Abstract
Mesenchymal stem cells/multipotent stromal cells (MSCs) are promising therapeutics for a variety of conditions. However, after transplantation, cell retention remains extremely challenging. Given that many hypoxic signals are transitory and that the therapeutic administration of MSCs is typically into tissues that are normally hypoxic, we studied the effect of hypoxic preconditioning (HP) prior to new exposure to hypoxia. We show that preincubation for 2 days or more in 1% oxygen reduces serum deprivation-mediated cell death, as observed by higher cell numbers and lower incorporation of EthD-III and Annexin V. Consistently, HP-MSCs expressed significantly lower levels of cytochrome c and heme oxygenase 1 as compared to controls. Most importantly, HP-MSCs showed enhanced survival in vivo after intramuscular injection into immune deficient NOD/SCID-IL2Rgamma−/− mice. Interestingly, HP-MSCs consume glucose and secrete lactate at a slower rate than controls, possibly promoting cell survival, as glucose remains available to the cells for longer periods of time. In addition, we compared the metabolome of HP-MSCs to controls, before and after hypoxia and serum deprivation, and identified several possible mediators for HP-mediated cell survival. Overall, our findings suggest that preincubation of MSCs for 2 days or more in hypoxia induces metabolic changes that yield higher retention after transplantation.
Keywords: Mesenchymal stromal cells, Hypoxia, Metabolism, Survival
Introduction
Mesenchymal stem cells/multipotent stromal cells (MSCs) have become widely used in the field of regenerative medicine to treat a variety of conditions [1, 2]. In some cases, such as in bone and cartilage repair, they are used to directly differentiate and replace damaged tissue. But many other clinical applications of MSCs rely on the paracrine activity of the cells, for example, to modulate immune response, induce angiogenesis, or promote wound repair [3, 4]. In this context, a major limitation is the poor retention of the cells after administration [5].
MSCs reside in tissues such as bone marrow or adipose tissue, which exhibit low oxygen tension [6, 7], but are commonly expanded in the laboratory under normoxic conditions (20.8% atmospheric oxygen, 160 mmHg oxygen in solution). Thus, it has been of great relevance to address the effects of hypoxia on MSCs (reviewed in [8–10]). Conversely, it also has to be considered that, in therapeutic applications, MSCs are typically transplanted into hypoxic sites; oxygen tension in tissues is 4–20 mmHg, while arterial oxygen is 104 mmHg [11]. Here, we examine the effects of exposing MSCs to various degrees of hypoxia as well as the effects of hypoxic exposure prior to their administration to a hypoxic site, which we refer to as hypoxic preconditioning (HP). It has been previously demonstrated that HP increases the therapeutic potential of MSCs in applications such as treatment of cardiac ischemia [12, 13], critical limb ischemia [14–17], traumatic brain injury [18], and in liver regeneration [19]. It has also been proposed that increased retention of MSCs induced by HP is a key factor in enhancing tissue repair [12, 15–17], but the underlying mechanisms remain elusive.
We sought to determine the optimal hypoxia levels and incubation times to promote survival of MSCs in an environment of low oxygen and nutrients. In addition, we hypothesized that HP promotes metabolic adaptations in MSCs that allow increased survival under conditions of limited nutrients and oxygen.
Materials and Methods
MSC Isolation and Culture
Bone marrow aspirates from human donors were purchased from Lonza (#1M-125) or Stem Express (#BM0901F). As previously described [20], bone marrow aspirates were passed through 90 μm pore strainers for isolation of bone spicules. Then, the strained bone marrow aspirates were diluted with an equal volume of phosphate-buffered saline (PBS) and centrifuged over Ficoll (#17-1440-03, GE Healthcare) for 30 minutes at 700g. Next, mononuclear cells and bone spicules were plated in plastic culture flasks using standard culture medium (minimum essential medium a [MEMa] #SH30265.01, HyClone) supplemented with 10% fetal bovine serum (FBS; #S11550, Atlanta Biologicals). After 2 days, nonadherent cells were removed by washing twice with PBS. MSCs from passages 3–6 were used for experimentation, where each passage implies 5–7 days in culture (i.e., approximately three population doublings). For the immune characterization of MSCs by flow cytometry, cells were lifted by trypsin treatment and incubated for 45 minutes with the fluorophore-conjugated antibodies (diluted 1:100) indicated in Supporting Information Figure S1. For studies with hypoxia, cell cultures were performed in incubators (MCO-18M, Sanyo) at 37°C with 5% CO2, humidified atmosphere, and dedicated oxygen level (20% [atmospheric], 10%, 5%, or 1% O2), as established by replacement with nitrogen injections.
Cell Proliferation and Cell Cycle Analysis
For proliferation assays, MSCs were seeded into 12-well-plates at 1,000 cells per square centimeter. At every time point (2–3 days), cells were lifted with trypsin treatment and counted using Trypan blue exclusion dye (#15250–061, Gibco) and a hemocytometer.
To determine the status in cell cycle, MSCs were seeded into 75 cm2 culture flasks at 500 cells per square centimeter and incubated in standard culture medium with varying levels of hypoxia for 4 days, with media change at day 2. Then, cells were lifted by trypsin treatment and permeabilized with ice-cold 70% ethanol overnight. After 45 minutes incubation with a 50 μg/ml propidium iodide solution (#13301000, Roche) containing 20 ng/ml RNase A (#19101, Qiagen), followed by a washing step with PBS, samples were inspected by flow cytometry and analyzed using ModFit LT (Verity Software House).
Osteogenic and Adipogenic Differentiation Assays
For both osteogenesis and adipogenesis, 10,000 MSCs/cm2 were cultured for 14 days in incubators with varying hypoxia levels (20%, 10%, 5%, and 1% O2), with media changes every 3 days. Osteogenic medium consisted of standard culture medium supplemented with 0.2 mM ascorbic acid, 0.1 μM dexamethasone, and 20 mM β-glycerolphosphate. Adipogenic medium was standard culture medium with 0.5 mM isobutylmethylxanthine, 50 μM indomethacin, and 0.5 μM dexamethasone.
To quantify osteogenesis, alkaline phosphatase (ALP) activity was measured by lifting the cells with trypsin and lysing the cells for protein extraction using 1.5 mM Tris-HCl solution containing 1.0 mM ZnCl2, 1.0 mM MgCl2, and 1% Triton X-100 for 15 minutes. Lysates were then centrifuged at 12,000g for 10 minutes and incubated with p-nitrophenylphosphate liquid substrate solution (#3368-04-5, Sigma-Aldrich) for 30 minutes. Released p-nitrophenolate was determined spectrophotometrically at 405 nm. Calcium precipitation was determined using Alizarin Red S indicator (ARS; #500–4, Ricca Chemicals). Cells were fixed with 10% vol/vol formalin solution for 15 minutes, washed once with PBS, and stained for 20 minutes with 1% wt/vol ARS over gentle shaking. Samples were then photographed for visual documentation and then incubated with 10% vol/vol acetic acid for 30 minutes, the cell layer scraped, vortexed for 30 seconds, then centrifuged at 12,000g for 10 minutes. Optic density of the supernatants was measured at 405 nm. For both, ALP and ARS, total protein concentration was determined with Coomassie staining and measured at 595 nm. To determine expression of osteogenic markers, total RNA was extracted using a Direct-zol RNA MiniPrep kit (#R2050, Zymo Research). Reverse transcription was performed using Taqman Reverse Transcription Reagents (#N808–0234, Life Technologies), mRNA levels measured by real-time PCR using SYBRGreen (#4385612, Life Technologies), and primers indicated in the legend of Supporting Information Figure S3.
For adipogenesis, cells were fixed with 10% vol/vol formalin for 15 minutes, rinsed once with PBS, and stained for 30 minutes with oil red O (#26609–01, Electron Microscopy Sciences). Cells were then washed three times with PBS, photographed, and incubated with 4% Tween 20 (#9005-64-5, Affymetrix) in isopropanol for 5 minutes, in order to release the dye. Optic density of supernatants was then measured at 490 nm. To measure expression of adipogenic markers, total RNA and reverse transcription were performed as described above. Real-time PCR was done using Applied Biosystems taq-man, using the sets shown in the respective figure legend.
In Vitro Cell Survival and Apoptosis Assays
To determine survival of MSCs in hypoxia and serum deprivation we first incubated MSCs (10,000 cells per square centimeter) in 12-well plates in standard culture medium and 1% O2, for varying times (0, 16, 48, or 96 hours). Then, media were changed to MEMa alone (without FBS) and all plates were transferred to 1% O2 for up to 12 days with no additional media changes. Every 3 days, cells were detached by trypsin treatment and counted using Trypan blue exclusion dye and a hemocytometer.
To quantify the percentage of dead cells, MSCs were cultured on glass coverslips (10,000 cells per square centimeter) and incubated for 48 hours in standard culture medium in 20% O2 (control) or 1% O2 (HP). Then, medium was changed to serum-free medium and plates were transferred to 1% O2 for 9 days with no additional media changes. Coverslips were then incubated in 4 μM Ethidium homodimer III (EthD-III; #99905, Biotium) in PBS for 30 minutes and then mounted on slides with Vectashield Mounting Medium with DAPI (#H-1200, Vector Laboratories). Images were acquired and processed for large-field merge using a BZ-9000 (BIOREVO) fluorescence microscope (Keyence) and analyzed for automated counting with NIS-Elements BR software (Nikon).
To quantify the percentage of apoptotic cells, MSCs were cultured as described above for EthD-III, but in six-well plates. At day 9, cells were lifted using trypsin and stained with PE Annexin V Apoptosis Detection Kit I (#559763, BD Pharmin-gen) following manufacturer’s instructions and measured by flow cytometry.
Apoptosis Array and Western Blot
To determine differences in apoptotic pathways, samples were prepared as follows: MSCs were incubated in standard culture medium in 20% or 1% O2 for 48 hours, then medium was changed to MEMa alone (without FBS) and all plates transferred to 1% O2. After 3 days, cells were lysed for protein extraction using RIPA Buffer (#89901, Thermo Scientific) containing 1% Halt Proteinase and Phosphatase Inhibitor Cocktail (#1861281, Thermo Scientific). Proteins were further extracted by strong agitation for 20 minutes on ice, then centrifuged at 12,000g for 10 minutes and stored at −80°C. Protein extracts were then analyzed using a Human Apoptosis Antibody Array (#ARY009, R&D Systems) following manufacturer’s instructions.
For Western blots, MSC culture and protein extracts were performed as described for the Apoptosis Proteome Array. Protein extracts (30 μg) were loaded into polyacrylamide gels (#456–8094, BioRad) and transferred into polyvinylidene fluoride membranes (#162–0174, BioRad). Blots were then incubated with anti-cytochrome c (1:200, #SC-7159, Santa Cruz) and anti-heme oxygenase 1 (1:200, #SC-10789, Santa Cruz) overnight. After protein detection, both membranes were stripped (#46430, Thermo Scientific) and incubated with anti-actin (1:1,000, #A5441, Sigma-Aldrich).
In Vivo Retention Study
In order to generate luciferase-expressing MSCs, we used a third generation lentiviral vector with the general form pCCLc-MNDU3-Luciferase-PGK-eGFP-WPRE. We transduced the cells using protamine sulfate (20 μg/ml) and a quantity of virus equivalent to a multiplicity of infection of 20. Using this protocol, more than 90% of cells were eGFP positive. For cell administration, immune compromised NOD/SCID-IL2Rγ−/− (NSG) mice were anesthetized using inhaled isoflurane and then injected with the luciferase-expressing MSCs in 20 μl of HyStem C (#G5131, BioTime) in the medial hamstring muscles. To generate the standard curve, increasing number of cells were used (from 12,500 to 100,000 cells), while 200,000 cells were injected for the retention studies. For imaging, animals were injected intraperitoneally with 100 μl of 20 mg/ml d-Luciferin Firefly (#122796, Perkin Elmer) 10 minutes before bioluminescence detection via IVIS Spectrum imaging for 5 minutes exposure time. LivingImage software was used to quantify MSCs bioluminescence (Perkin Elmer). All animal procedures were performed as approved by the Institutional Animal Care and Use Committee at UC Davis.
Glucose and Lactate Measurements
Supernatants were collected every 3 days from MSCs cultured under identical conditions to the in vitro survival assay (in 12-well plates) described above. Supernatants for glucose measurement were stored at −20°C and those for lactate at −80°C. Glucose and lactate concentrations of supernatants were determined using a Glucose Colorimetric/Fluorometric Assay Kit (#K606–100, BioVision) and a Lactate Colorimetric Assay Kit (#K607–100, BioVision), respectively, following their provided protocols.
Metabolome Analysis
One million MSCs were cultured in 225 cm2 culture flasks for 48 hours in either 20% O2 or 1% O2 or for an additional 3 days in 1% O2 in serum-free medium (Fig. 2A). Cells were then lifted with trypsin treatment, centrifuged at 500g, and washed once with PBS. After a second centrifugation and removal of excess liquid, tubes were snap-frozen in liquid nitrogen and stored at −80°C. Samples were then washed one more time with water (to minimize salts that could affect data acquisition), centrifuged, and submitted to the West Coast Metabolomics Center at University of California Davis. There, samples were processed and analyzed by gas chromatography—time of flight mass spectrometry (GCTOF MS) as previously described [21]). A heat map and cluster analysis were performed on the processed and normalized data using PermutMatrix software [22]. Significant differences in levels of metabolites were identified using Excel, using a paired t test.
Figure 2.
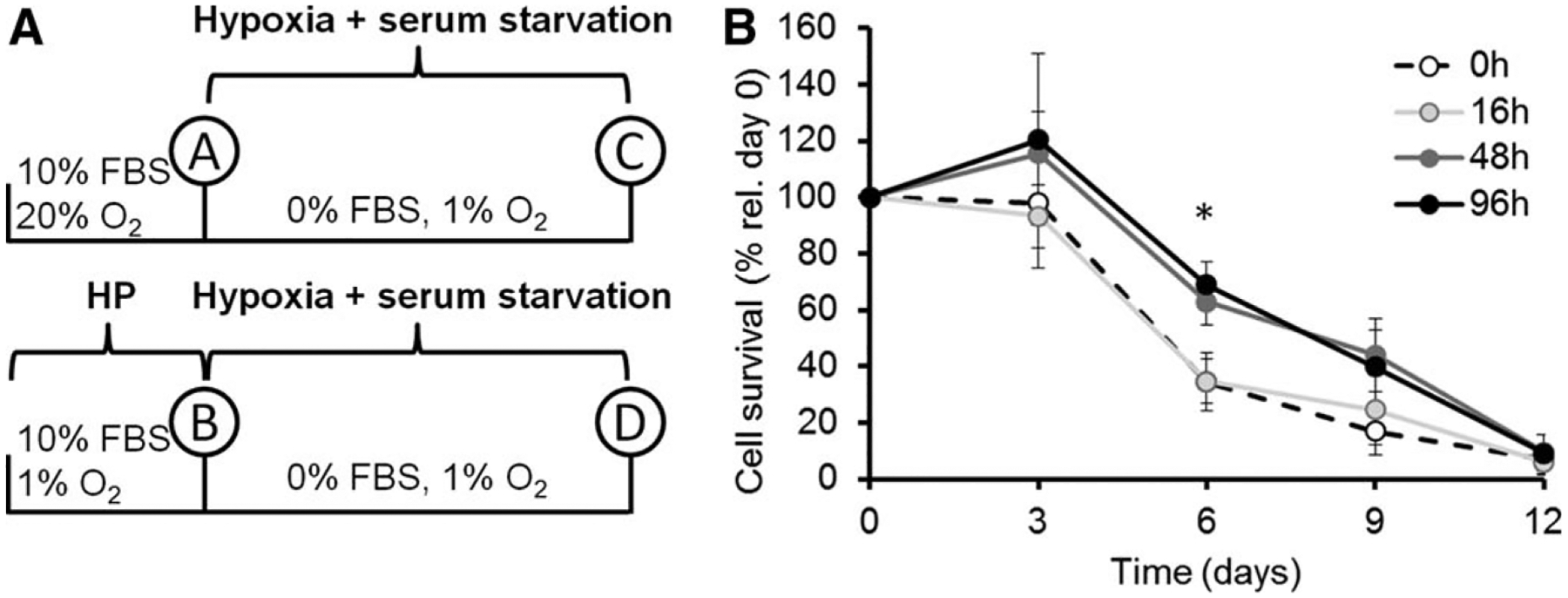
Hypoxic preconditioning increases cell survival in vitro. (A): Schematic overview of experimental design: mesenchymal stem cells/multipotent stromal cells (MSCs) were cultured in complete culture media (supplemented with 10% FBS) for varying times in hypoxia (1% O2). Then (time points A and B), culture media were changed to MEMa without supplements (0% FBS), with no further media changes, and incubated in 1% O2 for up to 12 days. (B): After preincubation of MSCs for 0, 16, 48, or 96 hours in hypoxia, cells were changed to the nutrient and oxygen limited environment. Cells were counted using a hemocytometer and Trypan blue exclusion dye every 3 days. Cell survival is expressed as a percentage of remaining cells relative to day 0, after HP (n = 6). Statistical analysis was performed by ANOVA; *, p < .05. Abbreviations: FBS, fetal bovine serum; HP, hypoxic preconditioning.
Data Presentation and Statistical Analysis
All experiments were performed at least three times with MSCs derived from different donors. The specific number of biological replicates for each experiment is indicated in the respective figure legend as n. In accordance, results are shown as average with the SEM as error bars. Throughout this manuscript, when only two conditions were tested, a Student’s t test was used, where significant differences are denoted as: *, p < .05 and **, p < .005. In contrast, in experiments where four conditions were tested, ANOVA or a Student’s t test with Bonferroni correction was applied, with significant differences from control indicated as *, p < .0125.
Results
Proliferation and Differentiation of MSCs Are Inhibited by Hypoxia in a Dose-Dependent Manner
The effect of hypoxia on basic stem cell properties, such as proliferation and differentiation, has been subject to debate. This variation may be attributed to the wide variety of conditions being tested, including time of exposure (ranging from minutes to days) and level of hypoxia (from 0.1% to 10% O2) [8, 10]. Therefore, we first tested the effects of varying degrees of hypoxia (1%–20% O2) on proliferation and differentiation of MSCs. Of note, MSCs were isolated and cultured under atmospheric oxygen (approximately 20% O2) for 2 weeks or more prior to experimentation, and exhibit a common immune phenotype [23] (Supporting Information Fig. S1). In addition, we monitored oxygen diffusion in culture media and found that, after placing cell cultures in the respective incubator, full hypoxic levels in the media are reached within 1 hour (Supporting Information Fig. S2). Taking these conditions into account, we observed that proliferation of MSCs is decreased proportionally to the degree of hypoxia, as assessed by both cell cycle analysis and proliferation assays (Fig. 1A, 1B). Viability of MSCs was not affected by any of these conditions (not shown) and, notably, MSCs did not become fully quiescent even after culture for weeks in 1% O2, as cells retained their proliferative and differentiation properties when placed back to 20% O2 (not shown).
Figure 1.
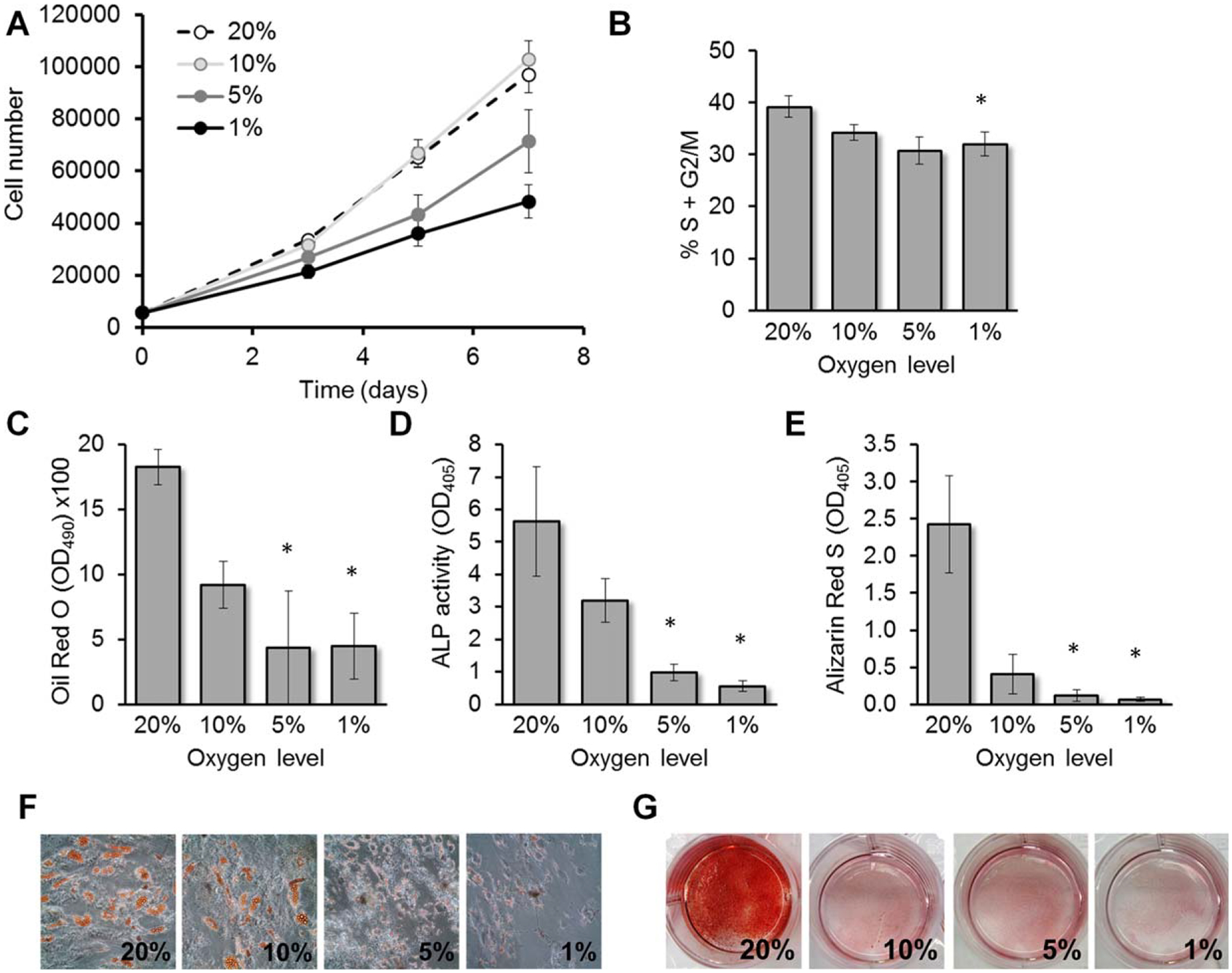
Hypoxia inhibits both proliferation and differentiation of mesenchymal stem cells/multipotent stromal cells (MSCs). (A): MSCs were cultured in incubators with the indicated oxygen level and cell numbers were determined using a hemocytometer and Trypan blue exclusion dye (n = 3). (B): Similarly, MSCs were cultured for 3 days in the respective incubators prior to cell cycle analysis using propidium iodide staining and flow cytometry (n = 5). (C): After 14 days in the respective incubators in presence of adipogenic media, oil red O staining was performed to quantify triglyceride content (n = 3). After 14 days in osteogenic media, alkaline phosphatase activity (D; n = 5) and calcium precipitation (E; Alizarin Red S staining, n = 5) were measured. (F): Representative images of oil red O staining after 14 days for adipogenesis (original magnification 3100). (G): Selected images of culture wells after Alizarin Red S staining for osteogenesis. Statistical analysis was performed by paired Student’s t test for (B) and ANOVA for (C, D, E); *, p < .05.
Next, we tested whether incubation in varying degrees of hypoxia affected osteogenic or adipogenic differentiation of MSCs, and found a strong inhibitory effect proportional to the degree of hypoxia. Adipogenesis (measuring triglyceride content of cells using oil red O) was inhibited to a similar degree with 5% and 1% O2 (Fig. 1C, 1F). Osteogenesis was measured by ALP activity and calcium precipitation (Alizarin Red S staining). For both parameters, we observed a reduction proportional to the degree of hypoxia (Fig. 1D, 1E, 1G). We also measured expression of osteogenic markers runx2, osteopontin, and bsp and adipogenic markers ppar-γ, adipsin, and adiponectin by real-time PCR, and confirmed an inhibitory effect proportional to the degree of hypoxia (Supporting Information Fig. S3). Altogether, we concluded that 1% O2 (equivalent to 10 mmHg in the culture media) exerted the strongest inhibitory effects on proliferation and differentiation, especially during osteogenesis.
HP Increases Survival of MSCs In Vitro
Given that many hypoxia-associated signals such as HIF-1α respond in a transitory manner [24] and that the therapeutic administration of MSCs is typically into tissues that are hypoxic [11], it was important to us to evaluate not only the effect of hypoxia but also the role of HP prior to new exposure to hypoxia. To mimic an environment poor in nutrients and oxygen similar to the sites where MSCs are administered in clinical applications, we performed the following set of experiments by preincubating MSCs in standard culture media in either 20% O2 (Control) or 1% O2 (HP). Then medium was changed to serum-free media and all cells were transferred to 1% O2 (Fig. 2A). Since media was not changed afterward, MSCs experienced starvation due to nutrient deficiency [25]. Figure 2B shows the decline of MSC survival over time. For all conditions, no living cells could be detected after 12 days under serum deprivation. However, MSCs that received HP for 48 or 96 hours showed an approximately twofold increase in survival at 6 days under serum deprivation as compared to control MSCs (Fig. 2B), while 16 hours-HP had no effect on survival as compared to controls. These results suggest that HP for 48 hours or more transitorily promotes cell survival in environments with limited oxygen and nutrients.
To further support these results, MSCs were stained with EthD-III, which is only able to penetrate dead cells. We found that after 9 days, 48 hours HP-MSCs showed over a twofold reduction in dead cells as compared to controls (Fig. 3A, 3B). We then measured apoptotic cells by Annexin V staining and further confirmed that after 9 days under serum deprivation, HP-MSCs were significantly less apoptotic as compared to control MSCs (Fig. 3C). To identify the possible apoptotic signaling pathway(s) involved in HP-mediated rescue of the cells under serum deprivation, we used an apoptosis proteome array. After culture for 6 or 9 days under nutrient and oxygen deficient conditions no significant differences were found in apoptosis-related signals (not shown). In contrast, on day 3, significantly lower levels of cytochrome c and heme oxygenase 1 (HO-1) were detected in HP-MSCs as compared to controls (Fig. 3D, 3E; Supporting Information Table S1). These differences were further confirmed by Western blot (Fig. 3F). These results suggest that HP induces early changes in MSCs that promote survival under serum deprivation and hypoxia.
Figure 3.
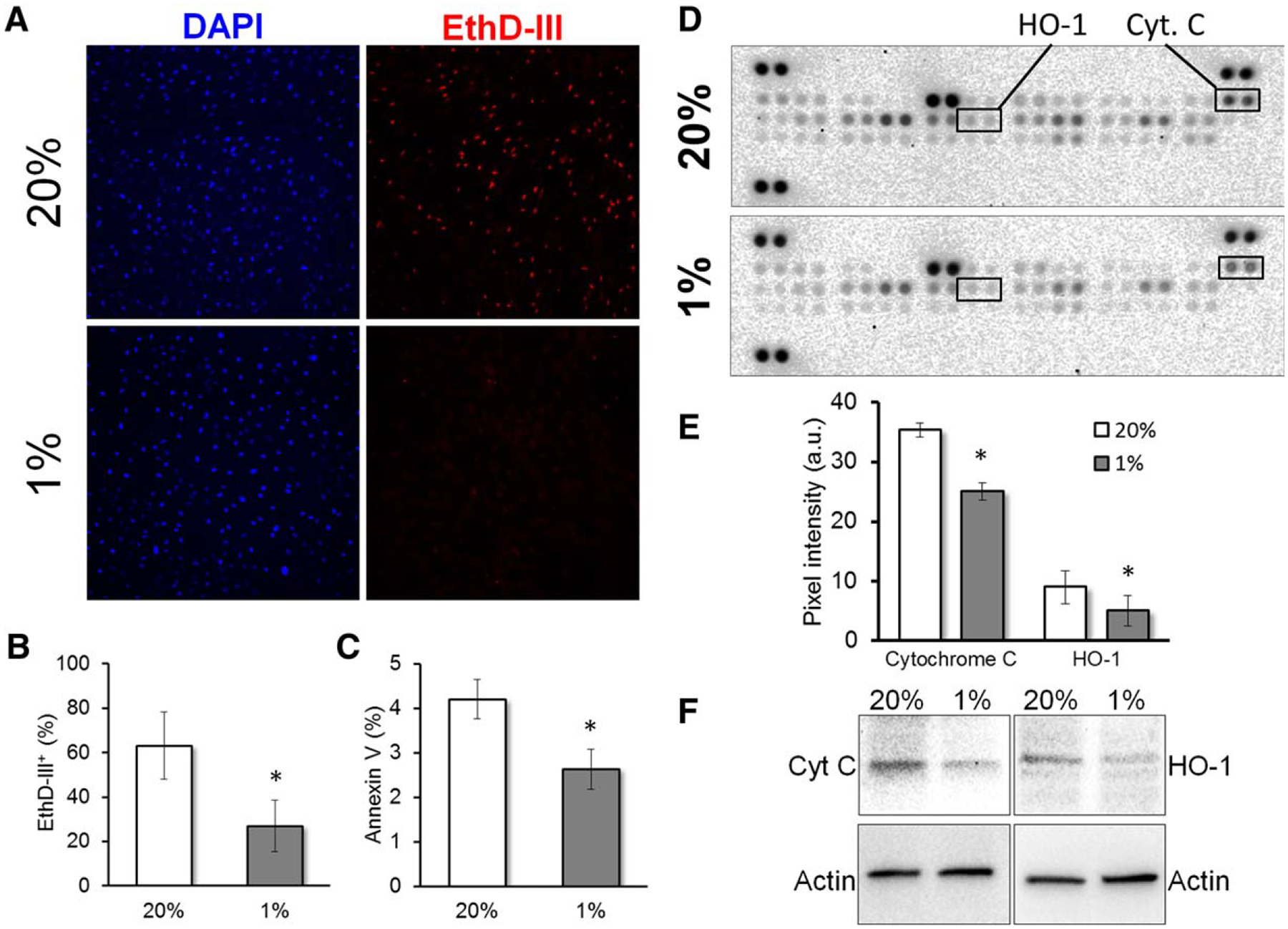
Hypoxic preconditioning reduces apoptosis of mesenchymal stem cells/multipotent stromal cells (MSCs) in vitro. After hypoxic preconditioning for 48 hours and culture for further 9 days, as described in Figure 3, MSCs were stained with EthD-III (dead cells) and DAPI (total nuclei). (A): Representative images of EthD-III and DAPI staining. (B): Quantification of A (n = 5). (C): Also after 9 days in culture, cells were stained with Annexin V and measured by flow cytometry (n = 5). After 3 days in culture under serum deprivation and hypoxia, total proteins were extracted and expression of apoptosis-related proteins detected using an antibody-based array. (D): Representative images. (E): Quantification of significant differences detected in the antibody array (n = 4). (F): These results were further confirmed by Western blot (n = 3). Statistical analysis was performed by paired Student’s t test; *, p < .05.
HP Enhances Retention of MSCs In Vivo
To evaluate whether HP of MSCs could enhance cell survival in vivo, we intramuscularly injected luciferase-expressing MSCs into immune deficient mice and followed their retention over time. First, to establish a correlation between IVIS-detected luminescence and cell number, we generated a standard curve by injecting increasing numbers of MSCs into the medial hamstring muscles of immune-deficient NSG mice and measured luminescence immediately thereafter (Fig. 4A). In addition, we confirmed the detection limit of this method and found that injection of 5,000 MSCs or more emit a robust signal, while the signal of 1,000 luciferase-expressing MSCs was barely detectable above background (not shown). We then addressed cellular retention with and without HP using the standard curve to determine the number of retained cells after injection over time. We found that MSCs without HP (controls) are retained for 1 week after transplantation, but rapidly decrease thereafter to undetectable levels 28 days after transplantation (Fig. 4B). In contrast, MSCs with 48 hours HP remained detectable after 28 days. Cell retention of HP-MSCs was also significantly better 14 and 21 days after transplantation as compared to control MSCs.
Figure 4.
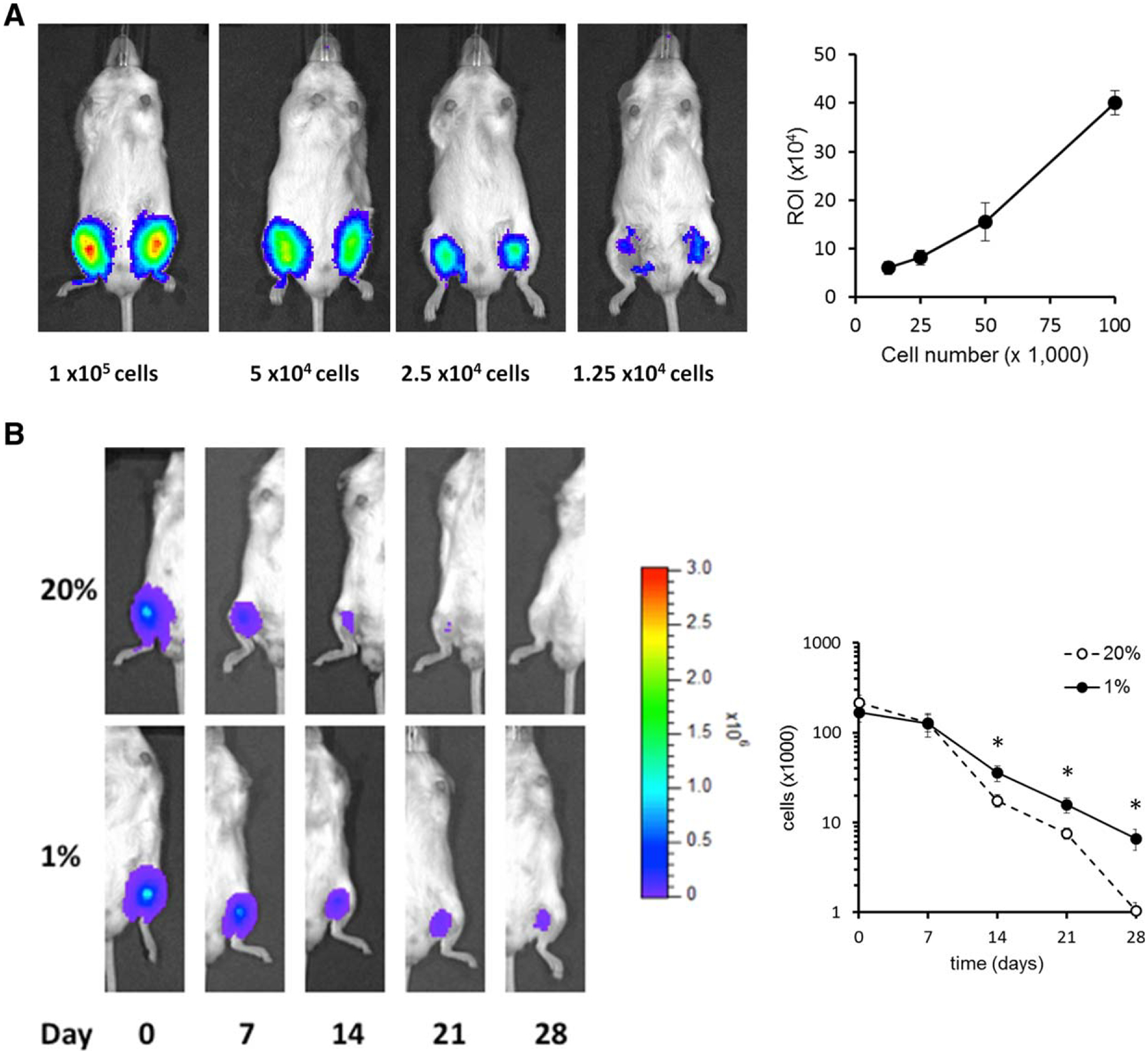
Hypoxic preconditioning promotes cell retention in vivo. (A): In order to establish a correlation between cell number and luminescence intensity (exerted by luciferase-expressing mesenchymal stem cells/multipotent stromal cells [MSCs]), varying amounts of cells were injected into NSG mice and measured immediately after (n = 3 animals per cell dose). (B): After preincubating MSCs for 48 hours in 20% or 1% oxygen, 200,000 cells were injected intramuscularly into NSG mice and luminescence was measured weekly (n = 14 animals per group, with MSCs derived from three different donors). For both (A) and (B), panels on the left show representative images, while average of total experiments is shown on right. Statistical analysis was performed by paired Student’s t test to each individual time point; *, p < .05.
Hypoxic Preconditioned MSCs Have More Glucose Available During Serum Deprivation In Vitro
We next sought to determine the underlying mechanism for the HP-induced survival of MSCs and hypothesized that metabolic changes play a critical role in this regard. We first measured glucose in the supernatants of MSCs and found significantly higher glucose levels in MSCs cultured in hypoxia for 96 hours (Fig. 5A), suggesting that, under this condition, MSCs consume less glucose as compared to controls. In hypoxia, most glucose is consumed through glycolysis, which leads to secretion of lactate into the supernatant. Consequently, higher lactate levels were found in MSCs cultured in hypoxia as compared to controls (Fig. 5B).
Figure 5.
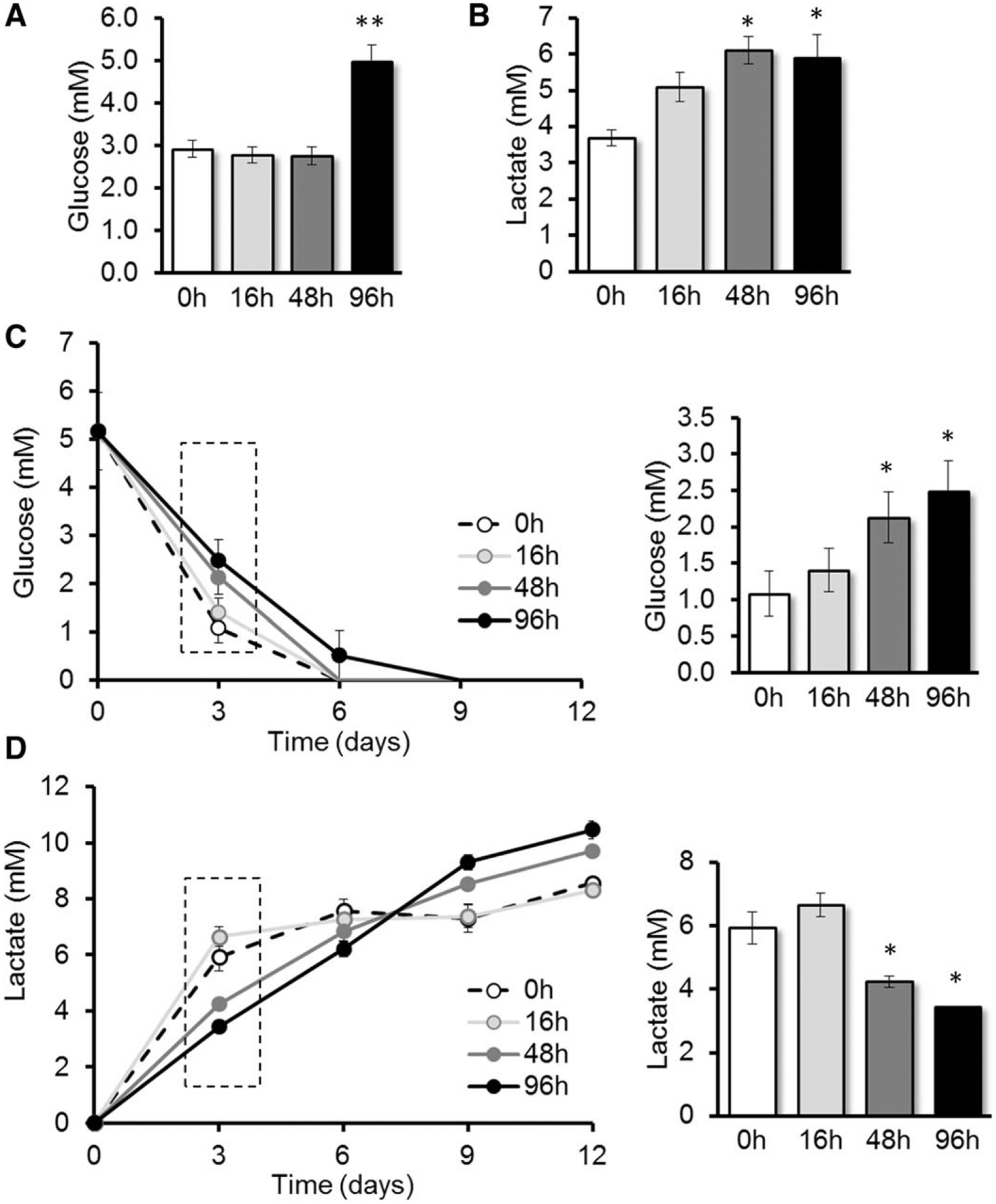
Hypoxic preconditioning reduces glucose consumption and lactate secretion. Mesenchymal stem cells/multipotent stromal cells (MSCs) were cultured for 96 hours with a single medium change after 48 hours and in 1% O2 for the indicated time periods. Then, supernatants were collected to measure glucose (A; n = 5) and lactate (B; n = 4) as described in Materials and Methods. Then, MSCs were cultured as shown in the scheme of Figure 2A. After HP, MSCs were cultured under serum deprivation and 1% O2 for up to 12 days with no further medium changes. Every 3 days, supernatants were collected to determine glucose (C; n = 3) and lactate (D; n = 3). Histograms to the right of (C) and (D) highlight glucose and lactate levels at day 3. Statistical analysis was performed by paired Student’s t test with Bonferroni correction; *, p < .05; **, p < .005.
After respective preconditioning, we measured glucose and lactate under serum deprivation and hypoxia (Fig. 2A). After 3 days, glucose levels were more than twofold higher in supernatants of 48 and 96 hours HP-MSCs, as compared to controls (without HP) (Fig. 5C), a result that is in line with the transitory prosurvival effect of HP. We also found increasing levels of lactate, until day 9, by which time most MSCs have died (Fig. 5D). After 3 days, lactate levels were significantly lower in 48 and 96 hours HP-MSCs as compared to controls. Taken together, these results suggest that HP reduces glucose consumption in MSCs.
HP Changes the Metabolome of MSCs
In order to generate a comprehensive and unbiased view of the changes that occur in MSCs under HP, we performed mass spectrometry analysis to compare the metabolomes of MSCs under the following four conditions (Fig. 2A): MSCs cultured for 48 hours in normoxia (A), MSCs cultured for 48 hours in 1% O2 (HP-MSCs; B) and MSCs cultured as in A and B, with an additional 3 days under hypoxia and serum starvation conditions (C and D, respectively). This time point was chosen based on the differences in glucose and lactate levels detected in the supernatant of MSCs and HP-MSCs (Fig. 5). Overall, we identified over 260 different metabolites, from which approximately 50% are identified solely by a BinBase number [26], as they do not have a common name.
The heat map in Figure 6 shows the metabolites (with common names) and highlights strong differences in their relative abundance. In particular, several amino acids, pyrophosphate, cholesterol, and lactic acid were found to be extremely abundant. The cluster analysis 6 and Table 1 show that the metabolomes of control MSCs (A) and HP-MSCs (B) are more similar to each other than to their counterparts after 3 days under starvation conditions (C and D), suggesting that nutrient deprivation causes greater metabolic changes than hypoxia. Table 1 shows the metabolites that were consistently changed in the MSCs derived from all four different donors (as described in Materials and Methods). Often the magnitude of these fold-differences was small (as low as 10%). Of note, when comparing control MSCs to HP-MSCs before and after starvation conditions (A vs. B and C vs. D), most differentially expressed metabolites were downregulated in HP-MSCs as compared to their controls. It is also remarkable that after 3 days under starvation conditions, the metabolites 21811, beta-alanine, lysine, serine, tyrosine, and uracil were consistently changed in MSCs with and without HP-MSCs to almost identical magnitudes (fold differences). In general, our metabolome analysis reveals both changes that occur as consequence from HP and/or serum deprivation and differences that are potentially the cause for increased survival of HP-MSCs, suggesting that some of these metabolites could become targets for future approaches to enhance cellular survival and retention after transplantation.
Figure 6.

Metabolome profile of hypoxic preconditioned mesenchymal stem cells/multipotent stromal cells (MSCs). Following the experimental design schematized in Figure 2A, MSCs were cultured for 48 hours in standard culture media in either 20% or 1% O2 (A and B, respectively), or for an additional 3 days under serum deprivation and 1% O2 (C and D). Then, samples were processed and total metabolites determined by mass spectrometry (see Materials and Methods). Cluster analysis (upper left corner) was performed with total metabolites detected, while heat map shows only metabolites with common names.
Table 1.
Differentially expressed metabolites in hypoxic preconditioned mesenchymal stem cells/multipotent stromal cells (MSCs) as detected by mass spectrometry analysis
| BinBase name | Fold change | p value |
|---|---|---|
| A versus B (>1 higher in B) | ||
| 31547 | 0.7 | .002 |
| 42445 | 0.6 | .030 |
| Adenosine | 0.6 | .040 |
| Aspartic acid | 0.5 | .026 |
| Creatinine | 2.1 | .045 |
| Fumaric acid | 0.7 | .022 |
| Lactose | 1.4 | .014 |
| Malic acid | 0.7 | .050 |
| A versus C (>1 higher in C) | ||
| 21682 | 1.8 | .041 |
| 21811 | 2.3 | .015 |
| 31224 | 1.6 | .028 |
| Adenine | 0.5 | .016 |
| Aspartic acid | 0.6 | .037 |
| Beta-alanine | 0.5 | .018 |
| Citrulline | 1.9 | .029 |
| Creatinine | 0.5 | .012 |
| Glucose-6-phosphate | 0.6 | .026 |
| Guanosine | 0.5 | .034 |
| Isoleucine | 1.1 | .017 |
| Lactose | 1.7 | .036 |
| Leucine | 1.4 | .039 |
| Lysine | 1.6 | .042 |
| Phenylalanine | 1.4 | .040 |
| Serine | 1.4 | .012 |
| Threonic acid | 2.3 | .047 |
| Tyrosine | 1.4 | .014 |
| Uracil | 0.2 | .030 |
| Valine | 1.4 | .027 |
| C versus D (>1 higher in D) | ||
| 17068 | 0.8 | .007 |
| 34097 | 1.4 | .008 |
| Citrulline | 0.7 | .022 |
| Fumaric acid | 0.8 | .009 |
| Histidine | 1.1 | .012 |
| Lactose | 0.4 | .051 |
| Sorbitol | 0.7 | .008 |
| B versus D (>1 higher in D) | ||
| 47 | 1.1 | .034 |
| 257 | 1.4 | .049 |
| 657 | 1.2 | .016 |
| 1709 | 1.5 | .007 |
| 1875 | 1.2 | .013 |
| 1878 | 1.2 | .024 |
| 2810 | 0.7 | .011 |
| 18520 | 1.6 | .042 |
| 21664 | 1.2 | .020 |
| 21811 | 1.9 | .027 |
| 31222 | 2.6 | .002 |
| 100721 | 2.7 | .012 |
| 100768 | 1.4 | .050 |
| Beta-alanine | 0.5 | .010 |
| Creatinine | 0.3 | .036 |
| Glucose | 2.0 | .008 |
| Glycine | 1.2 | .008 |
| Glycyl proline | 2.3 | .042 |
| Guanine | 0.5 | .041 |
| Histidine | 1.4 | .018 |
| Inosine 5′-monophosphate | 2.1 | .049 |
| Lauric acid | 0.9 | .004 |
| Lysine | 1.6 | .004 |
| Nicotinamide | 0.7 | .006 |
| Serine | 1.3 | .047 |
| Trans-4-hydroxy-l-proline | 1.4 | .050 |
| Tyrosine | 1.4 | .025 |
| UDP-N-acetylglucosamine | 0.6 | .010 |
| Uracil | 0.2 | .037 |
Conditions (schematically described in Fig. 2A) are as follows: (A) MSCs cultured for 2 days in standard culture media in 20% O2; (B) MSCs cultured for 2 days in standard culture media in 1% O2; (C) MSCs cultured as in (A) and additional 3 days under hypoxia and serum deprivation; (D) MSCs cultured as in (B) and additional 3 days under hypoxia and serum deprivation. Only statistically significant differences are shown, where values of detected metabolites are expressed as ratios of one experimental condition over another.
Discussion
The effects of varying oxygen environments on MSCs have been previously studied, typically comparing a single level of hypoxia to atmospheric levels (normoxia). The effects of hypoxia on proliferation and differentiation of MSCs have been controversial as they depend on multiple parameters, including hypoxia level, tissue, and species source of the MSCs, time of exposure to hypoxia, cell density, and others [8, 10]. Under our experimental settings, hypoxia exerted an inhibitory effect on both proliferation and differentiation on human bone marrow-derived MSCs, proportional to the degree of hypoxia. For osteogenesis, our results support the notion that MSC-mediated bone repair is strongly dependent on oxygen supply [9, 27, 28]. Our results also suggest that, among the different levels of hypoxia tested, 1% O2 (equivalent to 10 mmHg in the culture media of cells) exerted the strongest effects on MSCs. These results were further confirmed by measuring secreted vascular endothelial growth factor (VEGF) levels in MSCs, where 1% O2 clearly induced the strongest increase of VEGF secretion (not shown).
Since hypoxic signaling is considered transcient [24], it is critical to address survival of MSCs after HP followed by additional exposure to hypoxia. Using similar experimental conditions, Chacko et al. showed that HP (24 hours in 0.5% O2) promoted survival of rat MSCs in vitro in a near anoxic environment [29], and recently Zhu et al. demonstrated that HP (24 hours in 1% O2) promotes survival of mouse MSCs in vitro by favoring glycogen storage [17]. Our results confirmed that 1% O2 is an optimal hypoxic level to favor cell survival. In addition, we established a time threshold, where 16 hours are insufficient to promote survival, while 48 and 96 hours promote survival to a similar degree.
Our in vitro survival assay used cell counts with a hemocytometer, which allowed a direct quantification of surviving cells over time as a fraction of the original cell number. In contrast, EthD-III incorporation directly measured the number of dead cells at a given time point (day 9) while Annexin V confirmed that the cell death was caused by apoptosis. We found a quantitative correlation between cell counts and EthD-III staining. However, the percentage of Annexin V-positive cells was remarkably lower than the number of dead cells. We speculate that this is because Annexin V only represents an early event in the apoptotic cascade, while, under starvation, apoptosis is gradually triggered in the cells. The apoptosis array performed showed a small but significant decrease in cytochrome c and HO-1 in HP-MSCs as compared to controls, which were confirmed by Western blot. In addition, the apoptosis proteome array showed significantly lower levels of HIF-1α in HP-MSC (Supporting Information Table S1). Release of cytochrome c from mitochondria is an essential step to induce apoptosis [30]. During apoptosis, cytochrome c activates caspases, which have been shown to increase under serum-deprivation in severe hypoxia (anoxia) in MSCs [31]. HIF-1α levels quickly increase after exposure to hypoxia, while the rate of decrease is rather slow. We speculate that since HP-MSCs had higher HIF-1α levels earlier in time, residual HIF-1α after 3 days are lower than controls. In line with this hypothesis, HO-1, which is a direct target gene of HIF-1α [32], was also found decreased in HP-MSCs.
Next, we confirmed that HP also enhanced cell retention in vivo. We first addressed a correlation between luminescence (luciferase signal) and cell number, and established that, within the range studied, signal intensity is proportional to the number of cells injected, while a signal of less than 103 intramuscularly injected cells is barely detectable above background signal, and is too weak to be properly quantified (not shown). We then compared retention of human MSCs preincubated for 48 hours in 20% O2 (control) or 1% O2 (HP) in immune-deficient mice and found better persistence of the cells that received HP. Of note, this method for tracking cell retention only detects living cells, implying that HP increases cell survival in vivo and may thereby yield greater therapeutic benefit over time. This result is in line with previous studies performed with intramyocardial injection of green fluorescent protein-labeled MSCs into wild-type mice [12]. Also, Huang et al. recently showed that HP of MSCs leads to enhanced retention after transplantation by attenuating the response of the host natural killer cells against the transplanted cells [15]. In our system, the immune component was minimized by the use of NSG mice, one of the most immune deficient strains available. Importantly, Deschepper et al. recently showed that glucose supplementation increased retention of MSCs in vivo [33], suggesting that after transplantation of MSCs, glucose availability may limit cellular retention. Conversely, it has been shown that MSCs from low passage are better retained than late-passage MSCs [34]. It is therefore feasible that an optimal retention strategy for MSCs could be obtained by a combination of these and other approaches, including optimal vehicle, route of administration, and site of injection. Future experiments should address the effects of HP-induced cell retention for specific therapeutic applications.
Different mechanisms have been proposed as underlying mechanisms for the HP-mediated increase in cell retention in vivo, including enhanced secretion of angiogenic factors [33], improved migration potential [35], increased expression of prosurvival signaling pathways such as Akt [14] and miR-210 [36], and increased glycogen storage [17]. Our results support the notion that cell survival is highly dependent on glucose availability. It has been shown that MSCs can survive long term in severe hypoxia when glucose is available [37], while MSCs cultured in glucose-free medium die within hours (our observations and [25]). We measured glucose in supernatants of MSCs cultured under identical conditions as our survival assays and found a strong correlation in time between the depletion of glucose and cell death: shortly after glucose was virtually depleted, the majority of cells had died. In hypoxia, MSCs switch their metabolic pathway to anaerobic glycolysis [25, 38–40]. Notably, many genes related to glucose metabolism are regulated by HIF-1 during hypoxia [41, 42].
The authors declare no conflicts of interest.ur results shown in Figure 5 suggest that exposure to hypoxia for a short time period does not affect glucose consumption but induces lactate secretion. However, long exposure to hypoxia induces MSCs to adapt by decreasing their glucose consumption (and consequently lactate secretion). In our experiments, cell starvation was induced under hypoxia such that, over time, HP of 48 or 96 hours induced a reduction in glucose consumption and lactate secretion. These results suggest that, under limited glucose availability, HP-MSCs survive for longer due to a lower rate of glucose consumption, thereby allowing more glucose availability over a longer period of time.
To identify metabolic changes involved in HP-mediated cell survival, we performed a mass spectrometry approach. Both the cluster analysis and number of differentially expressed metabolites shown in Table 1 suggest that the greatest metabolic differences are induced after serum deprivation. For example, after starvation both control MSCs and HP-MSCs showed an increase of specific amino acids including beta-alanine, lysine, and serine, most likely through the starvation-induced general amino acid control pathway [43]. Since some of the increased amino acids are essential (i.e., cannot be synthesized de novo by human cells), it is possible that autophagy is an important mechanism promoting survival of MSCs [44].
Both malic acid and fumaric acid were found lower in HP-MSCs than in control MSCs, which could be explained by the fact that both are intermediates of the citric acid cycle, which only occurs in the presence of oxygen. It is interesting that fumaric acid was also significantly lower in HP-MSCs 3 days after hypoxia and serum deprivation. Of note, fumaric acid stabilizes HIF-1α by inhibiting the hydroxylases responsible for HIF-1α degradation [45]. Lactose levels were first found increased by HP, while after serum deprivation lactose was lower in HP-MSCs. We consider these results in line with our observation of glucose consumption, as lactose is a disaccharide made from glucose.
Conclusion
Overall, our results strongly encourage, at both the clinical and research level, preincubation of MSCs in hypoxia prior to transplantation to enhance their retention. We propose that HP of MSCs limits glucose consumption, transitorily allowing higher availability of glucose in their environment. Finally, our general metabolome analysis of MSCs reveals new metabolites, including several amino acids, fumaric acid, lactose, and others as possible candidates for new interventions that could lead to higher cell retention.
Supplementary Material
Acknowledgments
This work was supported by Early Translational Grant TR2–01787 from the California Institute for Regenerative Medicine (CIRM) and NIH Transformative Grant 1R01GM099688 (J.N.). F.F., J.N., and J.B. are also funded by CIRM Disease Team Grant DR2A-05423 for Critical Limb Ischemia. We would like to thank Jane Wanyera, who, as part of the University of California Davis Teen Biotech Challenge Program and the CIRM Creativity Program, measured oxygen levels in solution from the different hypoxic incubators.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Bianco P, Cao X, Frenette PS et al. The meaning, the sense and the significance: Translating the science of mesenchymal stem cells into medicine. Nat Med 2013;19:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keating A. Mesenchymal stromal cells: New directions. Cell Stem Cell 2012;10:709–716. [DOI] [PubMed] [Google Scholar]
- 3.Caplan AI, Correa D. The MSC: An injury drugstore. Cell Stem Cell 2011;9:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008;8:726–736. [DOI] [PubMed] [Google Scholar]
- 5.Mangi AA, Noiseux N, Kong D et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med 2003;9:1195–1201. [DOI] [PubMed] [Google Scholar]
- 6.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell 2010;7:150–161. [DOI] [PubMed] [Google Scholar]
- 7.Grant JL, Smith B. Bone marrow gas tensions, bone marrow blood flow, and erythropoiesis in man. Ann Intern Med 1963;58: 801–809. [DOI] [PubMed] [Google Scholar]
- 8.Das R, Jahr H, van Osch GJ et al. The role of hypoxia in bone marrow-derived mesenchymal stem cells: Considerations for regenerative medicine approaches. Tissue Eng Part B Rev 2010;16:159–168. [DOI] [PubMed] [Google Scholar]
- 9.Ma T, Grayson WL, Frohlich M et al. Hypoxia and stem cell-based engineering of mesenchymal tissues. Biotechnol Prog 2009; 25:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai CC, Yew TL, Yang DC et al. Benefits of hypoxic culture on bone marrow multipotent stromal cells. Am J Blood Res 2012;2: 148–159. [PMC free article] [PubMed] [Google Scholar]
- 11.Brahimi-Horn MC, Pouyssegur J. Oxygen, a source of life and stress. FEBS Lett 2007; 581:3582–3591. [DOI] [PubMed] [Google Scholar]
- 12.Hu X, Yu SP, Fraser JL et al. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg 2008; 135:799–808. [DOI] [PubMed] [Google Scholar]
- 13.He A, Jiang Y, Gui C et al. The antiapoptotic effect of mesenchymal stem cell transplantation on ischemic myocardium is enhanced by anoxic preconditioning. Can J Cardiol 2009;25:353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosova I, Dao M, Capoccia B et al. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells 2008;26:2173–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang WH, Chen HL, Huang PH et al. Hypoxic mesenchymal stem cells engraft and ameliorate limb ischaemia in allogeneic recipients. Cardiovasc Res 2014;101:266–276. [DOI] [PubMed] [Google Scholar]
- 16.Leroux L, Descamps B, Tojais NF et al. Hypoxia preconditioned mesenchymal stem cells improve vascular and skeletal muscle fiber regeneration after ischemia through a Wnt4-dependent pathway. Mol Ther 2010; 18:1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu H, Sun A, Zou Y et al. Inducible metabolic adaptation promotes mesenchymal stem cell therapy for ischemia: A hypoxia-induced and glycogen-based energy prestorage strategy. Arterioscler Thromb Vasc Biol 2014;34:870–876. [DOI] [PubMed] [Google Scholar]
- 18.Chang CP, Chio CC, Cheong CU et al. Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin Sci 2013;124:165–176. [DOI] [PubMed] [Google Scholar]
- 19.Yu J, Yin S, Zhang W et al. Hypoxia preconditioned bone marrow mesenchymal stem cells promote liver regeneration in a rat massive hepatectomy model. Stem Cell Res Ther 2013;4:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fierro FA, Kalomoiris S, Sondergaard CS et al. Effects on proliferation and differentiation of multipotent bone marrow stromal cells engineered to express growth factors for combined cell and gene therapy. Stem Cells 2011;29:1727–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiehn O, Wohlgemuth G, Scholz M et al. Quality control for plant metabolomics: Reporting MSI-compliant studies. Plant J 2008;53:691–704. [DOI] [PubMed] [Google Scholar]
- 22.Caraux G, Pinloche S. PermutMatrix: A graphical environment to arrange gene expression profiles in optimal linear order. Bioinformatics 2005;21:1280–1281. [DOI] [PubMed] [Google Scholar]
- 23.Horwitz EM, Le Blanc K, Dominici M et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005;7:393–395. [DOI] [PubMed] [Google Scholar]
- 24.Park IH, Kim KH, Choi HK et al. Constitutive stabilization of hypoxia-inducible factor alpha selectively promotes the self-renewal of mesenchymal progenitors and maintains mesenchymal stromal cells in an undifferentiated state. Exp Mol Med 2013;45:e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mylotte LA, Duffy AM, Murphy M et al. Metabolic flexibility permits mesenchymal stem cell survival in an ischemic environment. Stem Cells 2008;26:1325–1336. [DOI] [PubMed] [Google Scholar]
- 26.Skogerson K, Wohlgemuth G, Barupal DK et al. The volatile compound BinBase mass spectral database. BMC Bioinformatics 2011; 12:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Ippolito G, Diabira S, Howard GA et al. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone 2006;39:513–522. [DOI] [PubMed] [Google Scholar]
- 28.D’Ippolito G, Diabira S, Howard GA et al. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of post-natal young and old human cells with extensive expansion and differentiation potential. J Cell Sci 2004;117:2971–2981. [DOI] [PubMed] [Google Scholar]
- 29.Chacko SM, Ahmed S, Selvendiran K et al. Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am J Physiol Cell Physiol 2010;299:C1562–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, Kim CN, Yang J et al. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 1996;86:147–157. [DOI] [PubMed] [Google Scholar]
- 31.Zhu W, Chen J, Cong X et al. Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem Cells 2006;24: 416–425. [DOI] [PubMed] [Google Scholar]
- 32.Lee PJ, Jiang BH, Chin BY et al. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J Biol Chem 1997;272: 5375–5381. [PubMed] [Google Scholar]
- 33.Deschepper M, Manassero M, Oudina K et al. Proangiogenic and prosurvival functions of glucose in human mesenchymal stem cells upon transplantation. Stem Cells 2013;31: 526–535. [DOI] [PubMed] [Google Scholar]
- 34.Jin J, Zhao Y, Tan X et al. An improved transplantation strategy for mouse mesenchymal stem cells in an acute myocardial infarction model. PLoS One 2011;6:e21005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu H, Liu S, Li Y et al. The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PLoS One 2012;7:e34608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim HW, Haider HK, Jiang S et al. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem 2009;284:33161–33168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deschepper M, Oudina K, David B et al. Survival and function of mesenchymal stem cells (MSCs) depend on glucose to overcome exposure to long-term, severe and continuous hypoxia. J Cell Mol Med 2011;15:1505–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Folmes CD, Dzeja PP, Nelson TJ et al. Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell 2012;11: 596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dos Santos F, Andrade PZ, Boura JS et al. Ex vivo expansion of human mesenchymal stem cells: A more effective cell proliferation kinetics and metabolism under hypoxia. J Cell Physiol 2010;223:27–35. [DOI] [PubMed] [Google Scholar]
- 40.Papandreou I, Cairns RA, Fontana L et al. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab 2006;3:187–197. [DOI] [PubMed] [Google Scholar]
- 41.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell 2012;148: 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carmeliet P, Dor Y, Herbert JM et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998;394:485–490. [DOI] [PubMed] [Google Scholar]
- 43.Chen R, Zou Y, Mao D et al. The general amino acid control pathway regulates mTOR and autophagy during serum/glutamine starvation. J Cell Biol 2014;206:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herberg S, Shi X, Johnson MH et al. Stromal cell-derived factor-1beta mediates cell survival through enhancing autophagy in bone marrow-derived mesenchymal stem cells. PLoS One 2013;8:e58207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koivunen P, Hirsila M, Remes AM et al. Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: Possible links between cell metabolism and stabilization of HIF. J Biol Chem 2007; 282:4524–4532. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


