Abstract
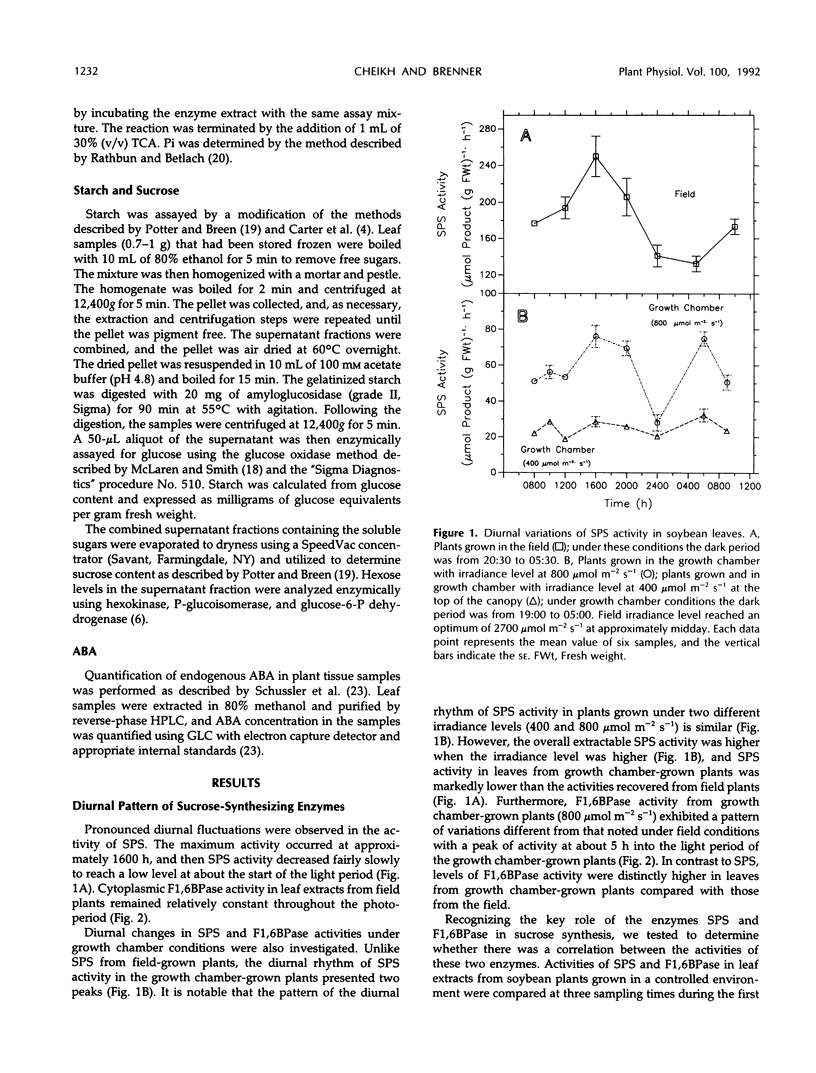
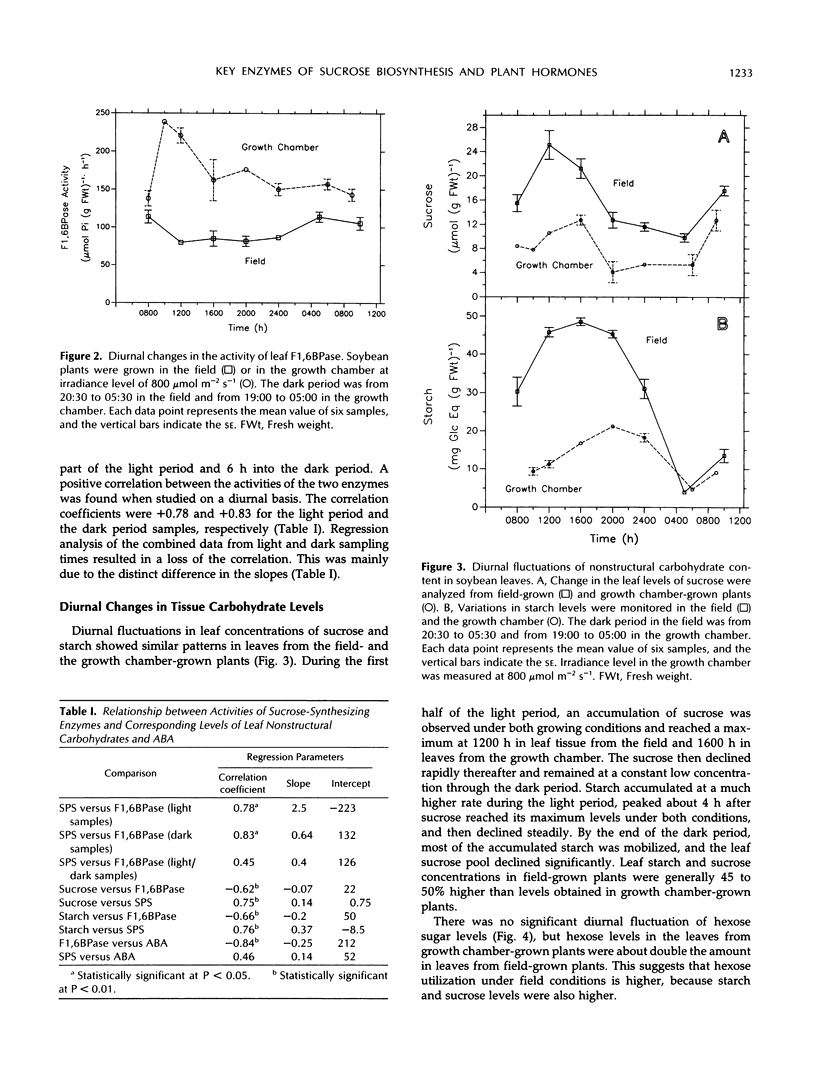
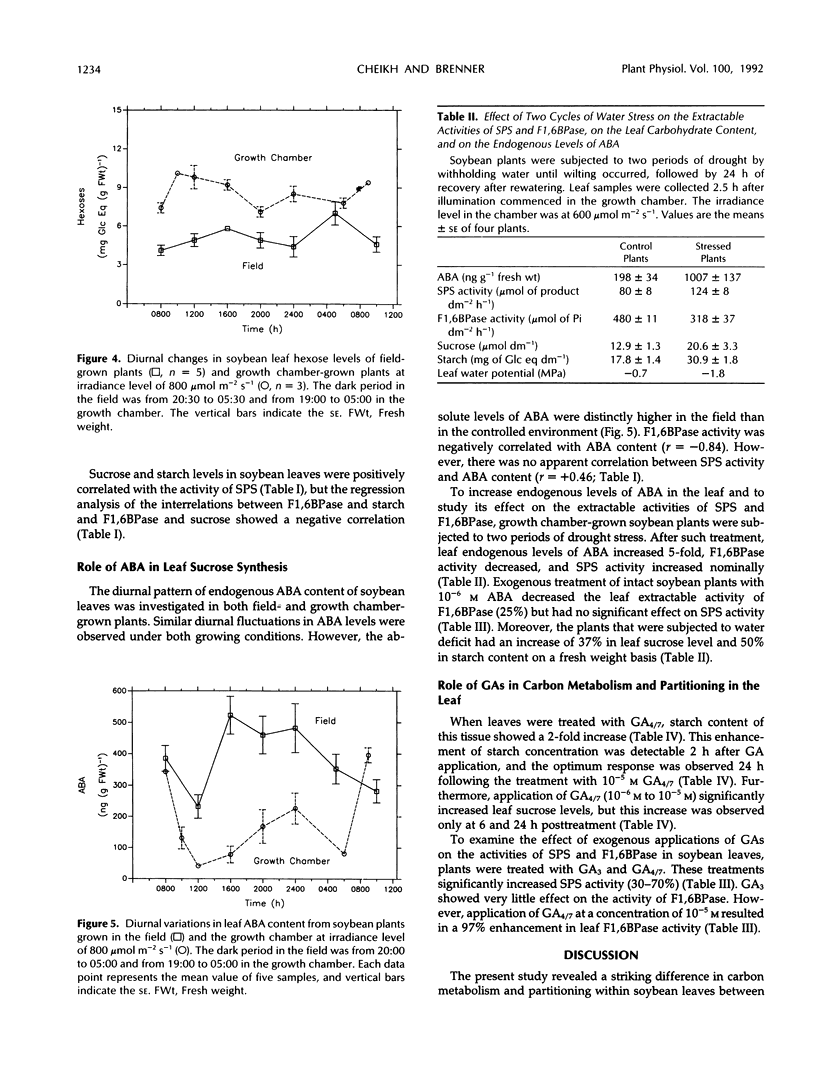
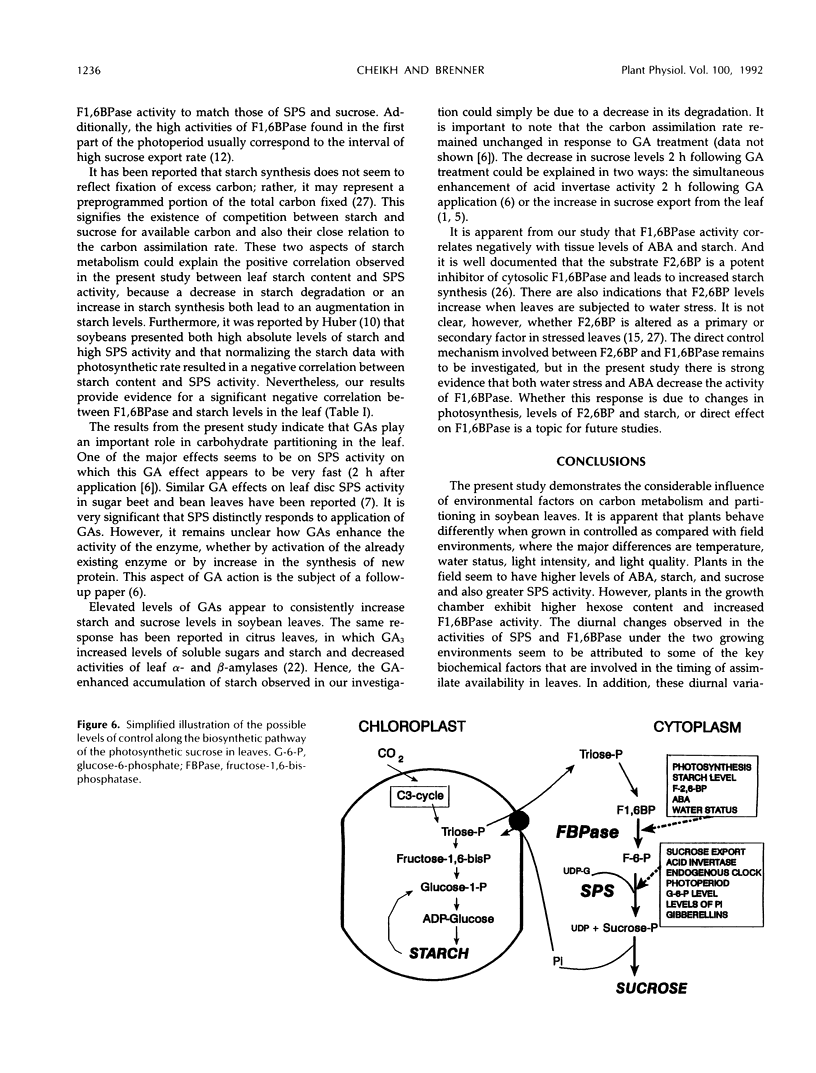
An important part in the understanding of the regulation of carbon partitioning within the leaf is to investigate the endogenous variations of parameters related to carbon metabolism. This study of diurnal changes in the activities of sucrose-synthesizing enzymes and levels of nonstructural carbohydrates in intact leaves of field-grown soybean plants (Glycine max [L.]) showed pronounced diurnal fluctuations in sucrose phosphate synthase (SPS) activity. However, there was no distinct diurnal change in the activity of fructose-1,6-bisphosphatase (F1,6BPase). SPS activity in leaves from plants grown in controlled environments presented two peaks during the light period. In contrast to field-grown plants, F1,6BPase activity in leaves from growth chamber-grown plants manifested one peak during the first half of the light period. In plants grown under both conditions, sucrose and starch accumulation rates were highest during early hours of the light period. By the end of the dark period, most of the starch was depleted. A pattern of diurnal fluctuations of abscisic acid (ABA) levels in leaves was also observed under all growing conditions. Either imposition of water stress or exogenous applications of ABA inhibited F1,6BPase activity. However, SPS-extractable activity increased following water deficit but did not change in response to ABA treatment. Gibberellin application to intact soybean leaves increased levels of both starch and sucrose. Both gibberellic acid (10−6m) and gibberellins 4 and 7 (10−5m) increased the activity of SPS but had an inconsistent effect on F1,6BPase. Correlation studies between the activities of SPS and F1,6BPase suggest that these two enzymes are coordinated in their function, but the factors that regulate them may be distinct because they respond differently to certain environmental and physiological changes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni B., Daie J., Wyse R. E. Enhancement of [C]Sucrose Export from Source Leaves of Vicia faba by Gibberellic Acid. Plant Physiol. 1986 Dec;82(4):962–966. doi: 10.1104/pp.82.4.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Israel D. W. Biochemical Basis for Partitioning of Photosynthetically Fixed Carbon between Starch and Sucrose in Soybean (Glycine max Merr.) Leaves. Plant Physiol. 1982 Mar;69(3):691–696. doi: 10.1104/pp.69.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C. Role of sucrose-phosphate synthase in partitioning of carbon in leaves. Plant Physiol. 1983 Apr;71(4):818–821. doi: 10.1104/pp.71.4.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. G., Outlaw W. H., Lowry O. H. Enzymic assay of 10 to 10 moles of sucrose in plant tissues. Plant Physiol. 1977 Sep;60(3):379–383. doi: 10.1104/pp.60.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalt-Torres W., Kerr P. S., Usuda H., Huber S. C. Diurnal changes in maize leaf photosynthesis : I. Carbon exchange rate, assimilate export rate, and enzyme activities. Plant Physiol. 1987 Feb;83(2):283–288. doi: 10.1104/pp.83.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr P. S., Huber S. C., Israel D. W. Effect of N-source on soybean leaf sucrose phosphate synthase, starch formation, and whole plant growth. Plant Physiol. 1984 Jun;75(2):483–488. doi: 10.1104/pp.75.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson D. T., Bunce J. A., Alberte R. S., Van Volkenburgh E. Photosynthesis in relation to leaf characteristics of cotton from controlled and field environments. Plant Physiol. 1977 Mar;59(3):384–387. doi: 10.1104/pp.59.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter J. R. Maintenance of High Photosynthetic Rates during the Accumulation of High Leaf Starch Levels in Sunflower and Soybean. Plant Physiol. 1980 Sep;66(3):528–531. doi: 10.1104/pp.66.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbun W. B., Betlach M. V. Estimation of enzymically produced orthophosphate in the presence of cysteine and adenosine triphosphate. Anal Biochem. 1969 Apr 4;28(1):436–445. doi: 10.1016/0003-2697(69)90198-5. [DOI] [PubMed] [Google Scholar]
- Rufty T. W., Kerr P. S., Huber S. C. Characterization of diurnal changes in activities of enzymes involved in sucrose biosynthesis. Plant Physiol. 1983 Oct;73(2):428–433. doi: 10.1104/pp.73.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schussler J. R., Brenner M. L., Brun W. A. Abscisic Acid and its relationship to seed filling in soybeans. Plant Physiol. 1984 Oct;76(2):301–306. doi: 10.1104/pp.76.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setter T. L., Brun W. A. Abscisic Acid Translocation and Metabolism in Soybeans following Depodding and Petiole Girdling Treatments. Plant Physiol. 1981 Apr;67(4):774–779. doi: 10.1104/pp.67.4.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicher R. C., Kremer D. F., Harris W. G. Diurnal carbohydrate metabolism of barley primary leaves. Plant Physiol. 1984 Sep;76(1):165–169. doi: 10.1104/pp.76.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Herzog B., Heldt H. W. Control of Photosynthetic Sucrose Synthesis by Fructose 2,6-Bisphosphate : I. Coordination of CO(2) Fixation and Sucrose Synthesis. Plant Physiol. 1984 Jul;75(3):548–553. doi: 10.1104/pp.75.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann G., Kelly G. J., Latzko E. Purification and properties of spinach leaf cytoplasmic fructose-1,6-bisphosphatase. J Biol Chem. 1978 Sep 10;253(17):5952–5956. [PubMed] [Google Scholar]


