Abstract
Background
Serum uric acid (UA) is correlated closely with traditional cardiovascular risk factors, which might interfere with the action of UA, in patients with coronary artery disease. We performed this study to evaluate the prognostic effect of UA levels in individuals with different numbers of standard modifiable cardiovascular risk factors (SMuRFs).
Methods and Results
In this prospective study, we consecutively enrolled 10 486 patients with coronary artery disease. They were stratified into 3 groups according to the tertiles of UA concentrations and, within each UA tertile, further classified into 3 groups by the number of SMuRFs (0–1 versus 2–3 versus 4). The primary end point was major adverse cardiovascular and cerebrovascular events (MACCEs), including death, myocardial infarction, stroke, and unplanned revascularization. Over a median follow‐up of 2.4 years, 1233 (11.8%) MACCEs were recorded. Patients with high UA levels developed significantly higher risk of MACCEs than those with low UA levels. In addition, UA levels were positively associated with MACCEs as a continuous variable. More importantly, in patients with 0 to 1 SMuRF, the risks of MACCEs were significantly higher in the high‐UA‐level group (adjusted hazard ratio [HR], 1.469 [95% CI, 1.197–1.804]) and medium‐UA‐level group (adjusted HR, 1.478 [95% CI, 1.012–2.160]), compared with the low‐UA‐level group, whereas no significant association was found between UA levels and the risk of MACCEs in participants with 2 to 3 or 4 SMuRFs.
Conclusions
In patients with coronary artery disease who received evidence‐based secondary prevention therapies, elevated UA levels might affect the prognosis of individuals with 0 to 1 SMuRF but not that of individuals with ≥2 SMuRFs.
Keywords: cardiovascular events, coronary artery disease, standard modifiable cardiovascular risk factors, uric acid
Subject Categories: Risk Factors, Metabolism, Coronary Artery Disease, Quality and Outcomes, Clinical Studies
Nonstandard Abbreviations and Acronyms
- CAMI
China Acute Myocardial Infarction
- LURIC
Ludwigshafen Risk and Cardiovascular Health
- MACCE
major adverse cardiovascular and cerebrovascular event
- SMuRF
standard modifiable cardiovascular risk factor
- UA
uric acid
- URRAH
Uric Acid Right for Heart Health
Clinical Perspective.
What Is New?
The prognostic effect of serum uric acid levels in patients with coronary artery disease with different numbers of standard modifiable cardiovascular risk factor (SMuRFs) has never been evaluated.
This study, for the first time, indicated that elevated uric acid levels might affect the prognosis of individuals with 0 to 1 SMuRF but not that of individuals with ≥2 SMuRFs.
What Are the Clinical Implications?
This study provides convincing evidence for the role of elevated UA levels for patients with coronary artery disease with no or few SMuRFs who received evidence‐based secondary prevention therapies.
Further randomized trials are needed to specify the effect of UA‐lowering therapy on prognosis in individuals with coronary artery disease and hyperuricemia, especially in those with no or few SMuRFs.
Uric acid (UA) is an end product of purine metabolism functioning as a vehicle eliminating purine waste from human body. 1 , 2 , 3 It is generally believed that accumulation of UA causes monosodium urate crystal deposition in the joints and kidneys, leading to gout and nephrolithiasis. 4 Given that hyperuricemia provokes endothelial dysfunction via increasing oxidative stress and inflammation, the roles of UA in the incidence of coronary artery disease (CAD) have raised great interest in the past 2 decades. Although mounting evidence from epidemiological studies have demonstrated a positive association between UA levels and CAD, 2 , 3 medical societies have not recognized elevated serum UA as an independent cardiovascular risk factor, 1 , 2 , 3 with a large number of observational studies and Mendelian randomization studies showing conflicting results. 5 , 6 , 7 , 8 , 9 , 10 Regarding individuals with established CAD, evidence for the positive association of high serum UA levels with poor prognosis seems to be stronger than that in the general population, 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 yet there were still some studies reporting inconsistent results. 20 , 21 , 22 , 23
Actually, elevated UA levels are often associated with various cardiovascular risk factors such as abnormal glucose regulation, hypertension, lipid disorder, and obesity, which might interfere with the action of serum UA. 1 , 2 , 3 Therefore, it is important to effectively eliminate or reduce the influence of traditional risk factors when exploring the relationship between UA and cardiovascular events. Standard modifiable cardiovascular risk factors (SMuRFs), which include diabetes, hypertension, dyslipidemia, and smoking, were considered as pivotal drivers triggering the pathogenesis and development of CAD. 24 , 25 To better specify the roles of UA in the secondary prevention population, we performed this study to evaluate the prognostic effect of serum UA levels in patients with CAD with different numbers of SMuRFs from a large prospective study in Asia.
Methods
We will make the data, methods used in the analysis, and materials used to conduct the research available to any researcher for purposes of reproducing the results or replicating the procedure. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design and Population
This was a single‐center prospective cohort study, and the details of the study design were reported previously. 26 , 27 , 28 Briefly, 10 724 patients with CAD who underwent percutaneous coronary intervention (PCI) were consecutively enrolled between January 2013 and December 2013 from Fuwai Hospital, National Center for Cardiovascular Diseases. This study complied with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Fuai Hospital. All participants provided written informed consent before enrollment. Of note, 66 participants with missing UA data before PCI; 4 participants with severe renal insufficiency; and 128 participants with left ventricular ejection fraction ≤40%, having a history of heart failure, or using diuretics due to heart failure were excluded. In addition, we also excluded 41 participants who suffered major adverse events during hospitalization (death, myocardial infarction [MI], stroke, or urgent revascularization). Eventually, a total of 10 486 participants were analyzed (Figure S1).
Study Procedures and Biochemical Analysis
After fasting for ≥12 hours before angiography, laboratory samples were obtained from each of the participants, and all tests were conducted through the clinical chemistry department at Fuwai Hospital. Concentrations of serum UA, creatinine, triglyceride, total cholesterol, low‐density lipoprotein cholesterol, and high‐density lipoprotein cholesterol were analyzed by an automated biochemical analyzer (Hitachi 7150, Tokyo, Japan). UA was measured using a UA Assay Kit (uricase–peroxidase method). A validated standard of UA was used for calibration, and the coefficient of variation of repetitive measurements was <10%. Angiographic and procedural data were collected from catheter laboratory records by 3 experienced interventional cardiologists. 28 During hospitalization, all procedures and medical therapies were performed according to guideline recommendations and cardiologist's discretion. Demographics, cardiovascular risk factors, clinical parameters, laboratory results, coronary angiographic and procedural details, and medications were prospectively collected with standardized questionnaires by independent research personnel.
All patients were stratified into 3 groups according to the tertiles of UA concentrations. Consistent with previous studies, the SMuRFs refers to 4 traditional risk factors including diabetes, hypertension, dyslipidemia, and current smoking. 24 , 25 Participants who had a history of diabetes, received hypoglycemic therapy, or had a fasting blood glucose ≥7.0 mmol/L, hemoglobin A1c ≥6.5%, or 2‐hour plasma glucose ≥11.1 mmol/L in an oral glucose tolerance test were regarded as having diabetes. 29 Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive treatment. 30 Dyslipidemia was defined as an increase in triglyceride (≥150 mg/dL), total cholesterol (≥200 mg/dL), or low‐density lipoprotein cholesterol (≥130 mg/dL); a decrease in high‐density lipoprotein cholesterol (<40 mg/dL); or use of cholesterol‐lowering medication. 31 Current smoking was defined as smoking regularly within the last month before admission. We classified patients into 3 groups on the basis of the number of SMuRFs (ie, 0–1, 2–3, and 4) to avoid a small sample size within groups and permit evaluation of trends according to SMuRF numbers. 32 , 33 Finally, patients were divided into 9 groups according to UA levels and SMuRF numbers.
Follow‐Up and End Points
After discharge, patients were followed up at 6‐month intervals until January 31, 2016. Data for end points were collected from medical records, clinical visit, or telephone interviews by trained investigators who were blind to the clinical data. The primary end point was major adverse cardiovascular and cerebrovascular events (MACCEs), including all‐cause death, nonfatal MI, stroke, or unplanned revascularization. Secondary end points consisted of cardiac death and the components of the primary end point. Death was considered cardiac unless an unequivocal noncardiac cause could be established. MI was defined in compliance with the Third Universal Definition of MI. 34 Stroke was defined as a new focal neurological deficit lasting >24 hours and confirmed by imaging evidence. Unplanned revascularization was repeat PCI or surgery after discharge excluding staged PCI. All events must be validated by source documents.
Statistical Analysis
Continuous variables were expressed as mean±SD if they conformed to the normal distribution; otherwise, they were shown as median (interquartile range), while categorical variables were expressed as frequencies (percentages). Differences between groups were compared using 1‐way ANOVA, the Kruskal–Wallis H test, Pearson's chi‐square test, or Fisher's exact test, as appropriate. The cumulative incidence of clinical events was estimated using Kaplan–Meier curves, and differences were assessed with the log‐rank test. Univariable and multivariable Cox regression analyses were performed to calculate hazard ratios (HRs) and 95% CIs. The optimal cutoff value of UA for predicting MACCEs were identified by the Youden Index with receiver operating characteristic curve analysis. On a continuous scale, restricted cubic splines were also used to examine the potential nonlinear relationships between UA levels and MACCEs. 35 Of note, most variables have no missing values, with a few exceptions. Missing information on covariates for statistical adjustment, that is, for systolic blood pressure (5.1%), triglyceride (2.2%), low‐density lipoprotein cholesterol (2.2%), minimum stent diameter (4.3%), and total stent length (4.3%), were imputed with a sequential regression multiple imputation method. All statistical analyses were conducted in SPSS 23.0 (SPSS Inc., Chicago, IL) and STATA 12.0 (StataCorp, College Station, TX). A 2‐sided P value of <0.05 was considered statistically significant.
Results
Baseline Characteristics
Of the 10 486 participants, 77.2% were men, the median age was 59 years, and the median UA level was 5.6 mg/dL, while 417 (4.0%), 1860 (17.7%), 3494 (33.3%), 3451 (32.9%), and 1264 (12.1%) patients combined with 0, 1, 2, 3, and 4 SMuRFs, respectively. Based on the tertiles of UA levels, participants were divided into 3 groups: <5.1 mg/dL (n=3495), 5.1 to 6.2 mg/dL (n=3497), and ≥6.2 mg/dL (n=3494). In addition, participants were also classified into 1 of 3 groups: 0 to 1 SMuRF (n=2277), 2 to 3 SMuRFs (n=6945), and 4 SMuRFs (n=1264).
Baseline characteristics of the participants according to UA levels are listed in Table 1. The percentage of male patients, prevalence of cardiovascular risk factors, and previous MI increased with UA levels. From the low‐UA‐level group to the high‐UA‐level group, there was an ascending gradient regarding body mass index, triglyceride concentrations, minimum stent diameter, and total stent length, whereas there was a descending gradient with respect to age, systolic blood pressure, high‐density lipoprotein cholesterol levels, and hemoglobin A1c levels. In Table S1, the prevalence of male patients, previous MI, previous stroke, peripheral vascular disease, estimated glomerular filtration rate <60 mL/min per 1.73 m2, 3‐vessel disease, and total stent length increased with the number of SMuRFs. In addition, there was an ascending gradient regarding body mass index, systolic blood pressure, triglyceride levels, hemoglobin A1c levels, and total stent length, whereas there was a descending gradient with respect to minimum stent diameter and the concentrations of total cholesterol, low‐density lipoprotein cholesterol, and high‐density lipoprotein cholesterol.
Table 1.
Baseline Patient, Angiographic, and Procedural Characteristics According to Uric Acid Levels
| Variable | Overall (n=10 486) | Low uric acid level (n=3495) | Medium uric acid level (n=3497) | High uric acid level (n=3494) | P value |
|---|---|---|---|---|---|
| Age, y | 59 (51–65) | 60 (54–67) | 58 (51–65) | 56 (49–64) | <0.001 |
| Male, n (%) | 8094 (77.2) | 2151 (61.5) | 2827 (80.8) | 3116 (89.2) | <0.001 |
| Body mass index, kg/m2 | 25.9 (23.9–27.8) | 25.3 (23.2–27.4) | 25.8 (23.9–27.7) | 26.5 (24.5–28.4) | <0.001 |
| Current smoker, n (%) | 5990 (57.1) | 1624 (46.5) | 2070 (59.2) | 2296 (65.7) | <0.001 |
| Diabetes, n (%) | 4430 (42.2) | 1695 (48.5) | 1441 (41.2) | 1294 (37.0) | <0.001 |
| Hypertension, n (%) | 6755 (64.4) | 2185 (62.5) | 2210 (63.2) | 2360 (67.5) | <0.001 |
| Dyslipidemia, n (%) | 7068 (67.4) | 2283 (65.3) | 2337 (66.8) | 2448 (70.1) | <0.001 |
| Previous myocardial infarction, n (%) | 1965 (18.7) | 571 (16.3) | 679 (19.4) | 715 (20.5) | <0.001 |
| Previous PCI, n (%) | 2553 (24.3) | 816 (23.3) | 891 (25.5) | 846 (24.2) | 0.113 |
| Previous CABG, n (%) | 423 (4.0) | 115 (3.3) | 150 (4.3) | 158 (4.5) | 0.021 |
| Previous stroke, n (%) | 1120 (10.7) | 390 (11.2) | 371 (10.6) | 359 (10.3) | 0.482 |
| Peripheral vascular disease, n (%) | 282 (2.7) | 96 (2.7) | 100 (2.9) | 86 (2.5) | 0.570 |
| eGFR <60 mL/min per 1.73 m2, n (%) | 309 (3.1) | 56 (1.7) | 71 (2.1) | 182 (5.4) | <0.001 |
| COPD, n (%) | 241 (2.3) | 80 (2.3) | 78 (2.2) | 83 (2.4) | 0.920 |
| LVEF <50%, n (%) | 410 (4.0) | 118 (3.5) | 123 (3.6) | 169 (5.0) | 0.002 |
| Acute coronary syndrome, n (%) | 6272 (59.8) | 2149 (61.5) | 2056 (58.8) | 2067 (59.2) | 0.045 |
| Systolic blood pressure, mm Hg | 125 (120–140) | 130 (120–140) | 125 (120–140) | 122 (115–139) | <0.001 |
| Laboratory data | |||||
| Triglyceride, mmol/L | 1.53 (1.14–2.10) | 1.40 (1.05–1.89) | 1.51 (1.13–2.03) | 1.72 (1.28–2.37) | <0.001 |
| Total cholesterol, mmol/L | 4.05 (3.44–4.81) | 4.06 (3.46–4.81) | 4.01 (3.40–4.77) | 4.09 (3.46–4.84) | 0.026 |
| LDL‐C, mmol/L | 2.35 (1.86–3.01) | 2.35 (1.86–2.97) | 2.32 (1.83–3.00) | 2.38 (1.88–3.06) | 0.119 |
| HDL‐C, mmol/L | 0.99 (0.84–1.18) | 1.06 (0.89–1.26) | 1.00 (0.84–1.17) | 0.94 (0.80–1.10) | <0.001 |
| Hemoglobin A1c, % | 6.2 (5.8–7.0) | 6.3 (5.9–7.4) | 6.2 (5.8–6.9) | 6.1 (5.8–6.7) | <0.001 |
| Uric acid, mg/dL | 5.64 (4.78–6.58) | 4.40 (3.89–4.78) | 5.64 (5.36–5.91) | 7.04 (6.58–7.73) | <0.001 |
| Radial artery access, n (%) | 8853 (91.4) | 2957 (90.8) | 2948 (91.5) | 2948 (91.8) | 0.394 |
| 3‐vessel disease, n (%) | 4539 (43.3) | 1489 (42.6) | 1515 (43.3) | 1535 (43.9) | 0.608 |
| SYNTAX score | 10 (6–17) | 10 (6–17) | 10 (6–17) | 10 (6–17) | 0.731 |
| Type B2 or C lesion, n (%) | 8063 (76.9) | 2685 (76.8) | 2679 (76.6) | 2699 (77.2) | 0.813 |
| No. lesions treated ≥3, n (%) | 714 (6.8) | 239 (6.8) | 235 (6.7) | 240 (6.9) | 0.967 |
| No. stents ≥3, n (%) | 2327 (22.2) | 743 (21.3) | 776 (22.2) | 808 (23.1) | 0.172 |
| Use of EES/ZES, n (%) | 5477 (52.2) | 1818 (52.0) | 1841 (52.6) | 1818 (52.0) | 0.835 |
| Minimum stent diameter, mm | 2.75 (2.50–3.00) | 2.75 (2.50–3.00) | 2.75 (2.50–3.00) | 2.75 (2.50–3.00) | <0.001 |
| Total stent length, mm | 36 (23–55) | 33 (23–54) | 35 (23–55) | 37 (23–57) | 0.001 |
| Medications at discharge | |||||
| Aspirin, n (%) | 10 348 (98.7) | 3444 (98.5) | 3453 (98.7) | 3451 (98.8) | 0.658 |
| P2Y12 receptor inhibitor, n (%) | 10 326 (98.5) | 3438 (98.4) | 3443 (98.5) | 3445 (98.6) | 0.734 |
| β‐Blockers, n (%) | 9447 (90.1) | 3160 (90.4) | 3132 (89.6) | 3155 (90.3) | 0.433 |
| Statins, n (%) | 10 056 (95.9) | 3335 (95.4) | 3371 (96.4) | 3350 (95.9) | 0.121 |
| Calcium channel blockers, n (%) | 5138 (49.0) | 1764 (50.5) | 1691 (48.4) | 1683 (48.2) | 0.101 |
CABG indicates coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; EES, everolimus‐eluting stent; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; SYNTAX, synergy between percutaneous coronary intervention with TAXUS and cardiac surgery; and ZES, zotarolimus‐eluting stent.
Data were presented as median (interquartile range), mean±SD or frequencies (percentages).
UA Levels and 2.4‐Year Clinical Outcomes
Over a median follow‐up of 2.4 (interquartile range, 2.2–2.6) years, 1233 (11.8%) MACCEs were recorded, including 121 (1.2%) deaths, 95 (0.9%) nonfatal MIs, 171 (1.6%) strokes, and 948 (9.0%) unplanned revascularizations. As shown in Table S2 and Figure 1A, participants with high UA levels had significantly higher risk of MACCEs than those with low UA levels in univariable and multivariable Cox regression analyses (13.1% versus 10.7%; adjusted HR, 1.103 [95% CI, 1.016–1.198]), whereas the incidence of MACCEs did not significantly differ between patients with medium UA levels and low UA levels. In addition, UA levels were positively associated with MACCEs as a continuous variable even after adjusting for potential confounders (adjusted HR, 1.057 [95% CI, 1.011–1.106]). In restricted cubic spline analysis, a linear positive association was seen between UA levels and MACCEs in the overall population (Figure S2). Based on receiver operating characteristic curve analysis, the optimal cutoff value of UA for predicting MACCEs was 6.17 mg/dL (Figure S3). With respect to cardiac death, MI, and unplanned revascularization, the results were in line with that of MACCEs (Table S2 and Figure S4).
Figure 1. Kaplan–Meier survival curves for major adverse cardiovascular and cerebrovascular events according to uric acid tertiles (A) and number of SMuRFs (B).
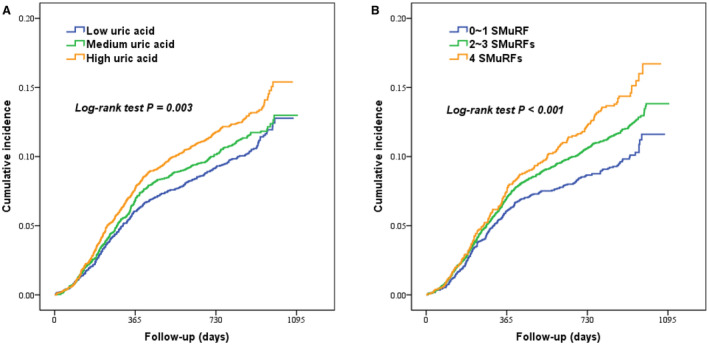
SMuRFs indicates standard modifiable cardiovascular risk factors.
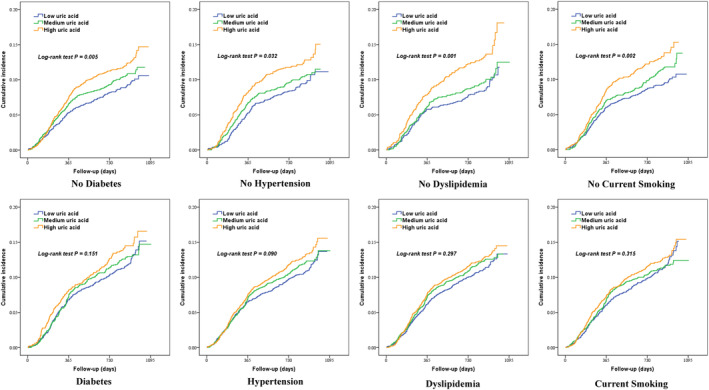
Subgroup analysis showed that the high‐UA‐level group presented an elevated incidence of MACCEs compared with that in the low‐UA‐level group in participants without diabetes (adjusted HR, 1.122 [95% CI, 1.001–1.257]). Similar results were obtained in participants without hypertension (adjusted HR, 1.190 [95% CI, 1.025–1.381]), dyslipidemia (adjusted HR, 1.307 [95% CI, 1.127–1.516]), current smoking (adjusted HR, 1.188 [95% CI, 1.042–1.354]), and metabolic syndrome (adjusted HR, 1.208 [95% CI, 1.060–1.377]). Nevertheless, the incidence of MACCEs did not significantly differ among low‐, medium‐, and high‐UA‐level groups in patients with diabetes, hypertension, dyslipidemia, current smoking (Tables S3 through S6 and Figure 2), and metabolic syndrome (Table S7). In addition, female patients in the high‐UA‐level group had a significantly higher incidence of MACCEs than those in the low‐UA‐level group, whereas there was a statistically nonsignificantly increased incidence of MACCEs in the high‐UA‐level group compared with the low‐UA‐level group in male patients (Table S8).
Figure 2. Kaplan–Meier survival curves for major adverse cardiovascular and cerebrovascular events according to uric acid tertiles in individuals with or without diabetes, hypertension, dyslipidemia, and current smoking.
SMuRFs and 2.4‐Year Clinical Outcomes
In Figure 1B and Table S9, the incidence of MACCEs was significantly higher in participants with 4 SMuRFs than that in participants with 0 to 1 SMuRF (HR, 1.227 [95% CI, 1.112–1.353]). Additional adjustment for other variables in Cox regression analysis obtained similar results (adjusted HR, 1.159 [95% CI, 1.027–1.308]). In addition, this significant association was also observed between participants with 2 to 3 SMuRFs and those with 0 to 1 SMuRF. Secondary outcomes according to the number of SMuRFs were shown in Table S9 and Figure S5.
UA Levels, SMuRFs and Clinical Outcomes
In participants with 0 to 1 SMuRF, the risks of MACCEs were significantly higher in the high‐UA‐level group (adjusted HR, 1.469 [95% CI, 1.197–1.804]) and medium UA‐level group (adjusted HR, 1.478 [95% CI, 1.012–2.160]) compared with the low‐UA‐level group in univariable and multivariable Cox regression analyses. Furthermore, UA levels were positively associated with MACCEs as a continuous variable even after adjusting for potential confounders (adjusted HR, 1.207 [95% CI, 1.082–1.347]). In participants with 2 to 3 SMuRFs, the risk of MACCEs was higher in the high‐UA‐level group than that in the low‐UA‐level group in the univariable model (HR, 1.087 [95% CI, 1.000–1.180]), whereas this association disappeared after adjusting for other variables in the multivariable model (adjusted HR, 1.057 [95% CI, 0.958–1.167]). Inversely, no significant association was found between UA levels and the incidence of MACCEs in participants with 4 SMuRFs (Table 2, Figure 3, and Figure S6). In addition, restricted cubic spline analysis showed a linear positive association between UA levels and MACCE using smoothed restricted cubic spline plots in participants with 0 to 1 SMuRF and 2 to 3 SMuRFs (Figure S2). The optimal cutoff values of UA for predicting MACCEs were 5.14 mg/dL and 6.18 mg/dL in participants with 0 to 1 SMuRF and 2 to 3 SMuRFs, respectively (Figure S3).
Table 2.
2.4‐Year Major Adverse Cardiovascular and Cerebrovascular Events According to Serum Uric Acid Tertiles in Patients With Different Numbers of SMuRFs
| No. SMuRFs | Group | Event, n (%) | Crude HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|
| 0–1 | Low uric acid | 63 (7.2) | 1 (reference) | 1 (reference) |
| Medium uric acid | 80 (10.2) | 1.444 (1.038–2.009)* | 1.478 (1.012–2.160)* | |
| High uric acid | 78 (12.6) | 1.359 (1.151–1.604)* | 1.469 (1.197–1.804)* | |
| Per 1 mg/dL increase | 221 (9.7) | 1.157 (1.056–1.269)* | 1.207 (1.082–1.347)* | |
| 2–3 | Low uric acid | 254 (11.4) | 1 (reference) | 1 (reference) |
| Medium uric acid | 259 (11.3) | 0.998 (0.840–1.187) | 0.957 (0.788–1.162) | |
| High uric acid | 317 (13.1) | 1.087 (1.000–1.180)* | 1.057 (0.958–1.167) | |
| Per 1 mg/dL increase | 830 (12.0) | 1.057 (1.008–1.132)* | 1.044 (0.988–1.104) | |
| 4 | Low uric acid | 57 (14.6) | 1 (reference) | 1 (reference) |
| Medium uric acid | 63 (15.0) | 1.038 (0.725–1.485) | 0.983 (0.665–1.453) | |
| High uric acid | 62 (13.7) | 0.972 (0.812–1.163) | 0.971 (0.795–1.185) | |
| Per 1 mg/dL increase | 182 (14.4) | 0.980 (0.888–1.082) | 0.979 (0.877–1.092) |
Adjusted for age, sex, body mass index, previous myocardial infarction, previous stroke, acute coronary syndrome, systolic blood pressure, triglyceride, low‐density lipoprotein cholesterol, minimum stent diameter, total stent length, and use of statin at discharge. HR indicates hazard ratio; and SMuRFs, standard modifiable cardiovascular risk factors.
P<0.05.
Figure 3. The adjusted HRs for major adverse cardiovascular and cerebrovascular events according to serum uric acid levels in patients with different numbers of SMuRFs.
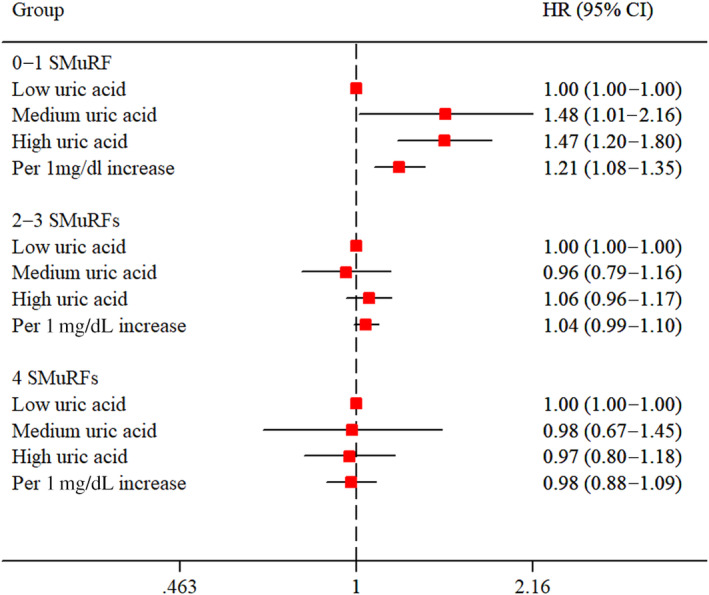
HR indicates hazard ratio; and SMuRFs, standard modifiable cardiovascular risk factors.
Discussion
This large‐scale cohort study confirmed that high UA levels were significantly associated with increased risk of 2.4‐year MACCEs, in conjunction with high risk of cardiac death, nonfatal MI, and unplanned revascularization in individuals with CAD who received evidence‐based secondary prevention therapies after PCI. Moreover, this study, for the first time, demonstrated that the predictive value of UA levels on MACCEs was more evident in individuals with 0 to 1 SMuRF, whereas no statistically significant association was found between UA levels and MACCEs in individuals with 2 to 3 or 4 SMuRFs.
UA per se exerts a plethora of deleterious effects in cells and it may be directly involved in the pathophysiology of cardiovascular disease through increased oxidative stress and inflammation, reduced availability of nitric oxide, endothelial dysfunction, vasoconstriction, proliferation of vascular smooth muscle cells, insulin resistance, and metabolic dysregulation. 2 , 3 Moreover, elevated UA levels may be a marker or a consequence of upregulated or increased xanthine oxidoreductase activity and increased oxidative stress. 2 Of note, extracellular UA can also act as an antioxidant, which might be beneficial for people with cardiovascular disease. Though having been extensively discussed, the association between UA levels and cardiovascular events has not been definitely established in primary and secondary prevention populations. For individuals with CAD, plenty of observational studies demonstrated that high UA levels were significantly associated with poor prognosis in individuals with stable CAD 11 , 12 and acute coronary syndrome 13 , 14 , 15 , 16 and in individuals who underwent PCI 15 , 16 , 17 , 18 or coronary artery bypass grafting. 19 However, in a recent study involving 5070 patients with chronic coronary syndrome, scholars found that patients with high UA levels did not significantly influence the rate of cardiovascular death and hospitalization of heart failure during 1‐year follow‐up. 20 Lazzeri et al 21 demonstrated that hyperuricemia was not independently associated with early death in 856 patients with ST‐segment–elevation MI. An analysis of the LURIC (Ludwigshafen Risk and Cardiovascular Health) study demonstrated that high UA levels were not significantly associated with a higher risk of 7.3‐year all‐cause death after adjusting for age, sex, traditional cardiovascular risk factors, the severity of coronary atherosclerosis, and medication use in subjects referred for coronary angiography. 22 In addition, a study with 647 patients with angiographically proven CAD found that UA ≥6.4 mg/dL was not significantly associated with a higher risk of cardiovascular death at 5‐year follow‐up. 23
Our study presented a consistent result with most of the studies displayed above, as the increased risk of MACCEs was documented in the high‐UA group even after fully adjusting for potential confounders, indicating that hyperuricemia might be a pivotal risk factor for recurrent cardiovascular events. Notably, this study found a linear positive association between UA levels and MACCEs in patients with CAD who underwent PCI. In contrast, Zheng et al 36 indicated a U‐shaped relationship between UA and 4.9‐year all‐cause death in patients with CAD (P for nonlinearity <0.05). In a study by Matsumoto et al, 18 patients with UA levels of ≤4.0 mg/dL had a statistically nonsignificantly increased risk of cardiovascular events compared with those with UA levels of 4.0 to 5.1 mg/dL after PCI. Further studies are needed to determine whether excessively low UA levels are associated with increased cardiovascular risk. Moreover, the prognostic effect of high UA levels for MACCE was more evident in women than in men. Consistently, several studies also reported a stronger association between UA levels and cardiovascular events in women than in men or the existence of such an association in women only. 37 , 38 , 39 , 40 In addition, we also found that the positive association between UA levels and cardiovascular events was more pronounced in participants without metabolic syndrome compared with that in participants with metabolic syndrome. However, Pugliese et al 41 reported that increasing UA levels were significantly associated with higher 11.8‐year cardiovascular death irrespective of the presence of metabolic syndrome. Actually, the follow‐up time was relatively short in our study; thus, the prognostic effect of UA for MACCE has not been fully reflected.
The URRAH (Uric Acid Right for Heart Health) study from Italy reported that the optimal cutoff values of UA able to discriminate all‐cause death, cardiovascular death, fatal MI, and stroke were 4.7 mg/dL, 5.6 mg/dL, 5.7 mg/dL, and 4.8 mg/dL, respectively, indicating that individuals with normal UA levels were also associated with a higher risk of cardiovascular events. 40 , 42 , 43 The present study identified 6.17 mg/dL as the optimal cutoff value of UA for predicting MACCE. The different optimal cutoff values of UA between the present study and the URRAH study may be due to the different population, follow‐up time, and end point. Nonetheless, UA as a predictor of recurrent cardiovascular disease in patients with CAD with moderate to high UA levels would not change the criteria for therapeutic intervention since there is a lack of clear evidence of its benefit.
It is well known that SMuRFs are positively related to the increased risk of cardiovascular events in individuals with CAD, which is confirmed in the present study. However, UA has not been regarded as an independent risk factor for cardiovascular disease. In clinical practice, UA is correlated closely with almost all known cardiovascular risk factors, and teasing out the individual contribution of each factor has proven difficult. 1 , 2 , 3 For example, previous studies revealed that elevated UA levels in metabolic syndrome have been attributed to hyperinsulinemia, as insulin reduces renal excretion of UA. 41 , 44 Therefore, some expert groups argued that studies indicating UA as an independent risk factor did not sufficiently control for other traditional risk factors. To well elucidate the roles of UA in patients with CAD who underwent PCI, the relationship between high UA levels and prognosis was also analyzed in individuals with different numbers of SMuRFs (0–1, 2–3, and 4), especially in those who had no or few SMuRFs.
Potentially the most important finding of this study is that UA levels were strongly and positively associated with the risk of MACCEs in individuals with 0 to 1 SMuRF, whereas this association was not found in individuals with 2 to 3 or 4 SMuRFs. In addition, elevated UA levels were also significantly associated with higher risk of MACCEs in individuals without diabetes, hypertension, dyslipidemia, and current smoking, other than in those who had these cardiovascular risk factors. Of note, patients with more SMuRFs will attach more medical attention and receive tight evidence‐based secondary prevention therapies compared with those with no or few SMuRFs. This action will reduce the overall risk of cardiovascular events and attenuate the effect of UA levels on the prognosis of individuals with more SMuRFs.
In clinical practice, individuals with no or few SMuRFs, who are perceived to be of low risk, are often ignored. However, previous studies enrolling individuals with acute coronary syndrome, especially those with ST‐segment–elevation MI, reported that patients without SMuRFs presented higher risk of unadjusted in‐hospital or 30‐day death than those with SMuRFs. 25 , 32 , 33 After adjusting for potential confounders including medication use, this association changed. In the Swedish Web‐System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART) registry, the increased risk of 30‐day death in SMuRF‐less patients remained significant after adjusting for age, sex, left ventricular ejection fraction, creatinine, and blood pressure but was attenuated or lost on inclusion of pharmacotherapy prescription (angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker, β‐blocker, or statin) at discharge, while the long‐term mortality rate remained increased in individuals without SMuRFs. 25 In the China Acute Myocardial Infarction (CAMI) registry, a higher risk‐factor burden with SMuRFs was associated with poor prognosis among patients with ST‐segment–elevation MI after multivariate adjustment including evidence‐based medications. 33 The potential causes of this paradox might be suboptimal prescription and neglect of nontraditional risk factors. According to the results of the present study, UA was a potential independent predictor for poor prognosis in patients with CAD with no or fewer SMuRFs. Thus, rigorous secondary prevention strategies including UA‐lowering therapy might be beneficial for patients with CAD with high UA levels and no or few SMuRFs.
Given that UA is closely related to systemic inflammation, an anti‐inflammatory combined with UA‐lowering therapy may be an effective method to improve the prognosis of patients. 45 Allopurinol is a xanthine oxidase inhibitor that lowers UA levels and is licensed for the prevention of gout rather than cardiovascular events. 45 Several studies have already suggested a benefit of allopurinol on endothelial function, flow‐mediated dilatation, blood pressure, left ventricular mass, carotid intimal thickness, and arterial stiffness. 2 , 3 Nevertheless, the ALL‐HEART (Allopurinol versus usual care in UK patients with ischaemic heart disease) trial involving 5721 individuals with ischemic heart disease reported that the primary outcome of cardiovascular death, nonfatal MI, or stroke was not significantly reduced when treated with allopurinol therapy (HR, 1.04 [95% CI, 0.89–1.21]). 46 It should be noted that most of the participants in that trial had normal UA levels, with a median concentration of 5.7 to 5.9 mg/dL. Thus, UA levels may have a weak effect on cardiovascular events, and the benefit of UA‐lowering therapy was not significant in this population. However, whether UA‐lowering therapy will provide a beneficial effect on patients with CAD with hyperuricemia, especially those with no or few SMuRFs, remains to be investigated.
There are several limitations that cannot be ignored. First, this is a single‐center, observational study that offers extremely low evidence in the evidence hierarchy, given the highly significant baseline imbalances among different groups. Although multivariable‐adjusted analysis was performed, it was difficult to control the unmeasured confounders. The results of the present study should be interpreted as hypothesis generating. Second, the lack of data on UA‐lowering therapy and changes of UA levels during follow‐up potentially conferred biases to the results. In addition, UA levels might also be affected by lifestyle modifications including diet, exercise habits, and alcohol intake during follow‐up. Third, it is not clear whether the increase of UA levels in participants was due to decreased excretion or overproduction. More detailed phenotypic characterization of patients will help to determine the relationship between UA levels and cardiovascular events and identify potential mechanisms accounting for it. Fourth, previous studies have documented a high risk of atrial fibrillation in individuals with hyperuricemia 47 , 48 ; however, the incidence of atrial fibrillation was not collected during follow‐up. Fifth, we did not have data on long‐term adherence to evidence‐based secondary prevention therapies. Last but not least, these findings should be applied with caution to ethnicities other than Chinese.
Conclusions
This large‐scale study of patients with CAD who received evidence‐based secondary prevention therapies, for the first time, indicated that elevated UA levels might affect the prognosis of individuals with 0 to 1 SMuRF but not the prognosis of those with ≥2 SMuRFs. Further randomized trials are needed to specify the effect of UA‐lowering therapy on prognosis in individuals with CAD and hyperuricemia, especially in those with no or few SMuRFs.
Sources of Funding
This work was supported by Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS: 2021‐I2M‐1‐008, 2020‐I2M‐C&T‐B‐056) and Beijing Municipal Health Commission‐Capital Health Development Research Project (20201‐4032).
Disclosures
None.
Supporting information
Tables S1–S9
Figures S1–S6
Acknowledgments
Concept and design: Kefei Dou and Qiuting Dong; acquisition, analysis, or interpretation of data: Kongyong Cui, Yanjun Song, Yuejin Yang, Dong Yin, Weihua Song, Hongjian Wang, Chenggang Zhu, Lei Feng, Rui Fu, Lei Jia, Ye Lu, Dong Zhang, and Chenxi Song; drafting of the manuscript: Kongyong Cui and Yanjun Song; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: Ye Lu, Kongyong Cui, and Yanjun Song; administrative, technical, or material support: Kefei Dou and Qiuting Dong; supervision: Kefei Dou and Qiuting Dong.
This manuscript was sent to Yen‐Hung Lin, MD, PhD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.030625
For Sources of Funding and Disclosures, see page 10.
Contributor Information
Qiuting Dong, Email: blue1005dqt@163.com.
Kefei Dou, Email: drdoukefei@126.com.
References
- 1. Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–1821. doi: 10.1056/NEJMra0800885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ndrepepa G. Uric acid and cardiovascular disease. Clin Chim Acta. 2018;484:150–163. doi: 10.1016/j.cca.2018.05.046 [DOI] [PubMed] [Google Scholar]
- 3. Saito Y, Tanaka A, Node K, Kobayashi Y. Uric acid and cardiovascular disease: a clinical review. J Cardiol. 2021;78:51–57. doi: 10.1016/j.jjcc.2020.12.013 [DOI] [PubMed] [Google Scholar]
- 4. Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, Pillinger MH, Merill J, Lee S, Prakash S, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64:1431–1446. doi: 10.1002/acr.21772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wannamethee SG, Shaper AG, Whincup PH. Serum urate and the risk of major coronary heart disease events. Heart. 1997;78:147–153. doi: 10.1136/hrt.78.2.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999;131:7–13. doi: 10.7326/0003-4819-131-1-199907060-00003 [DOI] [PubMed] [Google Scholar]
- 7. Moriarity JT, Folsom AR, Iribarren C, Nieto FJ, Rosamond WD. Serum uric acid and risk of coronary heart disease: Atherosclerosis Risk in Communities (ARIC) study. Ann Epidemiol. 2000;10:136–143. doi: 10.1016/S1047-2797(99)00037-X [DOI] [PubMed] [Google Scholar]
- 8. Wheeler JG, Juzwishin KD, Eiriksdottir G, Gudnason V, Danesh J. Serum uric acid and coronary heart disease in 9,458 incident cases and 155,084 controls: prospective study and meta‐analysis. PLoS Med. 2005;2:e76. doi: 10.1371/journal.pmed.0020076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zalawadiya SK, Veeranna V, Mallikethi‐Reddy S, Bavishi C, Lunagaria A, Kottam A, Afonso L. Uric acid and cardiovascular disease risk reclassification: findings from NHANES III. Eur J Prev Cardiol. 2015;22:513–518. doi: 10.1177/2047487313519346 [DOI] [PubMed] [Google Scholar]
- 10. Li X, Meng X, Timofeeva M, Tzoulaki I, Tsilidis KK, Ioannidis JP, Campbell H, Theodoratou E. Serum uric acid levels and multiple health outcomes: umbrella review of evidence from observational studies, randomised controlled trials, and Mendelian randomisation studies. BMJ. 2017;357:j2376. doi: 10.1136/bmj.j2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang R, Song Y, Yan Y, Ding Z. Elevated serum uric acid and risk of cardiovascular or all‐cause mortality in people with suspected or definite coronary artery disease: a meta‐analysis. Atherosclerosis. 2016;254:193–199. doi: 10.1016/j.atherosclerosis.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 12. Ndrepepa G, Braun S, King L, Hadamitzky M, Haase HU, Birkmeier KA, Schömig A, Kastrati A. Association of uric acid with mortality in patients with stable coronary artery disease. Metabolism. 2012;61:1780–1786. doi: 10.1016/j.metabol.2012.05.014 [DOI] [PubMed] [Google Scholar]
- 13. Lopez‐Pineda A, Cordero A, Carratala‐Munuera C, Orozco‐Beltran D, Quesada JA, Bertomeu‐Gonzalez V, Gil‐Guillen VF, Bertomeu‐Martinez V. Hyperuricemia as a prognostic factor after acute coronary syndrome. Atherosclerosis. 2018;269:229–235. doi: 10.1016/j.atherosclerosis.2018.01.017 [DOI] [PubMed] [Google Scholar]
- 14. Levantesi G, Marfisi RM, Franzosi MG, Maggioni AP, Nicolosi GL, Schweiger C, Silletta MG, Tavazzi L, Tognoni G, Marchioli R. Uric acid: a cardiovascular risk factor in patients with recent myocardial infarction. Int J Cardiol. 2013;167:262–269. doi: 10.1016/j.ijcard.2011.12.110 [DOI] [PubMed] [Google Scholar]
- 15. Kaya MG, Uyarel H, Akpek M, Kalay N, Ergelen M, Ayhan E, Isik T, Cicek G, Elcik D, Sahin O, et al. Prognostic value of uric acid in patients with ST‐elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol. 2012;109:486–491. doi: 10.1016/j.amjcard.2011.09.042 [DOI] [PubMed] [Google Scholar]
- 16. Tscharre M, Herman R, Rohla M, Hauser C, Farhan S, Freynhofer MK, Huber K, Weiss TW. Uric acid is associated with long‐term adverse cardiovascular outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Atherosclerosis. 2018;270:173–179. doi: 10.1016/j.atherosclerosis.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 17. Song X, Wang Y, Hou X, Che K, Wang R, Liu Y, Wang Y, Sun W. Association between hyperuricemia and clinical adverse outcomes after percutaneous coronary intervention: a meta‐analysis. Int J Cardiol. 2015;201:658–662. doi: 10.1016/j.ijcard.2015.07.074 [DOI] [PubMed] [Google Scholar]
- 18. Matsumoto I, Moriya S, Kurozumi M, Namba T, Takagi Y. Relationship between serum uric acid levels and the incidence of cardiovascular events after percutaneous coronary intervention. J Cardiol. 2021;78:550–557. doi: 10.1016/j.jjcc.2021.08.005 [DOI] [PubMed] [Google Scholar]
- 19. Shi Y, Zhang X, Du J, Chen S, Zhang H, Yang L, Zheng Z. Elevated postoperative serum uric acid is associated with major adverse events following coronary artery bypass grafting. J Card Surg. 2020;35:2559–2566. doi: 10.1111/jocs.14845 [DOI] [PubMed] [Google Scholar]
- 20. De Luca L, Gulizia MM, Gabrielli D, Meessen J, Mattei L, D'Urbano M, Colivicchi F, Temporelli PL, Borghi C, Desideri G, et al. Impact of serum uric acid levels on cardiovascular events and quality of life in patients with chronic coronary syndromes: insights from a contemporary, prospective, nationwide registry. Nutr Metab Cardiovasc Dis. 2022;32:393–401. doi: 10.1016/j.numecd.2021.09.034 [DOI] [PubMed] [Google Scholar]
- 21. Lazzeri C, Valente S, Chiostri M, Picariello C, Gensini GF. Uric acid in the early risk stratification of ST‐elevation myocardial infarction. Intern Emerg Med. 2012;7:33–39. doi: 10.1007/s11739-011-0515-9 [DOI] [PubMed] [Google Scholar]
- 22. Silbernagel G, Hoffmann MM, Grammer TB, Boehm BO, Marz W. Uric acid is predictive of cardiovascular mortality and sudden cardiac death in subjects referred for coronary angiography. Nutr Metab Cardiovasc Dis. 2013;23:46–52. doi: 10.1016/j.numecd.2011.02.008 [DOI] [PubMed] [Google Scholar]
- 23. Tsai TH, Chen YL, Chen SM, Yang CH, Fang CY, Hsieh YK, Wu CJ, Yip HK, Hang CL, Fu M, et al. Uric acid is not an independent predictor of cardiovascular death in patients with angiographically proven coronary artery disease. Chang Gung Med J. 2009;32:605–613. [PubMed] [Google Scholar]
- 24. Khot UN, Khot MB, Bajzer CT, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. 2003;290:898–904. doi: 10.1001/jama.290.7.898 [DOI] [PubMed] [Google Scholar]
- 25. Figtree GA, Vernon ST, Hadziosmanovic N, Sundström J, Alfredsson J, Arnott C, Delatour V, Leósdóttir M, Hagström E. Mortality in STEMI patients without standard modifiable risk factors: a sex‐disaggregated analysis of SWEDEHEART registry data. Lancet. 2021;397:1085–1094. doi: 10.1016/S0140-6736(21)00272-5 [DOI] [PubMed] [Google Scholar]
- 26. Cui K, Yin D, Zhu C, Song W, Wang H, Jia L, Zhang R, Wang H, Cai Z, Feng L, et al. How do lipoprotein(a) concentrations affect clinical outcomes for patients with stable coronary artery disease who underwent different dual antiplatelet therapy after percutaneous coronary intervention? J Am Heart Assoc. 2022;11:e023578. doi: 10.1161/JAHA.121.023578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. He J, Bian X, Song C, Zhang R, Yuan S, Yin D, Dou K. High neutrophil to lymphocyte ratio with type 2 diabetes mellitus predicts poor prognosis in patients undergoing percutaneous coronary intervention: a large‐scale cohort study. Cardiovasc Diabetol. 2022;21:156. doi: 10.1186/s12933-022-01583-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang D, Yan R, Gao G, Wang H, Fu R, Li J, Yin D, Zhu C, Feng L, Song W, et al. Validating the performance of 5 risk scores for major adverse cardiac events in patients who achieved complete revascularization after percutaneous coronary intervention. Can J Cardiol. 2019;35:1058–1068. doi: 10.1016/j.cjca.2019.02.017 [DOI] [PubMed] [Google Scholar]
- 29. American Diabetes Association . 2. Classification and diagnosis of diabetes: standards of medical care in diabetes‐2018. Diabetes Care. 2018;41:S13–S27. doi: 10.2337/dc18-S002 [DOI] [PubMed] [Google Scholar]
- 30. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 31. Rabar S, Harker M, O'Flynn N, Wierzbicki AS; Guideline Development Group . Lipid modification and cardiovascular risk assessment for the primary and secondary prevention of cardiovascular disease: summary of updated NICE guidance. BMJ. 2014;349:g4356. doi: 10.1136/bmj.g4356 [DOI] [PubMed] [Google Scholar]
- 32. Wang JY, Goodman SG, Saltzman I, Wong GC, Huynh T, Dery JP, Leiter LA, Bhatt DL, Welsh RC, Spencer FA, et al. Cardiovascular risk factors and in‐hospital mortality in acute coronary syndromes: insights from the Canadian Global Registry of Acute Coronary Events. Can J Cardiol. 2015;31:1455–1461. doi: 10.1016/j.cjca.2015.04.007 [DOI] [PubMed] [Google Scholar]
- 33. Li S, Gao X, Yang J, Xu H, Wang Y, Zhao Y, Yin L, Wu C, Wang Y, Zheng Y, et al. Number of standard modifiable risk factors and mortality in patients with first‐presentation ST‐segment elevation myocardial infarction: insights from China Acute Myocardial Infarction registry. BMC Med. 2022;20:217. doi: 10.1186/s12916-022-02418-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction ; Thygesen K, Alpert JS, White HD, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551–2567. doi: 10.1093/eurheartj/ehs184 [DOI] [PubMed] [Google Scholar]
- 35. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504 [DOI] [PubMed] [Google Scholar]
- 36. Zheng Y, Ou J, Huang D, Zhou Z, Dong X, Chen J, Liang D, Liu J, Liu Y, Chen J, et al. The U‐shaped relationship between serum uric acid and long‐term all‐cause mortality in coronary artery disease patients: a cohort study of 33,034 patients. Front Cardiovasc Med. 2022;9:858889. doi: 10.3389/fcvm.2022.858889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ndrepepa G, Cassese S, Braun S, Fusaro M, King L, Tada T, Schömig A, Kastrati A, Schmidt R. A gender‐specific analysis of association between hyperuricaemia and cardiovascular events in patients with coronary artery disease. Nutr Metab Cardiovasc Dis. 2013;23:1195–1201. doi: 10.1016/j.numecd.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 38. Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. Uric acid and risk of myocardial infarction, stroke and congestive heart failure in 417,734 men and women in the Apolipoprotein MOrtality RISk study (AMORIS). J Intern Med. 2009;266:558–570. doi: 10.1111/j.1365-2796.2009.02133.x [DOI] [PubMed] [Google Scholar]
- 39. Hoieggen A, Alderman MH, Kjeldsen SE, Julius S, Devereux RB, De Faire U, Fyhrquist F, Ibsen H, Kristianson K, Lederballe‐Pedersen O, et al. The impact of serum uric acid on cardiovascular outcomes in the LIFE study. Kidney Int. 2004;65:1041–1049. doi: 10.1111/j.1523-1755.2004.00484.x [DOI] [PubMed] [Google Scholar]
- 40. Casiglia E, Tikhonoff V, Virdis A, Masi S, Barbagallo CM, Bombelli M, Bruno B, Cicero AFG, Cirillo M, Cirillo P, et al. Serum uric acid and fatal myocardial infarction: detection of prognostic cut‐off values: the URRAH (Uric Acid Right for Heart Health) study. J Hypertens. 2020;38:412–419. doi: 10.1097/HJH.0000000000002287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pugliese NR, Mengozzi A, Virdis A, Casiglia E, Tikhonoff V, Cicero AFG, Ungar A, Rivasi G, Salvetti M, Barbagallo CM, et al. The importance of including uric acid in the definition of metabolic syndrome when assessing the mortality risk. Clin Res Cardiol. 2021;110:1073–1082. doi: 10.1007/s00392-021-01815-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Virdis A, Masi S, Casiglia E, Tikhonoff V, Cicero AFG, Ungar A, Rivasi G, Salvetti M, Barbagallo CM, Bombelli M, et al. Identification of the uric acid thresholds predicting an increased total and cardiovascular mortality over 20 years. Hypertension. 2020;75:302–308. doi: 10.1161/HYPERTENSIONAHA.119.13643 [DOI] [PubMed] [Google Scholar]
- 43. Tikhonoff V, Casiglia E, Spinella P, Barbagallo CM, Bombelli M, Cicero AFG, Cirillo M, Cirillo P, Desideri G, D'elia L, et al. Identification of a plausible serum uric acid cut‐off value as prognostic marker of stroke: the Uric Acid Right for Heart Health (URRAH) study. J Hum Hypertens. 2022;36:976–982. doi: 10.1038/s41371-021-00613-5 [DOI] [PubMed] [Google Scholar]
- 44. Quinones Galvan A, Natali A, Baldi S, Frascerra S, Sanna G, Ciociaro D, Ferrannini E. Effect of insulin on uric acid excretion in humans. Am J Phys. 1995;268:E1–E5. doi: 10.1152/ajpendo.1995.268.1.E1 [DOI] [PubMed] [Google Scholar]
- 45. Pugliese NR, Pellicori P, Filidei F, De Biase N, Maffia P, Guzik TJ, Masi S, Taddei S, Cleland JGF. Inflammatory pathways in heart failure with preserved left ventricular ejection fraction: implications for future interventions. Cardiovasc Res. 2023;118:3536–3555. doi: 10.1093/cvr/cvac133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mackenzie IS, Hawkey CJ, Ford I, Greenlaw N, Pigazzani F, Rogers A, Struthers AD, Begg AG, Wei L, Avery AJ, et al. Allopurinol versus usual care in UK patients with ischaemic heart disease (ALL‐HEART): a multicentre, prospective, randomised, open‐label, blinded‐endpoint trial. Lancet. 2022;400:1195–1205. doi: 10.1016/S0140-6736(22)01657-9 [DOI] [PubMed] [Google Scholar]
- 47. Tamariz L, Hernandez F, Bush A, Palacio A, Hare JM. Association between serum uric acid and atrial fibrillation: a systematic review and meta‐analysis. Heart Rhythm. 2014;11:1102–1108. doi: 10.1016/j.hrthm.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 48. Masi S, Pugliese NR, Taddei S. The difficult relationship between uric acid and cardiovascular disease. Eur Heart J. 2019;40:3055–3057. doi: 10.1093/eurheartj/ehz166 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S9
Figures S1–S6


