Abstract
Background
Limited data are available about clinical outcomes and residual mitral regurgitation (MR) after transcatheter edge‐to‐edge repair in the large Asian‐Pacific cohort.
Methods and Results
From the Optimized Catheter Valvular Intervention (OCEAN‐Mitral) registry, a total of 2150 patients (primary cause of 34.6%) undergoing transcatheter edge‐to‐edge repair were analyzed and classified into 3 groups according to the residual MR severity at discharge: MR 0+/1+, 2+, and 3+/4+. The mortality and heart failure hospitalization rates at 1 year were 12.3% and 15.0%, respectively. Both MR and symptomatic improvement were sustained at 1 year with MR ≤2+ in 94.1% of patients and New York Heart Association functional class I/II in 95.0% of patients. Compared with residual MR 0+/1+ (20.4%) at discharge, both residual MR 2+ (30.2%; P < 0.001) and 3+/4+ (32.4%; P = 0.007) were associated with the higher incidence of death or heart failure hospitalization (adjusted hazard ratio [HR], 1.59; P < 0.001, and adjusted HR, 1.73; P = 0.008). New York Heart Association class III/IV at 1 year was more common in the MR 3+/4+ group (20.0%) than in the MR 0+/1+ (4.6%; P < 0.001) and MR 2+ (6.4%; P < 0.001) groups, and the proportion of New York Heart Association class I is significantly higher in the MR 1+ group (57.8%) than in the MR 2+ group (48.3%; P = 0.02).
Conclusions
The OCEAN‐Mitral registry demonstrated favorable clinical outcomes and sustained MR reduction at 1 year in patients undergoing transcatheter edge‐to‐edge repair. Both residual MR 2+ and 3+/4+ after transcatheter edge‐to‐edge repair at discharge were associated with worse clinical outcomes compared with residual MR 0+/1+.
Registration Information
https://upload.umin.ac.jp. Identifier: UMIN000023653.
Keywords: edge‐to‐edge repair, mitral regurgitation, structural heart disease
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Valvular Heart Disease
Nonstandard Abbreviations and Acronyms
- COAPT
Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation
- EVEREST II
Endovascular Valve Edge‐to‐Edge Repair
- MitraClip EXPAND
A Contemporary, Prospective Study Evaluating Real‐World Experience of Performance and Safety for the Next Generation of MitraClip Devices
- MR
mitral regurgitation
- NYHA
New York Heart Association
- OCEAN‐Mitral
Optimized Catheter Valvular Intervention
- OCEAN‐SHD
Optimized Catheter Valvular Intervention–Structural Heart Disease
- TEER
transcatheter edge‐to‐edge repair
- TRAMI
Transcatheter Mitral Valve Interventions
- TVT
Transcatheter Valve Therapy
Clinical Perspective.
What Is New?
The Optimized Catheter Valvular Intervention (OCEAN‐Mitral) registry, a large‐scale Asian cohort registry of mitral transcatheter edge‐to‐edge repair, demonstrated favorable clinical outcomes with a mortality rate of 12.3%, a heart failure hospitalization rate of 15.0%, and sustained mitral regurgitation reduction in 2150 patients.
Both residual mitral regurgitation 2+ and 3+/4+ at discharge relative to residual mitral regurgitation 0+/1+ were associated with increased death or heart failure hospitalization and impaired heart failure symptoms, and the adverse effects of residual mitral regurgitation 2+ and 3+/4+ were more clearly observed in patients with a primary pathogenesis.
What Are the Clinical Implications?
Mitral transcatheter edge‐to‐edge repair was safe and effective for an Asian cohort with relatively small body size, and mitral regurgitation should be reduced as much as possible in both primary and secondary mitral regurgitation to improve the clinical outcomes after the procedure.
Severe mitral regurgitation (MR) is associated with reduced left ventricular (LV) function, progressive congestive heart failure (HF), and increased mortality rate. 1 , 2 , 3 Transcatheter edge‐to‐edge repair (TEER) with the MitraClip system (Abbott Vascular, Menlo Park, CA) has emerged as an effective therapeutic option for symptomatic patients with MR, who have suitable clinical and anatomic features. The MitraClip system obtained Food and Drug Administration approval for primary MR in 2013 and secondary MR in 2019. In Japan, the AVJ‐514 trial demonstrated the efficacy, safety, and clinical benefit of the MitraClip device in a Japanese population. 4 The MitraClip system has been commercially available since April 2018, and the new‐generation MitraClip G4 system has been available since September 2020.
Asian patients have a different anatomy with smaller heart and valve areas than Western patients. Although small studies in the Asia‐Pacific region and Japan have already reported the safety and feasibility of TEER, 5 , 6 the clinical outcomes and their predictors after TEER in a large cohort in the Asian‐Pacific region are unknown. Procedural failure with residual MR 3+/4+ at discharge has been reported to be a strong predictor of death and HF rehospitalization. 7 , 8 , 9 On the other hand, there was no significant difference in clinical outcomes between patients with residual MR 2+ and 1+ or less in the COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation) trial. 9 Therefore, whether residual MR 2+ can affect the clinical outcomes is still controversial. The objective of this study was to investigate 1‐year clinical outcomes after TEER with the MitraClip device and evaluate the impact of residual MR on the clinical outcomes.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population
The Optimized Catheter Valvular Intervention (OCEAN‐Mitral) registry is ongoing, prospective, investigator‐initiated, multicenter registry to assess the safety and efficacy of TEER for patients with significant MR. A total of 21 Japanese institutions have participated in this registry. Between April 2018 and June 2021, 2150 consecutive symptomatic patients with MR underwent TEER with the MitraClip device. The patients were reviewed by the multidisciplinary local heart team consisting of an interventional cardiologist, a cardiothoracic surgeon, and an echocardiologist. This study was registered with the University Hospital Medical Information Network Clinical Trials Registry, as accepted by the International Committee of Medical Journal Editors (UMIN000023653). All study participants provided informed consent, and the study protocol was approved by the institutional review board of each institution. The study was conducted in accordance with the provisions of the Declaration of Helsinki and the guidelines for epidemiological studies issued by the Ministry of Health, Labour, and Welfare of Japan.
TEER Procedure
TEER with the MitraClip device was performed as previously described. 10 The procedure is performed under general anesthesia with fluoroscopic and transesophageal echocardiographic guidance. After transseptal puncture using femoral vein access, a 24‐French guiding catheter is advanced into the left atrium. The clip delivery system is inserted above the origin of the MR jet and then advanced into the left ventricle. The mitral leaflets are grasped, and the clip is closed to approximate the leaflets. If adequate MR reduction is obtained, the clip is released. If further reduction of MR is necessary, a second clip implantation is considered.
Clinical Outcomes and Definition
Clinical follow‐up was scheduled by outpatient visits at 30 days and 1 year after the procedure. If patients were unable to present at follow‐up, telephone interviews were conducted with patients, their family members, or family physicians to collect information on survival status and clinical events. Clinical outcomes, including all‐cause death, cardiac death, single‐leaflet device attachment, leaflet tear, reintervention for mitral valve dysfunction, atrial septum defect closure, stroke, and HF hospitalization, were assessed. A composite of death and HF hospitalization was used to evaluate the impact of residual MR on 1‐year clinical outcomes. Transthoracic echocardiographic results at baseline, discharge, 1 month, and 1 year were analyzed in this study. MR severity at baseline was assessed on the basis of the guidelines of the American Society of Echocardiography, 11 and that after the MitraClip procedure was assessed as previously described. 12 The MR severity was classified into 0+ (none/trivial), 1+ (mild), 2+ (moderate), 3+ (moderate to severe), and 4+ (severe). In cases with secondary MR, atrial secondary MR was defined as secondary MR with atrial fibrillation, dilated left atrium (left atrial diameter ≥40 mm or left atrial volume index ≥34 mL/m2), and normal LV function (LV ejection fraction ≥50%) and size (LV end‐diastolic diameter ≤55 mm). Measurement of LV volumes and ejection fraction was performed according to the biplane Simpson method. If echocardiographic data at discharge were not available, those immediately after the MitraClip procedure were used.
Statistical Analysis
Categorical variables were reported as numbers with relative percentage and compared using a chi‐square test or Fisher's exact test. Continuous variables were expressed as mean±SD or median (interquartile range), and compared using unpaired Student's t‐test or Mann–Whitney U test on the basis of the distribution. The cumulative incidences of clinical events were estimated by the Kaplan–Meier method, and differences were assessed using the log‐rank test. A multivariable Cox proportional hazard model was used to assess the impact of residual MR on death or HF hospitalization. The following variables were entered in the multivariate model: residual MR severity, age >80 years, male sex, body size area <1.5 m2, hypertension, diabetes, history of smoking, chronic obstructive pulmonary disease, atrial fibrillation, peripheral artery disease, prior myocardial infarction, prior cardiac surgery, prior cardiac resynchronized therapy, prior stroke, HF hospitalization within 1 year, New York Heart Association (NYHA) functional class IV, hemoglobin <10 mg/dL, estimated glomerular filtration rate <30 mL/min per 1.73 m2, brain natriuretic peptide >500 pg/mL, renin–angiotensin inhibitor, aldosterone antagonists, beta‐blocker, secondary MR, baseline LV end‐diastolic diameter >60 mm, LV ejection fraction <30%, tricuspid regurgitation pressure gradient >35 mm Hg, and moderate or severe tricuspid regurgitation. Proportional hazard assumption was tested and confirmed using the log–log plot method curve and Schoenfeld residuals for each variable and verified to be acceptable. The results were expressed as adjusted hazard ratios (HRs) with associated 95% CIs. A P value of <0.05 was considered statistically significant. The IBM SPSS statistical software version 25 (International Business Machines Corp., Armonk, NY) was used for all statistical calculations.
Results
Baseline Patients and Procedural Characteristics
Baseline characteristics are shown in Table 1. The median age was 80 years (73–85), and 1209 patients (56.2%) were male. NYHA functional class III or IV was observed in 1359 patients (63.2%), and 1541 patients (71.7%) experienced HF hospitalization within 1 year before TEER. Degenerative or mixed pathogenesis was observed in 745 patients (34.6%). Among 1617 patients with secondary MR (75.2%), 1198 patients (55.7%) had ventricular secondary MR and 419 patients (19.5%) had atrial secondary MR. Most of the patients (89.0%) had MR 3+ or 4+ before the procedure. The median LV ejection fraction was 43% (31%–61%), and 489 patients (22.7%) had an LV ejection fraction <30%. The MitraClip G4 system was used in 611 patients (29.2%). Among 2135 patients (99.3%) undergoing at least 1 clip implantation, 1285 patients (59.8%) received 1 clip, 807 patients (37.5%) received 2 clips, and 43 patients (2.0%) received 3 clips. The residual MR severity at discharge was 0+ in 405 patients (18.8%), 1+ in 1225 patients (57.0%), 2+ in 440 patients (20.5%), 3+ in 51 patients (2.4%), and 4+ in 29 patients (1.3%). The medial postprocedural mitral valve mean pressure gradient was 2.2 mm Hg (2.0–3.1). The prevalence of missing data was from 26.8% to 0% in the baseline variables (Table 1).
Table 1.
Baseline Patient Characteristics
| N=2150 | |
|---|---|
| Age, y | 80 (73–85) |
| >80, n (%) | 1055 (49.1) |
| Male, n (%) | 1209 (56.2) |
| Body size area, m2 (n=2149, 99.9%) | 1.50 (1.37–1.65) |
| Coronary artery disease, n (%) | 774 (36.0) |
| Hypertension, n (%) | 1452 (67.5) |
| Dyslipidemia, n (%) | 1111 (51.7) |
| Diabetes, n (%) | 579 (26.9) |
| Smoker, n (%) | 145 (6.7) |
| Chronic obstructive pulmonary disease, n (%) | 215 (10.0) |
| Atrial fibrillation, n (%) | |
| Paroxysmal | 485 (22.6) |
| Persistent/chronic | 883 (41.1) |
| Prior stroke, n (%) | 247 (11.5) |
| Prior myocardial infarction, n (%) | 502 (23.3) |
| Prior cardiac surgery, n (%) | 298 (13.9) |
| Peripheral artery disease, n (%) | 224 (10.4) |
| Cardiac rhythm device implant, n (%) | |
| Pacemaker | 131 (6.1) |
| Implantable cardioverter–defibrillator | 105 (4.9) |
| Cardiac resynchronization therapy—pacemaker | 26 (1.2) |
| Cardiac resynchronization therapy—defibrillator | 196 (9.1) |
| Hemodialysis, n (%) | 113 (5.3) |
| Heart failure hospitalization within 1 year, n (%) | 1541 (71.7) |
| STS mortality score for mitral valve replacement (n=1858, 86.4%) | 9.10 (5.78–14.1) |
| Hemoglobin, g/L | 11.6 (10.4–12.9) |
| <10.0 g/L, n (%) | 385 (17.9) |
| Creatinine, mg/dL | 1.26 (0.96–1.76) |
| eGFR, mL/min/1.73 m2 | 38 (26–51) |
| <30 mL/min per 1.73 m2, n (%) | 721 (33.5) |
| Brain natriuretic peptide, pg/mL (n=1573, 73.2%) | 342 (170–675) |
| >500 pg/mL, n (%) | 569 (36.2) |
| NYHA functional class, n (%) | |
| I | 47 (2.2) |
| II | 744 (34.6) |
| III | 1047 (48.7) |
| IV | 312 (14.5) |
| Medication at discharge, n (%) | |
| Renin–angiotensin inhibitor | 1371 (63.9) |
| Beta‐blocker | 1614 (75.1) |
| Aldosterone antagonists, n (%) | 1170 (54.4) |
| Echocardiographic parameters | |
| Mitral regurgitation pathogenesis, n (%) | |
| Secondary | 1617 (75.2) |
| Atrial | 419 (19.5) |
| Primary | 639 (29.7) |
| Mixed | 106 (4.9) |
| Mitral regurgitation severity, n (%) | |
| 1+/2+ (mild/moderate) | 638 (11.0) |
| 3+ (moderate to severe) | 601 (28.0) |
| 4+ (severe) | 1311 (61.0) |
| Effective regurgitant orifice area, cm2 (n=1965, 91.4%) | 0.35 (0.25–0.47) |
| LV ejection fraction, % | 43 (31–61) |
| LV ejection fraction ≤30%, n (%) | 489 (22.7) |
| LV end‐systolic diameter, mm (n=2148, 99.9%) | 43 (33–54) |
| LV end‐diastolic diameter, mm (n=2147, 99.9%) | 57 (50–64) |
| LV end‐diastolic diameter>60 mm, n (%) | 759 (35.4) |
| LV end‐systolic volume, mL (n=2093, 97.3%) | 73 (40–126) |
| LV end‐diastolic volume, mL (n=2096, 97.5%) | 136 (96–190) |
| Left atrial diameter, mm (n=2143, 99.7%) | 49 (44–55) |
| Left atrial volume, mL (n=2057, 95.7%) | 116 (88–157) |
| Moderate/severe tricuspid regurgitation, n (%) | 766 (35.6) |
| Tricuspid regurgitation pressure gradient, mm Hg (n=2083, 96.9%) | 33 (25–44) |
| Tricuspid regurgitation pressure gradient >35 mm Hg, n (%) | 870 (40.5) |
| MV mean pressure gradient, mm Hg (n=1920, 89.3%) | 1.5 (1.0–2.1) |
| MV orifice area, cm2 (n=1769, 82.3%) | 5.1 (4.2–6.2) |
| Procedural results | |
| Number of clips implanted, n (%) | |
| 0 | 15 (0.7) |
| 1 | 1285 (59.8) |
| 2 | 807 (37.5) |
| 3 | 43 (2.0) |
| Procedural time, min (n=1973, 91.8%) | 87 (63–120) |
| Mitral regurgitation severity at discharge,* n (%) | |
| 0+ (none to trace) | 405 (18.8) |
| 1+ (mild) | 1225 (57.0) |
| 2+ (moderate) | 440 (20.5) |
| 3+ (moderate to severe) | 51 (2.4) |
| 4+ (severe) | 29 (1.3) |
| Postprocedural MV mean pressure gradient, mm Hg (n=2116, 98.4%) | 2.2 (2.0–3.1) |
eGFR indicates estimated glomerular filtration rate; LV, left ventricular; MV, mitral valve; NYHA, New York Heart Association; and STS, Society of Thoracic Surgeons.
If echocardiographic data at discharge were not available, those immediately after the MitraClip procedure were used.
One‐Year Outcomes
The 1‐year clinical follow‐up rate was 94.8%. The 1‐year clinical outcomes are summarized in Table 2. The 1‐year mortality rate was 12.3% (255 patients) (Figure 1), with 7.9% (159 patients) due to cardiovascular cause and 4.8% (96 patients) due to noncardiovascular cause. The incidences of single‐leaflet device attachment and leaflet tear were 1.7% (35 patients) and 1.2% (26 patients), respectively. Mitral valve reintervention was performed in 2.4% (48 patients) within 1 year. Among 48 patients who received reintervention, 12 patients underwent repeat TEER, 34 patients open heart surgery, and 2 patients both procedures. Regarding the surgical procedure, mitral valve replacement was performed in 28 patients and mitral valve repair in 8 patients. The incidence of HF hospitalization was 15.0% at 1 year (Figure 1). The cumulative incidence of death or HF hospitalization was 22.9% at 1 year (Figure 1).
Table 2.
One‐Year Outcomes
| Total cohort | Primary MR | Secondary MR | |
|---|---|---|---|
| All‐cause death, n (%) | 255 (12.3) | 71 (11.6) | 184 (12.6) |
| Cardiovascular | 159 (7.9) | 39 (6.5) | 120 (8.4) |
| Noncardiovascular | 96 (4.8) | 32 (5.4) | 64 (4.6) |
| Single‐leaflet device attachment, n (%) | 35 (1.7) | 16 (2.6) | 19 (1.3) |
| Leaflet tear, n (%) | 26 (1.2) | 5 (0.8) | 21 (1.4) |
| Mitral valve reintervention, n (%) | 48 (2.4) | 18 (3.0) | 30 (2.1) |
| Closure of atrial septal defect, n (%) | 23 (1.1) | 9 (1.4) | 14 (1.9) |
| Stroke, n (%) | 32 (1.6) | 11 (1.9) | 21 (1.5) |
| Heart failure hospitalization, n (%) | 297 (15.0) | 54 (9.3) | 243 (17.3) |
MR indicates mitral regurgitation.
Figure 1. Cumulative incidence of clinical outcomes.
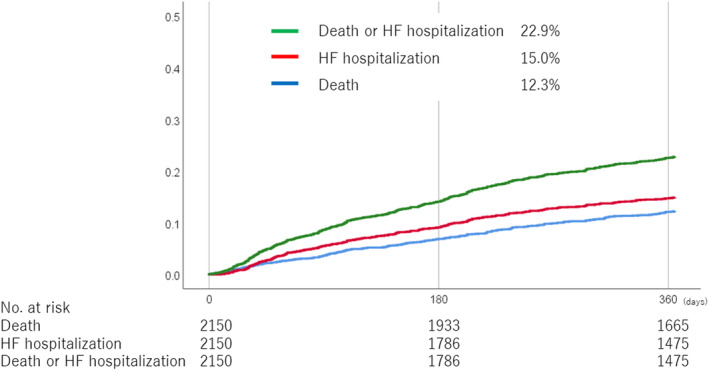
HF indicates heart failure.
Serial changes in MR severity and NYHA functional class were assessed in patients with available baseline, 1‐month, and 1‐year data. Figure 2A shows the serial changes in MR severity of 979 patients. At 1‐month follow‐up, MR ≤2+ was observed in 96.7% and 1+ or less in 75.1%. At 1‐year follow‐up, MR ≤2+ was observed in 94.1% and ≤1+ or less in 67.0%, indicating that MR reduction was sustained at 1 year after TEER. Figure 2B shows the serial changes in NYHA functional class of 1010 patients. NYHA functional class dramatically improved at 1 month, with 96.7% of patients achieving NYHA class I or II. The proportion of NYHA class I or II was 95.0% at 1 year, indicating that symptomatic improvement was also sustained at 1 year after TEER.
Figure 2. Serial changes in the MR severity and NYHA functional class.
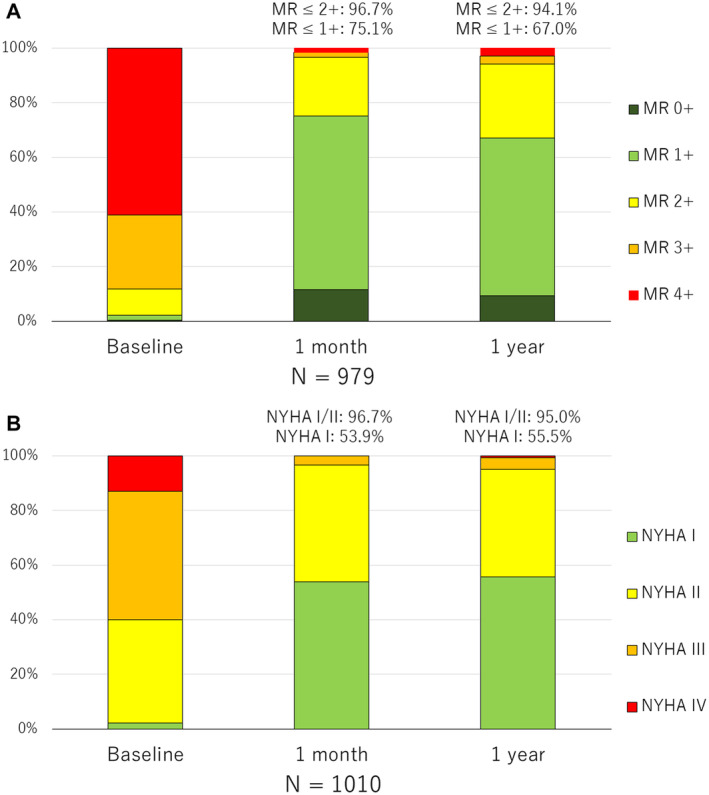
Serial MR severity in 979 patients (A) and NYHA functional class in 1010 patients (B) with available baseline, 1‐month, and 1‐year data. MR indicates mitral regurgitation; and NYHA, New York Heart Association.
Impact of Residual MR
All patients were classified into 3 groups according to the residual MR severity at discharge: 0+/1 (1630 patients), 2+ (440 patients), and 3+/4+ (80 patients). Table 3 shows the clinical outcomes compared between the MR 0+/1+, 2+, and 3+/4+ groups. The cumulative incidence of death or HF hospitalization at 1 year was significantly lower in the MR 0+/1+ group (20.4%) than in the MR 2+ group (30.2%; P<0.001) and MR 3+/4+ group (32.4%; P=0.007), but there was no significant difference between the MR 2+ and MR 3+/4+ groups (P=0.70) (Figure 3A). The mortality rate was also lower in the MR 0+/1+ group (10.3%) than in the MR 2+ group (18.9%; P<0.001) and MR 3+/4+ group (16.9%; P=0.06). Mitral valve reintervention was more frequently performed in the MR 3+/4+ group (19.0%) than in the MR 1+ group (1.6%; P<0.001) and MR 2+ group (2.4%; P<0.001). The incidence of HF hospitalization was also lower in the MR 0+/1+ group (13.4%) than in the MR 2+ group (19.8%; P<0.001) and MR 3+/4+ group (21.0%; P=0.06). In a multivariate analysis, the adjusted risk of residual MR 2+ and 3+/4+ relative to MR 0+/1+ for death or HF hospitalization at 1 year remained significant (adjusted HR, 1.59 [95% CI, 1.30–1.95]; P<0.001; and adjusted HR, 1.73 [95% CI, 1.15–2.60]; P=0.008) (Table 4).
Table 3.
One‐Year Outcomes According to the Residual MR Severity
| Total cohort | Primary MR | Secondary MR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MR 0+/1+ | MR 2+ | MR 3+/4+ | P value | MR 0+/1+ | MR 2+ | MR 3+/4+ | P value | MR 0+/1+ | MR 2+ | MR 3 + 4+ | P value | |
| Death or HF hospitalization, n (%) | 323 (20.4) | 128 (30.2) | 25 (32.3) | <0.001 | 55 (12.6) | 48 (34.0) | 10 (28.1) | <0.001 | 168 (23.5) | 80 (28.3) | 15 (36.0) | 0.01 |
| All‐cause death, n (%) | 162 (10.3) | 80 (18.9) | 13 (16.9) | <0.001 | 36 (8.2) | 21 (22.1) | 4 (11.3) | 0.001 | 126 (11.1) | 49 (17.3) | 9 (21.6) | 0.001 |
| HF hospitalization, n (%) | 204 (13.4) | 78 (19.8) | 15 (21.0) | 0.001 | 21 (5.1) | 26 (20.1) | 7 (20.9) | <0.001 | 183 (16.6) | 52 (19.7) | 8 (20.8) | 0.18 |
| Mitral valve reintervention, n (%) | 25 (1.6) | 9 (2.4) | 14 (19.0) | <0.001 | 10 (2.3) | 2 (1.5) | 6 (17.6) | <0.001 | 15 (1.4) | 7 (2.9) | 8 (20.8) | <0.001 |
HF indicates heart failure; and MR, mitral regurgitation.
Figure 3. Cumulative incidence of death or HF hospitalization according to the residual MR severity at discharge.
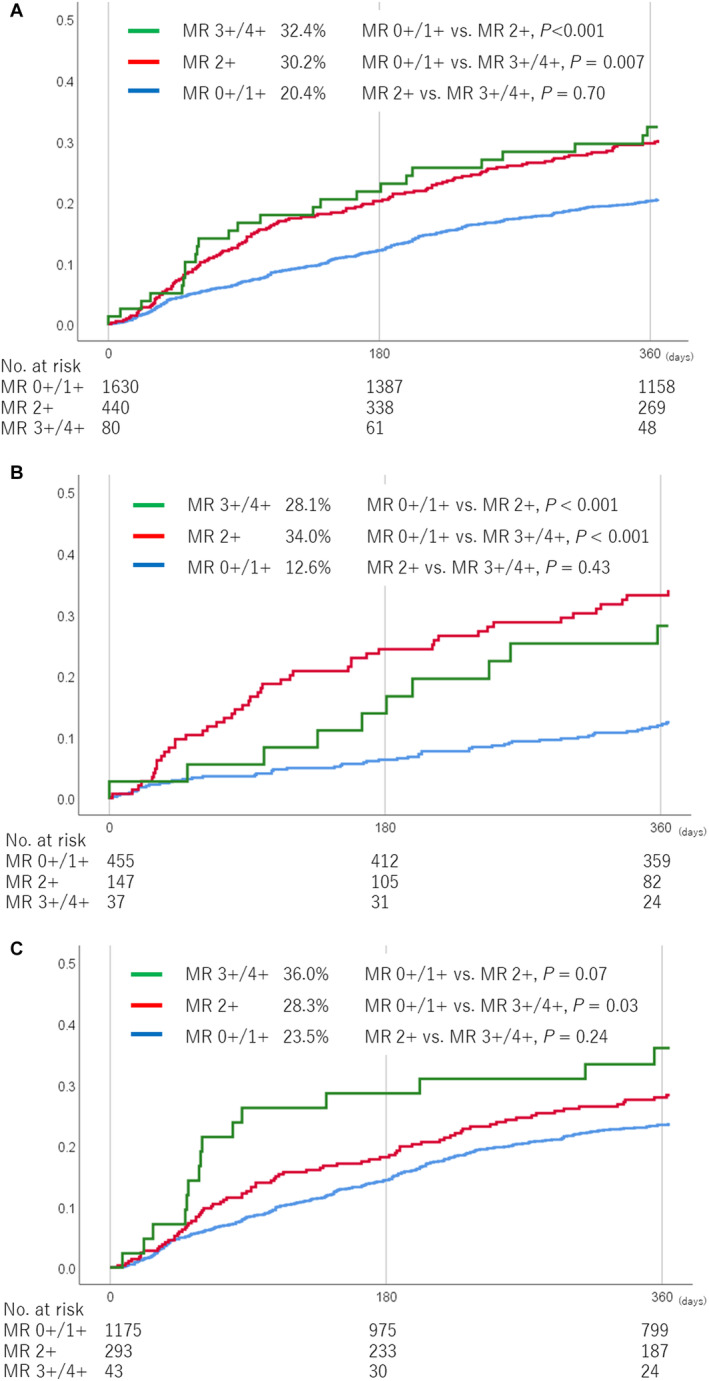
Cumulative incidence of death or HF hospitalization in all patients (A), patients with primary MR (B), and patients with secondary MR (C). HF indicates heart failure; and MR, mitral regurgitation.
Table 4.
Adjusted and Unadjusted Hazard Ratios of Death or HF Hospitalization
| Univariate model | Multivariable model | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
| Residual MR | <0.001 | 0.005 | ||
| MR 2+ vs MR 0+/1+ | 1.59 (1.30–1.95) | <0.001 | 1.40 (1.09–1.80) | 0.01 |
| MR 3+/4+ vs MR 0+/1+ | 1.73 (1.15–2.60) | 0.008 | 1.85 (1.14–2.99) | 0.01 |
| Age >80 y | 1.02 (0.85–1.22) | 0.82 | 1.25 (0.98–1.59) | 0.07 |
| Male sex | 1.29 (1.07–1.55) | 0.007 | 1.30 (1.03–1.65) | 0.03 |
| Hypertension | 0.92 (0.76–1.11) | 0.36 | 0.92 (0.73–1.17) | 0.50 |
| Diabetes | 1.26 (1.04–1.53) | 0.02 | 1.00 (0.79–1.28) | 0.97 |
| Smoker | 1.09 (0.77–1.55) | 0.62 | 0.80 (0.53–1.23) | 0.31 |
| Chronic obstructive pulmonary disease | 1.52 (1.17–1.97) | 0.002 | 1.23 (0.90–1.67) | 0.19 |
| Atrial fibrillation | 1.34 (1.10–1.63) | 0.003 | 1.39 (1.09–1.77) | 0.008 |
| Peripheral artery disease | 1.54 (1.19–1.99) | 0.001 | 1.12 (0.82–1.51) | 0.48 |
| Prior myocardial infarction | 1.47 (1.21–1.79) | <0.001 | 1.06 (0.81–1.37) | 0.68 |
| Prior cardiac surgery | 1.24 (0.97–1.58) | 0.09 | 1.28 (0.94–1.73) | 0.12 |
| Prior stroke | 1.31 (1.01–1.70) | 0.04 | 1.09 (0.79–1.50) | 0.62 |
| Prior cardiac resynchronization therapy | 1.44 (1.10–1.87) | 0.007 | 1.18 (0.84–1.65) | 0.35 |
| HF hospitalization within 1 year | 1.94 (1.53–2.45) | <0.001 | 1.86 (1.36–2.54) | <0.001 |
| NYHA functional class IV | 2.38 (1.94–2.92) | <0.001 | 1.83 (1.42–2.36) | <0.001 |
| Hemoglobin <10 g/L | 1.98 (1.62–2.42) | <0.001 | 1.67 (1.31–2.14) | <0.001 |
| eGFR <30 mL/min per 1.73 m2 | 1.70 (1.42–2.04) | <0.001 | 1.24 (0.98–1.56) | 0.07 |
| Brain natriuretic peptide >500 pg/dL | 2.34 (1.90–2.89) | <0.001 | 1.85 (1.46–2.35) | <0.001 |
| Renin–angiotensin inhibitor | 0.82 (0.68–0.98) | 0.03 | 1.04 (0.83–1.31) | 0.71 |
| Aldosterone antagonists | 1.03 (0.86–1.24) | 0.71 | 0.90 (0.71–1.14) | 0.37 |
| Beta‐blocker | 1.11 (0.89–1.37) | 0.35 | 0.93 (0.72–1.21) | 0.58 |
| Functional MR | 1.65 (1.30–2.09) | <0.001 | 1.15 (0.84–1.57) | 0.40 |
| LV end‐diastolic diameter >60 mm | 1.19 (0.99–1.43) | 0.06 | 0.88 (0.67–1.16) | 0.36 |
| LV ejection fraction <30% | 1.50 (1.23–1.82) | <0.001 | 1.48 (1.11–1.97) | 0.007 |
| TR pressure gradient >35 mm Hg | 1.21 (1.01–1.45) | 0.04 | 0.91 (0.72–1.14) | 0.42 |
| Moderate/severe TR | 1.20 (1.00–1.44) | 0.06 | 0.95 (0.75–1.21) | 0.68 |
eGFR indicates estimated glomerular filtration rate; HF, heart failure; LV, left ventricular; MR, mitral regurgitation; NYHA, New York Heart Association; and TR, tricuspid regurgitation.
The impact of residual MR on clinical outcomes was assessed according to the MR pathogenesis. In primary MR patients, the MR 0+/1+ group (12.6%) had a much lower incidence of death or HF hospitalization than the MR 2+ group (34.0%; P<0.001) and MR 3+/4+ group (28.1%; P<0.001; Figure 3B). In patients with secondary MR, the incidence of death or HF hospitalization in the MR 0+/1+ group (23.5%) tended to be lower than that in the MR 2+ group (28.3%; P=0.07) and was significantly lower than that in the MR 3+/4+ group (36.0%; P=0.03) (Figure 3C).
Figure 4A and 4B shows the MR severity and NYHA functional class at 1 year according to the residual MR severity. At 1 year, 3+/4+ MR was present in 2.5% of patients with 0/1+ residual MR at discharge, in 17.2% of patients with 2+ residual MR, and in 54.8% of patients with 3+/4+ residual MR. NYHA class III or IV at 1 year was more common in the MR 3+/4+ group (20.0%) than in the MR 0+/1+ group (4.6%; P<0.001) and MR 2+ group (6.4%; P<0.001). Furthermore, the proportion of NYHA class I was significantly higher in the MR 1+ group (57.8%) than in the MR 2+ group (48.3%; P=0.02).
Figure 4. MR severity and NYHA functional class at 1 year according to the residual MR severity at discharge.
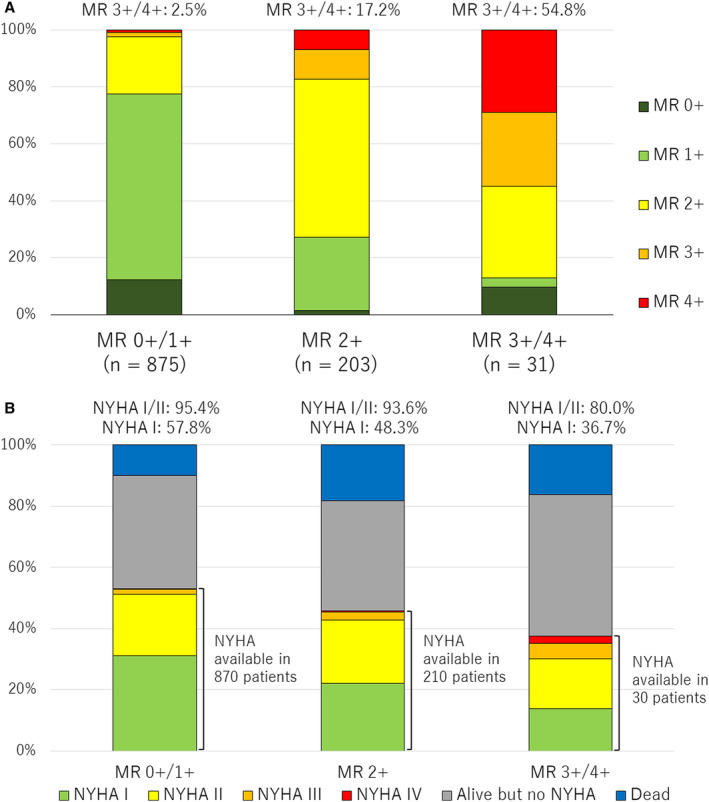
MR severity (A) and NYHA functional class (B) at 1 year according to the residual MR severity at discharge. MR indicates mitral regurgitation; and NYHA, New York Heart Association.
Discussion
The main findings of the present study were as follows: (1) The 1‐year clinical outcomes of 2150 patients undergoing TEER in Japan were favorable, with a mortality rate of 12.3% and an HF hospitalization rate of 15.0%; (2) MR reduction and symptomatic improvement were sustained up to 1 year in populations with available baseline, 1‐month, and 1‐year data; (3) both residual MR 2+ and 3+/4+ at discharge relative to residual MR 0+/1+ were associated with increased death or HF hospitalization and impaired HF symptoms, and the adverse effects of residual MR 2+ and 3+/4+ were more clearly observed in patients with a primary pathogenesis.
This study reported the 1‐year clinical outcomes of 2150 patients undergoing TEER with the MitraClip device from the largest cohort in the Asia‐Pacific region. The 1‐year mortality rate was 12.3%, which was comparable to those in 500 patients of the Japanese postmarketing surveillance study (14.9%), the device arm of the COAPT trial (18.8%), and the MitraClip EXPAND study (A Contemporary, Prospective Study Evaluating Real‐World Experience of Performance and Safety for the Next Generation of MitraClip Devices) (14.9%). 6 , 13 , 14 It was numerically lower than those in the independent German Transcatheter Mitral Valve Interventions (TRAMI) registry, (20.2%) and Transcatheter Valve Therapy (TVT) registry (25.8%). 15 , 16 In our study population, about 72% of the patients had been hospitalized for HF within 1 year. The HF hospitalization rate after TEER was only 15.0% at 1 year, indicating the efficacy of TEER as an HF therapy. In our cohort, single‐leaflet device attachment was observed in 1.7%, which was similar to that in the EXPAND registry. 14 Also, mitral valve reintervention, especially mitral valve surgery, is uncommon, and most patients who underwent surgery had mitral valve replacement rather than repair. Although our study period included only the first 3 years after the introduction of TEER to Japan, our clinical outcomes were comparable to the contemporary TEER results. Imaging techniques, procedural strategies, and patient management methods learned from Western countries, and also the teaching system we have constructed may have contributed to our favorable outcomes.
In our study, both MR reduction and NYHA improvement were sustained up to 1 year in the populations with available baseline, 1‐month, and 1‐year data. Notably, NYHA class I/II was achieved in 95.0% of patients at 1 year, and this was comparable to or better than those in previous studies. 6 , 13 , 14 Because these data suggest that MR reduction is directly related to symptomatic improvement, the current patient selection in Japan seems appropriate. MR reduction with MR ≤2+ in 94.1% of patients was better than that of the EVEREST II (Endovascular Valve Edge‐to‐Edge Repair) trials and compared well with that of the COAPT trial. 13 , 17 However, the EXPAND trial using the MitraClip G3 device with NTR and XTR clips provided MR ≤1+ of 79.2% of patients. Currently, the MitraClip G4 device with NT, NTW, XT, and XTW clips is available in Japan, and further improvement in MR reduction is expected in the future.
Currently, procedural failure is defined as residual MR 3+ or 4+ at discharge, and it has been reported to increase death and HF hospitalization. 18 Although residual MR 2+ is included in the procedural success, some small studies have reported that residual MR 2+ was a risk factor for adverse events. 19 , 20 , 21 A recent study of patients with secondary MR have reported that patients with residual 2+ MR at discharge had higher event rates than those with residual ≤1+. 22 However, the COAPT trial showed the similar mortality and HF hospitalization rates as well as clinical symptoms in patients with residual 2+ MR at 30 days after TEER for ventricular secondary MR compared with patients with residual 0+/1+ MR. 9 In our large‐cohort study, both residual MR 2+ and 3+/4+ were independently associated with worse clinical outcomes, and the adverse effects of residual MR 2+ relative to residual MR 1+ were more strongly observed in patients with primary MR. In patients with secondary MR, myocardial disease creates valvular disease, and not only MR severity but also advanced cardiomyopathy is related to the clinical outcomes after TEER. In patients with primary MR, residual MR caused by leaflet prolapse or degeneration, even MR 2+, is likely to affect the clinical outcomes in the chronic phase. Applying these results, it is recommended that MR be reduced as much as possible in both MR etiologies. Because the MR severity tends to be underestimated during general anesthesia, it should be assessed after the patient is brought close to the awake state by increasing blood pressure or adding fluid. After the introduction of the MitraClip G4 system, further MR reduction can be expected. One study reported that about 90% of patients achieved residual MR ≤1+ using the MitraClip G4 system. 23 As the device grows and MR reduction improves, the clinical outcomes will further improve.
Study Limitations
This study has several important limitations. First, this is a retrospective, observational study, and concomitant factors may have affected the results, even after adjusting for multivariate analysis. Second, the proportion of the MitraClip G4 system was small because of the study period. Therefore, we did not analyze the clinical impact of the MitraClip G4 system in this study. Third, echocardiographic data including the MR severity were not analyzed in an independent core laboratory. To standardize the MR grading, we established a consensus document on the echocardiographic MR assessment before and after TEER on the basis of the guidelines and shared it with the participating institutions before enrollment. In addition, a number of echocardiographic examinations for valvular heart disease had already been performed by experienced echocardiographers at the participating institutions when they started their TEER programs. Fourth, analysis of residual MRs was performed using the MR severity at discharge because there was no standardized method of assessing MR by transesophageal echocardiography. Fifth, the cumulative incidences of clinical outcomes assessed using Kaplan–Meier method with log‐rank test were unadjusted data. Finally, some patients were lost to follow‐up and lacked data for analysis, and serial changes in MR severity and NYHA functional class were assessed in 979 and 1010 of 2150 patients, respectively. Due to the missing value, multivariable analysis was performed in 1526 patients. Among variables in the model, the prevalence of missing value was 26.8% in “brain natriuretic peptide >500 pg/mL.” Even after excluding this variable (n=2082), the results were consistent (MR 0+/1+ versus MR 2+: adjusted HR, 1.55 [95% CI, 1.25–1.92]; P<0.001; and MR 0+/1+ versus MR 3+/4+: adjusted HR, 1.73 [95% CI, 1.15–2.60]; P=0.007).
Conclusions
The OCEAN‐Mitral registry demonstrated favorable clinical outcomes with a mortality rate of 12.3% and an HF hospitalization rate of 15.0% in 2150 patients undergoing TEER. MR reduction and symptomatic improvement were sustained up to 1 year. In our large‐scale cohort, both residual MR 2+ and 3+/4+ at discharge were associated with worse clinical outcomes and impaired NYHA functional class compared with residual MR 0+/1+.
Appendix
OCEAN‐Mitral Registry Investigators
Department of Cardiology, Keio University School of Medicine, Tokyo, Japan (Kentaro Hayashida); Department of Cardiology, Toyohashi Heart Center, Toyohashi, Japan (Yuya Adachi, Masanori Yamamoto); Department of Cardiology, Nagoya Heart Center, Nagoya, Japan (Masanori Yamamoto); Division of Cardiology, Kokura Memorial Hospital, Kitakyushu, Japan (Shinichi Shirai); Department of Cardiology, Teikyo University School of Medicine, Tokyo, Japan (Yusuke Watanabe); Department of Cardiology, New Tokyo Hospital, Chiba, Japan (Toru Naganuma); Department of Cardiology, Saiseikai Yokohama City Eastern Hospital, Kanagawa, Japan (Masahiro Yamawaki); Department of Cardiology, Sendai Kosei Hospital, Sendai, Japan (Yusuke Enta, Masaki Nakashima); Department of Cardiology, Shonan Kamakura General Hospital, Kanagawa, Japan (Shingo Mizuno); Second Department of Internal Medicine, Toyama University Hospital, Toyama, Japan (Hiroshi Ueno); Department of Cardiology, Tokai University School of Medicine, Isehara, Japan (Yohei Ohno); Division of Cardiology, Department of Internal Medicine, Iwate Medical University, Iwate, Japan (Yoshifumi Nakajima); Division of Cardiology, St. Marianna University School of Medicine Hospital, Kawasaki, Japan (Masaki Izumo); Department of Cardiology, Sapporo Higashi Tokushukai Hospital, Sapporo, Japan (Hiroki Bota); Division of Cardiology, Saiseikai Kumamoto Hospital Cardiovascular Center, Kumamoto, Japan (Kazuhisa Kodama); Department of Cardiology Tokyo Woman's Medical University, Tokyo, Japan (Junichi Yamaguchi); Department of Cardiology, Kurashiki Central Hospital, Kurashiki, Japan (Shunsuke Kubo); Department of Cardiology, National Cerebral and Cardiovascular Center, Suita, Japan (Makoto Amaki); Division of Cardiology, Mitsui Memorial Hospital, Tokyo, Japan (Masahiko Asami); Department of Cardiology, Sakakibara Heart Institute (Mike Saji); Division of Cardiology, Department of Medicine, Kinki University Faculty of Medicine, Osaka, Japan (Kazuki Mizutani).
Sources of Funding
The OCEAN‐Mitral registry, which is part of the Optimized Catheter Valvular Intervention–Structural Heart Disease (OCEAN‐SHD) registry, is supported by Edwards Lifesciences, Medtronic Japan, Boston Scientific, Abbott Medical Japan, and Daiichi‐Sankyo Company.
Disclosures
Drs Kubo, Saji, Izumo, Watanabe, and Amaki are clinical proctors of transcatheter edge‐to‐edge repair for Abbott Medical and have received consultant fees from Abbott Medical. Drs Asami and Kodama have received speaker fees from Abbott Medical. Drs Yamamoto and Nakajima are clinical proctors of transcatheter edge‐to‐edge repair for Abbott Medical and have received lecture fees from Abbott Medical. Dr Yamaguchi is a clinical proctor of transcatheter edge‐to‐edge repair for Abbott Medical and has received a lecture fee and a scholarship donation from Abbott Medical. Dr Ohno has received consultant, advisor, and speaker fees from Abbott Medical. Drs Enta, Shirai, Mizuno, and Bota are clinical proctors of transcatheter edge‐to‐edge repair for Abbott Medical. The remaining authors have no disclosures to report.
A complete list of the OCEAN‐Mitral Investigators can be found in the appendix at the end of the article.
This manuscript was sent to Amgad Mentias, MD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 13.
See Editorial by Krishnaswamy and Miyasaka.
Contributor Information
Shunsuke Kubo, Email: daba-kuboshun101@hotmail.co.jp.
OCEAN‐Mitral Investigators:
Kentaro Hayashida, Yuya Adachi, Masanori Yamamoto, Masanori Yamamoto, Shinichi Shirai, Yusuke Watanabe, Toru Naganuma, Masahiro Yamawaki, Yusuke Enta, Masaki Nakashima, Shingo Mizuno, Hiroshi Ueno, Yohei Ohno, Yoshifumi Nakajima, Masaki Izumo, Hiroki Bota, Kazuhisa Kodama, Junichi Yamaguchi, Shunsuke Kubo, Makoto Amaki, Masahiko Asami, Mike Saji, and Kazuki Mizutani
References
- 1. Ling LH, Enriquez‐Sarano M, Seward JB, Tajik AJ, Schaff HV, Bailey KR, Frye RL. Clinical outcome of mitral regurgitation due to flail leaflet. N Engl J Med. 1996;335:1417–1423. doi: 10.1056/NEJM199611073351902 [DOI] [PubMed] [Google Scholar]
- 2. Trichon BH, Felker GM, Shaw LK, Cabell CH, O'Connor CM. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol. 2003;91:538–543. doi: 10.1016/S0002-9149(02)03301-5 [DOI] [PubMed] [Google Scholar]
- 3. Agricola E, Ielasi A, Oppizzi M, Faggiano P, Ferri L, Calabrese A, Vizzardi E, Alfieri O, Margonato A. Long‐term prognosis of medically treated patients with functional mitral regurgitation and left ventricular dysfunction. Eur J Heart Fail. 2009;11:581–587. doi: 10.1093/eurjhf/hfp051 [DOI] [PubMed] [Google Scholar]
- 4. Hayashida K, Yasuda S, Matsumoto T, Amaki M, Mizuno S, Tobaru T, Jujo K, Ootomo T, Yamaguchi J, Fukuda K, et al. AVJ‐514 trial‐ baseline characteristics and 30‐day outcomes following MitraClip® treatment in a Japanese cohort. Circ J. 2017;81:1116–1122. doi: 10.1253/circj.CJ-17-0115 [DOI] [PubMed] [Google Scholar]
- 5. Yeo KK, Yap J, Yamen E, Muda N, Tay E, Walters DL, Santoso T, Liu X, Jansz P, Yip J, et al. Percutaneous mitral valve repair with the MitraClip: early results from the MitraClip Asia‐Pacific Registry (MARS). EuroIntervention. 2014;10:620–625. doi: 10.4244/EIJV10I5A107 [DOI] [PubMed] [Google Scholar]
- 6. Matsumoto T, Kubo S, Izumo M, Mizuno S, Shirai S; MitraClip Japan PMS Investigators . MitraClip treatment of moderate‐to‐severe and severe mitral regurgitation in high surgical risk patients ‐ real‐world 1‐year outcomes from Japan. Circ J. 2022;86:402–411. doi: 10.1253/circj.CJ-21-0309 [DOI] [PubMed] [Google Scholar]
- 7. Sürder D, Pedrazzini G, Gaemperli O, Biaggi P, Felix C, Rufibach K, der Maur CA, Jeger R, Buser P, Kaufmann BA, et al. Predictors for efficacy of percutaneous mitral valve repair using the MitraClip system: the results of the MitraSwiss registry. Heart. 2013;99:1034–1040. doi: 10.1136/heartjnl-2012-303105 [DOI] [PubMed] [Google Scholar]
- 8. Puls M, Tichelbäcker T, Bleckmann A, Hünlich M, von der Ehe K, Beuthner BE, Rüter K, Beißbarth T, Seipelt R, Schöndube F, et al. Failure of acute procedural success predicts adverse outcome after percutaneous edge‐to‐edge mitral valve repair with MitraClip. EuroIntervention. 2014;9:1407–1417. doi: 10.4244/EIJV9I12A238 [DOI] [PubMed] [Google Scholar]
- 9. Kar S, Mack MJ, Lindenfeld J, Abraham WT, Asch FM, Weissman NJ, Enriquez‐Sarano M, Lim DS, Mishell JM, Whisenant BK, et al. Relationship between residual mitral regurgitation and clinical and quality‐of‐life outcomes after transcatheter and medical treatments in heart failure: COAPT trial. Circulation. 2021;144:426–437. doi: 10.1161/CIRCULATIONAHA.120.053061 [DOI] [PubMed] [Google Scholar]
- 10. Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Siegel R, Rose GA, Engeron E, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–1406. doi: 10.1056/NEJMoa1009355 [DOI] [PubMed] [Google Scholar]
- 11. Zoghbi WA, Enriquez‐Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two‐dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3 [DOI] [PubMed] [Google Scholar]
- 12. Foster E, Wasserman HS, Gray W, Homma S, Di Tullio MR, Rodriguez L, Stewart WJ, Whitlow P, Block P, Martin R, et al. Quantitative assessment of severity of mitral regurgitation by serial echocardiography in a multicenter clinical trial of percutaneous mitral valve repair. Am J Cardiol. 2007;100:1577–1583. doi: 10.1016/j.amjcard.2007.06.066 [DOI] [PubMed] [Google Scholar]
- 13. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, et al. Transcatheter mitral‐valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–2318. doi: 10.1056/NEJMoa1806640 [DOI] [PubMed] [Google Scholar]
- 14. Kar S, von Bardeleben RS, Rottbauer W, Mahoney P, Price MJ, Grasso C, Williams M, Lurz P, Ahmed M, Hausleiter J, et al. Contemporary outcomes following transcatheter edge‐to‐edge repair: 1‐year results from the EXPAND study. JACC Cardiovasc Interv. 2023;16:589–602. doi: 10.1016/j.jcin.2023.01.010 [DOI] [PubMed] [Google Scholar]
- 15. Puls M, Lubos E, Boekstegers P, von Bardeleben RS, Ouarrak T, Butter C, Zuern CS, Bekeredjian R, Sievert H, Nickenig G, et al. One‐year outcomes and predictors of mortality after MitraClip therapy in contemporary clinical practice: results from the German transcatheter mitral valve interventions registry. Eur Heart J. 2016;37:703–712. doi: 10.1093/eurheartj/ehv627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sorajja P, Vemulapalli S, Feldman T, Mack M, Holmes DR Jr, Stebbins A, Kar S, Thourani V, Ailawadi G. Outcomes with transcatheter mitral valve repair in the United States: an STS/ACC TVT registry report. J Am Coll Cardiol. 2017;70:2315–2327. doi: 10.1016/j.jacc.2017.09.015 [DOI] [PubMed] [Google Scholar]
- 17. Kar S, Feldman T, Qasim A, Trento A, Kapadia S, Pedersen W, Lim DS, Kipperman R, Smalling RW, Bajwa T, et al. Five‐year outcomes of transcatheter reduction of significant mitral regurgitation in high‐surgical‐risk patients. Heart. 2019;105:1622–1628. doi: 10.1136/heartjnl-2017-312605 [DOI] [PubMed] [Google Scholar]
- 18. Stone GW, Adams DH, Abraham WT, Kappetein AP, Généreux P, Vranckx P, Mehran R, Kuck KH, Leon MB, Piazza N, et al. Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: part 2: endpoint definitions: a consensus document from the Mitral Valve Academic Research Consortium. J Am Coll Cardiol. 2015;66:308–321. doi: 10.1016/j.jacc.2015.05.049 [DOI] [PubMed] [Google Scholar]
- 19. Toggweiler S, Zuber M, Sürder D, Biaggi P, Gstrein C, Moccetti T, Pasotti E, Gaemperli O, Faletra F, Petrova‐Slater I, et al. Two‐year outcomes after percutaneous mitral valve repair with the MitraClip system: durability of the procedure and predictors of outcome. Open Heart. 2014;1:e000056. doi: 10.1136/openhrt-2014-000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paranskaya L, D'Ancona G, Bozdag‐Turan I, Akin I, Kische S, Turan GR, Rehders T, Ortak J, Nienaber CA, Ince H. Residual mitral valve regurgitation after percutaneous mitral valve repair with the MitraClip(R) system is a risk factor for adverse one‐year outcome. Catheter Cardiovasc Interv. 2013;81:609–617. doi: 10.1002/ccd.24586 [DOI] [PubMed] [Google Scholar]
- 21. Buzzatti N, De Bonis M, Denti P, Barili F, Schiavi D, Di Giannuario G, La Canna G, Alfieri O. What is a “good” result after transcatheter mitral repair? Impact of 2+ residual mitral regurgitation. J Thorac Cardiovasc Surg. 2016;151:88–96. doi: 10.1016/j.jtcvs.2015.09.099 [DOI] [PubMed] [Google Scholar]
- 22. Reichart D, Kalbacher D, Rübsamen N, Tigges E, Thomas C, Schirmer J, Reichenspurner H, Blankenberg S, Conradi L, Schäfer U, et al. The impact of residual mitral regurgitation after MitraClip therapy in functional mitral regurgitation. Eur J Heart Fail. 2020;22:1840–1848. doi: 10.1002/ejhf.1774 [DOI] [PubMed] [Google Scholar]
- 23. Chakravarty T, Makar M, Patel D, Oakley L, Yoon SH, Stegic J, Singh S, Skaf S, Nakamura M, Makkar RR. Transcatheter edge‐to‐edge mitral valve repair with the MitraClip G4 system. JACC Cardiovasc Interv. 2020;13:2402–2414. doi: 10.1016/j.jcin.2020.06.053 [DOI] [PubMed] [Google Scholar]


