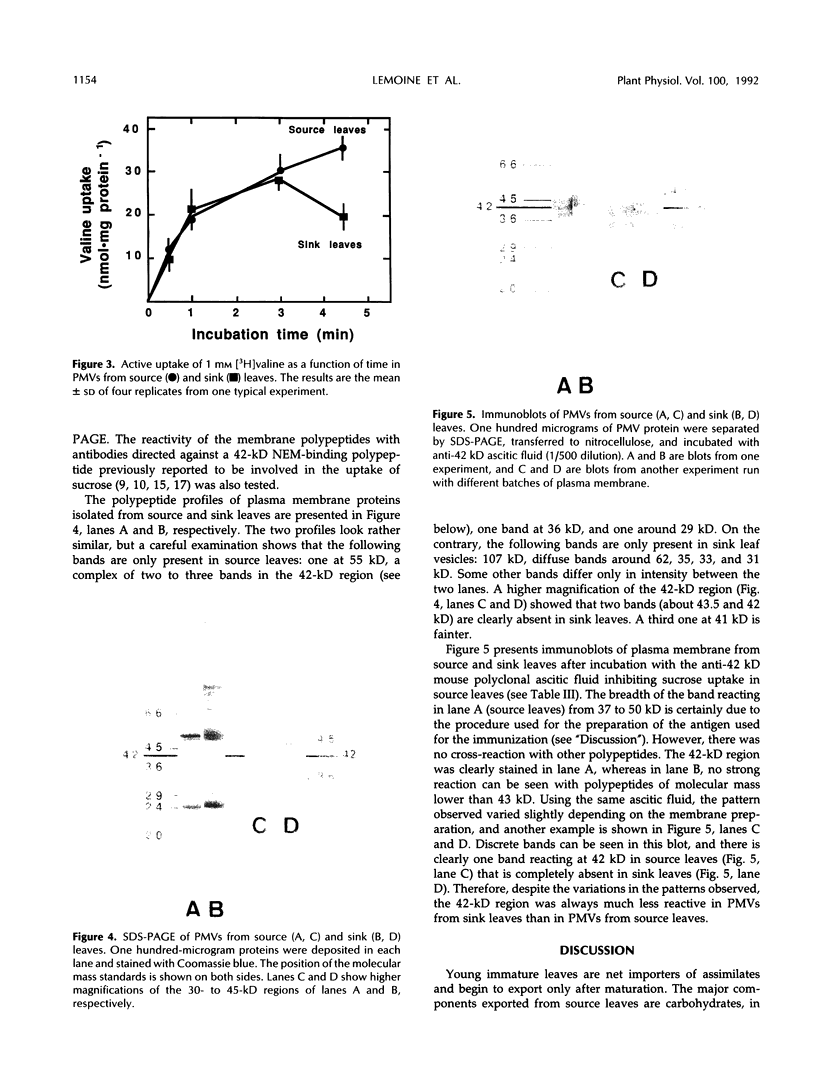
Abstract
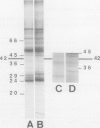
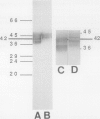
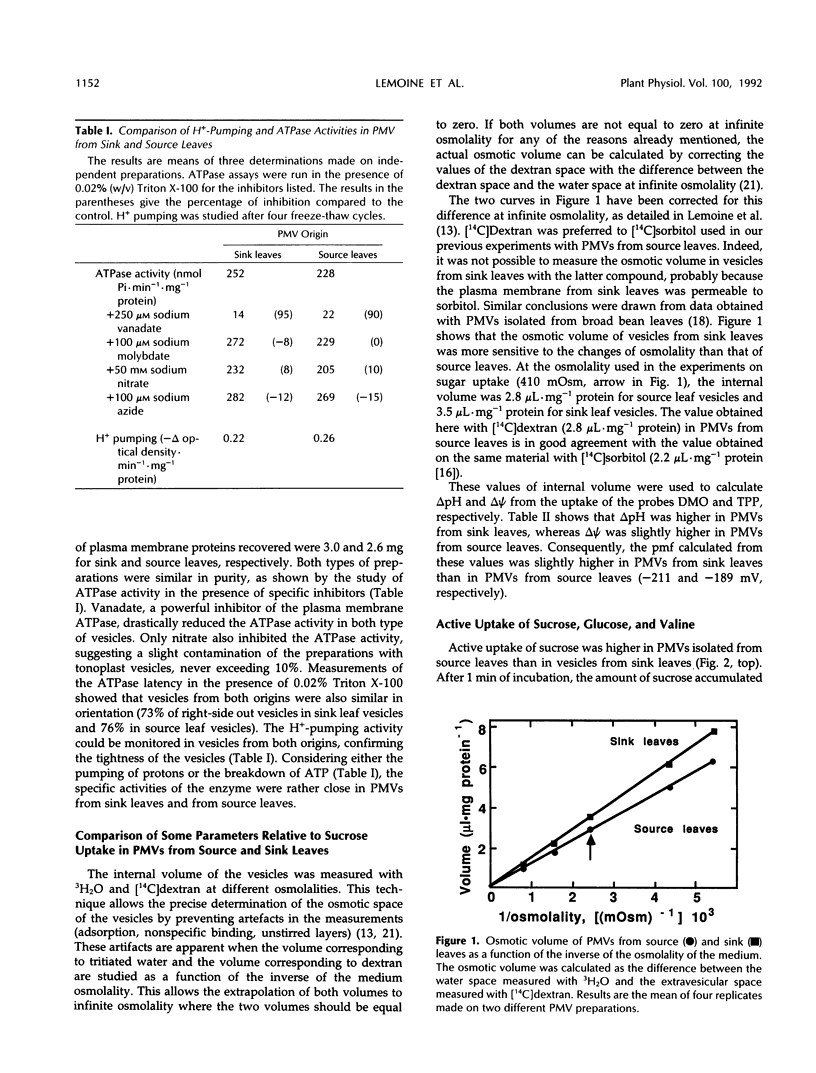
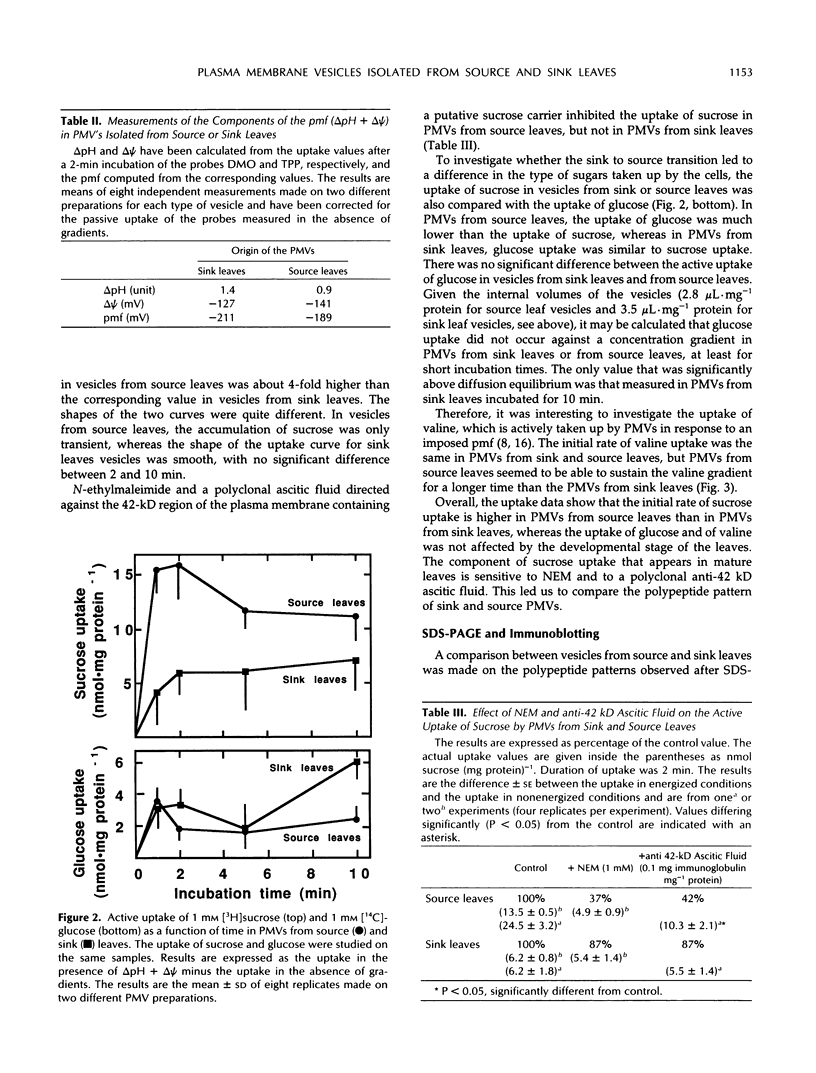
Plasma membrane vesicles (PMVs) were prepared by phase partitioning from microsomal fractions of either sink or source leaves of sugar beet (Beta vulgaris L.). The purity, the internal volume, the sidedness, and the sealingness of PMVs prepared from sink leaves did not differ from those measured with PMVs from source leaves. Yet, in response to an imposed proton motive force, PMVs from source leaves accumulated about 4-fold more sucrose than PMVs from sink leaves. The developmental stage did not affect the uptake of glucose and valine in PMVs prepared from leaf tissues. It was concluded that the sink/source transition is accompanied either by the incorporation into the plasma membrane of leaf cells of proteins mediating proton-sucrose cotransport, or by their activation. N-ethylmaleimide and a polyclonal ascitic fluid directed against the 42-kD region of the plasma membrane containing a putative sucrose carrier inhibited the uptake of sucrose in PMVs from source leaves, but not in PMVs from sink leaves. Sodium dodecyl sulfate gel electrophoresis and western blot suggested that the 42 polypeptide was more abundant in the PMVs from source leaves than in the PMVs from sink leaves.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bearden J. C., Jr Quantitation of submicrogram quantities of protein by an improved protein-dye binding assay. Biochim Biophys Acta. 1978 Apr 26;533(2):525–529. doi: 10.1016/0005-2795(78)90398-7. [DOI] [PubMed] [Google Scholar]
- Bennett A. B., O'neill S. D., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: I. Identification and Characterization of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):538–544. doi: 10.1104/pp.74.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourquin S., Bonnemain J. L., Delrot S. Inhibition of Loading of C Assimilates by p-Chloromercuribenzenesulfonic Acid : Localization of the Apoplastic Pathway in Vicia faba. Plant Physiol. 1990 Jan;92(1):97–102. doi: 10.1104/pp.92.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush D. R. Electrogenicity, pH-Dependence, and Stoichiometry of the Proton-Sucrose Symport. Plant Physiol. 1990 Aug;93(4):1590–1596. doi: 10.1104/pp.93.4.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush D. R. Proton-Coupled Sucrose Transport in Plasmalemma Vesicles Isolated from Sugar Beet (Beta vulgaris L. cv Great Western) Leaves. Plant Physiol. 1989 Apr;89(4):1318–1323. doi: 10.1104/pp.89.4.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows R. J., Geiger D. R. Structural and Physiological Changes in Sugar Beet Leaves during Sink to Source Conversion. Plant Physiol. 1974 Dec;54(6):877–885. doi: 10.1104/pp.54.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallet O., Lemoine R., Gaillard C., Larsson C., Delrot S. Selective Inhibition of Active Uptake of Sucrose into Plasma Membrane Vesicles by Polyclonal Sera Directed against a 42 Kilodalton Plasma Membrane Polypeptide. Plant Physiol. 1992 Jan;98(1):17–23. doi: 10.1104/pp.98.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Source and sink leaf metabolism in relation to Phloem translocation: carbon partitioning and enzymology. Plant Physiol. 1978 Mar;61(3):380–385. doi: 10.1104/pp.61.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Sucrose Hydrolysis in Relation to Phloem Translocation in Beta vulgaris. Plant Physiol. 1977 Sep;60(3):339–343. doi: 10.1104/pp.60.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. C., Bush D. R. DeltapH-Dependent Amino Acid Transport into Plasma Membrane Vesicles Isolated from Sugar Beet (Beta vulgaris L.) Leaves: II. Evidence for Multiple Aliphatic, Neutral Amino Acid Symports. Plant Physiol. 1991 Aug;96(4):1338–1344. doi: 10.1104/pp.96.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. S., Gallet O., Gaillard C., Lemoine R., Delrot S. The sucrose carrier of the plant plasmalemma. III. Partial purification and reconstitution of active sucrose transport in liposomes. Biochim Biophys Acta. 1992 Jan 31;1103(2):259–267. doi: 10.1016/0005-2736(92)90095-4. [DOI] [PubMed] [Google Scholar]
- Maurousset L., Lemoine R., Gallet O., Delrot S., Bonnemain J. L. Sulfur dioxide inhibits the sucrose carrier of the plant plasma membrane. Biochim Biophys Acta. 1992 Apr 13;1105(2):230–236. doi: 10.1016/0005-2736(92)90199-v. [DOI] [PubMed] [Google Scholar]
- Palmgren M. G., Sommarin M. Lysophosphatidylcholine stimulates ATP dependent proton accumulation in isolated oat root plasma membrane vesicles. Plant Physiol. 1989 Jul;90(3):1009–1014. doi: 10.1104/pp.90.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riens B., Lohaus G., Heineke D., Heldt H. W. Amino Acid and sucrose content determined in the cytosolic, chloroplastic, and vacuolar compartments and in the Phloem sap of spinach leaves. Plant Physiol. 1991 Sep;97(1):227–233. doi: 10.1104/pp.97.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H., Grunwald T., Avron M. Determination of pH in chloroplasts. I. Distribution of ( 14 C) methylamine. Eur J Biochem. 1972 Jan 31;25(1):54–63. doi: 10.1111/j.1432-1033.1972.tb01666.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Schmalstig J. G., Geiger D. R. Phloem Unloading in Developing Leaves of Sugar Beet : I. Evidence for Pathway through the Symplast. Plant Physiol. 1985 Sep;79(1):237–241. doi: 10.1104/pp.79.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalstig J. G., Geiger D. R. Phloem Unloading in Developing Leaves of Sugar Beet : II. Termination of Phloem Unloading. Plant Physiol. 1987 Jan;83(1):49–52. doi: 10.1104/pp.83.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turano F. J., Jordan R. L., Matthews B. F. Immunological characterization of in vitro forms of homoserine dehydrogenase from carrot suspension cultures. Plant Physiol. 1990 Feb;92(2):395–400. doi: 10.1104/pp.92.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R. Termination of nutrient import and development of vein loading capacity in albino tobacco leaves. Plant Physiol. 1984 Sep;76(1):45–48. doi: 10.1104/pp.76.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]