Abstract
Objective
Malignant biliary obstruction (MBO) is a rare disease with a poor prognosis. Recent studies have shown that endoscopic radiofrequency ablation (ERFA) may improve survival. We conducted a systematic review and meta-analysis of the efficacy of ERFA in combination with biliary stent placement for the treatment of MBO.
Methods
The study was registered in INPLASY (number 202340096). The PubMed, Cochrane Library, Web of Science, and Embase databases were searched from inception to April 2023. We selected studies comparing the efficacy of ERFA plus stent placement with stent placement alone. The primary outcomes were pooled hazard ratios (HRs) for overall survival and stent patency; the secondary outcomes were the odds ratios (ORs) for adverse events.
Results
Eleven studies (four randomized controlled trials and seven observational studies) were included in the meta-analysis. Pooled analysis showed a difference in survival time between the two groups (HR 0.65, 95% confidence interval [CI] 0.58–0.73, I2 = 40%). However, there were no differences in the duration of stent patency or the incidence of adverse events (HR 1.04, 95% CI 0.84–1.29, I2 = 46%; OR 1.41, 95% CI 1.02–1.96, I2 = 29%).
Conclusions
ERFA has a significant survival benefit for MBO, but does not increase the risk of adverse events.
Keywords: Meta-analysis, malignant biliary obstruction, endoscopic radiofrequency ablation, stent patency, adverse event, survival
Introduction
Malignant biliary obstruction (MBO) refers to a gradually progressive disease caused by various malignant tumors, including primary cancers and metastatic cancers, such as cholangiocarcinoma, gallbladder cancer, pancreatic cancer, and ampulla cancer. 1 Surgical resection is the conventional treatment for patients with MBO, but most patients do not show obvious symptoms during the early stages of the disease, which may mean that the ideal time for surgery is missed.1,2 Biliary stent placement is the conventional palliative treatment method for patients with advanced MBO, and this can be performed via percutaneous transhepatic puncture, endoscopic retrograde cholangiopancreatography (ERCP), or endoscopic ultrasonographic guidance, to relieve the symptoms of patients.3–5 However, tumors may grow along the stent and cause a further obstruction to the bile duct.
Radiofrequency ablation (RFA) can cause local or regional coagulation and necrosis through the application of high-energy radiation. It can be performed via percutaneous or endoscopic approaches to deliver energy to the bile duct.6,7 In recent years, endoscopic radiofrequency ablation (ERFA) in combination with biliary stent placement has been gradually introduced for the treatment of patients with inoperable MBO, but its efficacy has not been thoroughly evaluated. According to the results of a recent meta-analysis, ERFA can significantly prolong the survival of patients with unresectable cholangiocarcinoma. 8 Therefore, in the present study, we aimed to conduct a systematic review and meta-analysis regarding the efficacy of ERFA in combination with stent placement versus stent placement alone for the treatment of patients diagnosed with MBO.
Materials and Methods
We performed a systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines. 9 Because of the nature of the study, the requirement for ethics approval and informed consent was waived by the institutional ethics committee. The study was registered in INPLASY (202340096).
Literature search strategy
Two independent reviewers (CL and JD) searched PubMed, Embase, the Cochrane Library, and the Web of Science from their inception to April 2023. The detailed search items are presented in the Supplementary files. The language of the manuscripts was limited to English. Subsequently, the two reviewers checked each other’s lists and attempted to reach a consensus.
Selection criteria
Inclusion criteria
Studies that satisfied the following criteria were included in the meta-analysis: those including patients who were diagnosed with MBO, caused by lesions such as cholangiocarcinoma, pancreatic neoplasm, ampulla neoplasm, and gallbladder neoplasm; those including patients who were unable to undergo radical surgery at the time of diagnosis; those providing data from randomized controlled trials (RCTs) and observational studies (OSs, including prospective and retrospective studies) comparing the efficacy of RFA plus stent placement with stent placement alone; those of humans that were published in English; and those involving the use of endoscopic RFA (the ERFA procedure).
Exclusion criteria
Studies that satisfied the following criteria were excluded from the meta-analysis: those of patients who were diagnosed with non-MBO conditions, including benign and borderline diseases; reviews, case reports, single-arm studies, or protocols; those providing incomplete outcome data, including regarding overall survival, stent patency, and adverse events; animal studies and those published in other languages; and those in which a percutaneous or surgical RFA route was used.
Data extraction
Two investigators (JD and JL) independently extracted the following information from each study: the year of publication, country, study design, number of patients, age of patients, location of tumors, diameter of the biliary stricture, stent type, RFA device used, duration and amount of energy applied, overall survival, duration of stent patency, and incidence of adverse events (including cholangitis, bleeding, perforation, cholecystitis, and pancreatitis). When relevant survival data could not be acquired directly from the original study, survival was estimated using a Kaplan–Meier curve. 10 Coefficients were converted to the same format for use in the meta-analysis.11,12
Outcomes
The primary outcomes were pooled hazard ratios (HRs) for overall survival and stent patency, and the secondary outcomes were the odds ratios (ORs) for the post-ERCP adverse events listed above. Furthermore, we performed subgroup analyses according to the study type (RCT or OS), sample size (≥65 or <65), and the type of tumor (distal cholangiocarcinoma or non-distal cholangiocarcinoma).
Assessment of risk of bias
We used the Cochrane risk of bias (RoB) tool and the RoB in non-randomized studies of interventions (ROBINS-I) to evaluate RCTs and OSs, respectively.13,14 Two reviewers (CL and JD) independently assessed the RCTs with respect to random sequence generation, allocation concealment, the blinding of participants and personnel, the blinding of outcome assessment, incompleteness of the outcome data, and selective reporting. Each parameter for RCTs was classified as low risk, high risk, or unclear risk. 13 For OS, the assessment was similar to that for RCTs, with the addition of bias owing to confounding, 14 and each domain was graded as low, moderate, serious, or critical. Discussion and consultation with a third reviewer was used to resolve disagreements.
Statistical analysis
HRs were calculated for continuous datasets and ORs were calculated for categorical datasets, along with the 95% confidence intervals (CIs). Cochrane’s Q-test and the I2 statistic were used to assess statistical heterogeneity. The cut-off values used for low, moderate, and high heterogeneity were 25%, 50%, and 75%. 15 When the total heterogeneity exceeded 50%, we used a random-effect model; otherwise, a fixed-effect model was used. P < 0.05 was regarded as indicating statistical significance. To identify publication bias, we used funnel plots and Begg’s and Egger’s tests. All the analyses were conducted using Review Manager 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark).
Results
Study selection
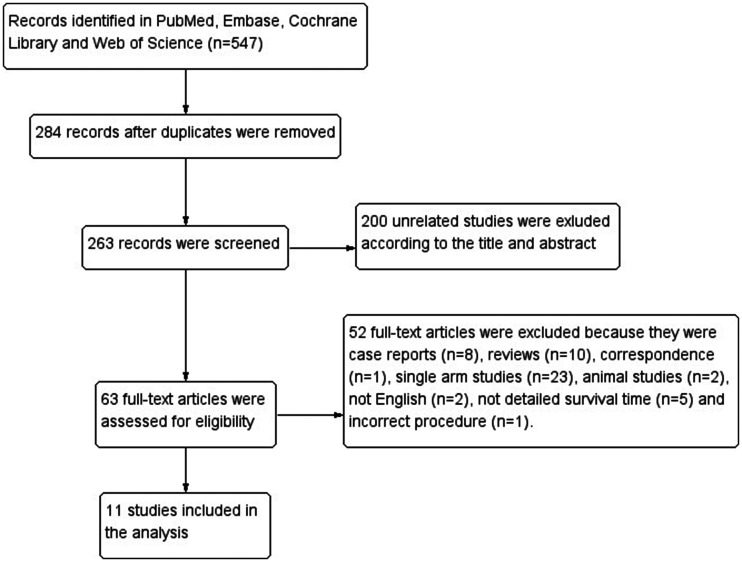
We initially identified 547 articles in PubMed, Embase, the Cochrane Library, and the Web of Science. After removing duplicates and irrelevant studies, 63 studies remained for the assessment of their full text for eligibility. Ultimately, 11 studies (4 RCTs16–19 and 7 OSs20–26) were found to be eligible for qualitative synthesis. The flow diagram of the selection process is shown in Figure 1.
Figure 1.
Flowchart of studies included in the meta-analysis.
Baseline characteristics and assessment of studies
A total of 1283 patients (434 who underwent ERFA plus stent placement and 849 who underwent stent placement only) were included in the meta-analysis. The constituent studies were published between 2015 and 2022. Cholangiocarcinoma, pancreatic neoplasm, and ampulla neoplasm accounted for 61.0%, 12.6%, and 8.7%, respectively, of the causative tumors. All of the studies used the Habib Endo HBP device (EMcision UK, London, UK; Boston Scientific, Marlborough, MA, USA) for ERFA, except one, in which the ELRA™ (EndoLuminal Radiofrequency Ablation; Taewoong Medical, Seoul, Korea) was used. 19 Metallic stents were inserted in five studies20,22–24,26 and plastic stents were inserted in six.16–19,21,25 All the studies recorded survival time, in different forms, but the duration of stent patency was recorded in only seven of the studies.16–21,26 Adverse events were recorded in nine studies.16–19,22–26 The detailed baseline characteristics of the participants are presented in Tables 1 and 2.
Table 1.
Characteristics of the included studies.
| Study | Country | Design | Group | Number of patients | Age (years) | RFA details | Diameter of stricture (mm), (median (IQR) or mean ± SD | Stent type |
|---|---|---|---|---|---|---|---|---|
| Xia et al. (2022) 23 | China | OS | R | 124 | 68 | Habib, 10–12 W, 60–120 s | NA | MS PS |
| S | 496 | 67 | NA | MS PS | ||||
| Xia et al. (2022) 24 | China | OS | R | 50 | 73 | Habib, 7–10 W, 60–120 s | NA | MS PS |
| S | 35 | 71 | NA | MS PS | ||||
| Sandru et al. (2022) 25 | Romania | OS | R | 8 | NA | Habib, 120 s | NA | PS |
| S | 17 | NA | NA | PS | ||||
| Oh et al. (2022) 26 | Korea | OS | R | 28 | 69 | Habib, 7–10 W, 120 s | NA | Uncovered SEMS |
| S | 51 | 65 | NA | Uncovered SEMS | ||||
| Kang et al. (2022) 19 | China | RCT | R | 15 | 76 | ELRA, 7 W, 60–120 s | 2.1 (1.5–2.9); 2.5 (1.5–3.0) | PS |
| S | 15 | 72 | 1.7 (1.2–2.2); 1.8 (1.3–2.6) | PS | ||||
| Gao et al. (2021) 18 | China | RCT | R | 87 | 69 | Habib, 7–10 W, 90 s | 2.5 (1.8–3.2) | PS |
| S | 87 | 68 | 2.6 (1.6–3.5) | PS | ||||
| Bokemeyer et al. (2019) 22 | Germany | OS | R | 20 | 68 | NA | NA | MS PS |
| S | 22 | 66 | NA | MS PS | ||||
| Dutta et al. (2017) 21 | England | OS | R | 15 | 78 | Habib, 7–10 W, 90 s | NA | MS |
| S | 16 | 76 | NA | MS | ||||
| Yang et al. (2017) 17 | China | RCT | R | 32 | 62 | Habib, 7–10 W, 90 s | 3.6 ± 0.5 | PS |
| S | 33 | 65 | 3.7 ± 0.9 | PS | ||||
| Hu et al. (2016) 16 | China | RCT | R | 32 | 72 | Habib, 7–10 W, 60–90 s | NA | PS |
| S | 31 | 71 | NA | PS | ||||
| Kallis et al. (2015) 20 | England | OS | R | 23 | 69 | Habib, 10 W, 120 s | NA | Uncovered SEMS |
| S | 46 | 70 | NA | Uncovered SEMS |
OS, observational study; RCT, randomized controlled study; R, radiofrequency; S, stent-alone; MS, metallic stent; PS, plastic stent; SEMS, self-expandable metallic stent; NA not available; IQR, interquartile range; SD, standard deviation.
Table 2.
Further characteristics of the included studies.
| Study | Group | Location of tumors | Concomitant Chemotherapy | OS (months) | HR for OS | SPT (months) | HR for SPT | Adverse events |
|---|---|---|---|---|---|---|---|---|
| Xia et al. (2022) 23 | R | CCA 79, GC 12, HCC 16, PC 8, ICC 7 | 12 (9.7%) | 9.5a | 0.72c | NA | NA | Cholangitis 8, Hemorrhage 2, Perforation 1, Cholecystitis 6, Pancreatitis 11 |
| S | CCA 256, GC 76, HCC 50, PC 70, ICC 37 | 72 (14.5%) | 6.1a | NA | Cholangitis 51, Hemorrhage 6, Cholecystitis 1, Pancreatitis 27 | |||
| Xia et al. (2022) 24 | R | AC 50 | 2 (4.0%) | 16.9a | 0.45c | NA | NA | Cholangitis 2, Hemorrhage 2, Pancreatitis 1 |
| S | AC 35 | 2 (5.7%) | 9.8a | NA | Cholangitis 1 | |||
| Sandru et al. (2022) 25 | R | CCA 8 | NA | 19a | 0.88c | NA | NA | Cholangitis 1 |
| S | CCA 17 | NA | 16a | NA | Cholangitis 0 | |||
| Oh et al. (2022) 26 | R | CCA 26, GC 2 | 20 (71.4%) | 10.4b | 1.16c | 4.7b | 1.14c | Cholangitis 2 |
| S | CCA 42, GC 9 | 41 (80.4%) | 10.4b | 6.4b | Cholangitis 1 | |||
| Kang et al. (2022) 19 | R | CCA 13, GC 2 | 2 (13.3%) | 7.7a | 0.65c | 5.9a | 0.53c | Cholangitis 3, Cholecystitis 1 |
| S | CCA 13, GC 2 | 8 (53.3%) | 4.8a | 4.1a | Cholangitis 5, Cholecystitis 1, Pancreatitis 1 | |||
| Gao et al. (2021) 18 | R | CCA 69, AC 18 | 0 (0.0%) | 14.3a | 0.49 | 13.3a | 1.07 | Cholangitis 10, Hemorrhage 1, Perforation 9, Pancreatitis 4 |
| S | CCA 78, AC 9 | 1 (1.1%) | 9.2a | 9.2a | Cholangitis 9, Hemorrhage 3, Pancreatitis 5 | |||
| Bokemeyer et al. (2019) 22 | R | CCA 20 | 6 (30.0%) | 11.4b | 0.60c | NA | NA | NA |
| S | CCA 22 | 7 (31.8%) | 7.4b | NA | NA | |||
| Dutta et al. (2017) 21 | R | CCA 7, PC 6, other 2 | NA | 7.3a | 0.53c | 7.3a | 2.40 | NA |
| S | CCA 6, PC 8, other 2 | NA | 4.9a | 3.6a | NA | |||
| Yang et al. (2017) 17 | R | CCA 10, DC 22 | 0 (0.0%) | 13.2b | 0.34c | 6.8a | NA | Cholangitis 2 |
| S | CCA 9, DC 24 | 0 (0.0%) | 8.3b | 3.4a | Cholangitis 1, Bleeding 1, Pancreatitis 1 | |||
| Hu et al. (2016) 16 | R | CCA 32 | NA | 10.4a | 0.47c | 5a | 0.90c | Cholangitis 4, Bleeding 1, Pancreatitis 2 |
| S | CCA 31 | NA | 5.7a | 3.9a | Cholangitis 6, Pancreatitis 2 | |||
| Kallis et al. (2015) 20 | R | PC 23 | 16 (69.6%) | 7.5a | 0.66 | 15.7a | 1.19 | NA |
| S | PC 46 | 24 (52.2%) | 4.1a | 10.8a | NA |
R, radiofrequency; S, stent-alone; CCA, cholangiocarcinoma; GC, gallbladder carcinoma; HCC, hepatocellular carcinoma; PC, pancreatic carcinoma; ICC, intrahepatic cholangiocarcinoma; AC, ampullary carcinoma; DC, duodenal carcinoma; OS, overall survival; HR, hazard ratio; SPT, duration of stent patency; NA, not available. aMedian; bMean; cHR calculated using the Kaplan–Meier curve.
All the RCTs were graded as having low overall risk of bias except for one (Hu et al. 16 ), which was graded as having an unclear risk, because of incomplete information. For the OSs, all the studies were graded having moderate risk of bias. The detailed results of the risk of bias analysis are shown in Tables S1 and S2.
Primary outcomes
Pooled HRs for overall survival
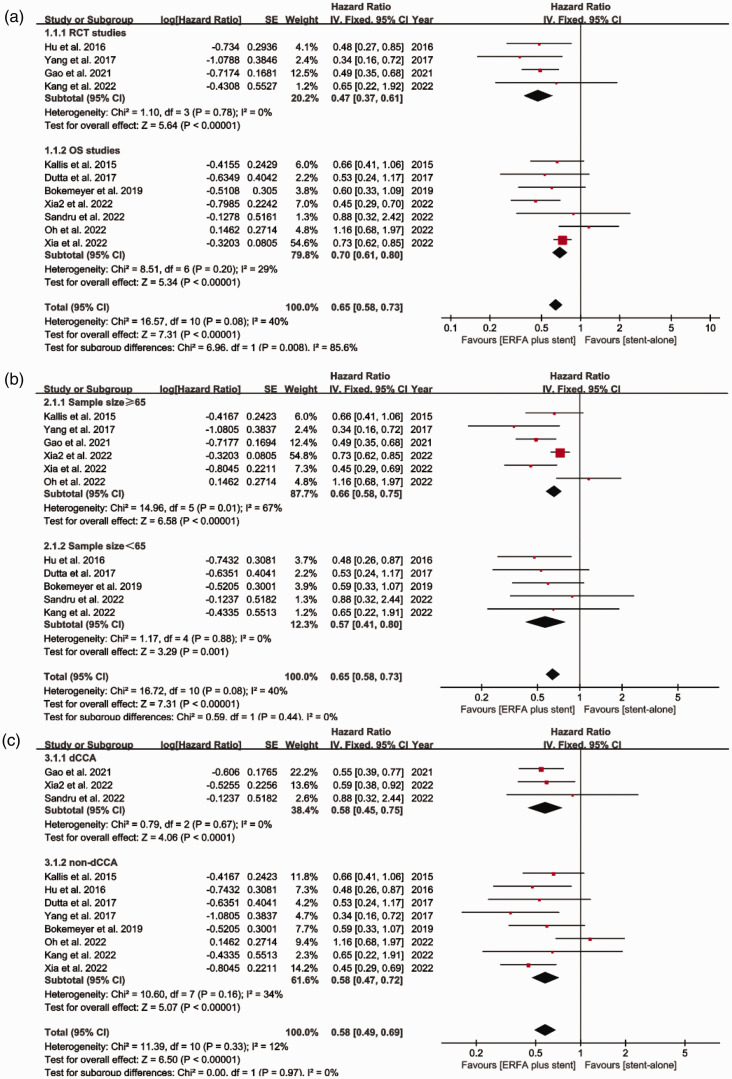
All the studies recorded the overall duration of survival of the participants. The pooled HR for overall survival was 0.65 [95% CI 0.58 to 0.73, I2 = 40%, P < 0.00001] (Figure 2). Subgroup analyses according to the type of study, the sample size, and the type of tumor also demonstrated that the use of ERFA plus stent implantation was associated with a significant survival advantage (Figure 3a–c).
Figure 2.
Forest plot for the comparison of the overall survival of patients who underwent ERFA plus stent placement or stent placement alone. ERFA, endoscopic radiofrequency ablation; SE, standard error; CI, confidence interval; IV, instrumental variable.
Figure 3.
Results of the subgroup analyses of the comparison between ERFA plus stent placement and stent placement alone with respect to overall survival, according to (a) Type of study, (b) Sample size, and (c) Type of tumor. ERFA, endoscopic radiofrequency ablation; SE, standard error; CI, confidence interval; IV, instrumental variable.
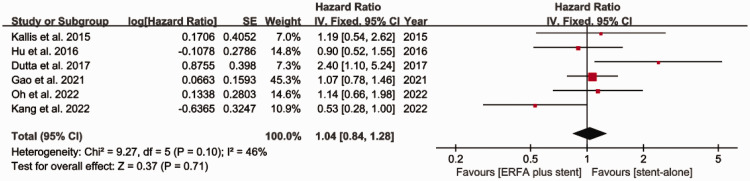
Pooled HRs for the duration of stent patency
Six studies (three RCTs and three OSs) compared the duration of stent patency of the two groups. The pooled HR for the duration of stent patency was 1.04 [95% CI 0.84 to 1.28, I2 = 46%], indicating no significant difference between the two groups (Figure 4). Subgroup analyses according to the type of study and the sample size generated similar results (Figure 5a and b).
Figure 4.
Forest plot for the comparison between ERFA plus stent placement versus stent placement alone with respect to the duration of stent patency. ERFA, endoscopic radiofrequency ablation; SE, standard error; CI, confidence interval; IV, instrumental variable.
Figure 5.
Results of the subgroup analyses of the comparison between ERFA plus stent placement and stent placement alone with respect to the duration of stent patency, according to (a) Type of study and (b) Sample size. ERFA, endoscopic radiofrequency ablation; SE, standard error; CI, confidence interval; IV, instrumental variable.
Secondary outcomes
Pooled ORs for post-ERCP adverse events
The meta-analysis showed no significant differences between the groups with respect to the incidences of pancreatitis [OR 1.32, 95% CI 0.75 to 2.32, I2 = 0%] (Figure 6a), cholangitis [OR 1.02, 95% CI 0.66 to 1.57, I2 = 19%,] (Figure 6b), hemorrhage [OR 1.04, 95% CI 0.39 to 2.76, I2 = 0%] (Figure 6c), and the adverse events as a whole [OR 1.41, 95% CI 1.02 to 1.96, I2 = 29%] (Figure 6e). However, there was a significant difference with respect to the incidence of cholecystitis [OR 11.34, 95% CI 2.88 to 44.59, I2 = 42%, P = 0.0005] (Figure 6d). The subgroup analyses according to the type of study, the sample size, and the type of tumor also showed no differences between the groups (Fig. S1−S5).
Figure 6.
Forest plot for the comparison between ERFA plus stent and stent alone with respect to post-ERCP adverse events. (a) Pancreatitis. (b) Cholangitis. (c) Hemorrhage. (d) Cholecystitis. (e) All adverse events. ERFA, endoscopic radiofrequency ablation. ERCP, endoscopic retrograde cholangiopancreatography; SE, standard error; CI, confidence interval; IV, instrumental variable.
Publication bias and the results of the sensitivity analysis
We used funnel plots and Egger’s test to evaluate the possibility of publication bias. The symmetry of the funnel plots implied no significant risk of publication bias (Fig. S6 and S7), and Egger’s test showed that the publication bias was minimal (survival, P = 0.062; duration of stent patency, P = 0.867) (Tables S3 and S4). A sensitivity analysis was conducted by reanalyzing the data after removing each study in turn, which did not affect the outcomes.
Discussion
MBO is a relatively rare malignancy-related disease, and its treatment has represented a challenge for clinicians all over the world for many years. Owing to its occult symptoms, MBO is often diagnosed when it has reached an advanced stage, meaning that the opportunity for treatment by means of radical surgical resection is often missed.1,2 Therefore, palliative options, including biliary stent placement, RFA, and photodynamic therapy (PDT), have been the conventional treatments for MBO. However, stent placement alone may be associated with a short duration of patency, owing to blockage. RFA is cheaper and is associated with fewer side effects than PDT, and therefore it has been gaining the attention of endoscopists and hepatobiliary surgeons during recent years. 27 In the present study, we compared the efficacy of ERFA in combination with biliary stent placement with stent placement alone for the treatment of MBO.
The present meta-analysis showed a statistically significant superiority with respect to survival time for the combination treatment, with a pooled HR of 0.65 [95% CI 0.58 to 0.73, P < 0.00001] vs. stent placement alone. Furthermore, the subgroup analyses according to the type of study, sample size, and type of tumor generated consistent results, which strengthens the reliability of the main finding. The significant survival advantage may be attributed to the ability of this combination treatment to prevent the recurrence of cholangitis and relieve biliary obstruction.
The efficacy of ERFA in combination with stent placement for the treatment of patients with MBO has also been assessed in previous meta-analyses. Zheng et al. 28 revealed that ERFA is effective and safe for the management of unresectable MBO. However, all the studies included in this analysis were OSs that had been performed many years ago, which reduced the reliability and generalizability of the conclusions. According to the study conducted by Cha et al. 29 , there is no survival advantage associated with endobiliary RFA vs. stent placement alone. We speculate that this was because relatively few data were included in this study. Finally, and consistent with the present findings, Rebhun et al. 8 found that the use of ERFA for the treatment of unresectable cholangiocarcinoma was associated with superior survival in a recent meta-analysis. In the present study, we found that this survival advantage extended to patients who had been diagnosed with MBO.
In terms of stent patency, we found no advantage of ERFA in combination with stent placement, with a pooled HR of 1.04 [95% CI 0.84 to 1.29]. We consider that there are two potential reasons for this. First, data from only six studies were included in the analysis, and some of these were obtained through Kaplan–Meier curve transformation, which may have affected the overall result. Second, in five of the studies, the stents placed in the bile ducts were made of plastic, which may have been associated with a shorter period of patency than the use of metal stents, thereby reducing the efficacy of ERFA. Therefore, in the future, additional large, multicenter RCTs should be performed to verify the efficacy of ERFA with respect to stent patency.
A careful review of all the included studies revealed apparent inconsistencies between the durations of survival and stent patency reported in the publications by Gao et al. 18 and Oh et al. 26 : the ERFA group in each survived longer but had a shorter duration of stent patency. Therefore, the survival advantage associated with ERFA may not be the result of a longer duration of stent patency. Although the mechanism by which ERFA prolongs survival remains unclear, we can speculate that it involves a specific anti-cancer effect. The ablative process may induce a systemic immune response that is amplified by immune-modulating agents, resulting in superior clinical outcomes. Such an effect has been demonstrated previously with respect to other cancers, such as colorectal liver metastasis and hepatocellular carcinoma.30,31
Because RFA is an emerging technique that involves the transmission of heat through a catheter into the bile duct, it carries the potential risk of damaging the walls of the bile duct and adjacent blood vessels. 32 Indeed, a few cases of thermal damage have been reported previously. For instance, Tal et al. 33 reported two cases of mortality associated with hemobilia, and as a result, recommended the use of metal stents in preference to plastic ones. In addition, Dolak et al. 34 reported a case of hepatic infarction caused by withdrawal hemorrhage during an RFA procedure. However, in the present pooled analysis of adverse events, including thermal injury, we did not find any significant differences between the combination treatment group and the stent placement group, except with respect to cholecystitis. This higher risk of cholecystitis was also found by Rebhun et al. 8 in their meta-analysis of cases of unresectable cholangiocarcinoma. We speculate that this higher risk may be associated with the inevitable damage caused to the wall of the bile duct during the RFA procedure. According to the results of the follow-up studies of Gao et al. 18 and Xia et al. 23 , all cases of RFA-induced cholecystitis can be successfully managed by timely treatment with antibiotics and percutaneous transhepatic gallbladder drainage, with a good prognosis. The other complications identified in the present study, including cholangitis, hemorrhage, and pancreatitis, were mild-to-moderate and could be treated successfully in a conservative fashion. Therefore, it is reasonable for us to conclude that ERFA is an effective and safe means of treating MBO. However, these findings require confirmation in large-scale RCTs.
The present meta-analysis had the following limitations. First, although most of the studies we included were recent and of high quality, they were few in number (four RCTs and seven OSs). Second, not all the survival and stent patency data were obtained directly from the original publications; some required conversion using a Kaplan−Meier survival curve. This would have affected the final pooled outcomes to some extent. Third, the use of concomitant anti-tumor therapies, such as palliative chemotherapy, was not described in some of the reports, which might also have influenced the results of the pooled analyses. Fourth, the subgroup analyses conducted were limited in number. Finally, owing to the lack of survival analysis data for all the types of tumors in the included studies, we only performed subgroup analyses based on a categorization of the type of tumor as distal cholangiocarcinoma or non-distal cholangiocarcinoma. The tumor site affects the clinical outcomes and survival of patients. In addition, we could not perform a subgroup analysis according to the type of stent used, which might have influenced the efficacy of ERFA.
Conclusion
Despite these limitations, in the present systematic review and meta-analysis of four RCTs and seven OSs, we have shown a significant survival advantage of ERFA in combination with stent placement for patients who are diagnosed with MBO. In addition, the duration of stent patency and the risk of postoperative adverse events were found to be comparable to those associated with stent placement alone. In the future, large, multicenter RCTs should be performed to confirm the benefits of ERFA in patients with tumors at various sites.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605231220825 for Is endoscopic radiofrequency ablation plus stent placement superior to stent placement alone for the treatment of malignant biliary obstruction? A systematic review and meta-analysis by Chenming Liu, Jiaming Dong, Yuxing Liu, Siyuan Zhang, Ruanchang Chen and Haijun Tang in Journal of International Medical Research
Author Contributions: CL wrote the manuscript. CL and JD searched the database. JD and YL extracted the data and conducted the statistical analysis. RC, SZ, and YL reviewed the manuscript. HT reviewed the manuscript and approved its submission.
The authors declare that there is no conflict of interest.
Funding: This work was funded by a Shaoxing Basic Public Welfare Project (No. 2022A14012) and a Shaoxing Health Science and Technology Project (Laboratory opening plan) (No. 2022SY013).
ORCID iDs: Chenming Liu https://orcid.org/0009-0003-3622-0400
Yuxing Liu https://orcid.org/0009-0008-7564-0092
Data Availability statement
Data for this study can be obtained from the corresponding author at reasonable request.
References
- 1.Sawas T, Al Halabi S, Parsi MA, et al. Self-expandable metal stents versus plastic stents for malignant biliary obstruction: a meta-analysis. Gastrointest Endosc 2015; 82: 256–267.e7. doi:10.1016/j.gie.2015.03.1980 [DOI] [PubMed] [Google Scholar]
- 2.Launois B, Reding R, Lebeau G, et al. Surgery for hilar cholangiocarcinoma: French experience in a collective survey of 552 extrahepatic bile duct cancers. J Hepatobiliary Pancreat Surg 2000; 7: 128–134. doi:10.1007/s005340050166 [DOI] [PubMed] [Google Scholar]
- 3.Kahaleh M, Tokar J, Conaway MR, et al. Efficacy and complications of covered Wallstents in malignant distal biliary obstruction. Gastrointest Endosc 2005, 61: 528–533. doi:10.1016/s0016-5107(04)02593-3 [DOI] [PubMed] [Google Scholar]
- 4.Mönkemüller K, Popa D, Wilcox CM. Endoscopic treatment options for cholangiocarcinomas. Expert Rev Anticancer Ther 2014; 14: 407–418. doi:10.1586/14737140.2014.870480 [DOI] [PubMed] [Google Scholar]
- 5.Rustagi T, Jamidar PA. Intraductal radiofrequency ablation for management of malignant biliary obstruction. Dig Dis Sci 2014; 59: 2635–2641. doi:10.1007/s10620-014-3237-9 [DOI] [PubMed] [Google Scholar]
- 6.Itoi T, Isayama H, Sofuni A, et al. Evaluation of effects of a novel endoscopically applied radiofrequency ablation biliary catheter using an ex-vivo pig liver. J Hepatobiliary Pancreat Sci 2012; 19: 543–547. doi:10.1007/s00534-011-0465-7 [DOI] [PubMed] [Google Scholar]
- 7.Shah DR, Green S, Elliot A, et al. Current oncologic applications of radiofrequency ablation therapies. World J Gastrointest Oncol 2013; 5: 71–80. doi:10.4251/wjgo.v5.i4.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rebhun J, Shin CM, Siddiqui UD, et al. Endoscopic biliary treatment of unresectable cholangiocarcinoma: A meta-analysis of survival outcomes and systematic review. World J Gastrointest Endosc 2023; 15: 177–190. doi:10.4253/wjge.v15.i3.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021; 372: n160. doi:10.1136/bmj.n160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8: 16. doi:10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo D, Wan X, Liu J, et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018; 27: 1785–1805. doi:10.1177/0962280216669183 [DOI] [PubMed] [Google Scholar]
- 12.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. doi:10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. doi:10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355: i4919. doi:10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. doi:10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 16.Hu B, Gao DJ, Zhang X, et al. Endobiliary radiofrequency ablation improve overall survival of cholangiocarcinoma: a multi-center randomized control study. Gastrointest Endosc 2016; 83: AB126. doi:10.1016/j.gie.2016.03.046 [Google Scholar]
- 17.Yang J, Wang J, Zhou H, et al. Efficacy and safety of endoscopic radiofrequency ablation for unresectable extrahepatic cholangiocarcinoma: a randomized trial. Endoscopy 2018; 50: 751–760. doi:10.1055/s-0043-124870 [DOI] [PubMed] [Google Scholar]
- 18.Gao DJ, Yang JF, Ma SR, et al. Endoscopic radiofrequency ablation plus plastic stent placement versus stent placement alone for unresectable extrahepatic biliary cancer: a multicenter randomized controlled trial. Gastrointest Endosc 2021; 94: 91–100.e2. doi:10.1016/j.gie.2020.12.016 [DOI] [PubMed] [Google Scholar]
- 19.Kang H, Han SY, Cho JH, et al. Efficacy and safety of temperature-controlled intraductal radiofrequency ablation in advanced malignant hilar biliary obstruction: A pilot multicenter randomized comparative trial. J Hepatobiliary Pancreat Sci 2022; 29: 469–478. doi:10.1002/jhbp.1082 [DOI] [PubMed] [Google Scholar]
- 20.Kallis Y, Phillips N, Steel A, et al. Analysis of endoscopic radiofrequency ablation of biliary malignant strictures in pancreatic cancer suggests potential survival benefit. Dig Dis Sci 2015; 60: 3449–3455. doi:10.1007/s10620-015-3731-8 [DOI] [PubMed] [Google Scholar]
- 21.Dutta AK, Basavaraju U, Sales L, et al. Radiofrequency ablation for management of malignant biliary obstruction: a single-center experience and review of the literature. Expert Rev Gastroenterol Hepatol 2017; 11: 779–784. doi:10.1080/17474124.2017.1314784 [DOI] [PubMed] [Google Scholar]
- 22.Bokemeyer A, Matern P, Bettenworth D, et al. Endoscopic radiofrequency ablation prolongs survival of patients with unresectable hilar cholangiocellular carcinoma-a case-control study. Sci Rep 2019; 9: 13685. doi:10.1038/s41598-019-50132-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia MX, Wang SP, Yuan JG, et al. Effect of endoscopic radiofrequency ablation on the survival of patients with inoperable malignant biliary strictures: a large cohort study. J Hepatobiliary Pancreat Sci 2022; 29: 693–702. doi:10.1002/jhbp.960 [DOI] [PubMed] [Google Scholar]
- 24.Xia MX, Shi ZM, Xing L, et al. Endoscopic radiofrequency ablation may improve overall survival in patients with inoperable ampullary carcinoma. Dig Endosc 2022; 34: 587–595. doi:10.1111/den.14078 [DOI] [PubMed] [Google Scholar]
- 25.Sandru V, Ungureanu BS, Stan-Ilie M, et al. Efficacy of endobiliary radiofrequency ablation in preserving survival, performance status and chemotherapy eligibility of patients with unresectable distal cholangiocarcinoma: a case-control study. Diagnostics (Basel) 2022; 12: 1804. doi:10.3390/diagnostics12081804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh D, Chong J, Song TJ, et al. The usefulness of endobiliary radiofrequency ablation before metal stent placement in unresectable malignant hilar obstruction. J Gastroenterol Hepatol 2022; 37: 2083–2090. doi:10.1111/jgh.15967 [DOI] [PubMed] [Google Scholar]
- 27.Takenaka M, Lee TH. Role of radiofrequency ablation in advanced malignant hilar biliary obstruction. Clin Endosc 2023; 56: 155–163. doi:10.5946/ce.2022.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng X, Bo ZY, Wan W, et al. Endoscopic radiofrequency ablation may be preferable in the management of malignant biliary obstruction: a systematic review and meta-analysis. J Dig Dis 2016; 17: 716–724. doi:10.1111/1751-2980.12429 [DOI] [PubMed] [Google Scholar]
- 29.Cha BH, Jang MJ, Lee SH. Survival benefit of intraductal radiofrequency ablation for malignant biliary obstruction: a systematic review with meta-analysis. Clin Endosc 2021; 54: 100–106. doi:10.5946/ce.2020.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.den Brok MH, Sutmuller RP, van der Voort R, et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res 2004; 64: 4024–4029. doi:10.1158/0008-5472.CAN-03-3949 [DOI] [PubMed] [Google Scholar]
- 31.Hansler J, Wissniowski TT, Schuppan D, et al. Activation and dramatically increased cytolytic activity of tumor specific T lymphocytes after radio-frequency ablation in patients with hepatocellular carcinoma and colorectal liver metastases. World J Gastroenterol 2006; 12: 3716–3721. doi:10.3748/wjg.v12.i23.3716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laquière A, Boustière C, Leblanc S, et al. Safety and feasibility of endoscopic biliary radiofrequency ablation treatment of extrahepatic cholangiocarcinoma. Surg Endosc 2016; 30: 1242–1248. doi:10.1007/s00464-015-4322-7 [DOI] [PubMed] [Google Scholar]
- 33.Tal AO, Vermehren J, Friedrich-Rust M, et al. Intraductal endoscopic radiofrequency ablation for the treatment of hilar non-resectable malignant bile duct obstruction. World J Gastrointest Endosc 2014; 6: 13–19. doi:10.4253/wjge.v6.i1.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dolak W, Schreiber F, Schwaighofer H, et al. Endoscopic radiofrequency ablation for malignant biliary obstruction: a nationwide retrospective study of 84 consecutive applications. Surg Endosc 2014; 28: 854–860. doi:10.1007/s00464-013-3232-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605231220825 for Is endoscopic radiofrequency ablation plus stent placement superior to stent placement alone for the treatment of malignant biliary obstruction? A systematic review and meta-analysis by Chenming Liu, Jiaming Dong, Yuxing Liu, Siyuan Zhang, Ruanchang Chen and Haijun Tang in Journal of International Medical Research
Data Availability Statement
Data for this study can be obtained from the corresponding author at reasonable request.