Abstract
Background:
The relationship between post-operative urine output (UO) following kidney transplantation and long-term graft function has not been well described.
Objective:
In this study, we examined the association between decreased UO on post-operative day 1 (POD1) and post-transplant outcomes.
Design:
This is a retrospective cohort study.
Setting:
Atlantic Canada.
Patients:
Patients from the 4 Atlantic Canadian provinces (Nova Scotia, New Brunswick, Newfoundland, and Prince Edward Island) who received a live or deceased donor kidney transplant from 2006 through 2019 through the multiorgan transplant program at the Queen Elizabeth II Health Sciences Centre (QEII) hospital in Halifax, Nova Scotia.
Measurements:
Using multivariable Cox proportional hazards models, we assessed the association of low POD1 UO (defined as ≤1000 mL) with death-censored graft loss (DCGL). In secondary analyses, we used adjusted logistic regression or Cox models as appropriate to assess the impact of UO on delayed graft function (DGF), prolonged length of stay (greater than the median for the entire cohort), and death.
Results:
Of the 991 patients included, 151 (15.2%) had a UO ≤1000 mL on POD1. Low UO was independently associated with DCGL (hazard ratio [HR] = 4.00, 95% confidence interval [CI] = 95% CI = 1.55-10.32), DGF (odds ratio [OR] = 45.25, 95% CI = 23.00-89.02), and prolonged length of stay (OR = 5.06, 95% CI = 2.95-8.69), but not death (HR = 0.81, 95% CI = 0.31-2.09).
Limitations:
This was a single-center, retrospective, observational study and therefore has inherent limitations of generalizability, data collection, and residual confounding.
Conclusions:
Overall, reduced post-operative UO following kidney transplantation is associated with an increased risk of DCGL, DGF, and prolonged hospital length of stay.
Keywords: kidney transplant, urine output, graft failure, delayed graft function, perioperative
Abrégé
Contexte:
Le lien entre la diurèse postopératoire après une transplantation rénale et la fonction du greffon à long terme n’a pas été bien décrit.
Objectif:
Dans cette étude, nous avons examiné l’association entre la diminution de la diurèse au jour 1 postopératoire et les résultats après la transplantation.
Conception:
Étude de cohorte rétrospective,
Cadre:
Canada atlantique
Patients:
Des patients des quatre provinces du Canada atlantique (Nouvelle-Écosse, Nouveau-Brunswick, Terre-Neuve et Île-du-Prince-Édouard) ayant reçu une greffe de rein provenant d’un donneur vivant ou décédé entre 2006 et 2019 dans le cadre du programme de transplantation multiorganes de l’hôpital QEII d’Halifax (Nouvelle-Écosse).
Mesures:
À l’aide de modèles à risques proportionnels de Cox multivariés, nous avons évalué l’association entre une faible diurèse (définie comme ≤ 1 000 ml) et la perte du greffon censurée par le décès (PGCD). Dans les analyses secondaires, nous avons utilisé des modèles de Cox ou des modèles de régression logistique ajustés, selon le cas, pour évaluer l’effet de la diurèse sur la fonction retardée du greffon, la durée prolongée du séjour (supérieure à la médiane pour l’ensemble de la cohorte) et le décès.
Résultats:
Des 991 patients inclus, 151 (15,2%) présentaient une diurèse inférieure à 1 000 ml au jour 1 postopératoire. Une faible diurèse a été indépendamment associée à la PGCD (rapport de risque [RR]: 4,00; IC 95 %: 1,55-10,32), à une fonction retardée du greffon (rapport de cotes [RC]: 45,25; IC 95 %: 23,00-89,02) et à un séjour prolongé à l’hôpital (RC: 5,06; IC 95 %: 2,95-8,69), mais pas au décès (RR: 0,81; IC 95 %: 0,31-2,09).
Limites:
Il s’agissait d’une étude observationnelle rétrospective monocentrique. L’étude présente ainsi des limites inhérentes à la généralisabilité, à la collecte des données et aux facteurs confondants résiduels.
Conclusion:
Dans l’ensemble, une diminution de la diurèse postopératoire après une transplantation rénale est associée à un risque accru de PGCD et de fonction retardée du greffon, ainsi qu’à un séjour prolongé à l’hôpital.
Introduction
Kidney transplantation is the gold standard for kidney replacement therapy in eligible patients with end-stage kidney disease, with well-established improvements in quality of life and survival.1 -3 Long-term graft survival rates have improved steadily over time in Canada, with 1-year graft survival rates for kidney transplants from deceased and living donors of 95% and 98%, respectively, and 5-year graft survival rates of 81% and 91%. 4 What predicts long-term graft survival is multifactorial and incompletely understood, but includes perioperative factors such as organ quality, cold and warm ischemia time (CIT and WIT),5,6 as well as post-transplant events, such as delayed graft function (DGF), 5 acute rejection7,8 and viral infections.9 -11
Early post-operative urine output (UO) is a marker of graft function and intuitively, decreased post-operative UO has been associated with an increased need for dialysis in the perioperative period. For example, Maier et al 12 showed that, in a cohort of 170 patients, a relatively higher UO on post-operative day 1 (POD1) was associated with lower risk of DGF in univariable analysis (odds ratio [OR] = 0.87; 95% confidence interval [CI] = 0.8-0.9 per 100 mL). Furthermore, there have been reports describing an association between UO on the first POD following kidney transplantation and graft function at 1 year and 5 years.13 -15 For example, Schnuelle et al 14 showed that, in a cohort of 300 patients, a UO >630 mL on POD1 was associated with a reduced risk of death-censored graft loss (DCGL) at 5 years (adjusted hazard ratio [HR] = 0.43; 95% CI = 0.26-0.72). The conclusions of these studies were limited by small sample sizes and were conducted in patient populations outside of North America. Whether low UO early post-transplant is a manifestation of poor post-operative graft function in a patient destined to experience DGF and/or graft failure, or represents a modifiable risk factor to be intervened on is unknown.
Ultimately, the associations between early post-operative UO and long-term outcomes including graft failure and death have not been fully explored. Therefore, the purpose of this study was to determine whether early post-operative UO following kidney transplantation is associated with subsequent graft failure. Secondary outcomes included the association of post-operative UO with DGF, hospital length of stay (LOS), and death.
Methods
Setting and Population
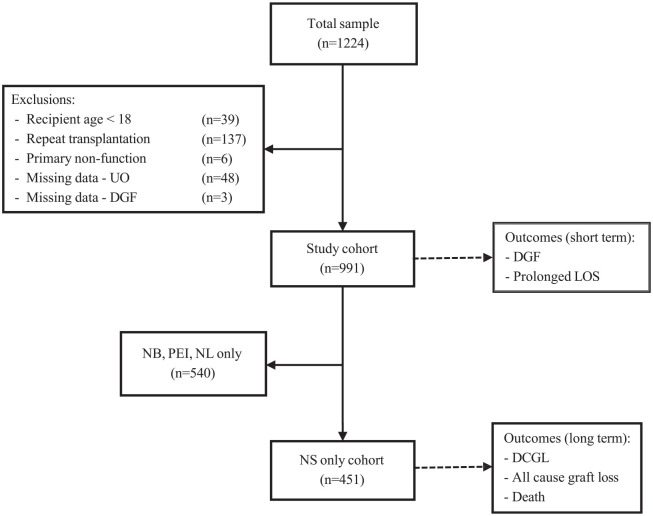
We conducted a retrospective cohort study at the Queen Elizabeth II Health Sciences Centre (QEII) in Halifax, Nova Scotia of patients who received a live or deceased donor kidney transplant from 2006 through 2019. The multiorgan transplant program at the QEII hospital performs kidney transplants for patients from 4 Atlantic Canadian provinces (Nova Scotia, New Brunswick, Newfoundland, and Prince Edward Island); however, follow-up data beyond the index hospitalization is only available for the cohort of patients from Nova Scotia. Patients were excluded from the study if they were <18 years of age, undergoing repeat kidney transplantation, or if they experienced primary non-function (PNF; defined as undergoing graft nephrectomy within 1 week of transplant; Figure 1). Importantly, those with follow-up outside of Nova Scotia were excluded in our analyses that examined long-term outcomes (graft loss and death), given that long-term outcomes would not be captured in Nova Scotia provincial records for these patients. Patients were identified using a pre-existing multiorgan transplant database at the QEII used for quality care in Atlantic Canada, which contains information on patient demographics, date of transplantation, and transplant outcomes such as death and graft failure. 16 Electronic health records were used to collect additional donor and recipient data for variables that were not included in the pre-existing database.
Figure 1.
Consort diagram of patients undergoing kidney transplantation in Atlantic Canada between 2006 and 2019.
UO = urine output; DGF = delayed graft function; LOS = length of stay; DCGL = death-censored graft loss; NB = New Brunswick; PEI = Prince Edward Island; NL = Newfoundland; NS = Nova Scotia.
Exposure
The exposure of interest was UO in the first day post-kidney transplantation defined as low (POD1 UO ≤1000 mL/day) or high (POD1 UO >1000 mL/day). Urine output data were recorded by nursing staff in the post-anesthetic care unit and on the ward on an hourly basis for the first 12 hours post-operatively, then on a 4-hourly basis. This was then documented as 12-hour intervals of UO on standardized fluid balance reporting sheets, which were used for data collection in our study. Post-operative day 1 refers to the day after surgery has taken place, and POD1 UO refers to UO from 07:00 hours POD1 until 07:00 hours the next day.
Outcomes
The primary outcome was DCGL (defined as either requiring kidney replacement therapy or pre-emptive re-transplant) in patients with follow-up data from Nova Scotia (“NS only cohort”; Figure 1). We also examined time to death and all-cause graft loss (ACGL) (the composite of death or graft loss) at 6 months, 1 year, and 5 years post-transplant in patients with follow-up in Nova Scotia (“NS only cohort”; Figure 1). Other secondary outcomes included DGF (defined as the need for dialysis within 7 days following transplantation) and prolonged LOS (defined as an index hospitalization at time of transplant longer than the median) 16 in all patients undergoing kidney transplant in Atlantic Canada (“Study cohort”; Figure 1).
Covariates
Data were collected for additional variables with known associations with the outcomes of interest, including recipient age, sex, weight, comorbidities (diabetes, hypertension, coronary artery disease, cerebrovascular disease, congestive heart failure), cause of end-stage kidney disease, residual patient-reported pre-transplant UO, pre-emptive status, dialysis modality (peritoneal or hemodialysis [HD]), human leukocyte antigen (HLA) mismatch with donor, WIT, CIT, and donor status (living donor, deceased donation after neurologic determination of death [NDD], or donation after cardio-circulatory death [DCD] status), donor age, donor sex, donor weight, and hemodynamic instability (defined as nadir systolic blood pressure <90 mm Hg in the first 48 hours post-operative following transplantation). Of note, volume of intravenous fluid administered was not included as a covariate due to expected collinearity with post-transplant UO, given the protocol at our institution is to determine the volume of intravenous fluid based on the preceding hour’s UO (see Supplemental Methods 1). No data were available for maintenance immunosuppression regimen, but our institution follows a standardized protocol following transplantation (see Supplemental Methods 2).
Analysis
Baseline characteristics for the cohort, stratified by POD1 UO ≤1000 mL or >1000 mL were described using means/standard deviations, medians/interquartile range, and number/proportion where applicable. The primary analysis used adjusted Cox proportional hazards models to examine the outcome of DCGL. Secondary analyses included adjusted Cox proportional hazards models for the outcome of death and adjusted logistic regression for the outcomes of DGF and prolonged LOS. To examine the temporal association with graft loss, we used adjusted logistic regression to assess the odds of ACGL at 6 months, 1 year, and 5 years post-transplant. The proportion of patients with DCGL, DGF, prolonged LOS, and death stratified by POD1 UO (<1000 mL, 1000-1500 mL, and >1500 mL) was also examined and depicted graphically.
We performed several sensitivity analyses by repeating our primary and secondary analyses with the following modifications:
Using an exposure variable of ≤500 mL of UO on POD1.
Using an exposure variable of ≤0.5 mL/kg/h of UO on POD1.
Excluding those with pre-emptive kidney transplantation.
Including self-reported residual UO (patients’ best estimate of their pre-transplant native UO, reported at the time of admission) as a covariate. Pre-transplant UO was averaged if patients provided a range for their best estimate.
Excluding patients with (rather than adjusting for) hypotension, defined as a systolic blood pressure <90 mm Hg in the first 48 hours post-op, given the confounding effect this might have on UO and DGF risk.
Using linear regression to estimate the reduced length of hospital stay associated with each additional 500 mL of UO on POD1.
Defining POD1 UO as a continuous variable (per 0.1 mL/kg/h).
Including DGF as a covariate (primary analysis only).
Using fewer covariates (5) in the multivariable model for the primary outcome.
All statistical analyses were performed using STATA (version 14.2, StataCorp, College Station, TX). For statistical comparisons, a P < .05 was deemed the threshold for statistical significance. Ethics approval for this study was provided though the Nova Scotia Health Research Ethics Board. The requirement for patient consent was waived as this was a retrospective chart review.
Results
Demographics
A total of 991 patients were included in the study (Figure 1), and baseline characteristics—stratified by UO are reported in Table 1. A total of 451 patients resided in Nova Scotia, which was the cohort used for the primary analysis (Figure 1). The low UO group had a higher median age (57 years vs 51 years in high UO group; P < .001), higher burden of comorbidities including diabetes (37.1% vs 24.5% in high UO group; P = .001) and heart failure (4.6% vs 1.1% in high UO group; P = .002), was more likely to be dialysis dependent prior to transplant (0.7% pre-emptive transplant vs 14.6% in high UO group; P < .001), and had longer median WIT (36 min vs 30 min in high UO group P < .001) and CIT (9.4 hours vs 7.8 hours in high UO group; P = .001). In terms of donor characteristics, the low UO group had a higher proportion of DCD donors (19.9% vs 5.2% in high UO group; P < .001), and a higher median donor age (54 years vs 48 years in high UO group; P < .001). Perioperative hypotension occurred more frequently in the low UO group (25.8% vs 7.7% in high UO group, P < .001).
Table 1.
Baseline Characteristics of Study Cohort by Post-operative Day 1 Urine Output Following Kidney Transplantation.
| Variable | UO > 1000 (n = 840) | UO ≤ 1000 (n = 151) | P |
|---|---|---|---|
| Age: median (IQR), years | 51 (41, 59) | 57 (47, 65) | <.001 |
| Sex | |||
| Male | 549 (65.4%) | 92 (60.9%) | .294 |
| Female | 291 (34.6%) | 59 (39.1%) | |
| Weight: median (IQR), kg | 80 (69, 91) | 83 (71, 96) | .075 |
| Comorbidities | |||
| Diabetes | 206 (24.5%) | 56 (37.1%) | .001 |
| Hypertension | 798 (95.0%) | 144 (95.4%) | .849 |
| Coronary artery disease | 38 (4.5%) | 10 (6.6%) | .269 |
| Heart failure | 9 (1.1%) | 7 (4.6%) | .002 |
| Cerebrovascular disease | 18 (2.1%) | 6 (4.0%) | .384 |
| Cause ESKD | .072 | ||
| PCKD | 170 (20.2%) | 25 (16.6%) | |
| Diabetes | 159 (18.9%) | 45 (29.8%) | |
| Glomerulonephritis | 259 (30.8%) | 36 (23.8%) | |
| Ischemic/hypertension | 61 (7.3%) | 14 (9.3%) | |
| Obstruction | 62 (7.4%) | 9 (6.0%) | |
| Other | 48 (5.7%) | 11 (7.3%) | |
| Inherited | 45 (5.4%) | 5 (3.3%) | |
| Unknown | 36 (4.3%) | 6 (4.0%) | |
| Residual UO: median (IQR), mL | 500 (225, 1000) | 250 (40, 500) | <.001 |
| Dialysis modality | |||
| Pre-emptive transplant | 123 (14.6%) | 1 (0.7%) | <.001 |
| Hemodialysis | 514 (61.2%) | 121 (80.1%) | |
| Peritoneal dialysis | 203 (24.2%) | 29 (19.2%) | |
| Number of HLA mismatches | |||
| 0 | 37 (4.4%) | 2 (1.3%) | .318 |
| 1 | 20 (2.4%) | 4 (2.7%) | |
| 2 | 90 (10.7%) | 12 (8.0%) | |
| 3 | 163 (19.4%) | 24 (15.9%) | |
| 4 | 178 (21.2%) | 38 (25.2%) | |
| 5 | 195 (23.2%) | 28 (23.2%) | |
| 6 | 113 (13.5%) | 8 (18.5%) | |
| WIT: median (IQR), minutes | 30 (24, 40) | 36 (26, 47) | <.001 |
| CIT: median (IQR), hours | 7.8 (4.0, 12.1) | 9.4 (6.9, 13.0) | .001 |
| <6 hours | 181 (21.6%) | 20 (13.3%) | <.001 |
| 6-12 hours | 173 (20.6%) | 47 (31.1%) | |
| >12 hours | 128 (15.2%) | 35 (23.2%) | |
| Donor status | |||
| Living | 305 (36.3%) | 11 (7.3%) | <.001 |
| DCD | 44 (5.2%) | 30 (19.9%) | <.001 |
| Donor age: median (IQR), years | 48 (34, 57) | 54 (45, 59) | <.001 |
| Donor sex—female | 468 (55.6%) | 73 (47.1%) | .279 |
| Induction Immunosuppression | |||
| Basiliximab | 669 (79.7%) | 105 (67.7%) | .001 |
| Anti-thymocyte globulin | 156 (18.6%) | 49 (31.6%) | |
| Methylprednisolone monotherapy | 14 (1.7%) | 1 (0.7%) | |
| Province or residence | |||
| Nova Scotia | 381 (45.4%) | 70 (46.4%) | .269 |
| New Brunswick | 229 (27.3%) | 49 (32.5%) | |
| Prince Edward Island | 70 (8.3%) | 7 (4.6%) | |
| Newfoundland | 160 (19.1%) | 25 (16.6%) | |
| Hypotension | 65 (7.7%) | 40 (25.8%) | <.001 |
| PACU | 26 (3.1%) | 22 (14.2%) | <.001 |
| Floor | 44 (5.2%) | 35 (22.6%) | <.001 |
Missing: heart failure 5/991 (0.5%), cerebrovascular disease 5/991 (0.5%), HLA mismatch 52/991 (5.3%), CIT 407/991 (41.1%), hypotension 49/1032 (4.8%), hypotension—PACU 40/1032 (3.9%), hypotension—floor 14/1032 (1.4%).
POD1 = post-operative day one; UO = urine output; BMI = body mass index; ESKD = end-stage kidney disease; PCKD = polycystic kidney disease; HLA = human leukocyte antigen; WIT = warm ischemia time; CIT = cold ischemia time; DCD = donation after circulatory death; PACU = post-anesthetic care unit; CI = confidence interval.
Of the total study cohort, 151 (15.2%) had a UO ≤1000 mL on POD1. Overall, the median UO was 2335 mL (Q1 = 1475; Q3 = 3500 mL); with median UO 520 mL (Q1 = 251; Q3 = 808 mL) in those with ≤1000 mL. One patient (0.1%) was completely anuric on POD1.
Graft Failure
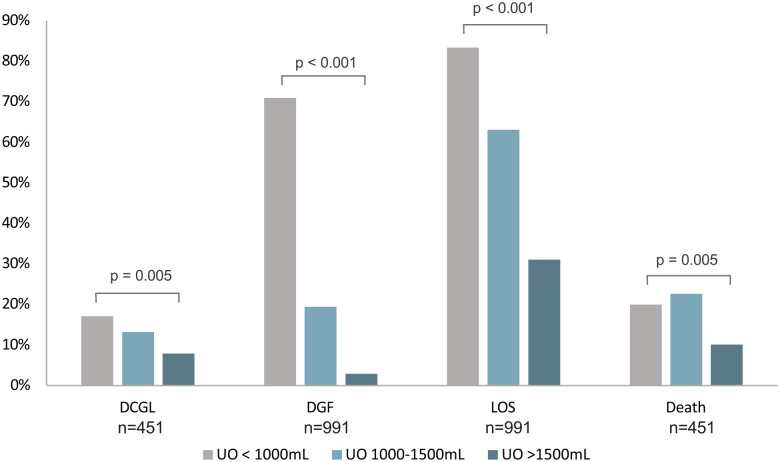
Amongst patients residing in Nova Scotia for whom long-term outcome data were available, 12/70 (17.1%) with UO ≤1000 mL developed DCGL over a median of 8.3 years (Q1 = 4.7; Q3 = 11.3) years compared with 7/53 (13.2%) with UO 1000 to 1500 mL, and 26/328 (7.9%) of those with UO >1500 mL (P = .005; Figure 2). In adjusted Cox regression analysis (Table 2) adjusting for the covariates listed above, UO ≤1000 mL was associated with DCGL (HR = 4.00, 95% CI = 1.55-10.32). In adjusted logistic regression analysis, UO ≤1000 mL was significantly associated with ACGL at 6 months (OR = 4.3, 95% CI = 1.12-16.4), but not at 1 year (OR = 2.72, 95% CI = 0.91-8.16) or 5 years (OR = 1.87, 95% CI = 0.87-4.02).
Figure 2.
Incidence of death-censored graft loss, delayed graft function, prolonged length of stay, and death following kidney transplantation—stratified by post-operative day 1 urine output.
DCGL = death-censored graft loss; DGF = delayed graft function; LOS = length of stay; UO = urine output.
Table 2.
Adjusted Risk of Death-Censored Graft Loss for Urine Output <1000 mL Post-operative Day 1 Following Kidney Transplantation.
| Variable | HR (n = 396) | 95% CI |
|---|---|---|
| UO ≤1000 mL POD1 | 4.00 | 1.55-10.32 |
| Age | 0.95 | 0.91-0.99 |
| Sex | ||
| Male (reference) | Reference | — |
| Female | 1.31 | 0.61-2.83 |
| Weight (kg) | 0.98 | 0.96-1.00 |
| Comorbidities | ||
| Diabetes | 0.30 | 0.08-1.14 |
| Coronary artery disease | 4.77 | 0.89-25.48 |
| Heart failure | 0.28 | 0.03-2.46 |
| Cerebrovascular disease | 1.43 | 0.16-12.92 |
| Cause ESKD | ||
| PCKD (reference) | Reference | — |
| Diabetes | 0.29 | 0.05-1.67 |
| Glomerulonephritis | 1.99 | 0.72-5.49 |
| Ischemic/hypertension | 1.15 | 0.26-5.07 |
| Other | 1.10 | 0.25-4.84 |
| Inherited | 2.68 | 0.65-11.14 |
| Unknown | 1.37 | 0.38-5.00 |
| Dialysis modality | ||
| Pre-emptive transplant (reference) | Reference | — |
| Hemodialysis | 0.87 | 0.29-2.63 |
| Peritoneal dialysis | 0.55 | 0.16-1.88 |
| Number of HLA mismatches | ||
| 0 (reference) | Reference | — |
| 1 | — | — |
| 2 | 0.50 | 0.04-5.67 |
| 3 | 0.68 | 0.07-6.65 |
| 4 | 0.73 | 0.07-7.42 |
| 5 | 1.30 | 0.14-12.10 |
| 6 | 0.81 | 0.08-8.15 |
| WIT | 1.01 | 0.04-5.67 |
| CIT | ||
| <6 hours (reference) | Reference | — |
| 6-12 hours | 1.49 | 0.46-4.79 |
| >12 hours | 1.13 | 0.33-3.83 |
| Donor status | ||
| Deceased (vs live) | 3.66 | 1.15-11.60 |
| DCD (vs non-DCD) | 0.96 | 0.28-3.24 |
| Donor age | 1.05 | 1.02-1.08 |
| Donor weight | 0.98 | 0.96-1.00 |
| Donor sex—female | 1.53 | 0.71-3.30 |
| Hypotension | ||
| PACU | 0.97 | 0.23-4.03 |
| Floor | 3.15 | 1.10-8.99 |
POD1 = post-operative day one; UO = urine output; BMI = body mass index; ESKD = end-stage kidney disease; PCKD = polycystic kidney disease; HLA = human leukocyte antigen; WIT = warm ischemia time; CIT = cold ischemia time; DCD = donation after circulatory death; PACU = post-anesthetic care unit; HR = hazard ratio; CI = confidence interval.
Delayed Graft Function
In the entire study cohort (those with follow-up in any Atlantic province), 107/151 (70.9%) of patients with UO ≤1000 mL had DGF compared with 20/103 (19.4%) with UO 1000 to 1500 mL, and 21/738 (2.9%) of those with UO >1500 mL (P < .001, Figure 2). In adjusted logistic regression analysis (Table 3), UO ≤1000 mL was strongly associated with DGF (OR = 45.25, 95% CI = 23.00-89.02).
Table 3.
Adjusted Risk of Delayed Draft Function, Prolonged Length of Stay, and Death, for Urine Output ≤1000 mL Post-operative Day 1 Following Kidney Transplantation.
| Variable | DGF OR (95% CI) n = 742 | LOS >9 days OR (95% CI) n = 851 | Death HR (95% CI) n = 396 |
|---|---|---|---|
| UO ≤ 1000 mL POD1 | 45.25 (23.00-89.02) | 5.06 (2.95-8.69) | 0.81 (0.31-2.09) |
| Age | 0.99 (0.96-1.01) | 1.01 (0.99-1.02) | 1.06 (1.02-1.10) |
| Sex | |||
| Male (reference) | Reference | Reference | Reference |
| Female | 1.77 (0.96-3.25) | 0.76 (0.54-1.06) | 2.16 (0.98-4.77) |
| BMI | |||
| “Body weight” | 1.00 (0.98-1.02) | 1.00 (0.99-1.01) | 1.01 (0.99-1.03) |
| Comorbidities | |||
| Diabetes | 0.55 (0.20-1.55) | 0.33 (0.17-0.63) | 0.48 (0.15-1.51) |
| Hypertension | 4.22 (0.98-18.11) | 0.46 (0.22-0.97) | 0.22 (0.06-0.83) |
| Coronary artery disease | 0.38 (0.09-1.61) | 0.77 (0.36-1.67) | 1.80 (0.56-5.79) |
| Heart failure | 1.89 (0.36-9.80) | 1.85 (0.48-7.10) | 0.30 (0.04-2.31) |
| Cerebrovascular disease | 1.50 (0.29-7.74) | 1.27 (0.45-3.56) | 1.21 (0.18-8.29) |
| Cause ESKD | |||
| PCKD (reference) | Reference | Reference | Reference |
| Diabetes | 0.90 (0.22-3.65) | 0.74 (0.33-1.66) | 0.80 (0.18-3.50) |
| Glomerulonephritis | 1.49 (0.58-3.85) | 1.21 (0.74-1.95) | 0.24 (0.07-0.85) |
| Ischemic/hypertension | 1.49 (0.43-5.15) | 1.61 (0.80-3.24) | 0.75 (0.20-2.80) |
| Obstruction | 3.13 (0.91-10.80) | 1.34 (0.66-2.72) | 0.96 (0.30-3.03) |
| Other | 4.85 (1.44-16.34) | 0.96 (0.44-2.09) | 1.57 (0.43-5.67) |
| Inherited | 0.78 (0.13-4.61) | 0.65 (0.27-1.57) | 0.60 (0.07-5.27) |
| Unknown | 2.40 (0.59-9.80) | 1.30 (0.58-2.88) | 0.45 (0.08-2.71) |
| Dialysis modality | |||
| Pre-emptive transplant (reference) | Reference | Reference | Reference |
| Hemodialysis | 3.65 (1.67-8.00) | 1.37 (0.78-2.40) | 2.87 (0.63-13.09) |
| Peritoneal dialysis | - | 1.36 (0.74-2.50) | 1.86 (0.35-9.79) |
| Number of HLA mismatches | |||
| 0 (reference) | Reference | Reference | Reference |
| 1 | 2.31 (0.13-40.66) | 1.42 (0.36-5.58) | 5.06 (0.45-57.56) |
| 2 | 1.65 (0.12-21.79) | 2.09 (0.73-5.98) | 1.74 (0.18-16.75) |
| 3 | 1.79 (0.15-21.73) | 1.70 (0.62-4.67) | 1.33 (0.14-12.89) |
| 4 | 2.11 (0.17-25.72) | 1.63 (0.59-4.51) | 1.97 (0.21-18.89) |
| 5 | 1.66 (0.14-20.51) | 2.34 (0.85-6.44) | 1.29 (0.13-12.59) |
| 6 | 2.75 (0.22-33.95) | 1.80 (0.63-5.15) | 2.90 (0.30-27.85) |
| WIT | 1.02 (1.00-1.03) | 1.02 (1.01-1.03) | 1.00 (0.98-1.02) |
| CIT | |||
| <6 hours (reference) | Reference | Reference | Reference |
| 6-12 hours | 1.36 (0.53-3.51) | 0.93 (0.53-1.64) | 0.61 (0.19-1.96) |
| >12 hours | 0.99 (0.37-2.70) | 1.13 (0.62-2.05) | 0.66 (0.20-2.16) |
| Donor status | |||
| Deceased (vs live) | 1.41 (0.54-3.69) | 1.59 (1.00-2.53) | 1.12 (0.40-3.17) |
| DCD (vs non-DCD) | 5.07 (2.08-12.36) | 10.26 (4.25-24.77) | |
| Donor age | 1.01 (0.99-1.03) | 1.00 (0.99-1.01) | 1.03 (1.00-1.06) |
| Donor weight | 1.01 (1.00-1.03) | 1.00 (0.99-1.01) | 0.99 (0.97-1.01) |
| Donor sex | |||
| Male (reference) | Reference | Reference | Reference |
| Female | 0.45 (0.22-0.94) | 1.20 (0.83-1.75) | 2.16 (0.98-4.77) |
| Hypotension | |||
| PACU | 2.28 (0.74-7.02) | 1.38 (0.61-3.13) | 0.77 (0.20-2.96) |
| Floor | 1.07 (0.41-2.84) | 2.30 (1.21-4.36) | 3.41 (1.31-8.89) |
POD1 = post-operative day one; UO = urine output; BMI = body mass index; ESKD = end-stage kidney disease; PCKD = polycystic kidney disease; HLA = human leukocyte antigen; WIT = warm ischemia time; CIT = cold ischemia time; DCD = donation after circulatory death; PACU = post-anesthetic care unit; DGF = delayed graft function; LOS = length of stay; CI = confidence interval.
Length of Stay
In the entire study cohort (those with follow-up in any Atlantic province), 126/151 (83.4%) patients with UO ≤1000 mL had an LOS greater than the median for the cohort (9 days) compared with 65/103 (63.1%) with UO 1000 to 1500 mL, and 229/737 (31.1%) of those with UO >1500 mL (P < .001, Figure 2). Median LOS was 16 (Q1 = 11, Q3 = 27) days in the low UO group and 8 days (Q1 = 7, Q3 = 11) in the high UO group. In adjusted logistic regression (Table 3), UO ≤1000 mL was associated with prolonged LOS (OR = 5.06, 95% CI = 2.95-8.69). Using linear regression, each additional 500 mL of UO on POD1 reduced the expected index hospitalization by 1.41 days (95% CI = 0.98-1.85 days).
Death
Amongst patients residing in Nova Scotia for whom long-term outcome data were available, 14/70 (20.0%) of patients with a UO <1000 mL died compared with 12/53 (22.6%) with UO 1000 to 1500 mL, and 33/328 (10.1%) of those with UO >1500 mL (P= .005, Figure 2). In adjusted Cox regression analysis (Table 3), UO ≤1000 mL was not associated with death (HR = 0.81, 95% CI = 0.31-2.09).
Sensitivity Analyses
Sensitivity analyses are reported in Supplemental Table 1. For our primary outcome, a significant association between POD1 UO and DCGL persisted when excluding pre-emptive transplantation (HR = 3.71, 95% CI = 1.39-9.92), when accounting for baseline patient-reported UO (HR = 4.38, 95% CI = 1.60-11.97), when excluding rather than adjusting for hypotension (HR = 4.34, 95% CI = 1.30-14.49), and when defining low POD1 UO as ≤0.5 mL/kg/h (HR = 3.17, 95% CI = 1.13-8.90). In a deviation from our previous results, when a threshold of POD1 UO ≤500 mL was used, there was a loss of significance for the association between UO and DCGL (HR = 3.73, 95% CI = 0.92-15.07) acknowledging low event rates (n = 5) in the ≤500 mL UO category, but a significant and strong association between UO and DGF (OR = 373.67, 95% CI = 69.07-2021.53) and LOS (OR = 11.99, 95% CI = 3.99-36.00) persisted. For the outcome of death, the results were similar to our initial analysis with no significant association. When including DGF as a covariate in our primary analysis, we found neither a statistically significant association between low UO and DCGL (HR = 2.84, 95% CI = 0.95-8.52) or between DGF and DCGL (HR = 2.04, 95% CI = 0.65-6.42). Each 0.1 mL/kg/h of UO on POD1 was independently associated with an HR for DCGL of 0.49 (95% CI = 0.27-0.90), with adjusted risk ratios for secondary outcome shown in Supplemental Table 2. When reducing the number of covariates included in our primary multivariable analysis to 5 variables, we found similar results (HR = 2.97, 95% CI = 1.38-6.39; Supplemental Table 3).
Discussion
In this study, we demonstrate that reduced early post-operative UO is associated with an increased risk of DCGL, DGF, and prolonged hospital LOS. These findings are consistent with previous studies that have demonstrated an association between early post-operative UO and graft function, although previous reports have been limited to small sample sizes (n ≤ 300) and restricted to regions outside of North America.13 -15 Even after accounting for traditional predictors of graft dysfunction including warm and cold ischemia time, DCD donor status, and dialysis modality,6,17 -19 we found that POD1 UO ≤1000 mL was the strongest predictor of DCGL, with a 4-fold increase in the hazard of DCGL. The independent effect of post-operative UO on DCGL was found to persist at 6 months, but not at 1 year, suggesting the association between early post-operative UO and DCGL relates to early graft loss events within the first year; notably, we excluded individuals with PNF. Our results were similar to previous studies examining POD1 UO and long-term graft outcomes, with some notable differences (see Table 4 for a summary of studies examining UO and graft outcomes).13 -15 In particular, Schnuelle et al 14 conducted a study in Germany of 300 NDD kidney transplant recipients from 1989 to 2005, and similarly reported higher POD1 UO (>630 mL) was protective against DCGL in adjusted analysis (HR = 0.44, 95% CI = 0.28-0.69). However, a number of covariates were not adjusted for, such as recipient comorbidity, residual UO, pre-emptive status, dialysis modality, WIT, CIT, or post-operative hypotension. In addition, this study was restricted to NDD donors, whereas our current study examined NDD, DCD, and live donors in a contemporary cohort. Other similar studies have demonstrated conflicting results, as described in Table 4, and there is a need for larger studies to clarify these discrepancies.
Table 4.
Summary of Studies Examining the Impact of Post-operative Urine Output Following Kidney Transplantation of Graft Outcomes.
| Study | Date | Location | N | Donor | Exposure | Outcome | Results |
|---|---|---|---|---|---|---|---|
| Schnuelle et al 14 | 1989-2005 | Germany | 300 | NDD only | UO >630 mL POD1 | DCGL at 5 years | Adjusted HR = 0.43 (95% CI = 0.26-0.72) |
| Lai et al 13 | 2006-2008 | Italy | 82 | Deceased | UO ≤500 mL POD1 | ACGL at 1 year | MV analysis P = .692 |
| UO ≤500 mL POD7 | ACGL at 1 year | MV analysis P < .001 |
|||||
| Kim et al 15 | 2008-2017 | South Korea | 291 | Living + deceased | UO POD1 | 1 year eGFR | P < .001* |
| UO POD1 | DGF | AUC = 0.913 | |||||
| Maier et al 12 | 2010-2012 | Austria | 170 | Living + deceased | POD1 UO per 100 mL | DGF | Unadjusted OR = 0.87 (95% CI = 0.8-0.9) |
| Parikh et al 20 | NA | USA | 53 | Living + deceased | UO <1 L POD1 | DGF | Adjusted OR = 11.7 (95% CI = 0.1-913) |
| Hall et al 21 | NA | USA | 91 | Deceased | UO <1 L POD1 | DGF | Adjusted OR = 2.8 (95% CI = 0.66-12.3) |
NDD = neurological determination of death; UO = urine output; POD = post-operative day; DCGL = death-censored graft loss; HR = hazard ratio; ACGL = all-cause graft loss; MV = multivariable; eGFR = estimated glomerular filtration rate; AUC = area under curve; NA = not available.
Details of statistical analysis are not available.
We also found that POD1 UO ≤1000 mL was an exceptionally strong predictor of DGF, with a 45-fold increased odds of DGF in those with low UO. The magnitude of this relationship is apparent when comparing this with previously studied predictors of DGF, such as those included in the calculator derived by Irish et al 19 —a well-known tool used to estimate risk of DGF. The strongest predictor of DGF included in this calculator was a DCD donor, with an OR of 3.06 (95% CI = 2.61-3.59) 19 and an OR of 5.07 (95% CI = 2.08-12.36) in our study. Therefore, the risk of DGF associated with early post-operative UO is likely 9- to 14-fold greater than that for DCD kidney donor status, which has been shown to influence decision making regarding induction immunosuppression regimens. 22
Reduced UO intuitively may predispose to DGF, but the mechanism underpinning this relationship is not entirely clear. Reduced UO may represent early graft injury through mechanisms of ischemia reperfusion or may simply represent a vulnerability to volume overload or a predisposition to metabolic derangements by way of reduced post-transplant kidney function, necessitating dialysis. Ultimately, a relationship between post-operative UO and DGF is not surprising and low post-operative UO likely contributes to DGF via multiple mechanisms.
Delayed graft function is known to increase the risk of long-term graft dysfunction.5,19,23,24 The mechanisms by which this occurs are not fully understood, but it has been postulated that increased allograft immunogenicity and chronic fibrosis may be contributing factors to long-term graft dysfunction. 25 Extrapolating from the DGF literature, similar mechanisms may explain the observed relationship between post-operative UO and long-term graft dysfunction in our study. A low post-operative UO may reflect perioperative ischemic injury with subsequent downstream effects on long-term graft function, regardless of whether that injury resulted in DGF. Independent of perioperative factors, decreased UO may rather represent a more vulnerable allograft, serving as a surrogate marker for inherent risk properties of the donor kidney. Our study controlled for donor status (living or DCD status), donor age, donor weight, and donor sex, but not other donor factors that could correlate with graft quality. Identifying UO as an independent predictor of long-term graft function may be advantageous over using DGF, given that there is a subjective component to DGF (clinician discretion is required to decide when to initiate dialysis post-transplant) versus the objective nature of POD1 UO. In addition, POD1 UO may serve as an earlier predictor than DGF, which can occur up to 7 days post-transplant.
Donation after cardio-circulatory death transplantation is associated with an increased risk of DGF, and therefore a risk of increased immunogenicity and subsequent risk of rejection.19,26 Given this, some institutions choose to use a more potent induction immunosuppression regimen that includes a lymphocyte depleting agent, such as rabbit anti-thymocyte globulin (ATG), rather than an interleukin antagonist, such as basiliximab, to mitigate this risk. 22 In addition, in some centers, if high immunological risk features develop after transplantation (such as DGF), converting from basiliximab induction (following administration of the first dose) to ATG induction post-operatively has been practiced. 27 Given the strong associations between UO and DGF demonstrated in this study, as well as UO and DCGL, an area of future research could be examining the role of converting to ATG from basiliximab when low post-operative urine occurs in an attempt to intervene in the pathway from low UO to graft loss. This would be particularly relevant as low UO may be an early harbinger of DGF that allows the opportunity for earlier intervention.
We also found that low post-operative UO was associated with a prolonged LOS with each additional 500 mL of UO on POD1 reducing the expected index hospitalization by 1.41 days. The average cost of a standard hospital stay in Canada is $7619, 28 and prolonged admission would be expected to increase these costs further. Prolonged hospitalization may also lead to decreased bed flow, greater risk of health care–associated infection, and impact patient quality of life.29,30
We conducted a number of sensitivity analyses examining differing UO cut-offs, differing methods of adjusting for baseline UO, and restricting to a hemodynamically stable cohort. Overall the results were similar for each outcome of DCGL, DGF, prolonged LOS, and death. Of note, using a UO threshold of ≤500 mL resulted in a loss of statistical significance for the association between POD1 UO and DCGL; however, this likely reflects the low number of event rates with only 27 patients total with follow-up in Nova Scotia recorded as having a POD1 UO ≤500. Notably, the magnitude of the relationship between UO and DGF was dramatically increased when using a UO cut-off of ≤500 mL, with an OR of 373 compared with an OR of 45 when using a cut-off of ≤1000 mL. This is intuitive as lower UO may reflect worse graft function and greater susceptibility to volume overload or lack of potassium clearance, but is nonetheless an impressive relationship. When using a UO cut-off of 0.5 mL/kg/h, a similar trend was seen to our primary and secondary analyses. Notably, a variety of definitions of “low UO” have been proposed, with previous studies using weight based and absolute cut-offs, including those using a cut-off of 1000 mL following kidney transplant.20,21 Regardless, the consistency in our sensitivity analyses supports similar clinical implications across these definitions.
In our primary analyses, we did not account for residual UO prior to transplantation. Despite this, our findings were unchanged in sensitivity models that accounted for pre-operative residual UO (as defined by patient best report) or that excluded pre-emptive transplants. This suggests that baseline native kidney function is not driving the association between post-operative UO and short and long-term outcomes.
In a sensitivity analysis, we repeated our primary analysis including DGF as a covariate and found neither a statistically significant association between low UO and DCGL or between DGF and DCGL. Of patients with UO <1000 mL on POD1, 70.8% went on to develop DCGL and a high degree of collinearity is expected. This collinearity may have led to the loss of statistical significance, acknowledging that the association between low UO and DCGL approached statistical significance.
When examining baseline characteristics of the UO ≤1000 mL and >1000 mL groups, there were a number of differences noted. Several of these differences have a plausible impact on the quality of the donor organ pre-operatively, such as a higher donor age and a greater proportion of DCD donors in the low UO group—known risk factors for graft dysfunction.19,24 Similarly, there were differences in perioperative factors in those with lower post-op UO that likely impact graft function, such as prolonged WIT, prolonged CIT, and greater incidence of hypotension.6,19,24,31 Finally, differences in recipient factors likely predisposed to worse graft outcomes, such as higher median age, higher burden of comorbidities including diabetes and heart failure, and greater likelihood of being dialysis dependent prior to transplant.19,32,33 However, we adjusted for these factors in our multivariable analyses and the signal of independent risk associated with low post-operative UO persisted despite accounting for these potential confounders.
Hypotension in the perioperative period may independently lead to reduced UO and serve as a trigger for clinicians to enact interventions such as IV fluid administration or vasopressors. In addition, hypotension may independently influence graft function. 31 Given this, our multivariable analyses were adjusted for hypotension, defined as a systolic blood pressure <90 mm Hg in the first 48 hours post-transplant. This decreases the likelihood that the risk of graft loss observed in our study is related to perioperative volume depletion or hemodynamic stress.
While our study has many strengths including being the largest to examine the implications of post-op UO on predicting short and long-term graft outcomes, there are important limitations to consider. We conducted a retrospective chart review with inherent limitations of data collection including potential misclassification and miscoding; however, we would anticipate any information bias to be distributed at random. Baseline UO was collected as a self-reported measure, which may correlate poorly with actual baseline UO, but it is reflective of data available to clinicians at the time of transplant and has been shown to be associated with outcomes such as mortality in HD patients. 34 Similarly, in-hospital UO measurements are subject to error as they rely on consistent and accurate recording by nursing staff. Data regarding rejection risk, cause of graft loss, and indication for dialysis in those with DGF were not available and therefore not incorporated into our analyses, but this represents an area for potential future investigation and may provide insight into the factors driving graft loss in our study. Although donor age, sex, weight, and donor status (DCD vs living donor data) were collected, comprehensive data regarding donors were not available, limiting our ability to evaluate the impact of additional pre-operative factors on graft loss. Data on the administration of diuretics were not available, but of note local practice at our institution is to avoid diuretics in the first 48 hours post-op, and thus, diuretic use is anticipated to have been inconsequential. In addition, data regarding the cause of graft loss were not available, and this would be an area to explore in future prospective studies. This was a single-center study; therefore, local protocols, such as diuretic use and intravenous fluid administration, may not be consistent with other centers and may impact generalizability. Although we included a number of covariates in our multivariable models to maximize control of confounding variables, we acknowledge that the event rate in our study was suboptimal for the number of covariates included. Despite this, we felt important confounding variables would be excluded were the number of covariates to be further reduced, and the signal for risk with low UO was preserved in a sensitivity analysis restricted to fewer variables. Further to this, the relatively small event rate may be contributing to the loss of statistical significance for ACGL at 1 and 5 years, limiting our ability to draw conclusions about timing of graft loss, and future prospective studies would be useful to explore this further. Finally, our study was not designed to assess whether interventions aimed at improving UO, such as diuretics, vasopressors, or intravenous fluid, would impact DCGL, DGF, or LOS and this represents an area for potential future study.
The results of this study help to inform clinicians of the significance of post-operative UO on both short-term and long-term graft outcomes in kidney transplant patients. These findings may be used to inform future prediction models for graft failure in kidney transplantation. Whether these findings serve in a predictive capacity alone or whether they may have a role to guide intervention remains to be seen. There are several aspects of this work that could be expanded upon further with future study, but it would be of particular interest to prospectively examine the role of UO as a predictor of long-term outcomes and explore the role of subsequent intervention, such as modification of immunosuppression regimen.
In summary, reduced post-operative UO following kidney transplantation is associated with an increased risk of DCGL, DGF, and prolonged hospital LOS. These findings highlight the impact of perioperative factors on not only short-term outcomes but also long-term graft function. To our knowledge, this is the largest and most comprehensive study to examine the impact of post-operative UO on long-term graft outcomes. Further work to investigate this finding prospectively and to explore possible interventions is warranted.
Supplemental Material
Supplemental material, sj-docx-1-cjk-10.1177_20543581231221630 for Association Between First Post-operative Day Urine Output Following Kidney Transplantation and Short-Term and Long-Term Outcomes: A Retrospective Cohort Study by Steven A. Morrison, Aran Thanamayooran, Karthik Tennankore and Amanda J. Vinson in Canadian Journal of Kidney Health and Disease
Footnotes
List of Abbreviations: CI, confidence interval; CIT, cold ischemia time; DCD, donation after circulatory death; DCGL, death-censored graft loss; DGF, delayed graft function; HLA, human leukocyte antigen; HR, hazard ratio; LOS, length of stay; OR, odds ratio; POD, post-operative day; QEII, Queen Elizabeth II Health Sciences Center; UO, urine output; WIT, warm ischemia time.
Author Contributions: S.A.M. participated in research design, data collection, data analysis, and manuscript preparation. A.T. participated in data collection and manuscript preparation. K.T. participated in research design, data analysis, and manuscript preparation. A.J.V. participated in research design, data analysis, and manuscript preparation.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.J.V. has received consultancy and fellowship grant funding through Paladin Labs Inc, and K.T. has received advisory board and CME support from Astra Zeneca, Baxter, Bayer, GSK, Otsuka, and Vifor Pharmaceuticals. However, the authors of this manuscript have no conflicts of interest to declare as described by the International Committee of Medical Journal Editors that are relevant to the current submission.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Steven A. Morrison  https://orcid.org/0000-0002-7669-0365
https://orcid.org/0000-0002-7669-0365
Karthik Tennankore  https://orcid.org/0000-0002-7919-6709
https://orcid.org/0000-0002-7919-6709
Amanda J. Vinson  https://orcid.org/0000-0002-9345-5252
https://orcid.org/0000-0002-9345-5252
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725-1730. [DOI] [PubMed] [Google Scholar]
- 2. Laupacis A, Keown P, Pus N, et al. A study of the quality of life and cost-utility of renal transplantation. Kidney Int. 1996;50(1):235-242. doi: 10.1038/KI.1996.307. [DOI] [PubMed] [Google Scholar]
- 3. Pauly RP. Survival comparison between intensive hemodialysis and transplantation in the context of the existing literature surrounding nocturnal and short-daily hemodialysis. Nephrol Dial Transplant. 2013;28(1):44-47. doi: 10.1093/NDT/GFS419. [DOI] [PubMed] [Google Scholar]
- 4. Wang JH, Skeans MA, Israni AK. Current status of kidney transplant outcomes: dying to survive. Adv Chronic Kidney Dis. 2016;23(5):281-286. doi: 10.1053/j.ackd.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 5. Quiroga I, McShane P, Koo DD, et al. Major effects of delayed graft function and cold ischaemia time on renal allograft survival. Nephrol Dial Transplant. 2006;21(6):1689-1696. doi: 10.1093/NDT/GFL042. [DOI] [PubMed] [Google Scholar]
- 6. Tennankore KK, Kim SJ, Alwayn IP, Kiberd BA. Prolonged warm ischemia time is associated with graft failure and mortality after kidney transplantation. Kidney Int. 2016;89(3):648-658. doi: 10.1016/J.KINT.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 7. El Ters M, Grande JP, Keddis MT, et al. Kidney allograft survival after acute rejection, the value of follow-up biopsies. Am J Transplant. 2013;13(9):2334-2341. doi: 10.1111/AJT.12370. [DOI] [PubMed] [Google Scholar]
- 8. Ferguson R. Acute rejection episodes: best predictor of long-term primary cadaveric renal transplant survival. Clin Transplant. 1994;8(3 pt 2):328-331. https://europepmc.org/article/med/8061375. Accessed January 4, 2023. [PubMed] [Google Scholar]
- 9. Stern M, Hirsch H, Cusini A, et al. Cytomegalovirus serology and replication remain associated with solid organ graft rejection and graft loss in the era of prophylactic treatment. Transplantation. 2014;98(9):1013-1018. doi: 10.1097/TP.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 10. Martin-Gandul C, Mueller NJ, Pascual M, Manuel O. The impact of infection on chronic allograft dysfunction and allograft survival after solid organ transplantation. Am J Transplant. 2015;15(12):3024-3040. doi: 10.1111/AJT.13486. [DOI] [PubMed] [Google Scholar]
- 11. Hariharan S, Israni AK, Danovitch G. Long-term survival after kidney transplantation. N Engl J Med. 2021;385(8):729-743. doi: 10.1056/nejmra2014530. [DOI] [PubMed] [Google Scholar]
- 12. Maier HT, Ashraf MI, Denecke C, et al. Prediction of delayed graft function and long-term graft survival by serum and urinary neutrophil gelatinase–associated lipocalin during the early postoperative phase after kidney transplantation. PLoS ONE. 2018;13(1):e0189932. doi: 10.1371/journal.pone.0189932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lai Q, Pretagostini R, Poli L, et al. Early urine output predicts graft survival after kidney transplantation. Transplant Proc. 2010;42(4):1090-1092. doi: 10.1016/j.transproceed.2010.03.088. [DOI] [PubMed] [Google Scholar]
- 14. Schnuelle P, Gottmann U, Köppel H, et al. Comparison of early renal function parameters for the prediction of 5-year graft survival after kidney transplantation. Nephrol Dial Transplant. 2007;22(1):235-245. doi: 10.1093/ndt/gfl530. [DOI] [PubMed] [Google Scholar]
- 15. Kim J, Pyeon T, Choi JI, et al. A retrospective study of the relationship between postoperative urine output and one year transplanted kidney function. BMC Anesthesiol. 2019;19(1):231. doi: 10.1186/s12871-019-0904-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bergman J, Tennankore K, Vinson A. Early and recurrent hospitalization after kidney transplantation: analysis of a contemporary Canadian cohort of kidney transplant recipients. Clin Transplant. 2020;34(8):e14007. doi: 10.1111/CTR.14007. [DOI] [PubMed] [Google Scholar]
- 17. Goldfarb-Rumyantzev AS, Hurdle JF, Scandling JD, Baird BC, Cheung AK. The role of pretransplantation renal replacement therapy modality in kidney allograft and recipient survival. Am J Kidney Dis. 2005;46(3):537-549. doi: 10.1053/J.AJKD.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 18. Mikhalski D, Wissing KM, Ghisdal L, et al. Cold ischemia is a major determinant of acute rejection and renal graft survival in the modern era of immunosuppression. Transplantation. 2008;85(7 suppl):S3-S9. doi: 10.1097/TP.0B013E318169C29E. [DOI] [PubMed] [Google Scholar]
- 19. Irish WD, Ilsley JN, Schnitzler MA, Feng S, Brennan DC. A risk prediction model for delayed graft function in the current era of deceased donor renal transplantation. Am J Transplant. 2010;10(10):2279-2286. doi: 10.1111/J.1600-6143.2010.03179.X. [DOI] [PubMed] [Google Scholar]
- 20. Parikh CR, Jani A, Mishra J, et al. Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant. 2006;6:1639-1645. doi: 10.1111/j.1600-6143.2006.01352.x. [DOI] [PubMed] [Google Scholar]
- 21. Hall IE, Yarlagadda SG, Coca SG, et al. IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol. 2010;21(1):189-197. doi: 10.1681/ASN.2009030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Asderakis A, Sabah TK, Watkins WJ, et al. Thymoglobulin versus alemtuzumab versus basiliximab kidney transplantation from donors after circulatory death. Kidney Int Rep. 2022;7(4):732-740. doi: 10.1016/J.EKIR.2022.01.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yarlagadda SG, Coca SG, Formica RN, Jr, Poggio ED, Parikh CR. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant. 2009;24(3):1039-1047. doi: 10.1093/NDT/GFN667. [DOI] [PubMed] [Google Scholar]
- 24. Hall IE, Reese PP, Doshi MD, et al. Delayed graft function phenotypes and 12-month kidney transplant outcomes HHS public access. Transplantation. 2017;101(8):1913-1923. doi: 10.1097/TP.0000000000001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perico N, Cattaneo D, Sayegh MH, Remuzzi G. Delayed Graft Function in Kidney Transplantation, Vol. 364. Amsterdam: Elsevier. [DOI] [PubMed] [Google Scholar]
- 26. Wu WK, Famure O, Li Y, Kim SJ. Delayed graft function and the risk of acute rejection in the modern era of kidney transplantation. Kidney Int. 2015;88(4):851-858. doi: 10.1038/KI.2015.190. [DOI] [PubMed] [Google Scholar]
- 27. Jeong R, Quinn RR, Lentine KL, et al. Incidence, risk factors, and outcomes of kidney transplant recipients treated with both basiliximab and antithymocyte globulin. Can J Kidney Health Dis. 2020;7:964061. doi: 10.1177/2054358120964061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Canadian Institute for Health Information. Cost of a standard hospital stay. 2022. https://yourhealthsystem.cihi.ca/hsp/inbrief?lang=en#!/indicators/015/cost-of-a-standard-hospital-stay-cshs/mapC1mapLevel2provinceC9001/. Accessed January 19, 2023.
- 29. Esther Babady N. Hospital-associated infections. Microbiol Spectr. 2016;4:735-758. doi: 10.1128/MICROBIOLSPEC.DMIH2-0003-2015. [DOI] [PubMed] [Google Scholar]
- 30. Siddique SM, Tipton K, Leas B, et al. Interventions to reduce hospital length of stay in high-risk populations: a systematic review. JAMA Netw Open. 2021;4:e25846. doi: 10.1001/JAMANETWORKOPEN.2021.25846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sandid MS, Assi MA, Hall S. Intraoperative hypotension and prolonged operative time as risk factors for slow graft function in kidney transplant recipients. Clin Transplant. 2006;20(6):762-768. doi: 10.1111/J.1399-0012.2006.00567.X. [DOI] [PubMed] [Google Scholar]
- 32. Gavela Martínez E, Pallardó Mateu LM, Sancho Calabuig A, et al. Delayed graft function after renal transplantation: an unresolved problem. Transplant Proc. 2011;43(6):2171-2173. doi: 10.1016/J.TRANSPROCEED.2011.06.051. [DOI] [PubMed] [Google Scholar]
- 33. Keith DS, Cantarovich M, Paraskevas S, Tchervenkov J. Duration of dialysis pretransplantation is an important risk factor for delayed recovery of renal function following deceased donor kidney transplantation. Transpl Int. 2008;21(2):126-132. doi: 10.1111/J.1432-2277.2007.00571.X. [DOI] [PubMed] [Google Scholar]
- 34. Shafi T, Jaar BG, Plantinga LC, et al. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis. 2010;56(2):348-358. doi: 10.1053/J.AJKD.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cjk-10.1177_20543581231221630 for Association Between First Post-operative Day Urine Output Following Kidney Transplantation and Short-Term and Long-Term Outcomes: A Retrospective Cohort Study by Steven A. Morrison, Aran Thanamayooran, Karthik Tennankore and Amanda J. Vinson in Canadian Journal of Kidney Health and Disease