Abstract
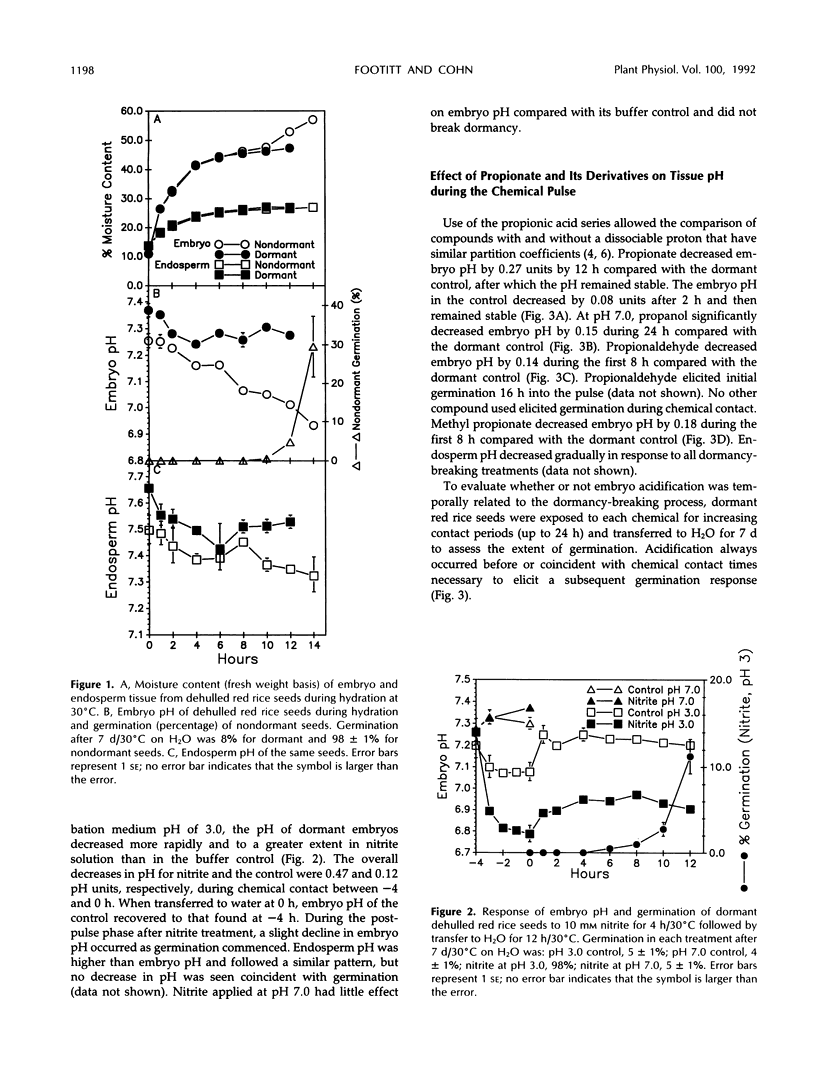
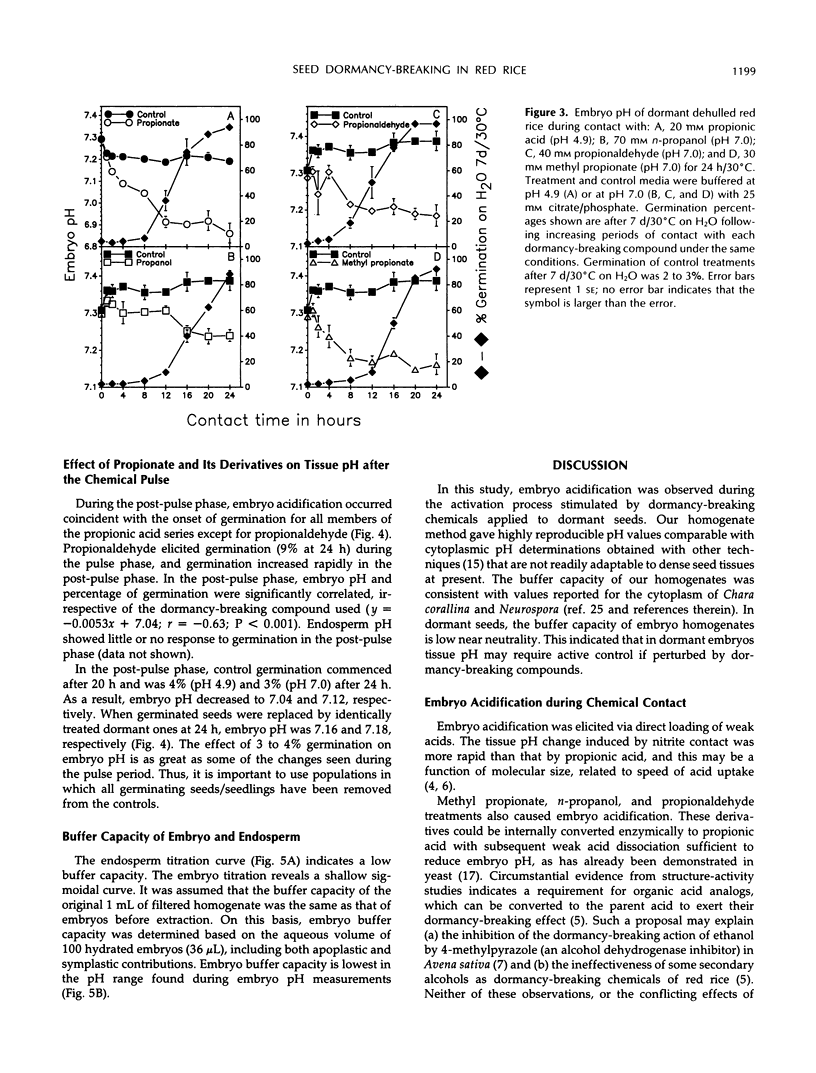
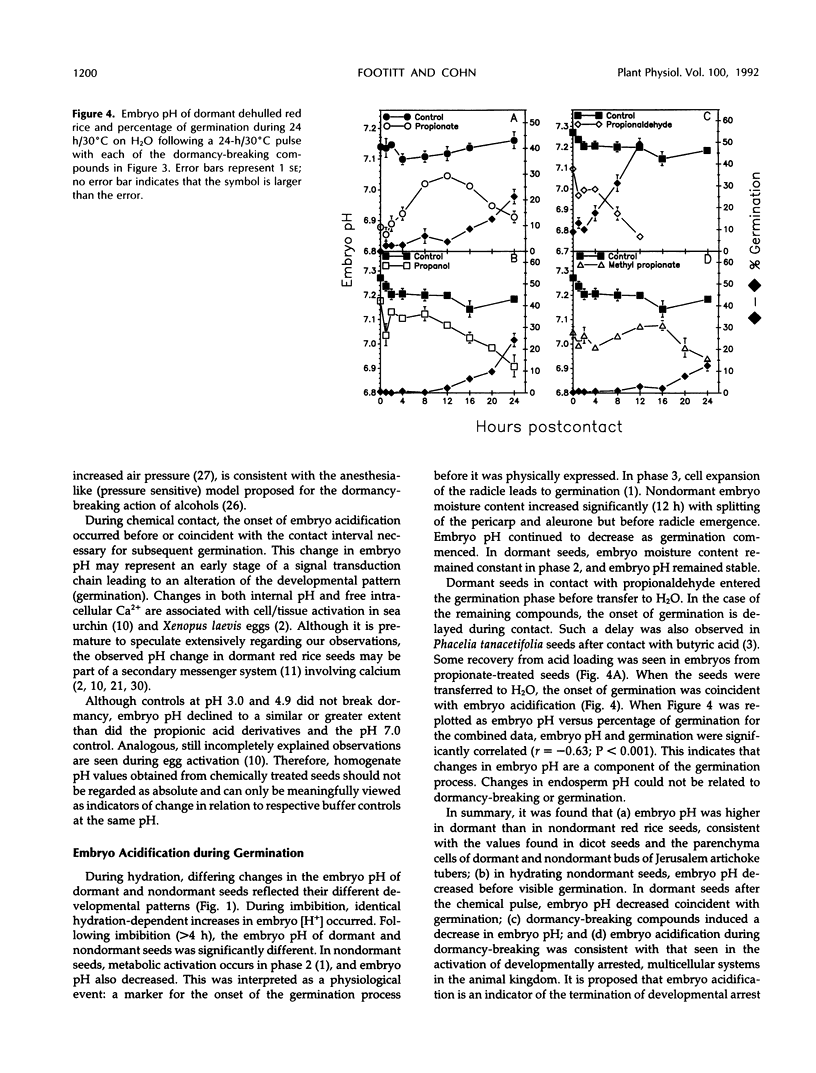
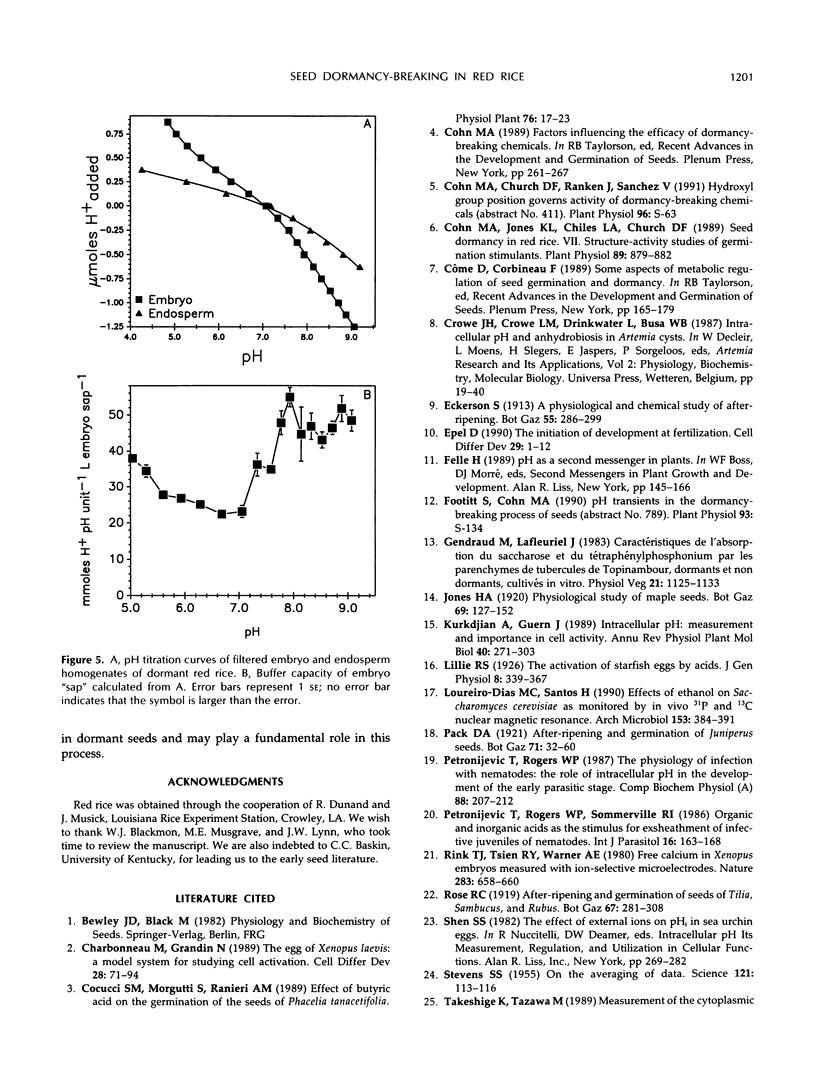
Exposure of dehulled, dormant red rice (Oryza sativa) seeds to dormancy-breaking treatments (10 mm sodium nitrite, 20 mm propionic acid, 30 mm methyl propionate, 40 mm propionaldehyde, or 70 mmn-propanol) induced tissue pH acidification during chemical contact at least 12 h before visible germination. During chemical contact, the onset of embryo acidification occurred before or coincident with the chemical contact interval necessary for subsequent germination. Upon seed transfer to H2O following chemical contact, embryo pH also decreased coincident with visible germination. During this period, the percentage of germination and embryo pH were closely linked irrespective of the dormancy-breaking compound used. Therefore, tissue acidification during the breaking of seed dormancy and the germination process may be analogous to similar tissue pH changes associated with the termination of developmental arrest in other multicellular systems, such as brine shrimp cysts and nematode larvae.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Charbonneau M., Grandin N. The egg of Xenopus laevis: a model system for studying cell activation. Cell Differ Dev. 1989 Nov;28(2):71–93. doi: 10.1016/0922-3371(89)90045-2. [DOI] [PubMed] [Google Scholar]
- Cohn M. A., Jones K. L., Chiles L. A., Church D. F. Seed Dormancy in Red Rice : VII. Structure-Activity Studies of Germination Stimulants. Plant Physiol. 1989 Mar;89(3):879–882. doi: 10.1104/pp.89.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel D. The initiation of development at fertilization. Cell Differ Dev. 1990 Jan;29(1):1–12. doi: 10.1016/0922-3371(90)90019-s. [DOI] [PubMed] [Google Scholar]
- Loureiro-Dias M. C., Santos H. Effects of ethanol on Saccharomyces cerevisiae as monitored by in vivo 31P and 13C nuclear magnetic resonance. Arch Microbiol. 1990;153(4):384–391. doi: 10.1007/BF00249010. [DOI] [PubMed] [Google Scholar]
- Petronijevic T., Rogers W. P., Sommerville R. I. Organic and inorganic acids as the stimulus for exsheathment of infective juveniles of nematodes. Int J Parasitol. 1986 Apr;16(2):163–168. doi: 10.1016/0020-7519(86)90101-3. [DOI] [PubMed] [Google Scholar]
- Petronijevic T., Rogers W. P. The physiology of infection with nematodes: the role of intracellular pH in the development of the early parasitic stage. Comp Biochem Physiol A Comp Physiol. 1987;88(2):207–212. doi: 10.1016/0300-9629(87)90471-3. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Warner A. E. Free calcium in Xenopus embryos measured with ion-selective microelectrodes. Nature. 1980 Feb 14;283(5748):658–660. doi: 10.1038/283658a0. [DOI] [PubMed] [Google Scholar]
- STEVENS S. S. On the averaging of data. Science. 1955 Jan 28;121(3135):113–116. doi: 10.1126/science.121.3135.113. [DOI] [PubMed] [Google Scholar]
- Takeshige K., Tazawa M. Measurement of the Cytoplasmic and Vacuolar Buffer Capacities in Chara corallina. Plant Physiol. 1989 Apr;89(4):1049–1052. doi: 10.1104/pp.89.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth W. G., Riddle D. L. Acidic intracellular pH shift during Caenorhabditis elegans larval development. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8435–8438. doi: 10.1073/pnas.85.22.8435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne R., Hepler P. K. Red Light Stimulates an Increase in Intracellular Calcium in the Spores of Onoclea sensibilis. Plant Physiol. 1985 Jan;77(1):8–11. doi: 10.1104/pp.77.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]


