Abstract
Background
Acute pericarditis (AP) is considered a cardiovascular complication in patients with COVID‐19. We aimed to ass‐ess the incidence, associated complications, and clinical impact of AP on hospitalized patients with COVID‐19.
Methods and Results
In this retrospective cohort study, International Classification of Diseases, Tenthth Revision, Clinical Modification (ICD‐10) codes were used to identify patients with COVID‐19 with or without AP in the National Inpatient Sample 2020 database. We compared outcomes between AP and non‐AP groups before and after propensity‐score matching for patient and hospital demographics and relevant comorbidities. A total of 211 619 patients with a primary diagnosis of COVID‐19 were identified, including 983 (0.46%) patients who had a secondary diagnosis of AP. Before matching, patients with COVID‐19 with AP were younger (59.93±19.24 years old versus 64.29±16.82 years old) and more likely to have anemia (40.5% versus 19.9%), cancer (6.7% versus 3.6%), and chronic kidney disease (29.3% versus 19.6%) (all P<0.05). After matching, patients with COVID‐19 with AP (n=980), when compared with the matched non‐AP group (n=2936), had higher rates of mortality (21.3% versus 11.1%, P<0.001), cardiac arrest (5.0% versus 2.6%, P<0.001), cardiogenic shock (4.2% versus 0.5%, P<0.001), ventricular arrhythmia (4.7% versus 1.9%, P<0.001), acute kidney injury (38.3% versus 28.9%, P<0.001), acute congestive heart failure (14.3% versus 4.8%, P<0.001), and longer length of stay (7.00±10.00 days versus 5.00±7.00 days, P<0.001) and higher total charges ($75066.5±$130831.3 versus $44824.0±$63660.5, P<0.001).
Conclusions
In hospitalized patients with COVID‐19, AP is a rare but severe in‐hospital complication and is associated with worse in‐hospital outcomes.
Keywords: acute pericarditis, COVID‐19, in‐hospital complications, mortality
Subject Categories: Big Data and Data Standards, Complications, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- ACHF
acute congestive heart failure
- AKI
acute kidney injury
- AP
acute pericarditis
- NIS
National Inpatient Sample
Clinical Perspective.
What Is New?
Hospitalized patients with COVID‐19 had a low incidence (0.46%) of the complication of acute pericarditis.
Hospitalized patients with COVID‐19 with acute pericarditis had higher in‐hospital mortality rates than did those without acute pericarditis.
Hospitalized patients with COVID‐19 with acute pericarditis had worse outcomes including a longer length of stay, higher health care costs, and a higher likelihood of cardiac arrest, cardiogenic shock, and acute kidney injury.
What Are the Clinical Implications?
Future studies should focus on developing risk stratification tools for targeting patients with COVID‐19 at high risk of developing acute pericarditis and assess the effect of early treatment on improving outcomes in hospitalized patients with COVID‐19.
Our study provides valuable insight into the complex relationship between acute pericarditis and COVID‐19 and will help guide future clinical studies.
Clinical trials are necessary to investigate the potential of using corticosteroids as first‐line therapy for acute pericarditis in hospitalized patients with COVID‐19 because of their benefit in treating severe COVID‐19, and because the frequency of acute kidney injury in this group may contraindicate nonsteroidal anti‐inflammatory drugs and colchicine use.
The global pandemic of COVID‐19 caused by severe acute respiratory syndrome‐coronavirus 2 has challenged health care services worldwide. 1 The infection not only caused respiratory complications but also resulted in extrapulmonary syndromes and risks to other systems, including the cardiovascular system. 2 Cardiovascular complications linked with COVID‐19 include myocarditis, myocardial infarction, cardiac arrest, and atrial fibrillation. 3 , 4 , 5 , 6
Most COVID‐19 reports have focused on myocardial involvement, whereas studies on pericardial disease, including acute pericarditis (AP), have been less common. 7 , 8 , 9 AP is the most common inflammatory heart disorder, and includes infectious and noninfectious forms. 10 Although the number of case reports suggesting the coexistence of AP and COVID‐19 is increasing, the incidence and effects of AP on the prognosis of patients with COVID‐19 are unclear. 11 , 12 Therefore, using the latest data from the National Inpatient Sample (NIS), we aim to investigate the incidence, associated adverse events, and impact of AP on hospitalized patients with COVID‐19.
Methods
The authors declare that all supporting data are available within the article and its supplemental material. Using the NIS 2020 database, we examined the association between AP and COVID‐19 outcomes. The NIS database includes >7 million hospital stays each year and, since 2016, has used the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM) codes for all in‐hospital diagnoses. 13 Beginning in 2020, COVID‐19‐related hospitalizations can be identified by the ICD‐10‐CM diagnosis code of “U071” (2019 novel coronavirus disease) in the NIS database. The ICD‐10‐CM codes used in this study are shown in Tables S1. Research using the NIS does not require Institutional Review Board approval or patient consent, given the deidentified nature of the database.
Study Population and Covariates
All patients in the 2020 NIS database with the primary diagnosis of COVID‐19 were identified. Patients without a discharge status were excluded from this study. We selected the following variables as covariates: patient and hospital demographics (age, sex, race, geographic location, household income, primary payer, and hospital type, region, and bed size), common cardiovascular comorbidities (hypertension, hyperlipidemia, diabetes, smoking, obesity, chronic obstructive pulmonary disease, chronic kidney disease, anxiety, depression, obstructive sleep apnea, anemia, and cancer), and autoimmune diseases (systemic lupus erythematosus, rheumatoid arthritis, systemic sclerosis, Sjögren syndrome, and inflammatory bowel disease).
We divided patients who had COVID‐19 into 2 groups: those who had AP as a comorbidity (n=983) and those who did not have AP (n=210 636). The detailed patient selection process is shown in Figure 1. To assess the correlation between waves of COVID‐19 infections and AP, we analyzed monthly trends of AP during the COVID‐19 hospitalization waves in 2020. We evaluated the incidence of COVID‐19‐related AP before and after June 2020 to assess the potential effect of corticosteroid treatment after preliminary results from the RECOVERY (Randomised Evaluation of COVID‐19 Therapy) trial were released on June 16, 2020. 14
Figure 1. Flow chart of the selection process for the final patient sample used in this study.
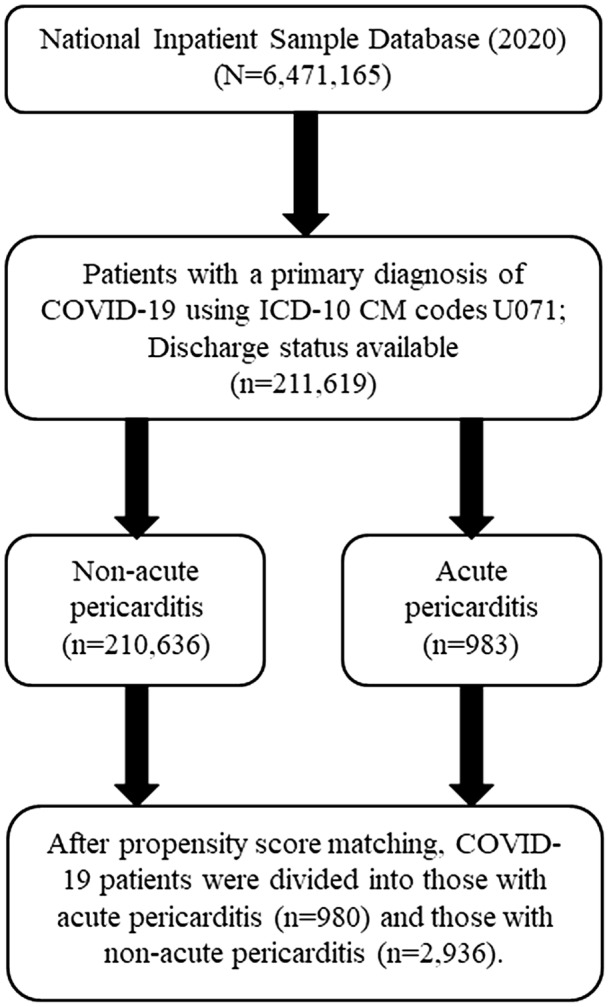
Inclusion criteria were applied to the National Inpatient Sample 2020 database. All eligible patients were matched 1:3 based on propensity scoring to generate the acute pericarditis versus nonacute pericarditis comparison cohorts. ICD‐10‐CM indicates International Classification of Diseases, Tenth Revision, Clinical Modification.
Outcomes
The primary outcomes were the rates of in‐hospital mortality and severe in‐hospital complications, including cardiogenic shock, cardiac arrest, ventricular arrhythmias (including ventricular tachycardia, ventricular flutter, and ventricular fibrillation), acute congestive heart failure (ACHF), acute respiratory failure, and acute kidney injury (AKI). Hospital length of stay (LOS) and total admission charges were also examined.
Statistical Analysis
We used mean and SD to express continuous variables and percentages to express categorical variables. We also provided the median and interquartile range for the LOS and total charges. Continuous variables were tested with a t test, and categorical variables were tested with a χ2 test. P values ≤0.05 were considered significant. All data analysis and statistical processes were performed using R statistics software (version 3.6.1, R Development Core Team).
To reduce selection bias in the unmatched cohort, we conducted a propensity‐score matching analysis in a 1:3 target ratio to match patients from the AP group and the non‐AP group. A multivariate logistic regression model was built and used to adjust for patient and hospital demographics (age, sex, race, geographic location, household income, primary payer, and hospital type, region, and bed size) and common cardiovascular comorbidities as mentioned above. This statistical method was consistent with the methodology used in previous studies. 15
Finally, we compared in‐hospital outcomes between the 2 groups before and after covariate adjustment to demonstrate the impact of AP on in‐hospital outcomes of COVID‐19.
To further explore the relationship between COVID‐19 and AP, we extracted data on all patients with the primary diagnosis of AP in the NIS database. We classified these patients into COVID‐19 and non‐COVID‐19 groups based on their COVID‐19 status. We performed a similar propensity‐score matching analysis in a 1:3 target ratio to match patients from the COVID‐19 group and the non‐COVID‐19 group using the abovementioned variables. The in‐hospital outcomes were compared between the 2 groups before and after matching.
Results
Comparison of Hospitalized Patients With COVID‐19 With and Without AP
Baseline Characteristics
The COVID‐19 cohort in this study included 211 619 patients; of those, 983 (0.46%) had AP, leaving 210 636 in the cohort of patients with COVID‐19 without AP. Baseline characteristics are provided in Table 1.
Table 1.
Baseline Characteristics
| Unmatched cohort | Propensity‐matched cohort | |||||
|---|---|---|---|---|---|---|
| Variables | COVID‐19 without acute pericarditis | COVID‐19 with acute pericarditis | P value | COVID‐19 without acute pericarditis | COVID‐19 with acute pericarditis | P value |
| N | 210 636 | 983 | 2936 | 980 | ||
| Age, mean (SD) | 64.29 (16.82) | 59.93 (19.24) | <0.001 | 60.46 (19.08) | 60.03 (19.18) | 0.54 |
| Sex, n (%) | 0.96 | 0.87 | ||||
| Male | 111 229 (52.8) | 516 (52.5) | 1551 (52.8) | 514 (52.4) | ||
| Female | 99 399 (47.2) | 467 (47.5) | 1385 (47.2) | 466 (47.6) | ||
| Unknown | 8 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Race and ethnicity, n (%) | <0.001 | 0.98 | ||||
| White | 107 236 (50.9) | 396 (40.3) | 1178 (40.1) | 395 (40.3) | ||
| Black | 37 642 (17.9) | 292 (29.7) | 873 (29.7) | 291 (29.7) | ||
| Hispanic | 42 256 (20.1) | 180 (18.3) | 547 (18.6) | 179 (18.3) | ||
| Asian or Pacific Islander | 6581 (3.1) | 31 (3.2) | 86 (2.9) | 31 (3.2) | ||
| Native American | 2126 (1.0) | 5 (0.5) | 14 (0.5) | 5 (0.5) | ||
| Other | 8385 (4.0) | 44 (4.5) | 117 (4.0) | 44 (4.5) | ||
| Unknown | 6410 (3.0) | 35 (3.6) | 121 (4.1) | 35 (3.6) | ||
| Patient location, n (%) | <0.001 | 0.99 | ||||
| “Central” counties of metro areas of ≥1 million population | 66 463 (31.6) | 359 (36.5) | 1064 (36.2) | 357 (36.4) | ||
| “Fringe” counties of metro areas of ≥1 million population | 48 936 (23.2) | 242 (24.6) | 739 (25.2) | 242 (24.7) | ||
| Counties in metro areas of 250 000–999 999 population | 39 346 (18.7) | 182 (18.5) | 532 (18.1) | 182 (18.6) | ||
| Counties in metro areas of 50 000–249 999 population | 18 683 (8.9) | 69 (7.0) | 205 (7.0) | 69 (7.0) | ||
| Micropolitan counties | 20 005 (9.5) | 65 (6.6) | 190 (6.5) | 65 (6.6) | ||
| Nonmetropolitan or micropolitan counties | 16 378 (7.8) | 60 (6.1) | 181 (6.2) | 59 (6.0) | ||
| NA | 825 (0.4) | 6 (0.6) | 25 (0.9) | 6 (0.6) | ||
| Mean household income, n (%) | 0.71 | 0.97 | ||||
| $1–$42 999 | 71 045 (33.7) | 340 (34.6) | 1037 (35.3) | 337 (34.4) | ||
| $43 000–$53 999 | 57 326 (27.2) | 279 (28.4) | 813 (27.7) | 279 (28.5) | ||
| $54 000–$70 999 | 45 435 (21.6) | 195 (19.8) | 575 (19.6) | 195 (19.9) | ||
| $71 000 or more | 33 530 (15.9) | 153 (15.6) | 458 (15.6) | 153 (15.6) | ||
| Unknown | 3300 (1.6) | 16 (1.6) | 53 (1.8) | 16 (1.6) | ||
| Primary payer, n (%) | 0.003 | 0.99 | ||||
| Medicare | 109 153 (51.8) | 491 (49.9) | 1471 (50.1) | 490 (50.0) | ||
| Medicaid | 25 350 (12.0) | 154 (15.7) | 437 (14.9) | 153 (15.6) | ||
| Private including HMO | 58 265 (27.7) | 255 (25.9) | 759 (25.9) | 255 (26.0) | ||
| Self‐pay | 7163 (3.4) | 27 (2.7) | 86 (2.9) | 27 (2.8) | ||
| No charge | 517 (0.2) | 4 (0.4) | 15 (0.5) | 4 (0.4) | ||
| Other | 9786 (4.6) | 47 (4.8) | 155 (5.3) | 47 (4.8) | ||
| Unknown | 402 (0.2) | 5 (0.5) | 13 (0.4) | 4 (0.4) | ||
| Hospital type, n (%) | <0.001 | 0.89 | ||||
| Rural | 24 605 (11.7) | 60 (6.1) | 168 (5.7) | 60 (6.1) | ||
| Urban nonteaching | 40 431 (19.2) | 155 (15.8) | 470 (16.0) | 155 (15.8) | ||
| Urban teaching | 145 600 (69.1) | 768 (78.1) | 2298 (78.3) | 765 (78.1) | ||
| Hospital region, n (%) | 0.21 | 0.71 | ||||
| Northeast | 37 206 (17.7) | 195 (19.8) | 550 (18.7) | 194 (19.8) | ||
| Midwest | 49 003 (23.3) | 210 (21.4) | 646 (22.0) | 209 (21.3) | ||
| South | 88 137 (41.8) | 416 (42.3) | 1284 (43.7) | 415 (42.3) | ||
| West | 36 290 (17.2) | 162 (16.5) | 456 (15.5) | 162 (16.5) | ||
| Hospital bed size, n (%) | <0.001 | 0.77 | ||||
| Small | 54 032 (25.7) | 206 (21.0) | 649 (22.1) | 206 (21.0) | ||
| Medium | 60 827 (28.9) | 258 (26.2) | 753 (25.6) | 257 (26.2) | ||
| Large | 95 777 (45.5) | 519 (52.8) | 1534 (52.2) | 517 (52.8) | ||
| Comorbidities, n (%) | ||||||
| Smoking | 56 403 (26.8) | 233 (23.7) | 0.03 | 722 (24.6) | 232 (23.7) | 0.59 |
| Hypertension | 87 811 (41.7) | 244 (24.8) | <0.001 | 728 (24.8) | 244 (24.9) | 0.98 |
| Diabetes | 85 215 (40.5) | 396 (40.3) | 0.94 | 1183 (40.3) | 396 (40.4) | 0.98 |
| Hyperlipidemia | 88 516 (42.0) | 373 (37.9) | 0.01 | 1080 (36.8) | 373 (38.1) | 0.50 |
| Obesity | 59 658 (28.3) | 247 (25.1) | 0.03 | 719 (24.5) | 247 (25.2) | 0.68 |
| Anxiety | 26 946 (12.8) | 103 (10.5) | 0.03 | 300 (10.2) | 103 (10.5) | 0.84 |
| Depression | 24 010 (11.4) | 91 (9.3) | 0.04 | 285 (9.7) | 91 (9.3) | 0.75 |
| OSA | 21 686 (10.3) | 77 (7.8) | 0.01 | 237 (8.1) | 77 (7.9) | 0.88 |
| Chronic kidney disease | 41 324 (19.6) | 288 (29.3) | <0.001 | 850 (29.0) | 286 (29.2) | 0.92 |
| COPD | 30 966 (14.7) | 141 (14.3) | 0.79 | 402 (13.7) | 141 (14.4) | 0.62 |
| Anemia | 41 838 (19.9) | 398 (40.5) | <0.001 | 1202 (40.9) | 395 (40.3) | 0.76 |
| Cancer | 7506 (3.6) | 66 (6.7) | <0.001 | 211 (7.2) | 66 (6.7) | 0.69 |
| SLE | 1130 (0.5) | 20 (2.0) | <0.001 | 48 (1.6) | 18 (1.8) | 0.78 |
| Rheumatoid arthritis | 4289 (2.0) | 25 (2.5) | 0.31 | 67 (2.3) | 24 (2.4) | 0.86 |
| Systemic sclerosis | 151 (0.1) | 4 (0.4) | 0.001 | 8 (0.3) | 3 (0.3) | 1 |
| Sjogren syndrome | 355 (0.2) | 1 (0.1) | 0.91 | 4 (0.1) | 1 (0.1) | 1 |
| IBD | 1153 (0.5) | 4 (0.4) | 0.71 | 12 (0.4) | 4 (0.4) | 1 |
COPD indicates chronic obstructive pulmonary disease; HMO, health maintenance organization; IBD, inflammatory bowel disease; NA, not available; OSA, obstructive sleep apnea; and SLE, systemic lupus erythematosus.
Before matching, patients in the AP cohort were younger than those in the non‐AP cohort (59.93±19.24 versus 64.29±16.82 years, respectively; P<0.001) and had higher rates of comorbidities including anemia, cancer, chronic kidney disease, systemic lupus erythematosus, and systemic sclerosis. In contrast, a higher proportion of patients in the non‐AP group had hypertension, obesity, hyperlipidemia, anxiety, depression, and obstructive sleep apnea. No differences were seen in the distribution of chronic obstructive pulmonary disease, diabetes, rheumatoid arthritis, Sjogren syndrome, and inflammatory bowel disease (Table 1).
Various differences were seen in socioeconomic factors, including race, patient location, primary payer, hospital type, and hospital size, in both groups before matching (Table 1). More patients in both groups were White (40.3% AP and 50.9% non‐AP). The analysis of hospital type and bed size suggested that most patients in both groups were admitted at urban teaching hospitals and clinics with a large bed size.
After matching, the non‐AP (n=2936) and AP (n=980) groups were well matched for all baseline characteristics (P>0.05; Table 1). All baseline variables in this study had standard mean differences <0.1 between 2 groups (Figure 2).
Figure 2. The standardized mean differences of covariates before and after propensity score matching between patients with COVID‐19 with and without acute pericarditis.
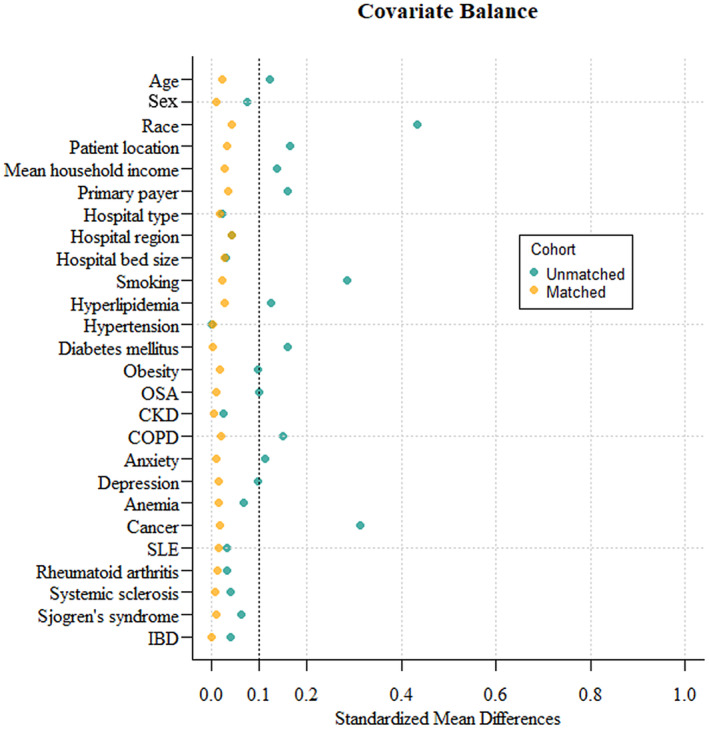
The standardized mean difference was used to examine the balance of the covariate distribution between the matched acute pericarditis and the nonacute pericarditis groups. All standardized mean differences of covariate distributions in this study were <0.1, which was considered balanced. CKD indicates chronic kidney disease; COPD, chronic obstructive pulmonary disease; IBD, inflammatory bowel disease; OSA, obstructive sleep apnea; and SLE, systemic lupus erythematosus.
In‐Hospital Complications
Before matching (Table 2), more patients with COVID‐19 in the AP group had complications of cardiac arrest, cardiogenic shock, ventricular arrhythmia, AKI, and ACHF than did those in the non‐AP group. These trends were observed after matching, with the AP group having a higher incidence of the same complications listed above (Table 2). Figure 3 presents the adjusted odds ratio for in‐hospital complications after matching.
Table 2.
In‐Hospital Outcomes
| Unmatched cohort | Propensity‐matched cohort | ||||||
|---|---|---|---|---|---|---|---|
| Variables | COVID‐19 without acute pericarditis | COVID‐19 with acute pericarditis | P value | COVID‐19 without acute pericarditis | COVID‐19 with acute pericarditis | P value | |
| n | 210 636 | 983 | 2936 | 980 | |||
| Outcomes | |||||||
| Death, n (%) | 23 246 (11.0) | 210 (21.4) | <0.001 | 325 (11.1) | 209 (21.3) | <0.001 | |
| Cardiac arrest, n (%) | 4437 (2.1) | 49 (5.0) | <0.001 | 75 (2.6) | 49 (5.0) | <0.001 | |
| Cardiogenic shock, n (%) | 799 (0.4) | 41 (4.2) | <0.001 | 14 (0.5) | 41 (4.2) | <0.001 | |
| Ventricular arrhythmia, n (%) | 3652 (1.7) | 46 (4.7) | <0.001 | 57 (1.9) | 46 (4.7) | <0.001 | |
| AKI, n (%) | 52 770 (25.1) | 375 (38.1) | <0.001 | 849 (28.9) | 375 (38.3) | <0.001 | |
| ARF, n (%) | 117 535 (55.8) | 518 (52.7) | 0.05 | 1564 (53.3) | 518 (52.9) | 0.85 | |
| ACHF, n (%) | 8308 (3.9) | 140 (14.2) | <0.001 | 141 (4.8) | 140 (14.3) | <0.001 | |
| LOS, d | |||||||
| Median (IQR) | 5.00 (6.00) | 7.00 (10.00) | <0.001 | 5.00 (7.00) | 7.00 (10.00) | <0.001 | |
| Mean (SD) | 7.43 (8.03) | 11.89 (14.07) | <0.001 | 8.05 (8.85) | 11.90 (14.08) | <0.001 | |
| Total charge, $ | |||||||
| Median (IQR) | 41 490 (54657) | 74 978 (130424) | <0.001 | 44 824 (63661) | 75 067 (130831) | <0.001 | |
| Mean (SD) | 78 063 (147908) | 178 424 (367509) | <0.001 | 91 539 (188695) | 178 758 (368009) | <0.001 | |
ACHF indicates acute congestive heart failure; AKI, acute kidney injury; ARF, acute respiratory failure; IQR, interquartile range; and LOS, length of stay.
Figure 3. Forest plot graph showing adjusted odds ratio for in‐hospital outcomes after propensity score matching.
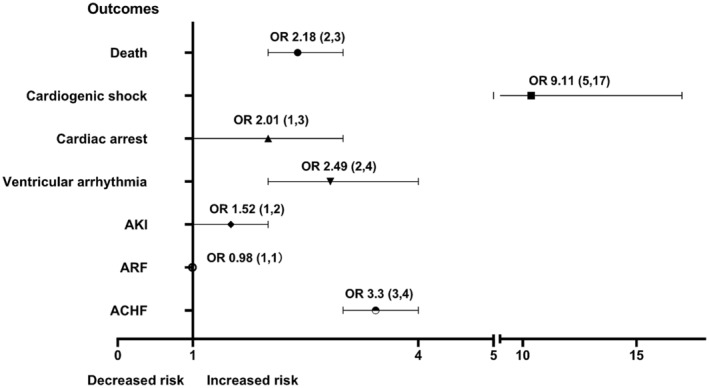
ACHF indicates acute congestive heart failure; AKI, acute kidney injury; ARF, acute respiratory failure; and OR, odds ratio.
Mortality, LOS, and Total Cost
In the unmatched cohort, patients with AP had a higher in‐hospital mortality rate (21.4% versus 11.0%, P<0.001), a longer LOS (7.00±10.00 days versus 5.00±6.00 days, P<0.001), and higher total charges ($74 978.0±130 424.0 versus $41 490.0±54 656.5, P<0.001) than did the non‐AP group (Table 2). The significant differences in all 3 of the above variables remained after matching (Table 2).
Trends of AP Cases During COVID Hospitalization Waves
Because several waves of COVID infection and hospitalization occurred during 2020, we analyzed the monthly trend of AP cases and COVID‐19 hospitalizations in 2020 (Figure 4). As expected, a rise in COVID‐19 hospitalizations corresponded to a rise in COVID‐19‐related AP cases. The highest numbers of both COVID‐19 cases and COVID‐19‐related AP cases were seen in November and December 2020. The highest incidence of COVID‐19‐related AP occurred in March, May, and August, and the lowest incidence occurred in September and October. The incidence of COVID‐19‐related AP decreased significantly after June 2020 when compared with before June 2020 (0.46% versus 0.51%, P<0.001).
Figure 4. Monthly trends of COVID‐19‐related acute pericarditis and COVID‐19 hospitalizations in 2020 in the United States.
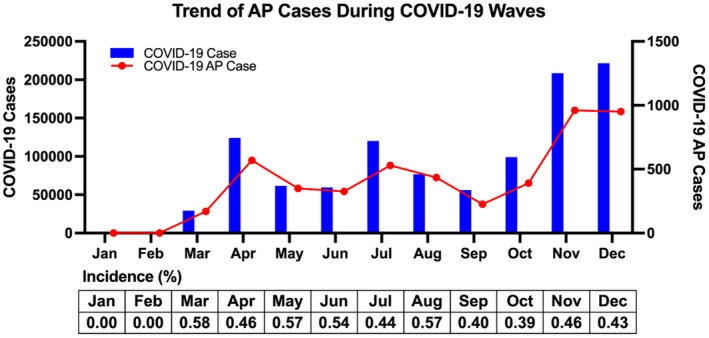
The monthly trends of COVID‐19‐related acute pericarditis and COVID‐19 hospitalizations show a similar wave pattern. AP indicates acute pericarditis.
Comparison of AP Cases With and Without COVID‐19
To gain a better understanding of the impact of COVID‐19 on AP, we studied patients with the diagnosis of AP with and without COVID‐19. Of the 46 913 patients with AP, 3.96% (n=1860) were infected with COVID‐19. The baseline characteristics of these patients are provided in Table S2.
Comparison of in‐hospital outcomes (Table S3) showed that the group of patients with AP with COVID‐19 had a higher rate of in‐hospital mortality than did the non‐COVID‐19 group in both the unmatched (23.6% versus 7.3%, P<0.001) and the matched (23.7% versus 7.5%, P<0.001) groups. Compared with patients with AP without COVID‐19, those with COVID‐19 had higher rates of complications including cardiac arrest, AKI, and acute respiratory failure. In contrast, the non‐COVID‐19 group had higher rates of ACHF and cardiac tamponade than did the COVID‐19 group. Compared with the non‐COVID‐19 group, the COVID‐19 group had a longer LOS and higher total charges in both unmatched and matched groups (Table S3). There were no statistically significant differences in the incidence of cardiogenic shock and ventricular arrhythmia.
Discussion
To our knowledge, this is the first study to examine the incidence and impact of AP in hospitalized patients with COVID‐19. The incidence of AP in hospitalized patients with COVID‐19 was low. However, when compared with the matched non‐AP cohort of patients with COVID‐19, those with AP had worse in‐hospital mortality, higher risks of complications (cardiac arrest, cardiogenic shock, ventricular arrhythmia, AKI, and ACHF), longer LOS, and more hospitalization charges. Our findings also indicated in‐hospital outcomes were significantly worse in patients who had AP that was complicated with COVID‐19 than in AP patients without COVID‐19.
The first reported study on infection‐related pericarditis was published in 1933. 16 Viral infections have been documented as the most common cause of AP in the general population. 17 Yet, pericardial disease studies on coronaviruses are limited to case reports and small prospective studies. 12 , 18 , 19 In our study, we identified 983 (0.46%) patients with AP in the COVID‐19 in‐hospital group. The low incidence is consistent with findings from previous prospective studies. 18 , 20 However, small retrospective studies have suggested a high incidence of pericardial disease (up to 90.7%) in critically ill patients with COVID‐19 in the intensive care unit. 21 , 22 , 23 The different study populations could explain the variations in the reported incidence of AP since our cohort was unselected hospitalized patients with COVID‐19, including both intensive care unit and non–intensive care unit settings, and may be more representative of the incidence of AP in the general hospitalized COVID‐19 population.
Hospitalized patients with COVID‐19 with AP had a higher mortality rate and incidence of complications, including cardiac arrest, cardiogenic shock, ventricular arrhythmia, and ACHF, than did those without AP. Similarly, previous reports found that patients with COVID‐19 with increased cardiovascular risks presented with worse clinical outcomes. 24 , 25 Although the interaction between severe acute respiratory syndrome‐coronavirus 2 and AP remains unclear, several mechanisms have been proposed. First, infection can trigger an overactive immune response, such as a cytokine storm, which could cause deterioration in pericardial structure and function. Pathological studies suggested that higher expression levels of cytokines were correlated with viral loads, and thus more severe infections. 26 This suggests that patients with AP may have a more severe overactive immune response than non‐AP patients. These chain reactions could accelerate disease progression and lead to poor outcomes. Moreover, acute respiratory distress syndrome and hypoxia caused by viral infections and inflammation can lead to pericardial damage. 18 , 27 , 28 With all these possible pathways to disease progression, a worse clinical outcome in patients with COVID‐19 with AP would be expected. Although patients with COVID‐19 with AP experienced worse outcomes than those without pericarditis, they had a lower incidence of cardiac tamponade and ACHF than did the non‐COVID pericarditis group. This observation suggests that AP could serve as an indicator of a broader immune response, rather than being the primary underlying issue itself.
In the outcome analysis, we found a higher incidence of AKI in the AP‐COVID‐19 group. This finding may be attributed to various pathophysiological events. First, the pericardial effusion caused by AP, if it overwhelmed the pericardial capacity to stretch and thus restrict the cardiac chamber size, could lead to hemodynamic compromise with reduced cardiac output and systemic hypotension, thereby triggering AKI. 29 The diagnosis and management of AP could also contribute to AKI. 30 At the diagnosis stage, the complication of AP in patients with COVID‐19 could mimic acute coronary syndrome. To differentiate acute coronary syndrome from pericarditis, coronary angiography with contrast dye may be used, which could cause contrast‐induced AKI. 31 From a management perspective, as the first‐line therapy indicated for AP, nonsteroidal anti‐inflammatory drugs could induce AKI. 32 Additionally, inflammatory events secondary to AP and viral infection can directly attack the kidneys and lead to AKI. 33
Hospitalized patients with COVID‐19 with AP have higher health care costs and a longer LOS as compared with patients with COVID‐19 without AP. Similar findings have been reported in previous studies of patients with COVID‐19 with other cardiovascular comorbidities. 34 , 35 Although it is implicit that treating AP in addition to COVID‐19 alone may result in higher costs generated from diagnosing and treating this condition, having more complications may have an exponential rather than linear effect on costs, as more comorbidities may reflect a more complex clinical condition and a sicker population. Furthermore, we found that the patients with COVID‐19 with AP have unique demographic and socioeconomic features when compared with the non‐AP group. We found a higher proportion of Black patients in the COVID‐19 group with AP. The exact reason is unknown, but this finding is consistent with that in a previous study of the American Heart Association's COVID‐19 Cardiovascular Disease Registry. 36 Further analysis from the same study identified that Black patients with COVID‐19 and cardiovascular diseases had higher mortality and morbidity. Patients with AP and COVID were seen more frequently in urban teaching hospitals or institutions with more beds, a finding also consistent with data from a previous study. 37 Higher rates of patients with AP and COVID in urban areas may reflect the demographics in the area and could also reflect a higher sensitivity for this diagnosis in teaching institutions and in hospitals with more resources. Overall, these findings may provide insight for additional financial support, resource allocation, and policy making.
The monthly trends of COVID‐19‐related AP and COVID‐19 hospitalizations show similar wave patterns. The incidence of COVID‐19‐related AP decreased significantly after June 2020 compared with before June 2020 (0.46% versus 0.51%, P<0.001). The use of corticosteroids may partially explain this finding. After the release of the dexamethasone trial (RECOVERY trial) 38 data in June 2020, several meta‐analyses 39 , 40 and randomized control trials 38 , 41 , 42 showed the benefits of corticosteroids in hospitalized patients with COVID, making low‐dose dexamethasone the standard treatment for patients with COVID‐19 who required respiratory support. Glucocorticoids 43 are usually used as second‐line therapy for AP in patients who cannot tolerate or who do not respond to aspirin/nonsteroidal anti‐inflammatory drugs and colchicine. The use of low‐dose dexamethasone in patients with severe COVID‐19 may result in a lower incidence of AP. This raises the question of whether corticosteroids could be used as first‐line therapy for AP in hospitalized patients with COVID‐19 because of their potential benefits for severe COVID‐19 and because of the high AKI incidence in COVID‐19‐AP patients, which may contraindicate the use of nonsteroidal anti‐inflammatory drugs. Clinical trials are needed to determine the optimal treatment approach for this patient group.
There are several strengths in our study. We have demonstrated, for the first time, the higher rates of mortality and higher incidences of complications in hospitalized patients with COVID‐19 with AP. The findings will offer important information for optimizing diagnostic guidelines and management plans for patients with COVID‐19 with AP. Also, the current retrospective study was conducted in a nationwide database with a large number of registries, which enhanced the power of our findings.
The present study has limitations. First, data extractions may be biased through the use of ICD‐10‐CM codes for selection. Moreover, the comorbidities identified with ICD‐10‐CM codes did not indicate the level of the severity of disease. Second, using the NIS database, we cannot acquire additional important information including treatment plan, multimodality imaging results, laboratory data, diagnostic features, and long‐term outcomes (eg, recurrence of pericarditis) for analyzing patients with COVID‐19 with AP. Third, although baseline characteristics were matched in both groups, we can only minimize the biased effects of confounding factors. Certain unidentified confounding factors still exist. Fourth, whether AP in hospitalized patients with COVID‐19 is simply a marker for severe systemic inflammation or a relatively independent process cannot be ascertained in the current study because of the lack of laboratory data. Fifth, data on AP patients treated as outpatients, which may represent a significant group, are lacking in the NIS database. Sixth, we were unable to evaluate the effect of the COVID‐19 variant (such as the Alpha variant that emerged at the end of 2020) or vaccination (which became available in December 2020) because this information was unavailable. Lastly, the use of retrospective data and diagnosis with ICD‐10‐CM codes may contribute to the underestimation of AP reported in the NIS database.
Conclusions
In the current large retrospective cohort of hospitalized patients with COVID‐19 infection, AP was not common. Nevertheless, it was associated with excess mortality, longer LOS, higher health care charges, and a greater chance of cardiac arrest, cardiogenic shock, ventricular arrhythmia, AKI, and ACHF. Our findings suggest that optimizing a diagnosis algorithm, especially focused on the early detection, prevention, and treatment of AP, should be considered during admission and monitoring of patients with COVID‐19. Future studies should be aimed at developing and assessing risk stratification tools to target patients with COVID‐19 with a high risk of developing AP and investigate whether early AP treatment can improve outcomes in hospitalized patients with COVID‐19. Moreover, the underlying mechanism of crosstalk between COVID‐19 and AP should be studied to increase our current understanding of the pathogenesis of both diseases, which may improve the current clinical protocol and give insight into new therapeutics.
Sources of Funding
This work was supported by the 2020 Li Ka Shing Foundation Cross–Disciplinary Research Grant (2020LKSFG04B).
Disclosures
None.
Supporting information
Tables S1–S3
This manuscript was sent to Sula Mazimba, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.028970
See Editorial by Alder.
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. Al‐Aly Z, Xie Y, Bowe B. High‐dimensional characterization of post‐acute sequelae of COVID‐19. Nature. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9 [DOI] [PubMed] [Google Scholar]
- 2. Hendren NS, Drazner MH, Bozkurt B, Cooper LT Jr. Description and proposed management of the acute COVID‐19 cardiovascular syndrome. Circulation. 2020;141:1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baldi E, Sechi GM, Mare C, Canevari F, Brancaglione A, Primi R, Klersy C, Palo A, Contri E, Ronchi V, et al. COVID‐19 kills at home: the close relationship between the epidemic and the increase of out‐of‐hospital cardiac arrests. Eur Heart J. 2020;41:3045–3054. doi: 10.1093/eurheartj/ehaa508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siripanthong B, Nazarian S, Muser D, Deo R, Santangeli P, Khanji MY, Cooper LT Jr, Chahal CAA. Recognizing COVID‐19‐related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17:1463–1471. doi: 10.1016/j.hrthm.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li Z, Shao W, Zhang J, Ma J, Huang S, Yu P, Zhu W, Liu X. Prevalence of atrial fibrillation and associated mortality among hospitalized patients with COVID‐19: a systematic review and meta‐analysis. Front Cardiovasc Med. 2021;8:720129. doi: 10.3389/fcvm.2021.720129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Capaccione KM, Leb JS, D'Souza B, Utukuri P, Salvatore MM. Acute myocardial infarction secondary to COVID‐19 infection: a case report and review of the literature. Clin Imaging. 2021;72:178–182. doi: 10.1016/j.clinimag.2020.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fattouh N, Hallit S, Salameh P, Choueiry G, Kazour F, Hallit R. Prevalence and factors affecting the level of depression, anxiety, and stress in hospitalized patients with a chronic disease. Perspect Psychiatr Care. 2019;55:592–599. doi: 10.1111/ppc.12369 [DOI] [PubMed] [Google Scholar]
- 8. Furqan MM, Verma BR, Cremer PC, Imazio M, Klein AL. Pericardial diseases in COVID19: a contemporary review. Curr Cardiol Rep. 2021;23:90. doi: 10.1007/s11886-021-01519-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pournazari P, Spangler AL, Ameer F, Hagan KK, Tano ME, Chamsi‐Pasha M, Chebrolu LH, Zoghbi WA, Nasir K, Nagueh SF. Cardiac involvement in hospitalized patients with COVID‐19 and its incremental value in outcomes prediction. Sci Rep. 2021;11:19450. doi: 10.1038/s41598-021-98773-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adler Y, Charron P, Imazio M, Badano L, Barón‐Esquivias G, Bogaert J, Brucato A, Gueret P, Klingel K, Lionis C, et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) endorsed by: the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2015;36:2921–2964. doi: 10.1093/eurheartj/ehv318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bhandari B, Neupane S, Khanal R, Lnu K, Wert Y, Komanduri S. COVID‐19 pericarditis mimicking an acute myocardial infarction: a case report and review of literature. J Community Hosp Intern Med Perspect. 2021;11:315–321. doi: 10.1080/20009666.2021.1896429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blagojevic NR, Bosnjakovic D, Vukomanovic V, Arsenovic S, Lazic JS, Tadic M. Acute pericarditis and severe acute respiratory syndrome coronavirus 2: case report. Int J Infect Dis. 2020;101:180–182. doi: 10.1016/j.ijid.2020.09.1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Overview of the National (Nationwide) Inpatient Sample (NIS). Agency for Healthcare Research and Quality. 2022. Accessed February 2, 2023. Last updated September 15, 2022. https://www.hcup‐us.ahrq.gov/nisoverview.jsp.
- 14. Chief Investigators of the Randomised Evaluation of COVid‐19 thERapY (RECOVERY) Trial . Low‐cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID‐19. Nuffield Department of Population Health. 2020. Accessed February 2, 2023. Last updated June 16, 2020. https://www.recoverytrial.net/news/low‐cost‐dexamethasone‐reduces‐death‐by‐up‐to‐one‐third‐in‐hospitalised‐patients‐with‐severe‐respiratory‐complications‐of‐covid‐19.
- 15. Lu X, Li P, Teng C, Cai P, Wang B. Anemia is associated with poor clinical outcomes in hospitalized patients with Takotsubo cardiomyopathy. Angiology. 2021;72:842–849. doi: 10.1177/0003319721999492 [DOI] [PubMed] [Google Scholar]
- 16. Bing HI. Epidemical pericarditis. Acta Med Scand. 1933;80:29–33. [Google Scholar]
- 17. Imazio M, Brucato A, Derosa FG, Lestuzzi C, Bombana E, Scipione F, Leuzzi S, Cecchi E, Trinchero R, Adler Y. Aetiological diagnosis in acute and recurrent pericarditis: when and how. J Cardiovasc Med (Hagerstown). 2009;10:217–230. doi: 10.2459/JCM.0b013e328322f9b1 [DOI] [PubMed] [Google Scholar]
- 18. Ghantous E, Szekely Y, Lichter Y, Levi E, Taieb P, Banai A, Sapir O, Granot Y, Lupu L, Hochstadt A, et al. Pericardial involvement in patients hospitalized with COVID‐19: prevalence, associates, and clinical implications. J Am Heart Assoc. 2022;11:e024363. doi: 10.1161/JAHA.121.024363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buckley BJR, Harrison SL, Fazio‐Eynullayeva E, Underhill P, Lane DA, Lip GYH. Prevalence and clinical outcomes of myocarditis and pericarditis in 718,365 COVID‐19 patients. Eur J Clin Investig. 2021;51:e13679. doi: 10.1111/eci.13679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu Y, Xie J, Gao P, Tian R, Qian H, Guo F, Yan X, Song Y, Chen W, Fang L, et al. Swollen heart in COVID‐19 patients who progress to critical illness: a perspective from echo‐cardiologists. ESC Heart Fail. 2020;7:3621–3632. doi: 10.1002/ehf2.12873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duerr GD, Heine A, Hamiko M, Zimmer S, Luetkens JA, Nattermann J, Rieke G, Isaak A, Jehle J, Held SAE, et al. Parameters predicting COVID‐19‐induced myocardial injury and mortality. Life Sci. 2020;260:118400. doi: 10.1016/j.lfs.2020.118400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Doyen D, Dupland P, Morand L, Fourrier E, Saccheri C, Buscot M, Hyvernat H, Ferrari E, Bernardin G, Cariou A, et al. Characteristics of cardiac injury in critically ill patients with coronavirus disease 2019. Chest. 2021;159:1974–1985. doi: 10.1016/j.chest.2020.10.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holman N, Knighton P, Kar P, O'Keefe J, Curley M, Weaver A, Barron E, Bakhai C, Khunti K, Wareham NJ, et al. Risk factors for COVID‐19‐related mortality in people with type 1 and type 2 diabetes in England: a population‐based cohort study. Lancet Diabetes Endocrinol. 2020;8:823–833. doi: 10.1016/S2213-8587(20)30271-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lindner D, Fitzek A, Bräuninger H, Aleshcheva G, Edler C, Meissner K, Scherschel K, Kirchhof P, Escher F, Schultheiss HP, et al. Association of cardiac infection with SARS‐CoV‐2 in confirmed COVID‐19 autopsy cases. JAMA Cardiol. 2020;5:1281–1285. doi: 10.1001/jamacardio.2020.3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID‐19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lewek J, Kaczmarek K, Cygankiewicz I, Wranicz JK, Ptaszynski P. Inflammation and arrhythmias: potential mechanisms and clinical implications. Expert Rev Cardiovasc Ther. 2014;12:1077–1085. doi: 10.1586/14779072.2014.942286 [DOI] [PubMed] [Google Scholar]
- 29. García‐Guimaraes M, Mojón D, Calvo A, Izquierdo A, Belarte‐Tornero L, Salvatella N, Llagostera M, Negrete A, Mas‐Stachurska A, Ruiz S, et al. Influence of cardiovascular disease and cardiovascular risk factors in COVID‐19 patients. Data from a large prospective Spanish cohort. REC: CardioClinics. 2021;56:108–117. doi: 10.1016/j.rccl.2020.11.004 [DOI] [Google Scholar]
- 30. Ismail TF. Acute pericarditis: update on diagnosis and management. Clin Med (Lond). 2020;20:48–51. doi: 10.7861/clinmed.cme.20.1.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Azzalini L, Candilio L, McCullough PA, Colombo A. Current risk of contrast‐induced acute kidney injury after coronary angiography and intervention: a reappraisal of the literature. Can J Cardiol. 2017;33:1225–1228. doi: 10.1016/j.cjca.2017.07.482 [DOI] [PubMed] [Google Scholar]
- 32. Zhang X, Donnan PT, Bell S, Guthrie B. Non‐steroidal anti‐inflammatory drug induced acute kidney injury in the community dwelling general population and people with chronic kidney disease: systematic review and meta‐analysis. BMC Nephrol. 2017;18:256. doi: 10.1186/s12882-017-0673-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rehman KA, Betancor J, Xu B, Kumar A, Rivas CG, Sato K, Wong LP, Asher CR, Klein AL. Uremic pericarditis, pericardial effusion, and constrictive pericarditis in end‐stage renal disease: insights and pathophysiology. Clin Cardiol. 2017;40:839–846. doi: 10.1002/clc.22770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pshenichnaya N, Lizinfeld I, Zhuravlev G. Factors influencing on hospitalization of COVID‐19 patients with comorbidity. Int J Infect Dis. 2022;116:S39. doi: 10.1016/j.ijid.2021.12.094 [DOI] [Google Scholar]
- 35. Hamdan M, Badrasawi M, Zidan S, Sayarah A, Zahra LA, Dana S, Almasry T. Risk factors associated with hospitalization owing to COVID‐19: a cross‐sectional study in Palestine. J Int Med Res. 2021;49:3000605211064405. doi: 10.1177/03000605211064405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rodriguez F, Solomon N, de Lemos JA, Das SR, Morrow DA, Bradley SM, Elkind MSV, Williams JH, Holmes D, Matsouaka RA, et al. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID‐19: findings from the American Heart Association's COVID‐19 Cardiovascular Disease Registry. Circulation. 2021;143:2332–2342. doi: 10.1161/CIRCULATIONAHA.120.052278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seringa J, Pedreiras S, Freitas MJ, Valente de Matos R, Rocha J, Millett C, Santana R. Direct costs of COVID‐19 inpatient admissions in a Portuguese tertiary care university centre. Port J Public Health. 2022;40:26–34. doi: 10.1159/000524368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. RECOVERY Collaborative Group ; Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, et al. Dexamethasone in hospitalized patients with Covid‐19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. WHO Rapid Evidence Appraisal for COVID‐19 Therapies (REACT) Working Group ; Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, Annane D, Azevedo LCP, Berwanger O, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID‐19: a meta‐analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li H, Yan B, Gao R, Ren J, Yang J. Effectiveness of corticosteroids to treat severe COVID‐19: a systematic review and meta‐analysis of prospective studies. Int Immunopharmacol. 2021;100:108121. doi: 10.1016/j.intimp.2021.108121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Angus DC, Derde L, Al‐Beidh F, Annane D, Arabi Y, Beane A, van Bentum‐Puijk W, Berry L, Bhimani Z, Bonten M, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID‐19: the REMAP‐CAP COVID‐19 corticosteroid domain randomized clinical trial. JAMA. 2020;324:1317–1329. doi: 10.1001/jama.2020.17022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dequin PF, Heming N, Meziani F, Plantefeve G, Voiriot G, Badie J, Francois B, Aubron C, Ricard JD, Ehrmann S, et al. Effect of hydrocortisone on 21‐day mortality or respiratory support among critically ill patients with COVID‐19: a randomized clinical trial. JAMA. 2020;324:1298–1306. doi: 10.1001/jama.2020.16761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chiabrando JG, Bonaventura A, Vecchie A, Wohlford GF, Mauro AG, Jordan JH, Grizzard JD, Montecucco F, Berrocal DH, Brucato A, et al. Management of acute and recurrent pericarditis: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2020;75:76–92. doi: 10.1016/j.jacc.2019.11.021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3


