Abstract
The expression of iron-regulated systems in gram-negative bacteria is generally controlled by the Fur protein, which represses the transcription of iron-regulated promoters by using Fe2+ as a cofactor. Mutational analysis of the Campylobacter jejuni fur gene was carried out by generation of a set of mutant copies of fur which had a kanamycin or chloramphenicol resistance gene introduced into the regions encoding the N and C termini of the Fur protein. The mutated genes were recombined into the C. jejuni NCTC 11168 chromosome, and putative mutants were confirmed by Southern hybridization. C. jejuni mutants were obtained only when the resistance genes were transcribed in the same orientation as the fur gene. The C. jejuni fur mutant grew slower than the parental strain. Comparison of protein profiles of fractionated C. jejuni cells grown in low- or high-iron medium indicated derepressed expression of three iron-regulated outer membrane proteins with molecular masses of 70, 75, and 80 kDa. Characterization by N-terminal amino acid sequencing showed the 75-kDa protein to be identical to CfrA, a Campylobacter coli siderophore receptor homologue, whereas the 70-kDa protein was identified as a new siderophore receptor homologue. Periplasmic fractions contained four derepressed proteins with molecular masses of 19, 29, 32, and 36 kDa. The 19-kDa protein has been previously identified, but its function is unknown. The cytoplasmic fraction contained two iron-repressed and two iron-induced proteins with molecular masses of 26, 55, 31, and 40 kDa, respectively. The two iron-repressed proteins have been previously identified as the oxidative stress defense proteins catalase (KatA) and alkyl hydroperoxide reductase (AhpC). AhpC and KatA were still iron regulated in the fur mutant, suggesting the presence of Fur-independent iron regulation. Further analysis of the C. jejuni iron and Fur regulons by using two-dimensional gel electrophoresis demonstrated the total number of iron- and Fur-regulated proteins to be lower than for other bacterial pathogens.
The microaerophilic enteric pathogen Campylobacter jejuni is one of the most frequently isolated causes of bacterial diarrhea; thus, Campylobacter infection is a major public health and economic burden (48). The clinical spectrum of Campylobacter enteritis ranges from a noninflammatory watery diarrhea to a severe inflammatory diarrhea. The mechanisms involved in pathogenesis are not well characterized but may include damage to the intestinal epithelium by host cell invasion and toxin production (27). Complications associated with Campylobacter infection include Guillain-Barré syndrome (4, 33).
When colonizing a host, C. jejuni must compete in the intestine with other bacteria for the available nutrients, including iron. In host tissues, the availability of free iron is very low, as most iron is complexed with host proteins such as hemoglobin and transferrin. This low iron concentration constitutes a nonspecific defense mechanism, but conversely, successful pathogens can use it as a stimulus to express virulence factors like toxins and other factors required for in vivo growth (7, 31).
Iron is an essential nutrient for almost all living organisms. It is needed as a cofactor by several enzymes and as a catalyst in electron transport processes. However, in the presence of oxygen, iron is also capable of generating oxygen radicals which can damage DNA, proteins, and membranes. Iron homeostasis therefore has to be carefully balanced, and this is achieved by tightly controlling the uptake, metabolism, and storage of iron. At a molecular level, gene expression is coupled to the intracellular concentration of Fe2+. Iron-regulated gene expression in gram-negative bacteria is generally under the control of the Fur (ferric uptake regulator) protein. This protein, encoded by the fur gene, represses the transcription of iron-regulated promoters in response to an increasing intracellular Fe2+ concentration. It has been suggested that Fur binds to control sequences (Fur boxes) overlapping Fur-regulated promoters when the intracellular Fe2+ concentration is high enough to allow the formation of a complex consisting of a Fur dimer and Fe2+ (13, 47). It is thought that the N terminus of Fur contains the DNA-binding domain, whereas the C terminus contains the dimerization and Fe2+-binding domains (47).
Fur was first identified in Salmonella typhimurium (15) and has been most extensively characterized in Escherichia coli (21, 43). Fur homologues have been identified in several other gram-negative bacteria, including Pseudomonas species (39, 57), Vibrio species (28, 30, 51), Neisseria species (3, 25, 49) and Yersinia pestis (45). The mutation of fur has enabled investigation of the function of Fur and identification of members of the Fur regulon. Insertional mutagenesis has been possible in only a few bacterial species, including E. coli (53), Vibrio cholerae (28, 29), Vibrio vulnificus (30), and Y. pestis (44); in most other bacterial species, including Pseudomonas spp. (39, 58), Neisseria gonorrhoeae (50), and Vibrio anguillarum (60), only strains with point mutations in the fur gene could be isolated. Upon growth in iron-depleted and iron-supplemented media, fur mutants show pleiotropic phenotypes, ranging from chromosomal deletions in Y. pestis (44) to delayed aerobic growth and impaired iron uptake in Pseudomonas aeruginosa (23).
Campylobacter species contain a fur homologue, which was described in 1994 by Wooldridge et al. (62) and later by another group (5, 10). Iron-regulated outer membrane proteins of C. jejuni were described by Field et al. (17), and the gene encoding a 75-kDa iron-regulated outer membrane protein (CfrA) of Campylobacter coli has been identified (20). Iron-regulated expression of two C. jejuni oxidative stress enzymes, alkyl hydroperoxide reductase (AhpC) and catalase (KatA), was demonstrated recently (2), but regulation of AhpC and KatA expression by Fur has not been reported.
In this study, we constructed the first mutation of a Campylobacter regulatory gene. Analysis of the strain carrying this mutation has enabled us to (i) determine if previously recognized iron-regulated proteins are part of the Fur regulon and (ii) identify new members of the iron and Fur regulons.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The strains and plasmids used in this study are listed in Table 1. C. jejuni was routinely maintained on Mueller-Hinton (MH) medium (Oxoid, Basingstoke, United Kingdom) supplemented with vancomycin (10 μg/ml) and trimethoprim (5 μg/ml) in a variable atmosphere incubator (Don Whitley, Shipley, United Kingdom) containing 85% N2, 10% CO2, and 5% O2. Low-iron conditions were achieved by supplementing MH medium with the iron chelator deferoxamine mesylate (Desferal; Sigma Chemical Co.) to a final concentration of 20 μM. High-iron conditions were achieved by supplementing MH medium with Fe(III)SO4 to a final concentration of 40 μM. Minimal essential medium, alpha modification (MEMα; Gibco), was used as a defined low-iron medium, as it contains no added iron source. E. coli was grown aerobically in Luria-Bertani medium (42) at 37°C. When antibiotic selection was necessary, the growth medium was supplemented with ampicillin (100 μg/ml), kanamycin (50 μg/ml), or chloramphenicol (20 μg/ml).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or relevant characteristics | Source or reference |
|---|---|---|
| C. jejuni strains | ||
| NCTC 11168 | Parental strain | National Collection of Type Cultures |
| AV17 | 11168 fur:Kanr (C-terminal insertion | This study |
| AV45 | 11168 fur:Kanr (N-terminal insertion) | This study |
| AV41 | 11168 fur:Cmr (C-terminal insertion) | This study |
| AV42 | 11168 fur:Cmr (N-terminal insertion)r | This study |
| E. coli strains | ||
| DH5α | F− φ80dlacZΔM15 | Gibco BRL |
| H1780 | MC4100 fiu::λplacMu53 fur | 22 |
| Plasmids | ||
| pBluescript II SK− | Cloning vector, Apr | Stratagene |
| pJMK30 | C. coli Kanr cassette in pUC19 | This study |
| pAV35 | C. coli Cmr cassette (64) in pBluescript | This study |
| pAV25 | 1.9-kb HindIII fragment of C. jejuni NCTC 11168 containing fur and part of lysS in pBluescript | This study |
| pAV57 | pAV25 with a BglII site introduced in fur | This study |
| pAV32/31 | Kanr cassette of pJMK30 cloned in the unique BclI site in pAV25 in fur | This study |
| pAV82/83 | Cmr cassette of pAV35 cloned in the unique BclI site in pAV25 in fur | This study |
| pAV62/61 | Kanr cassette of pJMK30 cloned in the unique BglII site in pAV57 in fur | This study |
| pAV80/81 | Cmr cassette of pAV35 cloned in the unique BglII site in pAV57 in fur | This study |
Recombinant DNA techniques.
Restriction enzymes and T4 DNA ligase were purchased from Gibco BRL. All enzymes were used according to the manufacturer’s instructions. Standard protocols were used for manipulation of DNA and transformation of E. coli (1, 42) and C. jejuni (32, 56, 59). Genomic DNA of C. jejuni was prepared as described by Ausubel et al. (1). Plasmid DNA was prepared by using affinity columns (Qiagen). DNA sequencing was performed with an Applied Biosystems model 377 DNA sequencing system and a Taq Dye Deoxy Terminator cycle sequencing kit (Applied Biosystems). Southern blots were prepared by standard protocols (42). Probes were labelled with a Gene Images fluorescein-based system (Amersham); hybridization and detection were performed with a Gene Images chemiluminescence detection module (Amersham).
PCR.
PCR was used for introduction of mutations in fur and was performed with Taq polymerase (Advanced Biotechnologies, Epsom, United Kingdom) on an Omnigene Thermal Cycler (Hybaid, Ashford, United Kingdom). A BglII restriction enzyme site was introduced in pAV25 in the region encoding the N terminus of Fur by inverse PCR mutagenesis by using primers 5′-ggaagatctCCACATTTTCTATCAGCAT and 5′-ggaagatctAAGACAAGGCGGACTTAA under standard conditions (63). The BglII restriction enzyme site added to the primers is in lowercase and underlined, the “clamp” sequence (not complementary to fur gene) is in lowercase, and the primer sequence complementary to the fur gene sequence is in uppercase.
Protein analysis.
C. jejuni wild-type and mutant strains were grown in iron-depleted or iron-supplemented medium, pelleted, washed with phosphate-buffered saline, and subjected to cell fractionation to produce periplasmic, cytoplasmic, and outer membrane fractions. C. jejuni cells were pelleted, washed twice with TES (10 mM Tris-HCl [pH 7.5], 10 mM EDTA, 25% [wt/vol] sucrose), subsequently resuspended in ice-cold H2O to a final optical density at 600 nm (OD600) of 30, and incubated 10 min on ice. After centrifugation at 13,000 × g for 5 min, the supernatant (periplasmic) fraction was removed. Spheroplasts were resuspended in an equal volume of 10 mM Tris-HCl (pH 7.5) and disrupted by sonication. Crude membranes (pellet) were separated from the cytoplasmic fraction (supernatant) by ultracentrifugation at 100,000 × g for 10 min. Inner membranes were solubilized in 10 mM Tris-HCl (pH 7.5)–7 mM EDTA–0.6% (wt/vol) sarcosyl for 20 min at 37°C; outer membranes were pelleted by ultracentrifugation and then washed twice with and finally resuspended in 10 mM Tris-HCl (pH 7.5). Samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subsequently stained with Coomassie brilliant blue. N-terminal amino acid sequences were determined from proteins transferred to Fluorotrans membranes (Flowgen Laboratories), using Edman degradation on an ABI model 476 sequencer (Applied Biosystems). Approximately 30 μg of protein was separated by two-dimensional (2-D) electrophoresis using a Multiphor II electrophoresis unit (Pharmacia LKB, Uppsala, Sweden). Isoelectric focusing was performed on 18-cm Immobiline DryStrips (Pharmacia) with a pH range of 3 to 10, and proteins thus focused were separated according to molecular weight (MW) on a SDS-containing ExcelGel (Pharmacia) with an acrylamide concentration gradient from 12 to 14%. Proteins were subsequently silver stained according to the manufacturer’s instructions.
Nucleotide sequence accession number.
The sequence of the pAV25 insert containing C. jejuni NCTC 11168 fur and upstream and downstream sequences has been deposited in the GenBank database under accession no. AF052056.
RESULTS
Isolation and site-directed mutagenesis of C. jejuni NCTC 11168 fur.
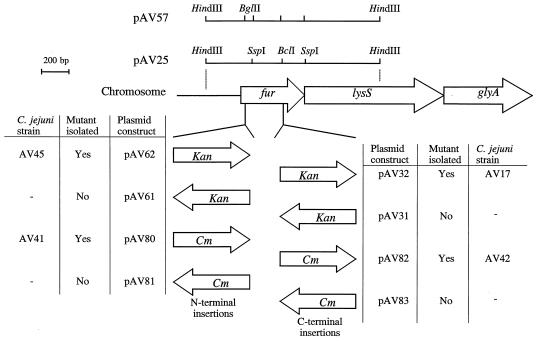
In C. jejuni, the fur gene is located in front of the lysS (9) and glyA (8) genes, and these three genes appear to be cotranscribed (10). To increase the efficiency of homologous recombination, we cloned the C. jejuni NCTC 11168 fur gene and flanking regions in pBluescript as a 1.9-kb HindIII fragment from a cosmid library of C. jejuni NCTC 11168 (26). Determination of the nucleotide sequence of this clone, designated pAV25, showed that it contained the fur gene of 474 bp, with 373 bp of upstream sequence and 1,058 bp of downstream sequence containing part of the lysS gene (Fig. 1). Two sets of constructs based on pAV25 were made by insertion of either a kanamycin resistance (Kanr) or chloramphenicol resistance (Cmr) cassette into the fur gene (Fig. 1). The first set of constructs introduce a mutation toward the 3′ end of the fur gene by insertion of the resistance gene into a unique BclI site in the fur gene. This interrupted fur gene would be predicted to encode a truncated protein of 101 amino acids. This truncated gene was not capable of complementing the fur mutation of E. coli H1780 (data not shown), unlike the intact gene (62). A second set of mutant constructs was generated by inverse PCR mutagenesis (63) which introduced a unique BglII site and stop codons in the region encoding the N terminus of the Fur protein. After introduction of the Kanr or Cmr cassette, these interrupted fur genes would be predicted to encode a truncated Fur protein of only seven amino acids. The interrupted fur genes with the antibiotic resistance genes in both transcriptional orientations were introduced into C. jejuni NCTC 11168 by electroporation, and selection for Kanr or Cmr was used to isolate clones resulting from allelic replacement of the wild-type fur gene by the mutagenized derivatives. Kanr or Cmr colonies were obtained only by using constructs containing the antibiotic resistance genes in the same transcriptional orientation as the fur gene. This observation suggests a polar effect of the mutation. Replacement of the wild-type genes by the mutated copies was confirmed by Southern hybridization (Fig. 2). An internal SspI fragment of C. jejuni fur (Fig. 1) was used as a probe and hybridized with HindIII-digested genomic DNA. In C. jejuni NCTC 11168, this probe hybridizes with a ∼1.9-kb fragment (Fig. 2, lane A). The inserted kanamycin resistance gene contains an HindIII site; thus, insertion of the Kanr cassette in fur will result in hybridization with fragments of ∼2.1 and ∼1.3 kb (Fig. 2, lane B). Similar experiments were performed to ensure the presence of the Kanr cassette and the removal of plasmid sequences (data not shown). Identical phenotypes were obtained with mutants with either the kanamycin or chloramphenicol resistance gene inserted in the regions encoding the N and C termini of Fur (data not shown). This result indicated that neither the presence nor the location of the resistance genes had any detectable influence on the phenotype of the fur mutants.
FIG. 1.
Strategy for the isolation of C. jejuni fur mutants. Schematic diagrams of the genomic restriction map of the region and all constructs and C. jejuni mutant strains described in this study are indicated. The position and orientation of the inserted antibiotic resistance cassette in fur for each construct are shown.
FIG. 2.
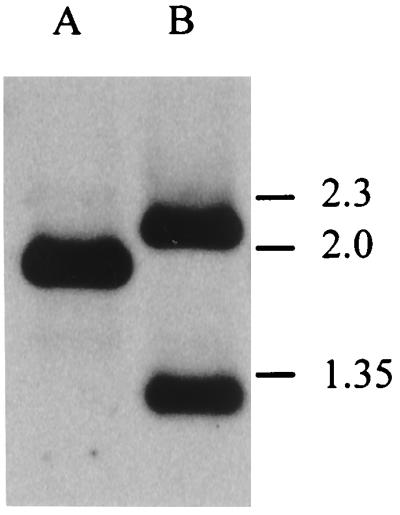
Confirmation of successful disruption of the C. jejuni fur gene by Southern hybridization. A probe consisting of an internal SspI fragment of C. jejuni fur (Fig. 1) was hybridized to HindIII-digested genomic DNA from C. jejuni NCTC 11168 (wild type; lane A) and AV17 (fur mutant; lane B). Marker sizes (in kilobases) are indicated on the right.
Growth of the C. jejuni fur mutant at different iron levels.
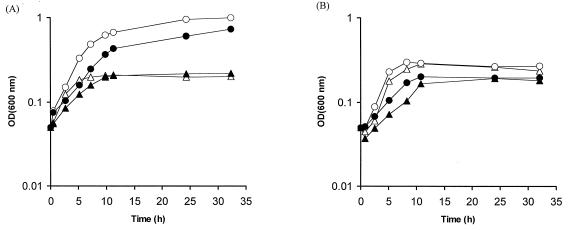
The effect of the fur mutation in C. jejuni AV17 on growth was determined in iron-supplemented and iron-restricted MH medium. Low-iron conditions were achieved by supplementation of MH medium with the iron chelator Desferal. High-iron conditions were achieved by the addition of FeSO4 to MH medium. To confirm the results obtained with MH medium, the experiments were repeated with a defined low-iron medium (MEMα), which was supplemented with FeSO4 to create high-iron conditions. Growth curves thus obtained are shown in Fig. 3. Growth characteristics of C. jejuni NCTC 11168 were similar to those of other C. jejuni strains tested previously under low- and high-iron conditions (17, 38). In MH media (Fig. 3A), chelation of iron had a strong inhibitory effect on the growth of both C. jejuni NCTC 11168 and C. jejuni AV17. In iron-supplemented medium, growth of both NCTC 11168 and AV17 was strongly stimulated. The growth rate of the fur mutant AV17 was lower than that of NCTC 11168 in both conditions, although the final OD600 levels achieved in this experiment were similar. In contrast to fur mutants in some other bacteria (44, 53), growth in high-iron conditions does not seem to have deleterious effects on the C. jejuni fur mutant. Growth in defined medium (MEMα [Fig. 3B]) gave similar results, as the growth rate of the fur mutant was lower. In contrast to MH media, supplementation of MEMα with FeSO4 did not stimulate growth.
FIG. 3.
Growth curves for C. jejuni wild-type strain NCTC 11168 and fur mutant strain AV17 in low- and high-iron MH media (A) and the defined medium MEMα (B) at 37°C under microaerophilic atmospheric conditions. ▵, NCTC 11168 in low-iron medium; ○, NCTC 11168 in high-iron medium; ▴, AV17 in low-iron medium; •, AV17 in high-iron medium.
Effect of the fur mutation on iron-regulated protein expression.
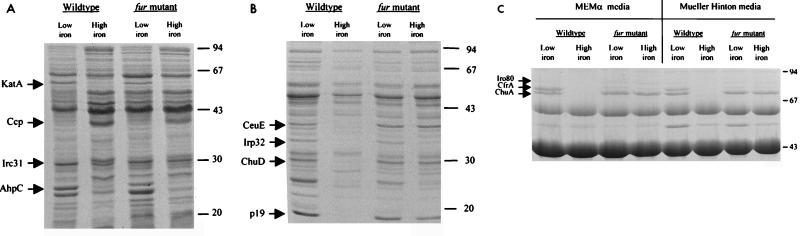
The protein profiles of C. jejuni NCTC 11168 and the fur mutant AV17 grown in low- and high-iron conditions were compared to identify proteins whose expression becomes derepressed in the fur mutant. C. jejuni was grown under the conditions described above and fractionated into cytoplasmic, periplasmic, and outer membrane fractions. Proteins were separated on SDS-polyacrylamide gels and stained with Coomassie brilliant blue. Protein profiles of all fractions of C. jejuni NCTC 11168 and AV17 are presented in Fig. 4. When not further identified, iron-regulated proteins observed in these fractions are designated Irc, Irp, and Iro (iron-regulated cytoplasmic, periplasmic, and outer membrane proteins, respectively).
FIG. 4.
Protein profiles of fractionated C. jejuni wild-type strain NCTC 11168 and fur mutant strain AV17 grown in low- and high-iron media upon separation of proteins by SDS-PAGE. Iron-regulated proteins (cytoplasmic [Irc], periplasmic [Irp], and outer membrane [Iro]) are highlighted by arrows. When further identified, the protein designation is given. (A) Cytoplasmic fraction; (B) periplasmic fraction; (C) outer membrane fraction. Sizes are indicated in kilodaltons on the right.
The cytoplasmic fraction contained two iron-repressed proteins with approximate molecular masses of 26 and 55 kDa (Fig. 4A). These proteins have been previously identified (2) as the small subunit of alkyl hydroperoxide reductase (AhpC; 26 kDa) and catalase (KatA; 55 kDa) (19). The genes encoding these proteins both have a putative Fur box in their promoter regions (2, 19). Surprisingly, the expression of these two proteins was not derepressed in the fur mutant but was lower in the fur mutant background. Two iron-induced proteins with molecular masses of 31 and 40 kDa (Irc31 and Ccp [Fig. 4A]) were also identified, and their expression was lower but not derepressed in the fur mutant. The 40-kDa protein has been identified as a cytochrome c peroxidase homologue (11, 41).
The periplasmic fraction was obtained by osmotic shock and contained at least four iron-repressed proteins of 36, 32, 29, and 19 kDa (Irp36 [CeuE], Irp32, Irp29 [ChuD], and p19), whose expression was derepressed in the fur mutant. These proteins are indicated in Fig. 4B. An iron-repressed protein of 26 kDa is probably identical to AhpC and derived from contamination with the cytoplasmic fraction.
The outer membrane protein fraction (Fig. 4C) of C. jejuni NCTC 11168 contained three iron-repressed outer membrane proteins with molecular masses of approximately 70, 75, and 80 kDa (ChuA, CfrA, and Iro80). The 75-kDa outer membrane protein was later identified as CfrA, whereas the ChuA protein represents a new siderophore receptor homologue (see below). Iron-repressed outer membrane proteins of similar molecular masses have been previously described as being iron regulated in other C. jejuni (17, 38) and C. coli (20) strains. All three iron-repressed outer membrane proteins showed derepressed expression in the fur mutant AV17 (Fig. 4C). The fur mutant also showed different levels of expression of ChuA and CfrA in comparison to the wild type. The fur mutant expressed ChuA at much higher levels than CfrA. In contrast, C. jejuni NCTC 11168 expressed ChuA and CfrA at similar levels in iron-restricted conditions. ChuA is expressed at higher levels in the fur mutant than in wild-type C. jejuni; conversely, CfrA is expressed at lower levels in the fur mutant than in the wild type. This finding suggests that in addition to being under Fur regulation, both ChuA and CfrA proteins may be subject to regulation by one or more other regulatory circuits. To confirm that expression of all of these proteins was indeed regulated by iron, protein profiles were also obtained from cells grown in MEMα and MEMα supplemented with FeSO4. Protein profiles thus obtained were identical to those obtained for cells grown in MH media (e.g., Fig. 4C).
Identification of novel C. jejuni Fur-regulated proteins.
To identify proteins whose expression was derepressed in the fur mutant, we analyzed their N-terminal amino acid sequences and compared them with entries in the GenBank/EMBL nucleotide and protein databases. The N-terminal amino acid sequence (QNVELDS) of the 75-kDa outer membrane protein was identical to that of the C. coli CfrA protein (20). The N-terminal amino acid sequence of the ChuA protein (QESNKAINLQKVVVSTD) did not show significant homology to any amino acid sequence in the database. In addition, an internal amino acid sequence (NTGKMSKN) was determined to allow cloning of the chuA gene. The N-terminal amino acid sequence of the 19-kDa periplasmic protein (GEVPIGDPKELNGMEIAAV) was identical to that of p19, a previously described 19-kDa periplasmic protein of C. jejuni (24).
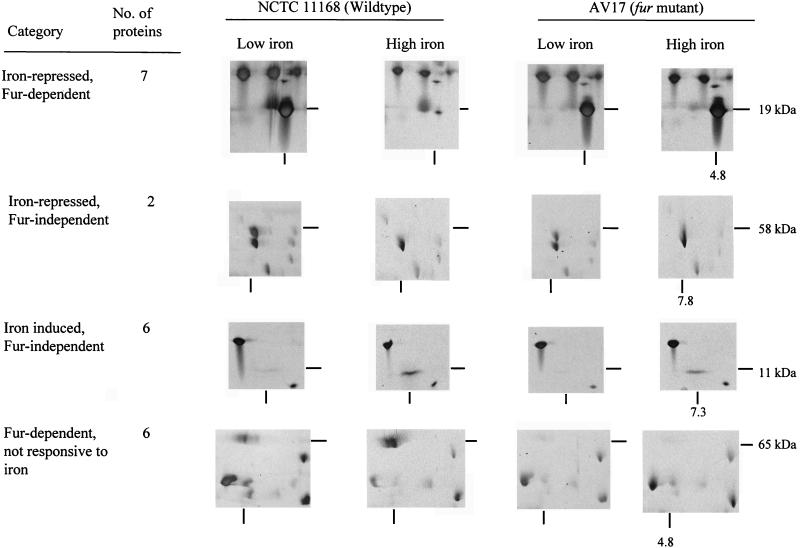
Further identification of a number of proteins in the C. jejuni iron and Fur regulons was performed by 2-D gel electrophoresis. Cells grown overnight in iron-restricted and iron-supplemented MH media were separated by pI, and MW and protein profiles were compared for differences in expression under these conditions. Proteins were designated as iron regulated only when a major difference in expression was noted. The total number of iron-regulated and Fur-regulated proteins and examples of four different classes of regulation are presented in Fig. 5.
FIG. 5.
Identification of members of the C. jejuni iron and Fur regulons by 2-D gel electrophoresis. Four different classes of regulation are illustrated, with the number of C. jejuni proteins belonging to each class indicated. From each class, one example from 2-D gels with the surrounding region is included, with the pI decreasing from left to right. The approximate pI of the regulated protein is indicated below the relevant photograph; the estimated molecular mass is shown at the right.
DISCUSSION
The Fur protein is a global regulator of gene expression in gram-negative bacteria, where it regulates gene expression in response to intracellular iron concentration. The iron concentration has to be monitored carefully in order to obtain enough iron to satisfy the cell’s metabolic requirements without overloading the cell with iron that could lead to oxidative stress. This is achieved by repressing the expression of genes involved in iron uptake once the intracellular Fe2+ reaches a critical level. The number of Fur-regulated genes in gram-negative bacteria varies considerably. This is probably a reflection of the size of the genome and the number of iron-containing compounds the organism can utilize. E. coli, for example, has eight different iron uptake systems (61) and can utilize iron-containing host compounds. It can also use siderophores (small secreted iron-scavenger molecules) produced by other microorganisms. In contrast, only some strains of C. jejuni secrete siderophores (38). The strains tested were capable of utilizing enterochelin, ferrichrome, hemin, and hemoglobin but not aerobactin, Desferal, ferritin, lactoferrin, or transferrin (17, 38).
Several techniques have been used to identify Fur-regulated genes. Stojiljkovic et al. (46) developed an E. coli-based Fur titration assay that detected putative Fur-regulated genes in S. typhimurium (54). However, we have been unsuccessful in adapting this assay to C. jejuni (55). Using selective amplification based on in vitro binding of Fur-regulated promoters to recombinant Fur, Ochsner and Vasil (35) isolated 20 putative Fur-regulated genes of P. aeruginosa. An adaptation of this approach for use in Campylobacter spp. is currently under investigation.
A third method has been the construction of fur mutants and subsequent identification of iron-regulated proteins whose expression is derepressed. We used insertional mutagenesis using antibiotic resistance genes, as this is, to date, the only way of constructing targeted mutations in Campylobacter. Insertional mutagenesis of fur has not been possible in several bacteria, including P. aeruginosa (39), N. gonorrhoeae (50), and V. anguillarum (60). Fur expression seems to be essential in these species. In C. jejuni, we were able to isolate insertional mutants in fur, but this was dependent on the orientation of the antibiotic resistance gene. It is most likely that the requirement for a specific orientation is due to polar effects on the expression of the two genes directly downstream of fur. The fur gene in both C. jejuni (10, 62) and Campylobacter upsaliensis (5) is followed by the lysS and glyA genes, which encode the housekeeping enzymes lysyl-tRNA synthetase and serine hydroxymethyltransferase, respectively (8, 9). So far, this gene organization seems to be unique to Campylobacter spp. The expression of lysS and glyA seems to be coupled to that of fur (10), and the start codon of lysS follows the stop codon of fur directly. The insertion of an antibiotic resistance gene in the direction opposite to that of fur could disrupt the formation of a multicistronic mRNA containing fur, lysS, and glyA. However, if the antibiotic resistance gene were inserted in the same direction as fur, it would allow the formation of a multicistronic mRNA containing the antibiotic resistance gene, lysS, and glyA, as neither of the antibiotic resistance genes has a transcriptional terminator downstream. The level of LysS and GlyA under these conditions may be suboptimal and deregulated; therefore, caution must be used in attributing the phenotype (especially the difference in growth rate from the wild type) exclusively to the absence of Fur.
C. jejuni is not amenable to the use of shuttle vectors for complementation experiments; thus, the introduction of either fur or lysS-glyA was not possible. Although NCTC 11168, like most C. jejuni strains, does not readily accept shuttle vectors, it is transformable and is being used for the genome sequencing project. The availability of the genome sequence facilitated the analysis of differential expression of proteins in fur mutants and was particularly important due to the significant variation of iron transport systems across different C. jejuni strains. As lysS and glyA are cotranscribed with fur and possibly other upstream members of the operon, it is likely that the growth phenotype observed with the fur mutant is due to uncontrolled and increased expression of LysS and GlyA. Meaningful complementation analysis of the polar effect on lysS-glyA would be complicated by the requirement to use a multicopy shuttle vector, plasmid selection, and an alternative non-fur promoter. Similar problems would be encountered during an attempt to complement the fur mutation. In addition, the use of a multicopy plasmid is likely to lead to compensatory mutations in the complementing fur sequence as observed in an E. coli background (55). As fur may be autoregulated (10) and we have not definitely identified the fur promoter, the expression of a complementary copy of fur would be uncontrolled. That the fur phenotype is not the result of an unknown unrelated mutation is supported by the fact that we have constructed several independent fur mutants in several strains that have identical phenotypes. Nevertheless, as LysS and GlyA are not expected to be involved in iron-responsive gene regulation, the derepressed expression of several iron-regulated genes can be attributed solely to the fur mutation.
The C. jejuni fur mutant strain AV17 shows changes in growth characteristics similar to those of a P. aeruginosa fur mutant (23). The growth rate of the fur mutant was clearly lower than that of the wild-type strain under both low- and high-iron conditions. The difference between the final OD600 under high-iron conditions is due to the fact that the late exponential growth phase is much longer under high-iron conditions than under low-iron conditions. This finding clearly indicates that the fur mutant does not suffer iron overload under iron-replete conditions, unlike the Y. pestis fur mutant (44). In the P. aeruginosa fur mutant, siderophore-mediated iron uptake was defective (23), which might explain the apparent absence of intracellular iron overload. Whether a similar phenomenon spares the C. jejuni fur mutant from high-iron stress remains to be determined.
By comparing protein profiles, we were able to identify seven proteins whose expression is iron regulated in C. jejuni NCTC 11168 but not in the fur mutant. The N-terminal amino acid sequences of three of these proteins were determined. The genes encoding two of these proteins have been described previously, whereas the N-terminal sequence of the third protein does not match any sequence in the databases.
The 75-kDa outer membrane protein was identical to C. coli CfrA (20). This protein shows significant homology to putative ferric siderophore receptors of Bordetella bronchiseptica and V. cholerae, though the cognate ligand of CfrA is unknown (20). Mutants in CfrA were still able to utilize enterochelin, hemin, and ferrichrome (20). Although CfrA has been previously shown to be iron regulated (17, 20, 38) and putative Fur boxes were identified within its putative promoter region, it had not been proven to be Fur regulated. While expression of CfrA is derepressed in the fur mutant, expression levels are not as high in the fur mutant as in the wild type. Thus, there might be other regulatory systems involved in repressing expression of CfrA. The N-terminal amino acid sequence of ChuA did not show any homology with sequences in the database. C. jejuni mutants lacking expression of this protein were shown to be unable to utilize hemin and hemoglobin (38), and so ChuA is likely to be the receptor for these compounds. We have identified most of the gene encoding ChuA (55) and have searched the preliminary C. jejuni genome sequence (http://www.sanger .ac.uk/Projects/C_jejuni/) for the regions upstream and downstream of chuA. The downstream genes resemble a typical ABC transporter operon containing a cytoplasmic membrane permease, ATP-binding protein and periplasmic binding protein (ChuB, ChuC, and ChuD, respectively). The predicted amino acid sequence of ChuA shows significant homology to outer membrane siderophore receptors of several other bacterial species and also to CfrA (55). The putative promoter region of ChuA contains a Fur box homologue, but this needs confirmation.
The 19-kDa periplasmic protein was identical to p19, a protein present in all C. jejuni and C. coli strains tested (24). It had not been identified previously as being iron regulated. While no function in either iron uptake or iron utilization can be attributed to this protein at this point, the N terminus shows homology with a 20-kDa protein of a magnetotactic bacterium which is involved in magnetosome synthesis (14). As for the identities of the three other periplasmic proteins, it is very likely that the Irp36 protein is CeuE (37, 40), the periplasmic component of an enterochelin uptake system. No attempt was made to sequence this protein, as CeuE is blocked at the N terminus (36). Further support for Irp36 being CeuE arises from the identification of an iron- and Fur-regulated 36-kDa protein by 2-D gel electrophoresis; this protein has a pI of approximately 9.5, whereas the calculated pI for CeuE is 9.18 (data not shown). Based on MW and pI (data not shown), it is likely that the Irp29 protein is the periplasmic binding protein, designated ChuD, whose gene was identified downstream of chuA. This awaits further confirmation.
Interestingly, the expression of two iron-regulated cytoplasmic proteins, alkyl hydroperoxide reductase (AhpC) and catalase (KatA), was not derepressed in the fur mutant. This was surprising, as the promoter regions of both the ahpC and katA genes have a sequence which closely resembles the E. coli Fur box consensus sequence (2, 19). This could mean that these genes are not in fact Fur regulated or that additional multiple levels of regulation exist. In several other bacteria, AhpC and KatA are positively regulated by the OxyR protein in response to oxidative stress (12, 16, 34). It is possible that C. jejuni does not have an oxyR homologue, as this gene is not present in the genome of Helicobacter pylori (52). H. pylori is a close relative of C. jejuni, and the two are similar with respect to genome size and codon usage. Whether multiple levels of regulation of oxidative stress defense proteins exist in C. jejuni remains to be elucidated. The fur mutant was slightly less resistant to the oxidative stress inducers hydrogen peroxide and cumene hydrogen peroxide (data not shown), which is consistent with the observation that AhpC and KatA are expressed at lower levels in the fur mutant. Regulation of oxidative stress genes by metal ions and a Fur homologue (designated PerR) has been recently described for Bacillus subtilis (6), and the identification of sequences encoding a putative second fur homologue of C. jejuni in the genome sequence makes it likely that C. jejuni possesses a homologous mechanism (55).
Further identification of members of the C. jejuni iron and Fur regulons by using 2-D gel electrophoresis showed few proteins to be regulated by iron or Fur compared to other bacteria (Fig. 5) (18, 29, 44, 50), possibly because Campylobacter species have comparatively small genomes (estimated to be 1,700 kb) and can utilize relatively few iron compounds. However, the detection of iron- and Fur-regulated proteins could be made more sensitive by incorporation of radioactive labels in proteins.
Knowledge of the mechanisms of virulence and their regulation in Campylobacter is scant. This study is the first description of a mutation in a regulatory gene in Campylobacter and the subsequent characterization of its regulon. Future work will focus on the cloning and characterization of the genes encoding the members of the iron and Fur regulons of C. jejuni.
ACKNOWLEDGMENTS
This study was supported by the Wellcome Trust and a Royal Society fellowship to J. M. Ketley.
We thank Vicky Wilson and John Henderson for constructing and screening the C. jejuni NCTC 11168 cosmid library, Kathryn Lilley for the N-terminal amino acid sequencing, and Marie-Louise Baillon and Charles Penn for performing oxidative stress experiments.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Short protocols in molecular biology. 2nd ed. New York, N.Y: John Wiley & Sons; 1992. [Google Scholar]
- 2.Baillon, M. L. A., A. H. M. van Vliet, J. M. Ketley, C. Constantinidou, and C. W. Penn. Characterisation and mutation of the iron-regulated Campylobacter jejuni small subunit of alkyl hydroperoxide reductase which confers resistance to oxidative stress. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 3.Berish S A, Subbarao S, Chen C Y, Trees D L, Morse S A. Identification and cloning of a fur homolog from Neisseria gonorrhoeae. Infect Immun. 1993;61:4599–4606. doi: 10.1128/iai.61.11.4599-4606.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaser M J, Taylor D N, Feldman R A. Epidemiology of Campylobacter jejuni infections. Epidemiol Rev. 1983;5:157–176. doi: 10.1093/oxfordjournals.epirev.a036256. [DOI] [PubMed] [Google Scholar]
- 5.Bourke B B, Al-Rashid S T, Bingham H L, Chan V L. Characterization of Campylobacter upsaliensis fur and its localization in a highly conserved region of the Campylobacter genome. Gene. 1996;183:219–224. doi: 10.1016/s0378-1119(96)00562-8. [DOI] [PubMed] [Google Scholar]
- 6.Bsat N, Herbig A, Casillas-Martinez L, Setlow P, Helmann J D. Bacillus subtilis contains multiple Fur homologues: identification of the iron-uptake (Fur) and peroxide regulon (PerR) repressors. Mol Microbiol. 1998;29:189–198. doi: 10.1046/j.1365-2958.1998.00921.x. [DOI] [PubMed] [Google Scholar]
- 7.Bullen J J. The significance of iron in infection. Rev Infect Dis. 1981;3:1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- 8.Chan V L, Bingham H L. Complete sequence of the Campylobacter jejuni glyA gene encoding serine hydroxymethyltransferase. Gene. 1991;101:51–58. doi: 10.1016/0378-1119(91)90223-x. [DOI] [PubMed] [Google Scholar]
- 9.Chan V L, Bingham H L. Lysyl-tRNA synthetase gene of Campylobacter jejuni. J Bacteriol. 1992;174:695–701. doi: 10.1128/jb.174.3.695-701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan V L, Louie H, Bingham H L. Cloning and transcription regulation of the ferric uptake regulatory gene of Campylobacter jejuni TGH9011. Gene. 1995;164:25–31. doi: 10.1016/0378-1119(95)00477-n. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee, S., K. G. Wooldridge, and J. M. Ketley. 1998. Unpublished results.
- 12.Christman M F, Storz G, Ames B N. OxyR, a positive regulator of hydrogen peroxide-inducible genes in Escherichia coli and Salmonella typhimurium, is homologous to a family of bacterial regulatory proteins. Proc Natl Acad Sci USA. 1989;86:3484–3488. doi: 10.1073/pnas.86.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coy M, Neilands J B. Structural dynamics and functional domains of the Fur protein. Biochemistry. 1991;30:8201–8210. doi: 10.1021/bi00247a016. [DOI] [PubMed] [Google Scholar]
- 14.Dubbels, B., and D. Bazylinski. Personal communication.
- 15.Ernst J F, Bennett R L, Rothfield L I. Constitutive expression of the iron-enterochelin and ferrichrome uptake systems in a mutant strain of Salmonella typhimurium. J Bacteriol. 1978;135:928–934. doi: 10.1128/jb.135.3.928-934.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farr S B, Kogoma T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol Rev. 1991;55:561–585. doi: 10.1128/mr.55.4.561-585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Field L H, Headley V L, Payne S M, Berry L J. Influence of iron on growth, morphology, outer membrane protein composition, and synthesis of siderophores in Campylobacter jejuni. Infect Immun. 1986;54:126–132. doi: 10.1128/iai.54.1.126-132.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster J W, Hall H K. Effect of Salmonella typhimurium ferric uptake regulator (fur) mutations on iron-regulated and pH-regulated protein synthesis. J Bacteriol. 1992;174:4317–4323. doi: 10.1128/jb.174.13.4317-4323.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant K A, Park S F. Molecular characterization of katA from Campylobacter jejuni and generation of a catalase-deficient mutant of Campylobacter coli by interspecific allelic exchange. Microbiology. 1995;141:1369–1376. doi: 10.1099/13500872-141-6-1369. [DOI] [PubMed] [Google Scholar]
- 20.Guerry P, Perez-Casal J, Yao R J, McVeigh A, Trust T J. A genetic locus involved in iron utilization unique to some Campylobacter strains. J Bacteriol. 1997;179:3997–4002. doi: 10.1128/jb.179.12.3997-4002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hantke K. Cloning of the repressor protein gene of iron-regulated systems in Escherichia coli K12. Mol Gen Genet. 1984;197:337–341. doi: 10.1007/BF00330982. [DOI] [PubMed] [Google Scholar]
- 22.Hantke K. Selection procedure for deregulated iron transport mutants (fur) in Escherichia coli K12—fur not only affects iron-metabolism. Mol Gen Genet. 1987;210:135–139. doi: 10.1007/BF00337769. [DOI] [PubMed] [Google Scholar]
- 23.Hassett D J, Sokol P A, Howell M L, Ma J F, Schweizer H T, Ochsner U, Vasil M L. Ferric uptake regulator (fur) mutants of Pseudomonas aeruginosa demonstrate defective siderophore-mediated iron uptake, altered aerobic growth, and decreased superoxide dismutase and catalase activities. J Bacteriol. 1996;178:3996–4003. doi: 10.1128/jb.178.14.3996-4003.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janvier, B., C. Constantinidou, P. Aucher, Z. V. Marshall, C. W. Penn, and J. L. Fauchere. Characterization and gene sequencing of a 19 kDa periplasmic protein of Campylobacter jejuni/coli. Res. Microbiol., in press. [DOI] [PubMed]
- 25.Karkhoff-Schweizer R R, Schryvers A B, Schweizer H P. Cloning and sequence analysis of the fur gene encoding an iron-regulatory protein of Neisseria meningitidis. Gene. 1994;141:139–140. doi: 10.1016/0378-1119(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 26.Karlyshev A V, Henderson J, Ketley J M, Wren B W. An improved physical and genetic map of Campylobacter jejuni NCTC 11168 (UA580) Microbiology. 1998;144:503–508. doi: 10.1099/00221287-144-2-503. [DOI] [PubMed] [Google Scholar]
- 27.Ketley J M. Pathogenesis of enteric infection by Campylobacter. Microbiology. 1997;143:5–21. doi: 10.1099/00221287-143-1-5. [DOI] [PubMed] [Google Scholar]
- 28.Litwin C M, Boyko S A, Calderwood S B. Cloning, sequencing, and transcriptional regulation of the Vibrio cholerae fur gene. J Bacteriol. 1992;174:1897–1903. doi: 10.1128/jb.174.6.1897-1903.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Litwin C M, Calderwood S B. Analysis of the complexity of gene regulation by Fur in Vibrio cholerae. J Bacteriol. 1994;176:240–248. doi: 10.1128/jb.176.1.240-248.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Litwin C M, Calderwood S B. Cloning and genetic analysis of the Vibrio vulnificus fur gene and construction of a fur mutant by in vivo marker exchange. J Bacteriol. 1993;175:706–715. doi: 10.1128/jb.175.3.706-715.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Litwin C M, Calderwood S B. Role of iron in regulation of virulence genes. Clin Microbiol Rev. 1993;6:137–149. doi: 10.1128/cmr.6.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller J F, Dower W J, Tompkins L S. High-voltage electroporation of bacteria: genetic transformation of Campylobacter jejuni with plasmid DNA. Proc Natl Acad Sci USA. 1988;85:856–860. doi: 10.1073/pnas.85.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mishu B, Blaser M J. Role of infection due to Campylobacter jejuni in the initiation of Guillain-Barré syndrome. Clin Infect Dis. 1993;17:104–108. doi: 10.1093/clinids/17.1.104. [DOI] [PubMed] [Google Scholar]
- 34.Mongkolsuk S, Loprasert S, Whangsuk W, Fuangthong M, Atichartpongkun S. Characterization of transcription organization and analysis of unique expression patterns of an alkyl hydroperoxide reductase C gene (ahpC) and the peroxide regulator operon ahpF-oxyR-orfX from Xanthomonas campestris pv. phaseoli. J Bacteriol. 1997;179:3950–3955. doi: 10.1128/jb.179.12.3950-3955.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ochsner U A, Vasil M L. Gene repression by the ferric uptake regulator in Pseudomonas aeruginosa—cycle selection of iron-regulated genes. Proc Natl Acad Sci USA. 1996;93:4409–4414. doi: 10.1073/pnas.93.9.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park, S. F. Personal communication.
- 37.Park S F, Richardson P T. Molecular characterization of a Campylobacter jejuni lipoprotein with homology to periplasmic siderophore-binding proteins. J Bacteriol. 1995;177:2259–2264. doi: 10.1128/jb.177.9.2259-2264.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pickett C L, Auffenberg T, Pesci E C, Sheen V L, Jusuf S S. Iron acquisition and hemolysin production by Campylobacter jejuni. Infect Immun. 1992;60:3872–3877. doi: 10.1128/iai.60.9.3872-3877.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prince R W, Cox C D, Vasil M L. Coordinate regulation of siderophore and exotoxin A production: molecular cloning and sequencing of the Pseudomonas aeruginosa fur gene. J Bacteriol. 1993;175:2589–2598. doi: 10.1128/jb.175.9.2589-2598.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson P T, Park S F. Enterochelin acquisition in Campylobacter coli—characterization of components of a binding protein dependent transport system. Microbiology. 1995;141:3181–3191. doi: 10.1099/13500872-141-12-3181. [DOI] [PubMed] [Google Scholar]
- 41.Ridout C J, James R, Greenwood C. Nucleotide sequence encoding the di-haem cytochrome c551 peroxidase from Pseudomonas aeruginosa. FEBS Lett. 1995;365:152–154. doi: 10.1016/0014-5793(95)00461-h. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 43.Schaeffer S, Hantke K, Braun V. Nucleotide sequence of the iron regulatory gene fur. Mol Gen Genet. 1985;200:110–113. doi: 10.1007/BF00383321. [DOI] [PubMed] [Google Scholar]
- 44.Staggs T M, Fetherston J D, Perry R D. Pleiotropic effects of a Yersinia pestis fur mutation. J Bacteriol. 1994;176:7614–7624. doi: 10.1128/jb.176.24.7614-7624.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Staggs T M, Perry R D. Identification and cloning of a fur regulatory gene in Yersinia pestis. J Bacteriol. 1991;173:417–425. doi: 10.1128/jb.173.2.417-425.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stojiljkovic I, Baumler A J, Hantke K. Fur regulon in gram-negative bacteria. Identification and characterization of new iron-regulated Escherichia coli genes by a fur titration assay. J Mol Biol. 1994;236:531–545. doi: 10.1006/jmbi.1994.1163. [DOI] [PubMed] [Google Scholar]
- 47.Stojiljkovic I, Hantke K. Functional domains of the Escherichia coli ferric uptake regulator protein (Fur) Mol Gen Genet. 1995;247:199–205. doi: 10.1007/BF00705650. [DOI] [PubMed] [Google Scholar]
- 48.Tauxe R V. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni: current status and future trends. Washington, D.C: American Society for Microbiology; 1992. pp. 9–19. [Google Scholar]
- 49.Thomas C E, Sparling P F. Identification and cloning of a fur homologue from Neisseria meningitidis. Mol Microbiol. 1994;11:725–737. doi: 10.1111/j.1365-2958.1994.tb00350.x. [DOI] [PubMed] [Google Scholar]
- 50.Thomas C E, Sparling P F. Isolation and analysis of a fur mutant of Neisseria gonorrhoeae. J Bacteriol. 1996;178:4224–4232. doi: 10.1128/jb.178.14.4224-4232.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tolmasky M E, Wertheimer A M, Actis L A, Crosa J H. Characterization of the Vibrio anguillarum fur gene: role in regulation of expression of the FatA outer membrane protein and catechols. J Bacteriol. 1994;176:213–220. doi: 10.1128/jb.176.1.213-220.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzegerald L M, Lee N, Adams M D, Venter J C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 53.Touati D, Jacques M, Tardat B, Bouchard L, Despied S. Lethal oxidative damage and mutagenesis are generated by iron in fur mutants of Escherichia coli: protective role of superoxide dismutase. J Bacteriol. 1995;177:2305–2314. doi: 10.1128/jb.177.9.2305-2314.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsolis R M, Baumler A, Stojiljkovic I, Heffron F. Fur regulon of Salmonella typhimurium: identification of new iron-regulated genes. J Bacteriol. 1995;177:4628–4637. doi: 10.1128/jb.177.16.4628-4637.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Vliet, A. H. M., and J. M. Ketley. Unpublished results.
- 56.van Vliet A H M, Wood A C, Henderson J, Wooldridge K G, Ketley J M. Genetic manipulation of enteric Campylobacter species. In: Williams P, Ketley J, Salmond G, editors. Methods in microbiology. 27. Bacterial pathogenesis. London, England: Academic Press; 1998. pp. 405–419. [Google Scholar]
- 57.Venturi V, Ottevanger C, Bracke M, Weisbeek P. Iron regulation of siderophore biosynthesis and transport in Pseudomonas putida WCS358: involvement of a transcriptional activator and of the Fur protein. Mol Microbiol. 1995;15:1081–1093. doi: 10.1111/j.1365-2958.1995.tb02283.x. [DOI] [PubMed] [Google Scholar]
- 58.Venturi V, Weisbeek P, Koster M. Gene regulation of siderophore-mediated iron acquisition in Pseudomonas: not only the Fur repressor. Mol Microbiol. 1995;17:603–610. doi: 10.1111/j.1365-2958.1995.mmi_17040603.x. [DOI] [PubMed] [Google Scholar]
- 59.Wassenaar T M, Fry B N, van der Zeijst B A M. Genetic manipulation of Campylobacter: evaluation of natural transformation and electrotransformation. Gene. 1993;132:131–135. doi: 10.1016/0378-1119(93)90525-8. [DOI] [PubMed] [Google Scholar]
- 60.Wertheimer A M, Tolmasky M E, Actis L A, Crosa J H. Structural and functional analyses of mutant Fur proteins with impaired regulatory function. J Bacteriol. 1994;176:5116–5122. doi: 10.1128/jb.176.16.5116-5122.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wooldridge K G, Williams P H. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol Rev. 1993;12:325–348. doi: 10.1111/j.1574-6976.1993.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 62.Wooldridge K G, Williams P H, Ketley J M. Iron-responsive genetic regulation in Campylobacter jejuni: cloning and characterization of a fur homolog. J Bacteriol. 1994;176:5852–5856. doi: 10.1128/jb.176.18.5852-5856.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wren B W, Henderson J, Ketley J M. A PCR-based strategy for the rapid construction of defined bacterial deletion mutants. BioTechniques. 1994;16:994–996. [PubMed] [Google Scholar]
- 64.Yao R, Alm R A, Trust T J, Guerry P. Construction of new Campylobacter cloning vectors and a new mutational cat cassette. Gene. 1993;130:127–130. doi: 10.1016/0378-1119(93)90355-7. [DOI] [PubMed] [Google Scholar]