Abstract
Regulatory T (Treg) cells play an essential role in maintaining immune balance across various physiological and pathological conditions. However, the mechanisms underlying Treg homeostasis remain incompletely understood. Here, we report that RIPK1 is crucial for Treg cell survival and homeostasis. We generated mice with Treg cell-specific ablation of Ripk1 and found that these mice developed fatal systemic autoimmunity due to a dramatic reduction in the Treg cell compartment caused by excessive cell death. Unlike conventional T cells, Treg cells with Ripk1 deficiency were only partially rescued from cell death by blocking FADD-dependent apoptosis. However, simultaneous removal of both Fadd and Ripk3 completely restored the homeostasis of Ripk1-deficient Treg cells by blocking two cell death pathways. Thus, our study highlights the critical role of RIPK1 in regulating Treg cell homeostasis by controlling both apoptosis and necroptosis, thereby providing novel insights into the mechanisms of Treg cell homeostasis.
Keywords: Treg cell, Homeostasis, Apoptosis, Necroptosis
Subject terms: Immune cell death, Cell death and immune response
Introduction
Regulatory T (Treg) cells, a subset of CD4+ T cells, are essential for maintaining immune homeostasis and self-tolerance [1]. While Treg cells are mainly generated in the thymus, they can also be induced from naive T cells in the periphery. In the steady state, Treg cells are a relatively stable population, and abnormalities in their homeostasis are often associated with autoimmune or immunodeficient diseases. To maintain this relative stability, Treg cells need to coordinate survival and death signals from cytokines, TCR/costimulatory signaling and anatomical location [2]. Similar to the role of IL-7 in conventional T-cell homeostasis, IL-2 plays a pivotal role in promoting Treg cell homeostasis by driving the upregulation of antiapoptotic proteins [3, 4]. However, not all Treg cells depend on IL-2 for homeostatic maintenance. For example, CD62LlowCD44high activated Treg (aTreg) cells rely on TCR signals and the costimulatory molecule ICOS to maintain their phenotypical identity and homeostasis [5, 6]. Sensing and responding to diverse survival or death signals, many intracellular factors are also involved in the maintenance of Treg population homeostasis. Some studies have shown that Bak- and Bax-mediated intrinsic apoptosis pathways help constrain the Treg population, but Mcl-1, upregulated by IL-2 signals, inhibits Bak- and Bax-mediated Treg cell apoptosis [3]. Compared to extrinsic signals, the intracellular factors that regulate Treg homeostasis remain poorly understood.
Receptor-interacting protein kinase 1 (RIPK1) plays a vital role in regulating apoptosis, necroptosis and inflammation, depending on the cellular context. Among these pathways, the TNFR1-mediated RIPK1 activation pathway has been extensively studied. Upon activation by TNF-α, TNFR1 recruits TNFR-associated death domain protein (TRADD), RIPK1, TRAF2, and cIAP1/2 to form complex I [7]. Within complex I, RIPK1 undergoes polyubiquitination, activating the pro-survival NF-kappa B (NF-κB) pathway. If complex I is disturbed, a new cytosolic complex IIa is formed by internalized TNFR1 and deubiquitinated RIPK1, resulting in cell apoptosis [8, 9]. In cases where apoptosis is inhibited, RIPK1 interacts with RIPK3 through the RIP homotypic interaction motif (RHIM) to form complex IIb, which facilitates the phosphorylation and oligomerization of mixed lineage kinase domain-like protein (MLKL), resulting in necroptosis [10, 11]. Consistent with these findings, genetic studies in mice have demonstrated the crucial role of RIPK1 in maintaining tissue homeostasis by regulating apoptosis, necroptosis, and inflammation. Specifically, Ripk1-deficient mice die at birth due to systemic inflammation, which can be prevented by inhibiting both FADD/Caspase8-dependent apoptosis and RIPK3/MLKL-dependent necroptosis [12]. Furthermore, studies on conditional Ripk1 knockout mice have shown that RIPK1 plays a critical role in regulating skin and intestinal inflammation, autoimmunity, and tissue fibrosis [13–15].
In addition, recent studies have identified individuals with biallelic loss-of-function mutations in the RIPK1 gene who displayed a range of clinical manifestations with lymphopenia [16, 17]. Consistently, mice with targeted deletion of RIPK1 in T cells also showed severe T lymphopenia [18]. These findings strongly indicate the importance of RIPK1 in T-cell function and highlight its relevance in the immune system. However, considering that Treg cells have unique homeostatic mechanisms distinct from those found in conventional T cells, there is still great interest in investigating whether RIPK1 plays a role in Treg cell homeostasis and function. In this study, we generated mice with specific Ripk1 ablation in Treg cells and found that these mice developed fatal systemic autoimmunity due to a significant reduction in the Treg cell compartment caused by excessive cell death. Unlike conventional T cells, which undergo FADD-dependent apoptosis when RIPK1 is absent, Treg cells with Ripk1 deficiency exhibit only partial rescue with Fadd deletion. Rather, we discovered that simultaneous inhibition of both FADD-dependent apoptosis and RIPK3-dependent necroptosis fully rescued Ripk1-deficient Treg cells from excessive death. These results highlight the critical role of RIPK1 in regulating Treg cell survival by controlling both apoptosis and necroptosis, providing new insights into the mechanisms underlying Treg cell homeostasis.
Results
RIPK1 is upregulated in activated Treg cells
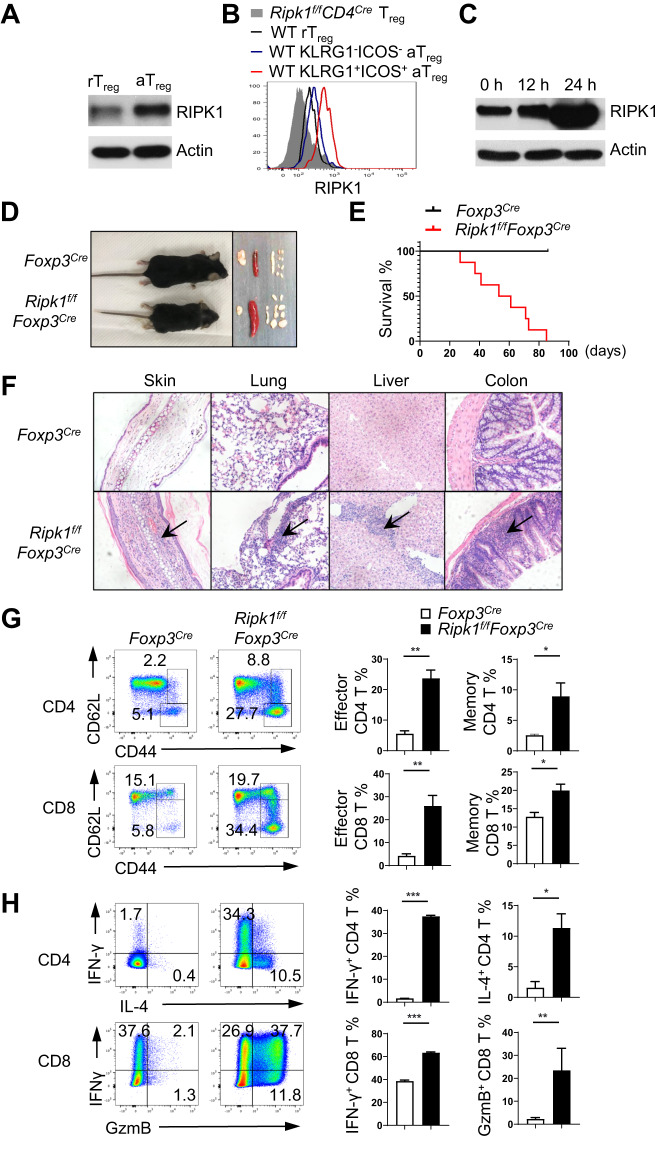
To gain a better understanding of the role of RIPK1 in Treg cells, we first examined its expression in these cells. Based on the expression levels of the cell surface markers CD44 and CD62L, Treg cells can be divided into two populations: a resting Treg cell population (rTreg) characterized by CD44lowCD62Lhigh and an activated Treg cell population (aTreg) characterized by CD44highCD62Llow [19, 20]. Both immunoblotting and intracellular staining results revealed that aTreg cells expressed higher levels of RIPK1 than quiescent rTreg cells (Fig. 1A, B). Interestingly, effector Treg cells, identified as KLRG1+ICOS+ aTreg cells, exhibited the highest level of RIPK1 among all Treg cells (Fig. 1B). TCR signals are important for the phenotypical identity of aTreg cells [5]. The high expression of RIPK1 in aTreg cells led us to suspect that it may be regulated by TCR signals. We observed that TCR stimulation in vitro for 24 h significantly increased the protein level of RIPK1 in Treg cells (Fig. 1C). Notably, IL-2 stimulation did not lead to an upregulation of RIPK1 expression in Treg cells (Supplementary Fig. 1A). These results suggest that RIPK1 is differentially expressed in Treg cells under different activation states and may have varying roles in different populations of Treg cells.
Fig. 1.
Treg cell-specific deletion of RIPK1 leads to fatal systemic autoimmunity. A Immunoblot analysis of RIPK1 expression in sorted YFP+CD4+CD44lowCD62Lhigh Treg cells (rTreg cells) and YFP+CD4+CD44highCD62Llow Treg cells (aTreg cells) from Foxp3Cre mice. B Intracellular staining of RIPK1 expression in rTreg cells, KLRG1lowICOSlow aTreg and KLRG1highICOShigh aTreg cells from wild-type (WT) mice and Treg cells from Ripk1f/fCD4Cre mice. C Immunoblotting of RIPK1 in sorted YFP+ WT Treg cells stimulated with plate-bound anti-CD3/CD28 for the indicated time points. D Representative images of 6-week-old Foxp3Cre and Ripk1f/fFoxp3Cre mice and the lymph organs isolated from these mice. E Survival curve of Foxp3Cre and Ripk1f/fFoxp3Cre mice at the indicated time points (n = 10). F Hematoxylin and eosin staining of the indicated organs from Foxp3Cre and Ripk1f/fFoxp3Cre mice. Arrows indicate the areas of immune cell infiltration. The magnification is 100×. G Flow cytometry analysis of CD44 and CD62L expression on T cells in the lymph nodes of 6-week-old Foxp3Cre and Ripk1f/fFoxp3Cre mice. Numbers adjacent to the outlined areas indicate the percentages of CD44highCD62Llow activated T cells. H Flow cytometry analysis of IFN-γ and IL-4 expression in CD4+ T cells and IFN-γ and granzyme B (GzmB) expression in CD8+ T cells in the lymph nodes of 6-week-old Foxp3Cre and Ripk1f/fFoxp3Cre mice after 6-h stimulation with PMA plus ionomycin. Numbers in quadrants indicate the percentage of cells in each respective quadrant. Data in (A–C) are representative of at least two independent experiments. Data in (D, F–H) are representative of at least three independent experiments
Treg cell-specific ablation of Ripk1 results in fatal systemic autoimmunity
To investigate the role of RIPK1 in Treg cells, we crossed mice with loxP-flanked Ripk1 alleles (Ripk1f/f) with Foxp3YFP-Cre knock-in mice (Foxp3Cre) to delete RIPK1 specifically in Treg cells (Ripk1f/fFoxp3Cre) (Supplementary Fig. 1B). The Ripk1f/fFoxp3Cre mice showed stunted growth and a markedly reduced lifespan, typically surviving only 3–12 weeks (Fig. 1D, E). These mice also manifested significant splenomegaly and lymphadenopathy (Fig. 1D). Histological analysis revealed extensive leukocyte infiltration in the skin, lung, liver, and colon of Ripk1f/fFoxp3Cre mice (Fig. 1F). Flow cytometry analysis consistently showed a substantially higher percentage of Gr-1+ myeloid cells in the lymph nodes of these mice (Supplementary Fig. 1C). Furthermore, there was a significant increase in CD44highCD62Lhigh memory or CD44highCD62Llow effector T cells in these mice, which produced increased levels of effector cytokines such as IFN-γ, IL-4 and IL-17A in CD4+ T cells, as well as IFN-γ and granzyme B in CD8+ T cells (Fig. 1G, H, Supplementary Fig. 1D). These phenotypes indicate severe defects in Treg cells resulting from Ripk1 deletion.
RIPK1 is crucial for maintaining peripheral Treg cell homeostasis
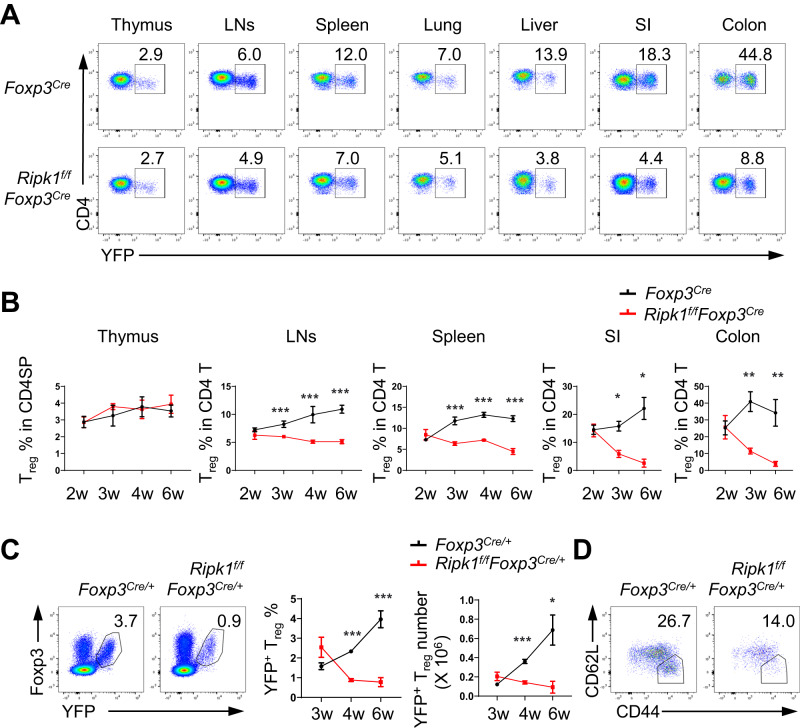
We proceeded to analyze the frequencies of Treg cells in the thymus, as well as various lymphoid and nonlymphoid organs, in both Ripk1f/fFoxp3Cre mice and control Foxp3Cre mice aged 2 to 6 weeks. Our findings revealed a significant reduction in the percentages of Treg cells in the spleens, lymph nodes, lungs, livers, small intestines and colons of Ripk1f/fFoxp3Cre mice (Fig. 2A, B). Interestingly, these decreases in Treg cell percentages were already observed in Ripk1f/fFoxp3Cre mice as early as 3 weeks of age (Fig. 2A, B). It is worth noting that these reductions in Treg cells were not attributed to developmental defects since the frequencies of Treg cells in the thymi of wild-type mice were comparable to those in the thymi of Ripk1f/fFoxp3Cre or Ripk1f/fCD4Cre mice (Fig. 2A, B, Supplementary Fig. 2A, B). These results strongly suggest that RIPK1 serves as a crucial regulator of Treg cell homeostasis in the periphery.
Fig. 2.
RIPK1 is critical for the maintenance of peripheral Treg homeostasis. A Flow cytometry analysis of YFP+ Treg cells in CD4SP thymocytes in the thymus or in CD4+ T cells in the lymph nodes (LNs), spleen, lungs, liver, small intestine and colon of 3-week-old Foxp3Cre and Ripk1f/fFoxp3Cre mice. Numbers adjacent to the outlined areas indicate the percentages of YFP+ Treg cells. B Kinetics of the frequencies of YFP+ Treg cells in the indicated organs from Foxp3Cre and Ripk1f/fFoxp3Cre mice at the indicated ages (n = 3–5). C Flow cytometry analysis of YFP+Foxp3+ Treg cells in the lymph nodes of female Foxp3Cre/+ and Ripk1f/fFoxp3Cre/+ mice. The left panel shows a representative flow cytometry plot highlighting YFP+Foxp3+ Treg cells in CD4+ T cells in the lymph nodes of 6-week-old female Foxp3Cre/+ and Ripk1f/fFoxp3Cre/+ mice, with numbers adjacent to the outlined areas indicating the percentages of YFP+Foxp3+ Treg cells. The right panel summarizes the frequencies and numbers of YFP+Foxp3+ Treg cells in the lymph nodes of female Foxp3Cre/+ and Ripk1f/fFoxp3Cre/+ mice at the indicated ages (n = 4). D Flow cytometry analysis of CD44highCD62Llow activated Treg (aTreg) cells among the YFP+ Treg cells in the lymph nodes of 6-week-old female Foxp3Cre/+ and Ripk1f/fFoxp3Cre/+ mice. The numbers adjacent to the outlined areas indicate the percentages of aTreg cells. Data in (A–D) are representative of at least two independent experiments. Data in (B, C: right panel) are mean ± s.e.m., ns not significant, *p < 0.05, **p < 0.01, ***p < 0.001 (two-tailed Student’s t test)
To investigate whether RIPK1 intrinsically regulates Treg cell homeostasis, we analyzed Treg cells in female Ripk1f/fFoxp3Cre/+ and Foxp3Cre/+ mice. In female Ripk1f/fFoxp3Cre/+ mice, a portion of Treg cells retained RIPK1 due to random X-chromosome inactivation, which prevented the development of autoimmune diseases. Our analysis revealed a gradual decrease in RIPK1-deficient YFP+ Treg cells starting at 4 weeks of age in female Ripk1f/fFoxp3Cre/+ mice compared to their counterparts in female Foxp3Cre/+ mice (Fig. 2C). Interestingly, the loss of CD44highCD62Llow aTreg cells was more pronounced in female Ripk1f/fFoxp3Cre/+ mice than the loss of CD44lowCD62Lhigh rTreg cells (Fig. 2D), which aligns with the higher RIPK1 protein expression in aTreg cells. Thus, our findings suggest that RIPK1 plays a crucial role in regulating Treg cell homeostasis in the periphery through an intrinsic cellular mechanism.
Treg cells lacking RIPK1 are more susceptible to cell death
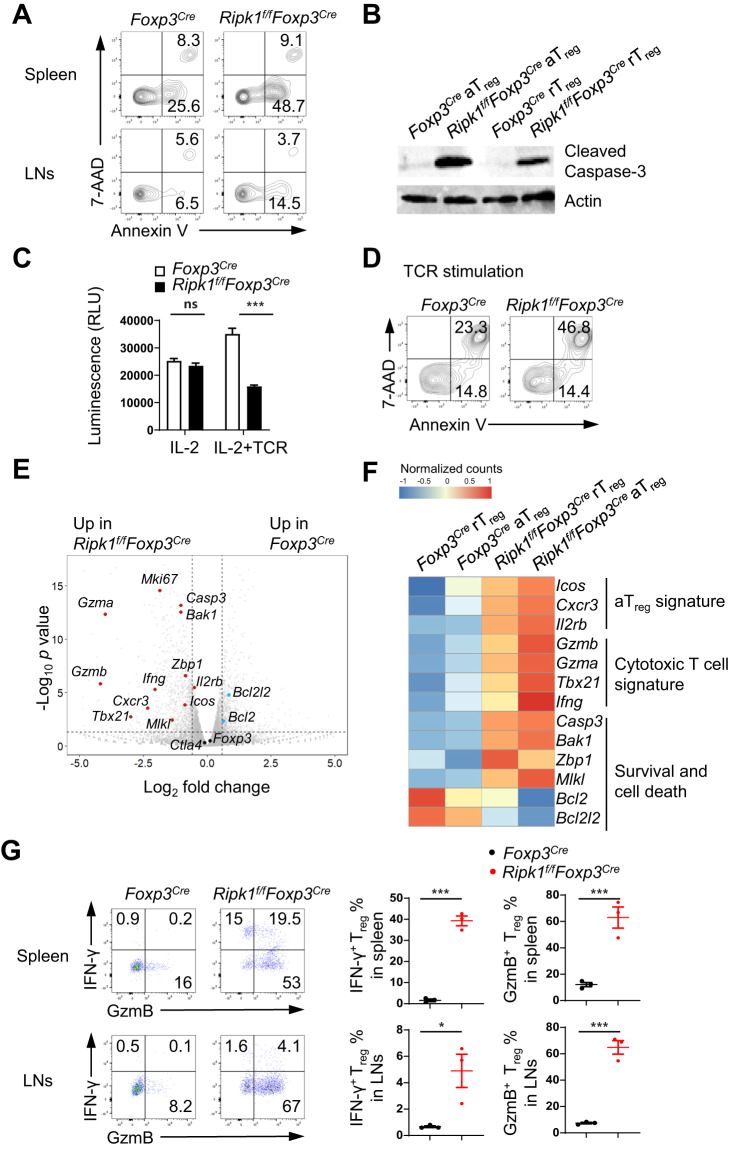
RIPK1 plays a dual role in regulating cell survival and death under different conditions. To investigate the primary cause of Treg cell reduction in Ripk1f/fFoxp3Cre mice, we first examined cell death in Treg cells, and we found that ex vivo RIPK1-deficient Treg cells exhibited a higher rate of apoptotic cell death, as indicated by the Annexin V+7-AAD− cell population (Fig. 3A). Similarly, both rTreg and aTreg cells from Ripk1f/fFoxp3Cre mice expressed elevated levels of cleaved caspase-3 protein compared with their wild-type counterparts (Fig. 3B, Supplementary Fig. 3A), indicating that RIPK1-deficient Treg cells are more susceptible to apoptosis. Although IL-2 and TCR signals are essential for Treg cell homeostasis, we observed no significant differences in cell death between RIPK1-sufficient and RIPK1-deficient cells when treated with IL-2 (Fig. 3C, Supplementary Fig. 3B). This suggests that RIPK1 may not be needed for IL-2-mediated Treg cell survival. However, TCR stimulation resulted in significantly increased cell death in RIPK1-deficient cells (Fig. 3C, D, Supplementary Fig. 3B), indicating that RIPK1 potentially offers crucial protection against activation-induced Treg cell death. Notably, we found no obvious defects in the proliferation of RIPK1-deficient Treg cells through Ki-67 protein staining and CellTrace dilution analysis (Supplementary Fig. 3C, D).
Fig. 3.
RIPK1-deficient Treg cells have a higher susceptibility to cell death. A Flow cytometry analysis of apoptotic and dead YFP+ Treg cells from 6-week-old Foxp3Cre and Ripk1f/fFoxp3Cre mice using Annexin V and 7-AAD staining. B Immunoblotting of cleaved Caspase-3 protein in sorted aTreg and rTreg cells from 3-week-old Foxp3Cre and Ripk1f/fFoxp3Cre mice. C Cell viability analysis of sorted Treg cells from Foxp3Cre and Ripk1f/fFoxp3Cre mice after stimulation with IL-2 (500 U/mL) or plate-bound anti-CD3/CD28 plus IL-2 for 48 h (n = 4). D Flow cytometry analysis of apoptotic and dead Treg cells sorted from Foxp3Cre and Ripk1f/fFoxp3Cre mice after stimulation with plate-bound anti-CD3/CD28 for 6 h using Annexin V and 7-AAD staining. E Volcano plot of RNA-seq data, with representative survival, activation/cytotoxic and Treg signature genes shown. The horizontal dotted line indicates a p value of 0.05, and the vertical dotted lines indicate a fold change of 1.5. F Heatmap analysis of representative gene expression in Foxp3Cre and Ripk1f/fFoxp3Cre Treg cells. G Flow cytometry analysis of IFN-γ+ or GzmB+ Treg cells in the spleen and LNs of 6-week-old Foxp3Cre and Ripk1f/fFoxp3Cre mice. The left panel shows representative flow cytometry plots of IFN-γ+ and GzmB+ Treg cells in the spleen and LNs. Numbers in quadrants indicate the percentage of cells in each respective quadrant. The right panel summarizes the percentages of IFN-γ+ and GzmB+ Treg cells in the spleen or LNs (n = 3). Data in (A–G) are representative of at least two independent experiments. Data in (C) are mean ± s.e.m., ns not significant, *p < 0.05, and ***p < 0.001 (two-tailed Student’s t test)
To gain further insights into the regulation of Treg cell homeostasis and functions by RIPK1, we performed a comprehensive analysis of the gene expression profiles of rTreg and aTreg cells from Ripk1f/fFoxp3Cre and wild-type mice using RNA-Seq and flow cytometry analysis. Our findings showed that the expression levels of Treg cell core signature genes and proteins such as Foxp3, Ctla4, Il10 and Tgfb1 in Ripk1f/fFoxp3Cre Treg cells were not lower than those in wild-type Treg cells (Fig. 3E, Supplementary Fig. 4A–D), indicating that the loss of RIPK1 does not disrupt Treg cell development. However, we observed that Ripk1f/fFoxp3Cre Treg cells exhibited some phenotypic and transcriptomic features of cytotoxic T cells. Specifically, the expression levels of Tbx21, Cxcr3, Ifng, Gzma and Gzmb were substantially higher in Ripk1f/fFoxp3Cre Treg cells than in wild-type Treg cells (Fig. 3E–G), suggesting that RIPK1 may play a critical role in maintaining Treg cell identity and function. As expected, Ripk1f/fFoxp3Cre Treg cells showed reduced expression levels of antiapoptotic genes, such as Bcl2 and Bcl2l2, while the levels of proapoptotic genes, such as Bak1 and Casp3, were elevated (Fig. 3E, F). However, we found no significant changes or only slight increases in the expression levels of apoptosis-related and necroptosis-related receptors such as TNFR1, CD95, and PD-1 (Supplementary Fig. 4A, B). This suggests that RIPK1 promotes Treg cell survival independent of the regulation of these cell death-related receptors. Interestingly, we also observed elevated expression levels of Mlkl and Zbp1, key molecules involved in the necroptosis pathway, in Ripk1f/fFoxp3Cre Treg cells (Fig. 3E, F). This suggests that, in addition to apoptosis, RIPK1 may also participate in regulating necroptosis in Treg cells.
Ablation of Fadd partially rescues RIPK1-deficient Treg cells from cell death
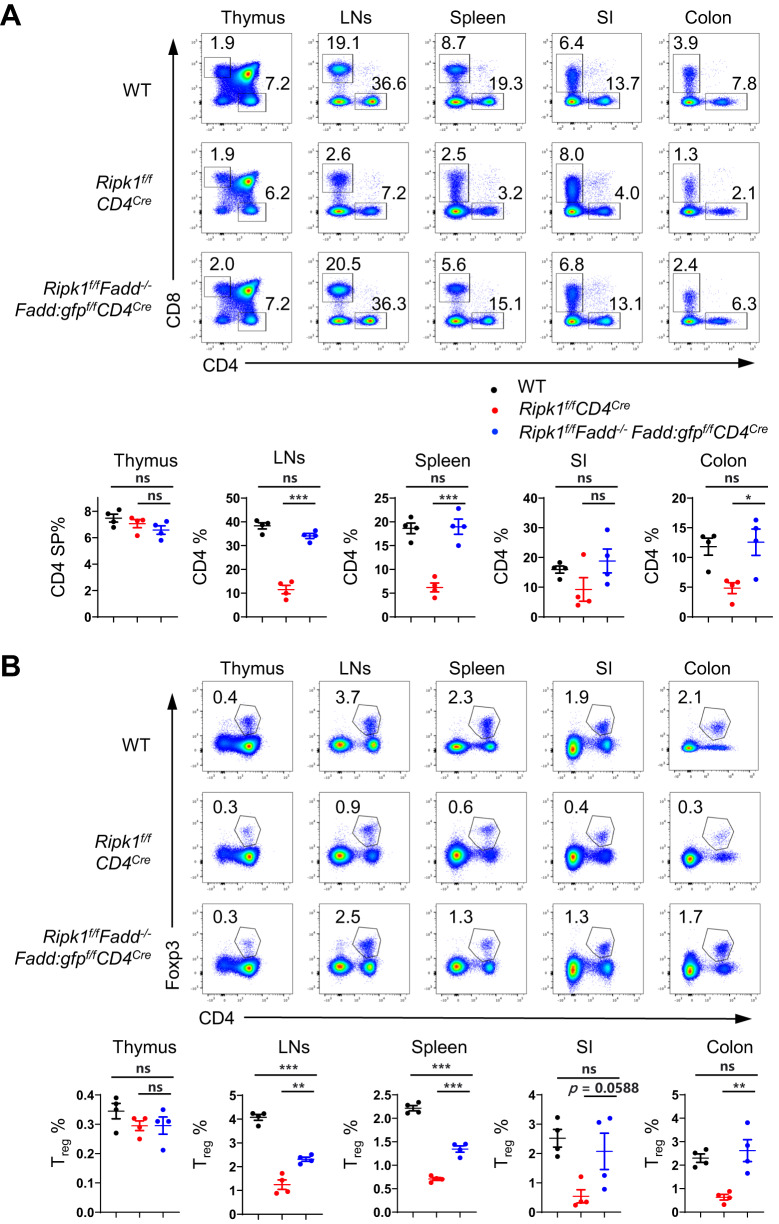
RIPK1 participates in multiple signaling pathways, including FADD/Caspase8-dependent apoptosis and RIPK3/MLKL-dependent necroptosis. To investigate the involvement of the FADD/Caspase8 apoptotic pathway in the functions of RIPK1 in Treg cells, we utilized Ripk1f/fFadd−/−Fadd:gfpf/fCD4Cre mice to evaluate the elimination of Fadd in RIPK1-deficient T cells (Supplementary Fig. 5). Previous studies have reported that RIPK1 is essential for maintaining homeostasis in conventional T cells by inhibiting apoptosis [18]. Consistent with these findings, we observed a significant reduction in CD4+ and CD8+ T cells in Ripk1f /fCD4Cre mice. Under lymphopenic conditions, there was an increase in the proportion of activated CD8+ T cells but not activated CD4+ T cells in the lymph nodes of Ripk1f/ fCD4Cre mice (Supplementary Fig. 6A). Despite the increase in activated CD8+ T cells, there were no evident signs of autoimmune diseases in young Ripk1f/fCD4Cre mice (Supplementary Fig. 6B). This might be attributed to the overall reduction in effector T cells, particularly CD4+ effector T cells, resulting from the deficiency of RIPK1. As expected, we observed that both CD4+ and CD8+ T cells in various organs of Ripk1f/fFadd−/−Fadd:gfpf/fCD4Cre mice were restored to the levels in wild-type mice (Fig. 4A). Furthermore, we observed that the proportions of naive (CD44lowCD62Lhigh) and activated (CD44highCD62Llow) T-cell subsets in Ripk1f/fFadd−/−Fadd:gfpf/fCD4Cre mice were similar to those in wild-type CD4+ and CD8+ T cells, respectively (Supplementary Fig. 6A). These findings indicate that the RIPK1-FADD pathway is vital for maintaining the homeostasis of conventional T cells.
Fig. 4.
Ablation of Fadd partially rescues RIPK1-deficient Treg cells from cell death. A Flow cytometry analysis of CD4SP and CD8SP thymocytes in the thymus and CD4+ and CD8+ T cells in the lymph nodes (LNs), spleen, small intestine (SI), and colon from 6-week-old WT, Ripk1f/fCD4Cre and Ripk1f/fFadd-/-Fadd:gfpf/fCD4Cre mice (upper panel); numbers adjacent to the outlined areas indicate the percentages of CD4SP and CD8SP in the thymus and CD4+ T and CD8+ T cells in the LNs, spleen, SI, and colon. The percentages of CD4SP or CD4+ T cells are summarized (n = 4, lower panel). B Flow cytometry analysis of Foxp3+ Treg cells in the thymus, LNs, spleen, SI and colon from 6-week-old WT, Ripk1f/fCD4Cre and Ripk1f/fFadd-/-Fadd:gfpf/fCD4Cre mice (upper panel). Numbers adjacent to the outlined areas indicate the percentages of Foxp3+ Treg cells in the thymus, LNs, spleen, SI and colon. The percentages of Foxp3+ Treg cells are summarized (n = 4, lower panel). Data in (A, B) are representative of at least two independent experiments. Data in (A: lower panel, B: lower panel) are mean ± s.e.m., ns, not significant, *p < 0.05, **p < 0.01, ***p < 0.001 (two-tailed Student’s t test)
Surprisingly, unlike conventional T cells, Treg cells were only partially restored in the spleens and lymph nodes in Ripk1f/fFadd−/−Fadd:gfpf/fCD4Cre mice, although Treg cells in the gut were rescued to wild-type levels (Fig. 4B). As the absence of RIPK1 and FADD in conventional T cells potentially impacts Treg homeostasis in these mice, we conducted cotransfer experiments to confirm whether the reduction in Treg cell number was intrinsic to Treg cells themselves. We cotransferred Treg cells from Ripk1f/fFadd−/−Fadd:gfpf/fCD4Cre mice and CD45.1+ wild-type Treg cells into CD45.1+CD45.2+ wild-type recipient mice and analyzed their proportions in recipient mice on Day 7 and Day 14 after transfer. During these time points, we observed a gradual decrease in the proportion of Treg cells from Ripk1f/fFadd−/−Fadd:gfpf/fCD4Cre mice (Supplementary Fig. 6C), suggesting that Treg cells deficient in RIPK1 and FADD do have inherent survival issues. Notably, despite only partial restoration of Treg cells in Ripk1f/fFadd−/−Fadd:gfpf/fCD4Cre mice, the extent of Treg cell restoration was sufficient to prevent autoimmune diseases (Supplementary Fig. 6B). Taken together, our findings demonstrate the critical role of the RIPK1-FADD pathway in maintaining Treg cell homeostasis and function, with other RIPK1-mediated pathways also being involved.
Pro-apoptotic BH3-only proteins have previously been reported to play a role in the regulation of Treg cell apoptosis [3]. Therefore, we proceeded to investigate whether Bim, one such protein, contributes to the cell death observed in RIPK1-deficient Treg cells using Bcl2l11−/−Ripk1f/fFoxp3Cre mice. We found that Bim ablation did not facilitate the restoration of the Treg population (Supplementary Fig. 7), which suggests that Bim may not be directly involved in the cell death of RIPK1-deficient Treg cells.
We subsequently investigated whether blocking RIPK3/MLKL can facilitate the restoration of the Treg population by utilizing Ripk3−/−Ripk1f/fFoxp3Cre mice, which have both Ripk3 and Ripk1 deletion in Treg cells. Compared to Ripk1f/fFoxp3Cre mice, Ripk3−/−Ripk1f/fFoxp3Cre mice displayed a significant reduction in the percentages of Treg cells in the spleen and lymph nodes (Supplementary Fig. 8A). As anticipated, Ripk3−/−Ripk1f/fFoxp3Cre mice showed severe inflammatory symptoms characterized by the activation of conventional CD4+ T cells and CD8+ T cells, as well as lymphocyte infiltration in various organs (Supplementary Fig. 8B, C). These findings suggest that solely blocking necroptosis is insufficient to rescue Treg cells from the death caused by RIPK1 deficiency.
Deletion of Fadd and Ripk3 completely rescues cell death in RIPK1-deficient Treg cells
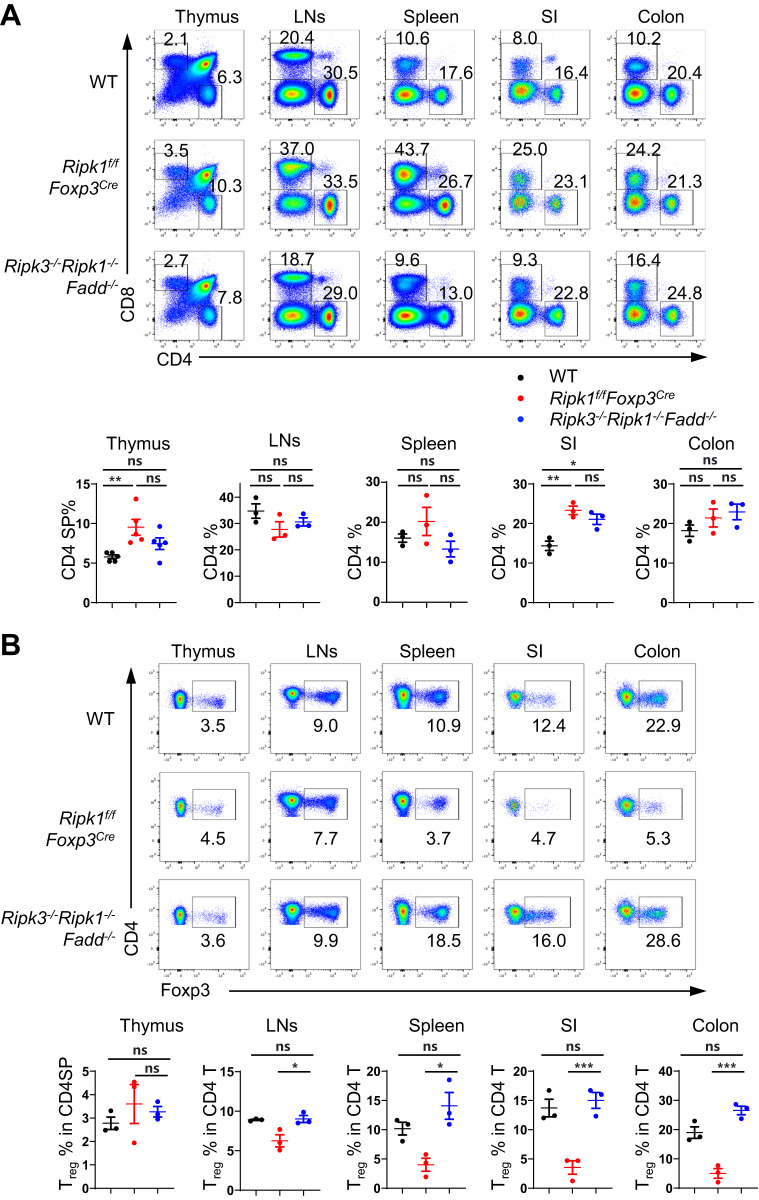
Since blocking either the Fadd pathway or the Ripk3 pathway alone cannot fully rescue Treg cells from the death caused by RIPK1 deficiency, we hypothesized that RIPK1 deficiency may lead to both necroptosis and apoptosis in Treg cells. To investigate this hypothesis, we utilized Ripk3−/−Ripk1−/−Fadd−/− mice, which lack both FADD-mediated apoptosis and RIPK3-mediated necroptosis [12]. As expected, these mice exhibited normal conventional CD4+ and CD8+ T compartments (Fig. 5A), similar to Ripk1 and Fadd double knockout mice. However, unlike Ripk1 and Fadd double knockout mice, Ripk3−/−Ripk1−/−Fadd−/− mice showed normal percentages of Treg cells in all examined organs, including the thymus, spleen, lymph nodes, small intestine and colon (Fig. 5B). Additionally, we observed that Treg cells from Ripk3−/−Ripk1−/−Fadd−/− mice recovered from cell death under in vitro TCR stimulation (Supplementary Fig. 9A) and exhibited normal suppressive function in vitro compared to wild-type Treg cells (Supplementary Fig. 9B). Moreover, no autoimmune diseases were detected in these mice (Supplementary Fig. 9C, D). These findings strongly suggest that the removal of Fadd and Ripk3 greatly improved the cell survival and functions of RIPK1-deficient Treg cells. Since triple knockout mice are not conditional knockout mice, we further analyzed Treg cells in chimeric mice reconstituted with Ripk3−/−Ripk1−/−Fadd−/− bone marrow to validate our observations in a more controlled model. Compared to wild-type bone marrow chimeras, Ripk3−/−Ripk1−/−Fadd−/− bone marrow chimeras also exhibited normal Treg cell percentages and numbers (Supplementary Fig. 10). Together, these results demonstrate that RIPK1 functions to inhibit both FADD-mediated apoptosis and RIPK3-mediated necroptosis in Treg cells.
Fig. 5.
Deletion of Fadd and Ripk3 completely rescues cell death in RIPK1-deficient Treg cells. A Flow cytometry analysis of CD4SP and CD8SP thymocytes in the thymus and CD4+ and CD8+ T cells in the lymph nodes (LNs), spleen, small intestine (SI), and colon from 6-week-old WT, Ripk1f/fFoxp3Cre and Ripk3-/-Ripk1-/-Fadd-/- mice (upper panel); numbers adjacent to the outlined areas indicate the percentages of CD4SP and CD8SP in the thymus and CD4+ T and CD8+ T cells in the LNs, spleen, SI, and colon. The percentages of CD4SP or CD4+ T cells are summarized in the lower panel (n = 5 in thymus; n = 3 in LNs, spleen, SI, colon). B Flow cytometry analysis of Foxp3+ Treg cells in CD4SP cells in the thymus and CD4+ T cells in the LNs, spleen, SI and colon from 6-week-old WT, Ripk1f/fFoxp3Cre and Ripk3-/-Ripk1-/-Fadd-/- mice (upper panel). Numbers adjacent to the outlined areas indicate the percentages of Foxp3+ Treg cells in CD4SP in the thymus and CD4+ T cells in the LNs, spleen, SI and colon. The percentages of Foxp3+ Treg cells are summarized (n = 3, lower panel). Data in (A, B) are representative of at least two independent experiments. Data in (A: lower panel, B: lower panel) are mean ± s.e.m., ns not significant, *p < 0.05, **p < 0.01, and ***p < 0.001 (two-tailed Student’s t test)
Discussion
T-cell homeostasis, achieved by a well-orchestrated balance of T-cell survival and death, is crucial for a functional immune system. Receptor-interacting protein kinase 1 (RIPK1) has been identified as a master regulator of apoptosis, necroptosis and inflammation, depending on cell type and context [14, 21–23]. Although RIPK1 is not essential for T-cell development in the thymus, it is crucial for conventional T-cell homeostasis because it inhibits apoptosis [18]. In our study, we observed an increase in the percentage of CD4SP thymocytes in 6-week-old Ripk1f/fFoxp3Cre mice compared to WT mice (Fig. 5A), which is likely due to a secondary effect of the severe autoimmune diseases present in Ripk1f/fFoxp3Cre mice. As a special subset of CD4+ T cells, Treg cells have quite different homeostasis mechanisms from conventional CD4+ T cells. It remains unclear whether RIPK1 has similar functions in Treg cell homeostasis. In this study, we found that Treg cell-specific ablation of Ripk1 leads to fatal systemic autoimmunity due to excessive Treg cell death. Unlike conventional T cells, Treg cells with RIPK1 deficiency are only partially rescued by Fadd deletion. However, the deletion of both Fadd and Ripk3 completely rescued Treg cells from death in Ripk1-deficient mice. Our results demonstrate that RIPK1 regulates Treg cell survival by controlling both necroptosis and apoptosis.
Mature T cells, including Treg cells, rely on TCR signaling to maintain their homeostasis. However, the mechanisms by which TCR regulates T-cell homeostasis remain to be elucidated. Our study reveals that TCR stimulation can induce the upregulation of RIPK1, which plays a vital role in Treg cell survival upon TCR stimulation. This indicates that RIPK1 function is essential for TCR-mediated T-cell homeostasis. Although there is currently no direct evidence implicating RIPK1 in TCR signal transduction, it has been demonstrated that MALT1, downstream of TCR signals, regulates the activation of caspase-8, which can form a complex with RIPK1 and c-FLIPs [24, 25]. Therefore, it is plausible that RIPK1 promotes Treg cell survival through its interaction with MALT1 and caspase-8, which warrants further investigation.
The RIPK1 protein comprises a kinase domain in its N-terminus, an RHIM domain, and a death domain in its C-terminus, each with distinct functional sites where posttranslational modifications such as ubiquitination and phosphorylation can occur [26]. Recent studies have demonstrated the critical role of RIPK1 ubiquitination at K376 in both apoptosis and necroptosis in vitro and in vivo [27–29]. However, in Ripk1f/K376RCD4Cre mice, we did not observe any abnormalities in Treg cell development or homeostasis (Supplementary Fig. 11). This suggests that while RIPK1 ubiquitination on the K376-mediated pathway is essential for suppressing cell death during embryogenesis and postnatal inflammation, it is not required for Treg cell homeostasis. Additionally, research has shown that phosphorylated RIPK1 can translocate to the nucleus, where it regulates chromatin remodeling and transcriptional control of inflammatory factors [30]. Therefore, in Treg cell homeostasis and during Treg activation, RIPK1 may serve not only as a vital protein for cell survival but also as a regulator of relevant gene expression. Our study revealed alterations in the expression levels of multiple genes in response to RIPK1 deficiency. Further investigation is necessary to determine whether these genes are directly regulated by phosphorylated RIPK1.
Treg cells are not a homogenous population but instead display some degree of heterogeneity in their phenotypes, functions, and lineage origins [4]. Our study has revealed intriguing insights into the differential roles played by RIPK1 in various subpopulations of Treg cells. Specifically, we found that RIPK1 deficiency leads to greater loss of activated Treg (aTreg) cells, suggesting that aTreg cells rely more on RIPK1 to maintain homeostasis than resting Treg (rTreg) cells. Within the gut mucosa, Treg cells include a significant proportion of peripherally induced Treg (pTreg) cells that are derived from conventional CD4+ T cells [31, 32]. Strikingly, our research demonstrated that under steady-state conditions, RIPK1-deficient Treg cells in the gut can be fully rescued through the deletion of the Fadd gene, whereas the same restorative effect is not observed in their counterparts within secondary lymphoid organs. These findings imply that while RIPK1 may have similar functions in pTreg cells and conventional CD4+ T cells, it exerts distinct effects on thymus-derived Treg (tTreg) cells.
Studies have provided evidence that the responsiveness of tumor-infiltrating Treg cells to microenvironmental signals can disrupt their stability and survival, thereby influencing the outcome of antitumor immune responses [33]. Our findings suggest that under steady-state conditions, the primary function of RIPK1 is to suppress Treg cell death and maintain self-tolerance. However, it remains to be determined whether manipulating RIPK1 expression in Treg cells within the tumor microenvironment or other pathological contexts could be leveraged as a strategic approach to modulate disease progression. Further investigations are warranted to shed light on this important aspect.
In summary, our study highlights the critical role of RIPK1 in preserving Treg cell homeostasis by restraining both RIPK3- and FADD-mediated cell death pathways. These findings provide fresh insights into the underlying mechanisms that govern the balance and stability of Treg cells.
Materials and methods
Mice
The Ripk1f/f mice were generated by a homologous recombination strategy (Shanghai Model Organisms Center, Inc.). In brief, hybrid mouse embryonic stem (ES) cells were electroporated with a targeting vector containing floxed exon 3. The Ripk3−/− mice were provided by Dr. Xiaodong Wang (National Institute of Biological Sciences, Beijing, China), and the Fadd−/−Fadd:gfpf/f mice were provided by Dr. Jianke Zhang (Thomas Jefferson University, Philadelphia, PA, USA), as previously described [34]. CD4Cre and Foxp3YFP-Cre (Foxp3Cre) mice were provided by Dr. Bin Li (Shanghai Institute of Immunology, Shanghai Jiao Tong University, Shanghai, China). Ripk1f/fCD4Cre and Ripk1f/fFoxp3Cre mice were generated by crossing Ripk1f/f mice with CD4cre or Foxp3Cre mice, respectively. Ripk1f/fFadd−/−Fadd:gfpf/fCD4Cre mice were obtained by breeding Fadd−/−Fadd:gfpf/f mice with Ripk1f/fCD4Cre mice. The CD45.1+ mice were provided by Dr. Qibin Leng (Institut Pasteur of Shanghai). All mice were maintained under specific pathogen-free conditions at the Institut Pasteur of Shanghai or Tongji University in China. All animal experiments were performed following the protocols approved by the Institutional Animal Care and Use Committee of the Institut Pasteur of Shanghai or Shanghai East Hospital, Tongji University.
Flow cytometry and cell sorting
For surface marker staining, cells were washed with staining buffer (PBS containing 1% FBS and 1 mM EDTA). To block nonspecific binding, anti-CD16/CD32 antibodies (2.4G2) were added, followed by a 30-minute incubation with surface antibodies on ice. The antibodies used for FACS analysis included APC-Cy7-anti-CD4 (GK1.5, Thermo Fisher Scientific), PE-Cy7-anti-CD4 (GK1.5, Thermo Fisher Scientific), PE-Cy7-anti-CD8 (53–6.7, Thermo Fisher Scientific), PerCP-Cy5.5-anti-CD8 (53–6.7, BioLegend), PE-anti-CD44 (IM7, Thermo Fisher Scientific), APC-anti-CD62L (MEL-14, Thermo Fisher Scientific), PE-Cy7-anti-CD25 (PC61.5, Thermo Fisher Scientific), PE-anti-TNFR1 (55R-286, BioLegend), PE-Cy7-anti-PD-1 (29 F.1A12, BioLegend), and AF647-anti-FAS (Jo2, BD Bioscience). Dead cells were excluded using a Live/Dead Fixable Aqua Dead Cell staining kit (Thermo Fisher Scientific). Samples were acquired using an LSRFortessa flow cytometer (BD PharMingen) and analyzed by FlowJo software (TreeStar).
For cytokine staining, cells were stimulated with 10 ng/mL PMA (phorbol 12-myristate 13-acetate, Sigma, St. Louis, MO) and 1 μg/mL ionomycin (Sigma) for 4 h. After treatment with 10 μg/mL brefeldin A (Sigma) for another 2 h, surface markers were stained, and cells were fixed with 3.7% formaldehyde for 15 min at room temperature. Subsequently, the cells were permeabilized with 0.2% saponin in PBS on ice for 20 min before intracellular staining. Samples were acquired using an LSRFortessa flow cytometer (BD PharMingen) and analyzed by FlowJo software (TreeStar).
To sort Tconv and Treg cells, CD4+ T cells were enriched by negative selection using biotin-labeled CD8a, TER-119 and B220 antibodies and streptavidin magnetic beads (Magnet sort, Thermo). CD44lowCD62Lhigh naive CD4+ T cells or CD4+YFP+ Treg cells were sorted by a FACS Aria II cell sorter (BD Bioscience). The sorted cell populations were >98% pure.
Isolation of tissue-resident lymphocytes
To isolate lamina propria (LP) mononuclear cells, small intestines and colons were dissected, and fat tissues and Peyer’s patches were removed. Predigestion was performed using PBS containing 1 mM DTT (Sigma) and 30 mM EDTA (AMResco) at 37 °C for 30 min. Then, the pretreated tissues were digested with Liberase (250 μg/mL, Roche) and DNase I (200 μg/mL, Sigma) in DMEM supplemented with 5% FBS at 37 °C for 30 min in a shaker. The digested tissues were then meshed in a 70 μm cell strainer and subjected to Percoll gradient (40%/80%) centrifugation. Mononuclear cells present in the interphase were collected, washed with PBS and resuspended in T-cell medium.
Construction of bone marrow chimeras
To generate bone marrow chimeras, bone marrow cells from either wild-type or Ripk3−/−Ripk1−/−Fadd−/− mice were transferred to 6- to 8-week-old TCRβ−/− recipient mice that had been irradiated with 450 rads. After 2 weeks of neomycin treatment, the chimeric mice were analyzed following a 6-week reconstitution period.
Treg cell cotransference
Treg cells were isolated from the spleens and lymph nodes of Ripk1f/fFadd−/−Fadd:gfpf/fCD4Cre mice or CD45.1+ wild-type mice using a Treg cell isolation kit (Miltenyi Biotec). Treg cells from Ripk1f/fFadd−/−Fadd:gfpf/fCD4Cre mice were then mixed with CD45.1+ wild-type cells at a 1:1 ratio and subsequently transferred into CD45.1+CD45.2+ wild-type recipient mice via intravenous injection. The proportions of transferred Treg cells in the recipient mice were analyzed 1 and 2 weeks after transfer.
In vitro Treg suppression assay
The in vitro Treg suppression assay was performed as previously described [35]. Briefly, CD4+CD25−CD45RBhigh naive CD4+ T cells and CD4+CD25+CD45RBlow Treg cells were sorted using fluorescence-activated cell sorting (FACS). Naive CD4+ T cells were labeled with CellTrace Violet (Thermo Fisher Scientific) and cocultured with Treg cells at a 1:1 ratio on a plate coated with anti-CD3/anti-CD28 antibodies for 72 h. Subsequently, the dilution of CellTrace was analyzed using flow cytometry.
Cell viability assay
Cell viability was measured using the CellTiter-Glo Luminescent Cell Viability Assay kit (Promega) according to the manufacturer’s instructions. The luminescence was recorded with a microplate luminometer (Thermo Scientific).
Histopathology
For histological analysis, the samples were fixed in 3.7% formaldehyde, dehydrated in ethanol, cleared with xylene, and embedded in paraffin. Thin sections, approximately five micrometers thick, were cut and mounted onto glass slides that had been precoated with poly-l-lysine. These sections were then stained with hematoxylin and eosin using standard procedures.
RNA-Seq analysis
Total RNA was extracted using TRIzol reagent (Life Technologies) following the manufacturer’s instructions. The quality of the RNA was evaluated, and sequencing was performed using the Illumina HiSeq X platform at Novogene (Beijing, China). Raw data from the sequencer underwent preprocessing, alignment, and deduplication before being exported as raw counts. The differential expression gene analysis was conducted using the R package DESeq2 [36]. For the generation of the heatmap, the raw counts were transformed using the variance stabilizing transformation method and used as input. The RNA-seq data have been deposited in the Gene Expression Omnibus under the primary accession code GSE230483.
Statistical analysis
Statistical analysis were performed using Prism 9 (GraphPad). Two-tailed Student’s t tests and paired t tests were used to calculate p values (*p < 0.05, **p < 0.01 and ***p < 0.001). The data presented in this article are the representative results of at least two independent experiments.
Supplementary information
Acknowledgements
This work was supported by the following grants: National Key Research and Development Program of China (2021YFA1301402), Shanghai Municipal Science and Technology Major Project (ZD2021CY001), National Key Research and Development Program of China (2021YFE0200900; 2022YFA0807300), National Natural Science Foundation of China (82101833, 82073901), Three-year Action Plan for Shanghai TCM Development and Inheritance Program [ZY(2021-2023)-0103], Top-level Clinical Discipline Project of Shanghai Pudong District (grant/award number: PWYgf 2021-01), and Training Plan for Discipline Leaders of Shanghai Pudong New Area Health Commission (grant/award number: PWRd2020-09).
Author contributions
HZ and HW conceived and supervised the research; XD, LW, YZ, ZY, YC, YS, YZ, HZ and HW contributed to the project design and discussions; XD, LW and YZ conducted the experiments; QL, YZ, WZ, YT, TW and JD helped with the mixed bone marrow chimera experiments and some phenotype analysis; FD and JR assisted with mouse models; PH did the bioinformatics analysis; XD, LW, YZ, ZY, YZ, HZ and HW wrote and edited the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Xiaoxue Deng, Lingxia Wang, Yunze Zhai.
Contributor Information
Yuejuan Zheng, Email: 13641776412@163.com.
Haibing Zhang, Email: hbzhang@sibs.ac.cn.
Haikun Wang, Email: 15001995464@163.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41423-023-01113-x.
References
- 1.Sakaguchi S, Mikami N, Wing JB, Tanaka A, Ichiyama K, Ohkura N. Regulatory T cells and human disease. Annu Rev Immunol. 2020;38:541–66. [DOI] [PubMed] [Google Scholar]
- 2.Smigiel KS, Srivastava S, Stolley JM, Campbell DJ. Regulatory T-cell homeostasis: steady-state maintenance and modulation during inflammation. Immunol Rev. 2014;259:40–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pierson W, Cauwe B, Policheni A, Schlenner SM, Franckaert D, Berges J, et al. Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3+ regulatory T cells. Nat Immunol. 2013;14:959–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liston A, Gray DHD. Homeostatic control of regulatory T cell diversity. Nat Rev Immunol. 2014;14:154–65. [DOI] [PubMed] [Google Scholar]
- 5.Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol. 2014;15:1070–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS Jr. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–3. [DOI] [PubMed] [Google Scholar]
- 8.Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, et al. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. [DOI] [PubMed] [Google Scholar]
- 10.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–27. [DOI] [PubMed] [Google Scholar]
- 11.Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, et al. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci USA. 2012;109:5322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kearney CJ, Cullen SP, Clancy D, Martin SJ. RIPK1 can function as an inhibitor rather than an initiator of RIPK3-dependent necroptosis. FEBS J. 2014;281:4921–34. [DOI] [PubMed] [Google Scholar]
- 14.Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, Wachsmuth L, et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature. 2014;513:90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan S, Zhao J, Sun Z, Cao S, Niu K, Zhong Y, et al. Hepatocyte-specific TAK1 deficiency drives RIPK1 kinase-dependent inflammation to promote liver fibrosis and hepatocellular carcinoma. Proc Natl Acad Sci USA. 2020;117:14231–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuchet-Lourenco D, Eletto D, Wu C, Plagnol V, Papapietro O, Curtis J, et al. Biallelic RIPK1 mutations in humans cause severe immunodeficiency, arthritis, and intestinal inflammation. Science. 2018;361:810–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Fuhrer M, Bahrami E, Socha P, Klaudel-Dreszler M, Bouzidi A, et al. Human RIPK1 deficiency causes combined immunodeficiency and inflammatory bowel diseases. Proc Natl Acad Sci USA. 2019;116:970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dowling JP, Cai Y, Bertin J, Gough PJ, Zhang J. Kinase-independent function of RIP1, critical for mature T-cell survival and proliferation. Cell Death Dis. 2016;7:e2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smigiel KS, Richards E, Srivastava S, Thomas KR, Dudda JC, Klonowski KD, et al. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J Exp Med. 2014;211:121–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo CT, Liao W, Dadi S, Toure A, Li MO. Graded Foxo1 activity in Treg cells differentiates tumour immunity from spontaneous autoimmunity. Nature. 2016;529:532–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi N, Vereecke L, Bertrand MJ, Duprez L, Berger SB, Divert T, et al. RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature. 2014;513:95–9. [DOI] [PubMed] [Google Scholar]
- 22.Roderick JE, Hermance N, Zelic M, Simmons MJ, Polykratis A, Pasparakis M, et al. Hematopoietic RIPK1 deficiency results in bone marrow failure caused by apoptosis and RIPK3-mediated necroptosis. Proc Natl Acad Sci USA. 2014;111:14436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mifflin L, Ofengeim D, Yuan J. Receptor-interacting protein kinase 1 (RIPK1) as a therapeutic target. Nat Rev Drug Discov. 2020;19:553–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su H, Bidere N, Zheng L, Cubre A, Sakai K, Dale J, et al. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science. 2005;307:1465–8. [DOI] [PubMed] [Google Scholar]
- 25.Misra RS, Russell JQ, Koenig A, Hinshaw-Makepeace JA, Wen R, Wang D, et al. Caspase-8 and c-FLIPL associate in lipid rafts with NF-kappaB adaptors during T cell activation. J Biol Chem. 2007;282:19365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shan B, Pan H, Najafov A, Yuan J. Necroptosis in development and diseases. Genes Dev. 2018;32:327–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Zhang H, Xu C, Li X, Li M, Wu X, et al. Ubiquitination of RIPK1 suppresses programmed cell death by regulating RIPK1 kinase activation during embryogenesis. Nat Commun. 2019;10:4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang Y, Tu H, Zhang J, Zhao X, Wang Y, Qin J, et al. K63-linked ubiquitination regulates RIPK1 kinase activity to prevent cell death during embryogenesis and inflammation. Nat Commun. 2019;10:4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kist M, Kőműves LG, Goncharov T, Dugger DL, Yu C, Roose-Girma M, et al. Impaired RIPK1 ubiquitination sensitizes mice to TNF toxicity and inflammatory cell death. Cell Death Differ. 2021;28:985–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li W, Shan B, Zou C, Wang H, Zhang MM, Zhu H, et al. Nuclear RIPK1 promotes chromatin remodeling to mediate inflammatory response. Cell Res. 2022;32:621–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol. 2012;30:733–58. [DOI] [PubMed] [Google Scholar]
- 32.Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbi J, Pardoll D, Pan F. Treg functional stability and its responsiveness to the microenvironment. Immunol Rev. 2014;259:115–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Rosenberg S, Wang H, Imtiyaz HZ, Hou YJ, Zhang J. Conditional Fas-associated death domain protein (FADD): GFP knockout mice reveal FADD is dispensable in thymic development but essential in peripheral T cell homeostasis. J Immunol. 2005;175:3033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collison LW, Vignali DA. In vitro Treg suppression assays. Methods Mol Biol. 2011;707:21–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.