e000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77-101. [Google Scholar]
- 24. Saunders B, Sim J, Kingstone T, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant. 2018;52(4):1893-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guest G, Bunce A, Johnson L. How many interviews are enough? an experiment with data saturation and variability. Field Methods. 2006;18(1):59-82. [Google Scholar]
- 26. Vasileiou K, Barnett J, Thorpe S, Young T. Characterising and justifying sample size sufficiency in interview-based studies: systematic analysis of qualitative health research over a 15-year period. BMC Med Res Methodol. 2018;18(1):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Francis JJ, Johnston M, Robertson C, et al. What is an adequate sample size? operationalising data saturation for theory-based interview studies. Psychol Health. 2010;25(10):1229-1245. [DOI] [PubMed] [Google Scholar]
- 28. Korstjens I, Moser A. Series: practical guidance to qualitative research. Part 4: trustworthiness and publishing. Eur J Gen Pract. 2018;24(1):120-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Forero R, Nahidi S, De Costa J, et al. Application of four-dimension criteria to assess rigour of qualitative research in emergency medicine. BMC Health Serv Res. 2018;18:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shenton AK. Strategies for ensuring trustworthiness in qualitative research projects. Educ Inf. 2004;22(2):63-75. [Google Scholar]
- 31. Grimshaw JM, Eccles MP, Lavis JN, Hill SJ, Squires JE. Knowledge translation of research findings. Implement Sci. 2012;7(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karam SL, Dendy J, Polu S, Blonde L. Overview of therapeutic inertia in diabetes: prevalence, causes, and consequences. Diabetes Spectr. 2020;33(1):8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mata-Cases M, Franch-Nadal J, Gratacòs M, Mauricio D. Therapeutic inertia: still a long way to go that cannot be postponed. Diabetes Spectr. 2020;33(1):50-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Government of Canada, Canadian Institutes of Health Research. More about knowledge translation at CIHR—long descriptions. https://cihr-irsc.gc.ca/e/46642.html. Published 2013. Accessed January 1, 2020.
- 35. Government of Canada, Canadian Institutes of Health Research. Knowledge translation. https://cihr-irsc.gc.ca/e/29418.html#4.2. Published 2005. Accessed October 9, 2023.
- 36. Gabbay RA, Kendall D, Beebe C, et al. Addressing therapeutic inertia in 2020 and beyond: a 3-year initiative of the American Diabetes Association. Clin Diabetes. 2020;38(4):371-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pimjai Sudsawad S. Center on Knowledge Translation for Disability and Rehabilitation Research (KTDRR) KT library—research quality. https://ktdrr.org/ktlibrary/articles_pubs/ktmodels/. Published 2007. Accessed June 2, 2023.
- 38. Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Waltz TJ, Powell BJ, Fernández ME, Abadie B, Damschroder LJ. Choosing implementation strategies to address contextual barriers: diversity in recommendations and future directions. Implement Sci. 2019;14(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Milder TY, Stocker SL, Day RO, Greenfield JR. Potential safety issues with use of sodium-glucose cotransporter 2 inhibitors, particularly in people with type 2 diabetes and chronic kidney disease. Drug Saf. 2020;43(12):1211-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Colacci M, Fralick J, Odutayo A, Fralick M. Sodium-glucose cotransporter-2 inhibitors and risk of diabetic ketoacidosis among adults with type 2 diabetes: a systematic review and meta-analysis. Can J Diabetes. 2022;46(1):10-15.e2. [DOI] [PubMed] [Google Scholar]
- 42. Lega IC, Bronskill SE, Campitelli MA, et al. Sodium glucose cotransporter 2 inhibitors and risk of genital mycotic and urinary tract infection: a population-based study of older women and men with diabetes. Diabetes Obes Metab. 2019;21(11):2394-2404. [DOI] [PubMed] [Google Scholar]
- 43. Liu J, Li L, Li S, et al. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: a systematic review and meta-analysis. Sci Rep. 2017;7:2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diab Vasc Dis Res. 2015;12(2):78-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347-357. [DOI] [PubMed] [Google Scholar]
- 46. Milder TY, Stocker SL, Baysari M, Day RO, Greenfield JR. Prescribing of SGLT2 inhibitors in primary care: a qualitative study of general practitioners and endocrinologists. Diabetes Res Clin Pract. 2021;180:109036. [DOI] [PubMed] [Google Scholar]
- 47. Carpenter CR, Sherbino J. How does an “opinion leader” influence my practice? CJEM. 2010;12(5):431-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Flodgren G, Parmelli E, Doumit G, et al. Local opinion leaders: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2011;(8):CD000125. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD000125.pub4/abstract. Accessed October 2, 2023. [DOI] [PMC free article] [PubMed]
- 49. Cervero RM. Changing continuing medical education. JAMA. 2015;314(10):1072. [DOI] [PubMed] [Google Scholar]
- 50. Cervero RM, Gaines JK. The impact of CME on physician performance and patient health outcomes: an updated synthesis of systematic reviews. J Contin Educ Health Prof. 2015;35(2):131-138. [DOI] [PubMed] [Google Scholar]
- 51. Canadian Cardiovascular Society. Guideline resources. Date unknown. https://ccs.ca/guideline-resources/. Accessed May 20, 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cjk-10.1177_20543581231217857 for Identifying Barriers and Facilitators for Increasing Uptake of Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors in British Columbia, Canada, using the Consolidated Framework for Implementation Research by Tae Won Yi, Daniel V. O’Hara, Brendan Smyth, Meg J. Jardine, Adeera Levin and Rachael L. Morton in Canadian Journal of Kidney Health and Disease