Abstract
Opioid use disorder (OUD), like other substance use disorders (SUDs), is widely understood to be a disorder of persistent relapse. Despite the use of three FDA-approved medications for OUD, typically in conjunction with behavioral treatments, relapse rates remain unacceptably high. Whereas medication assisted therapy (MAT) reduces the risk of opioid overdose mortality, the benefits of MAT are negated when people discontinue the medications. Currently approved medications present barriers to efficient use, including daily visits to a treatment center or work restrictions. With spiking increases in opioid relapse and death, it is imperative to identify new treatments that can reduce the risk of relapse. Recent evidence suggests that glucagon-like peptide-1 receptor agonists (GLP-1RAs), currently FDA-approved to treat obesity and type two diabetes, may be a promising candidate to reduce relapse. GLP-1RAs have been shown to reduce relapse in rats, whether elicited by cues, drug, and/or stress. However, GLP-1RAs also can cause gastrointestinal malaise, and therefore, in humans, the medication typically is titrated up to full dose when initiating treatment. Here, we used a rodent model to test whether cue- and drug-induced heroin seeking can be reduced by the GLP-1RA, liraglutide, when the dose is titrated across the abstinence period and prior to test. The results show this titration regimen is effective in reducing both cue-induced heroin seeking and drug-induced reinstatement of heroin seeking, particularly in rats with a history of high drug-taking. Importantly, this treatment regimen had no effect on either circulating glucose or insulin. GLP-1RAs, then, appear strong candidates for the non-opioid prevention of relapse to opioids.
Keywords: addiction, opioids, heroin, self-administration, rats, GLP-1RA, liraglutide, relapse
1. Introduction
During the first ten months of 2020, i.e., during the rapid growth of the COVID-19 pandemic, overdose deaths increased in almost every state in the nation.1 This increase can be attributed largely to a spike in deaths related to the use of opioids.1 Currently, there are three medication assisted treatments for opioid use disorder (OUD), naltrexone, buprenorphine/buprenorphine + naloxone (Suboxone), and methadone, yet relapse rates remain high.2–4 Compliance is low with extended-release naltrexone,4 treatment with Suboxone can elicit potent withdrawal in patients with fentanyl experience,5 and the full μ-agonist, methadone, requires daily visits to a treatment center and may pose work restrictions in certain industries (i.e. airlines, hospitals, work with heavy equipment, etc.). Given these limitations, and spiking opioid overdose deaths, it is imperative that we better understand OUD to identify new and effective avenues for treatment.
A great deal of attention in the substance use disorder (SUD) literature has focused on the reward pathway and the view that drugs of abuse hijack natural reward substrates.6,7 In 2008,8 we stated that “Drug-seeking animals and humans behave as though they need the drug. They seek to satisfy this need state much as they seek food when hungry, water when thirsty, and salt when sodium deficient. When these biological drives are activated, there is a single goal and there can be no substitute. This is the state of the [addicted individual] when actively engaged in drug-seeking.” If this need state hypothesis is correct, then treatment with a known ‘satiety’ signal, glucagon-like peptide-1 (GLP-1), should reduce drug seeking and taking in rats. GLP-1 is a hormone produced by L cells in the small intestine that reduces food intake, in part, by increasing insulin release, decreasing glucagon release, and decreasing gastric emptying.9 GLP-1 also is produced by neurons in the nucleus of the solitary tract (NST), and these neurons project widely throughout the brain, including to reward and feeding circuitry.10,11 In accordance, treatment with GLP-1 receptor agonists (GLP-1RA) inhibits the ingestion of sweets, water, and salt when made more palatable by need.10,12–13,18 Treatment with the GLP-1RA, Exendin-4 (Ex-4), also reduces a conditioned place preference for abused substances, blunts the resulting spike in dopamine release within the nucleus accumbens (NAc) from nicotine, cocaine, and amphetamine, and reduces cocaine self-administration in rodents.14–16,19–20 Likewise, treatment with Ex-4, in a dose that does not affect responding for sucrose, decreases fixed and progressive ratio responding for cocaine17 and, when administered centrally, cocaine-induced reinstatement of cocaine-seeking behavior.21
Regarding responding for opioids, one study conducted in mice failed to find Ex-4 effective in reducing remifentanil self-administration and other opioid-related behaviors.30 Since this report, however, supportive data have been obtained in rats. Ex-4 was found effective in reducing oxycodone self-administration and seeking in rats;31 we found Ex-4 [2.4 μg/kg intraperitoneal (i.p.)] effective in reducing cue-induced heroin seeking and drug-induced reinstatement of heroin seeking in rats.32 In addition, Ex-4 recently was reported to reduce fentanyl self-administration and fentanyl seeking in rats following a drug/cue challenge. This dose of Ex-4, however, also increased intake of the anti-emetic, kaolin, suggesting Ex-4 may cause gastrointestinal malaise.33
In a follow up study, we tested the effectiveness of a 0.1 mg/kg subcutaneous (sc) dose of the longer-acting GLP-1RA, liraglutide, administered beginning on the 11th day of a 22-day heroin self-administration regimen, throughout a subsequent two-week abstinence period, and 1 h prior to extinction testing. Results showed: (1) reduced heroin self-administration, (2) reduced escalation of heroin self-administration, and (3) a significant reduction of drug-induced reinstatement of heroin seeking behavior assessed 6 h later.41 Cue-induced heroin seeking, however, was not reduced when assessed 1 h following the administration of liraglutide. This likely was due to slower onset of the long-acting GLP-1RA compared with Ex-4. Indeed, the acute administration of a 0.3 mg/kg dose of liraglutide was highly effective in reducing both cue-induced heroin and fentanyl seeking and drug-induced reinstatement of heroin and fentanyl seeking when administered at least 6 h prior to test (Urbanik et.al., this issue).36,66 Importantly, we also demonstrated that the 0.3 mg/kg dose of liraglutide did not impair movement on a rotarod36 and, while liraglutide across a range of doses supported conditioned avoidance of a saccharin-paired cue, liraglutide, from the lowest to the highest dose tested, did not increase intake of the anti-emetic clay, kaolin.41
As such, GLP-1RAs are promising as a non-opioid treatment for OUD in humans. Importantly, GLP-1RAs can be readily applied for this new indication, as various formulations already are approved for the treatment of obesity and type 2 diabetes mellitus (T2DM) in humans.9,22 That said, the primary side effect of GLP-1RA treatment is gastrointestinal malaise.35 In an effort to reduce the negative impact of this unpleasant side effect on treatment, the dose of GLP-1RAs is titrated over a number of weeks in humans to reach the target dose. Here, we use an animal model to test whether such a treatment regimen (i.e., dose titration of liraglutide) will be effective in reducing both cue-induced heroin seeking and drug-induced reinstatement of heroin seeking in rats. In Experiment 1, the dose increased every three days from 0.06 mg/kg to 0.1 mg/kg, 0.3 mg/kg and finally 0.6 mg/kg. In Experiment 2, the dose increased similarly but did not exceed the 0.3 mg/kg dose. Additionally, as an incretin hormone, GLP-1 increases the secretion of insulin which leads to a reduction of blood glucose and to an increase in satiety. Whereas this is excellent for the treatment of T2DM,9,22 sudden onset of hypoglycemia could lead to a severe adverse event in otherwise normoglycemic individuals in treatment for OUD. Consequently, we also tested whether liraglutide, across a range of doses, alters levels of either plasma glucose or plasma insulin (Experiment 3).
2. Methods
Fifty-two naïve male outbred Sprague-Dawley rats delivered from Charles River (Wilmington, MA) at approximately 90 days of age, weighing between 300–400 g at the start of the experiment served as subjects. All subjects were housed individually in standard, suspended, stainless steel cages. The environment in the animal colony room had controlled humidity and temperature (21°C), with a 12/12 h light/dark cycle, and lights on at 7:00 am. All experimental manipulations began 2 h into the light phase of the cycle. Following one week of acclimation to their home cages, rats were habituated to experimenter handling by daily weighing. Food and water were available ad libitum, except where noted otherwise. All studies were approved by the Pennsylvania State University College of Medicine Institutional Animal Care and Use Committee and performed in accordance with the National Institutes of Health specifications outlined in their Guide for the Care and Use of Laboratory Animals.
2.1. Jugular Catheter Implantation Surgery
Rats in Experiment 1 (n=24) and Experiment 2 (n=24) were anesthetized with isoflurane (4% for induction and 2–3% for maintenance) and implanted with an intravenous (iv) jugular catheter (Instech Laboratories, Inc., Plymouth Meeting, PA) for drug self-administration as described previously.40 Rats in Experiment 3 (n=4) went through a similar surgery as rats in Experiments 1 and 2, but they also had a catheter placed in the carotid artery. Following surgery, rats received a sc injection of the non-steroidal anti-inflammatory drug (NSAID), carprofen, and the antibiotic, enrofloxacin, as post-operative care for at least two days, and were given a week to recover. Maintenance of jugular and carotid catheter patency included flushing catheters using heparinized saline (0.2 mL of 30 IU/mL heparin) once every four days. Catheter patency was verified at the end of each week of drug self-administration and the day before each test using 0.3 mL of propofol (Diprivan 1%).
2.2. Habituation
Rats in Experiments 1 and 2 experienced two days of habituation to the self-administration chambers. On the evening prior to the first habituation session, ad libitum water was removed overnight. The rats then underwent one 5-min habituation session per day for 2 days. During this 5-min period, rats were placed in the self-administration chambers and water was available in one of the two spouts (center future ‘inactive’ spout and rightmost future ‘active’ spout), counterbalanced across the first and second day. In order to maintain proper hydration during habituation, rats also received overnight access to 20 mL of filtered water at the front of the home cage beginning at 5 PM. Ad libitum access to water was resumed following the second habituation session.
2.3. Self-Administration (SA)
2.3.1. Apparatus
Twenty-four drug self-administration chambers (MED Associates, Inc., St. Albans, VT) were used as previously described.39 Each 30.5cm × 24.0cm × 29.0cm chamber was equipped with a 25 W light, tone generator, white noise speaker, and two empty, retractable spouts. A lickometer circuit was used to record contacts on both spouts. All recorded data from the lickometers and all events in the chamber (e.g., lights, tones, spout advancement and retraction) are controlled, measured and stored by a Pentium computer using the MED Associates programing language (MED Associates, Inc., St. Albans, VT).
2.3.2. Acquisition
After habituation, rats were placed in the self-administration chambers daily around 9 AM. They were then given 6 h to self-administer heroin (n=32) or saline (n=16) in Experiments 1 and 2. At the start of the session, the middle and rightmost empty spouts advanced, with the spouts centered in the aperture and flush with the Plexiglas wall. Contacts on the inactive empty spout (middle) led to no consequence. The availability of the rightmost active empty spout was signaled by a cue light located above and completion of a fixed ratio of 10 (FR10) contacts with this spout led to a 6 sec iv infusion of 0.06 mg/0.2 mL of heroin, previously shown to produce robust heroin self-administration and seeking in rats.32,36,38 Each infusion was accompanied by a 20 sec time-out period during which the cue light turned off, the house light turned on, the empty spouts retracted and was signaled by the sound of a tone. Self-administration occurred as described 5 days a week for 11 days. This paradigm was run for both Experiment 1 and Experiment 2.
2.3.3. Cue/Drug-induced Reinstatement Test 1
On the 12th day, the rats from both Experiments 1 and 2 were placed in their self-administration chambers beginning at 1PM, but no heroin was delivered across a 3 h extinction period. The number of contacts made on the right empty spout during the first h was an indication of cue-induced heroin seeking, with the behavior typically extinguishing across the 2nd and 3rd h of extinction. At the end of Hour 3, rats were given a computer-controlled, non-contingent iv administration of saline or heroin (as per predetermined group assignment) and drug-induced reinstatement of heroin seeking behavior was measured across an additional 60-min extinction session (i.e., in h 4). Home cage abstinence. Thereafter, all rats experienced 2 weeks of abstinence in their home cages, which can serve to augment heroin-seeking behavior.47
2.3.4. Liraglutide or vehicle treatment
Rats with a history of saline or heroin self-administration were matched for heroin taking and seeking behavior after Test Day 1 and injected sc daily (beginning at 7AM) with either vehicle (n=8/experiment) or liraglutide (n=16/experiment). The dose of liraglutide started at 0.06 mg/kg and increased every 3 days. For Experiment 1, the dose increased from 0.06 mg/kg to 0.1 mg/kg, 0.3 mg/kg and finally 0.6 mg/kg. For Experiment 2, the dose increased similarly but did not exceed the 0.3 mg/kg dose.
2.3.5. Cue/Drug-Induced Reinstatement Test 2
After 2 weeks of abstinence and daily vehicle or liraglutide treatment, the rats in both Experiment 1 and Experiment 2 underwent a second extinction test to measure the effect of daily treatment with vehicle or increasing doses of liraglutide (rats also received their daily sc administration of vehicle or liraglutide 6 h prior to Test 2) on cue-induced heroin seeking and drug-induced reinstatement of heroin seeking as described in Test 1 above.
2.3.6. Abstinence/Re-Test 0.3 mg/kg Liraglutide (Test 3: Experiment 1 only)
Given data suggesting that the 0.6 mg/kg dose of liraglutide may be too high (see below), rats in Experiment 1 were re-tested with a 0.3 mg/kg liraglutide challenge. Specifically, rats in Experiment 1 (i.e., those titrated to the 0.6 mg/kg dose of liraglutide), were given 3 additional days of home cage abstinence with daily vehicle or 0.3 mg/kg liraglutide injections. On the 4th day, the rats were injected sc with vehicle or 0.3 mg/kg liraglutide and returned 6 h later to the test chambers under extinction conditions to examine the effect of the lower dose on cue-induced heroin seeking and drug-induced reinstatement of heroin seeking as described in Test 1 above.
2.4. Experiment 3: Glucose and Insulin Levels
To assess the safety of liraglutide in maintaining glucose homeostasis, glucose and insulin levels were measured using a within subject design where rats (n=4) received a daily sc injection of liraglutide across increasing doses including 0.06, 0.1, 0.3, 0.6, and 1.0 mg/kg and compared to baseline (BL). The rats were ad libitum-fed overnight, but food was withheld following the 7 A.M. liraglutide injection and throughout sampling. Glucose was measured by a glucometer (Prodigy AutoCode, Charlotte, NC, USA) from arterial plasma samples taken at baseline and at 6, 8, and 10 h post-liraglutide administration. We examined only 6–10 h post-liraglutide since peak plasma concentrations of liraglutide occur in this time frame.59 Arterial plasma insulin concentrations were measured at these same time points using a rat ultrasensitive insulin ELISA kit (80-INSRTU-E01; Alpco Diagnostics, Salem, NH, USA).
2.5. Data Analysis
The data from Experiments 1 and 2 were analyzed using Statistica Version 13.5.0.17, TIBCO Software Inc. (Palo Alto, CA, USA). Significant mixed factorial Analysis of Variance (ANOVAs) were followed by Newman-Keuls post hoc tests to identify group differences. The data from Experiment 3 were analyzed using Prism Version 9.2.0, GraphPad Software (La Jolla, CA, USA). For glucose and insulin levels, data are presented as means ± standard error of the mean (SEM). Raw values for glucose and insulin levels are shown at each time point and an area under the curve (AUC) was calculated to summarize changes over time. Raw data were analyzed using a two-way repeated measures ANOVA to account for main effects of liraglutide dose, time, and their interaction. The data for the AUC were analyzed using a one-way repeated measures ANOVA.
3. Results
3.1. Experiment 1
3.1.1. Acquisition and Division of High and Low Heroin Takers
To identify high and low heroin takers, the number of infusions self-administered during terminal Trials 10 and 11 were averaged for all rats in the heroin condition (n=16). The median number of terminal infusions of heroin was 14.5. Thus, high drug-takers were identified as rats that self-administered more than the median (n=8) and low drug-takers, rats that self-administered less than the median (n=8).
3.1.2. Acquisition
Figure 2A shows the mean number of infusions per 6 h across 11 acquisition trials for low and high drug takers and for saline self-administering controls. The data were analyzed using a 3 × 11 mixed factorial ANOVA varying group × trials. Due to missing data from saline controls, 3 data points were added for 3 subjects via interpolation in order for Statistica to analyze the data. The results yielded a significant main effect of group, (F2,19=14.16, p=0.0002), with post hoc Newman-Keuls tests showing that high drug takers (her/high) took more infusions of heroin than low drug takers (her/low), p < 0.05, and group her/low took more than the saline self-administering controls, p < 0.05, overall. The group × trials interaction also was significant (F20,200=3.23, p<0.0001) and post hoc Newman-Keuls tests revealed that high drug takers took more infusions of heroin than controls took of saline from Trial 5 through Trial 11, p-values (ps) < 0.05. Otherwise, there were no significant differences between groups across trials. Such individual differences in drug self-administration behavior are consistent with our previous reports34,37–38,40 involving cocaine and heroin self-administration.
Figure 2: A. Heroin or Saline Acquisition Over 11 Trials; B. Cue-induced Seeking and Drug-induced Reinstatement Test 1.
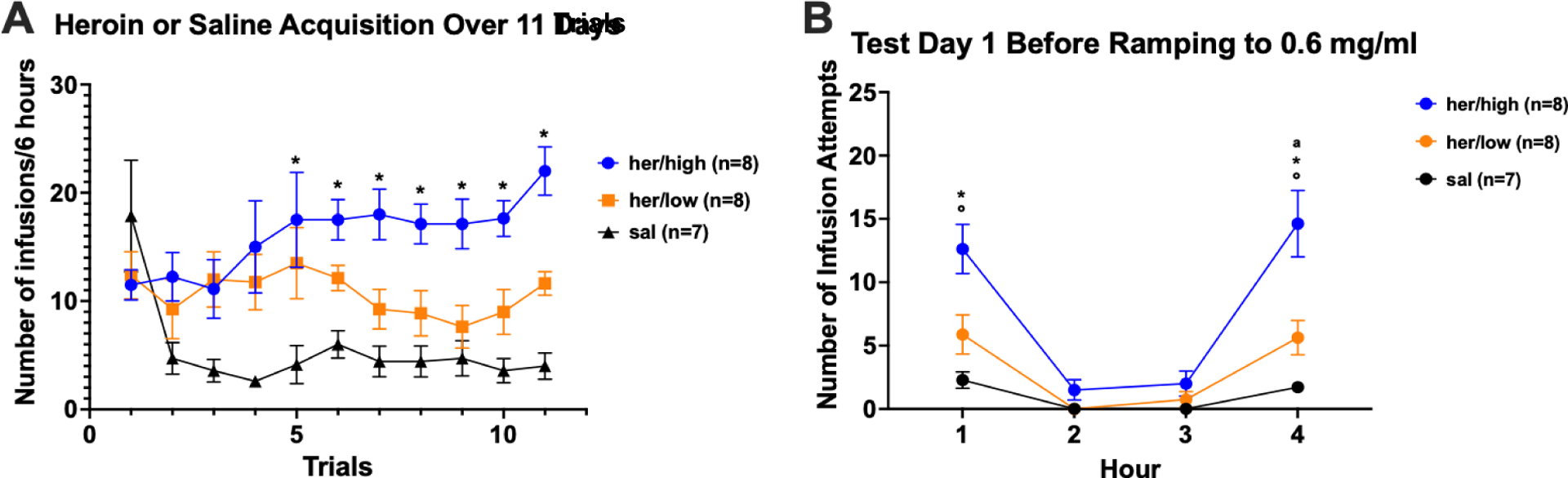
A) Acquisition: Mean (+/− SEM) number of infusions/6 hours across 11 daily trials for heroin high drug takers (blue), heroin low drug takers (orange), and saline controls. B) Test Day 1: Mean (+/− SEM) number of infusion attempts across Hours 1 – 4 of extinction for heroin high takers (blue), heroin low takers (orange) and saline controls (black). A single iv infusion of heroin was administered at the end of Hour 3. Cue-induced heroin seeking was assessed in Hour 1; Drug-induced reinstatement of heroin seeking was assessed in Hour 4. Symbols denote significance between groups (*=p<0.05 for her/high vs. sal; a= p<0.05 for her/low vs. sal; °=p<0.05 for her/high vs. her/low).
3.1.3. Cue/Drug-Induced Reinstatement Test 1
As described, on the 12th day, the rats were placed in the test chambers as usual, but under extinction conditions (i.e., with no drug delivered) and cue-induced seeking was measured in Hour 1 and drug-induced reinstatement of heroin seeking in Hour 4 (Figure 2B). Infusion attempts were analyzed using a 3 × 4 mixed factorial ANOVA varying group (high heroin-taker, low heroin-taker, or saline) and hour (1 – 4). The results showed a significant group × time interaction (F6,57=4.34, p=0.0011). Post hoc Newman-Keuls tests confirmed that, in Hour 1, greater cue-induced heroin seeking was evidenced by the high drug takers vs. the low drug takers and the saline self-administering controls, ps<0.05, which did not differ one from the other, p > 0.05 (see Figure 2, panel B). The high drug takers also evidenced greater drug-induced reinstatement of heroin-seeking behavior in Hour 4 than did the low drug takers, which exhibited greater seeking than did rats with a history of saline self-administration, ps < 0.05.
3.1.4. Cue/Drug-Induced Reinstatement Test 2
Following 2 weeks of home cage abstinence and daily sc injections of vehicle or increasing doses of liraglutide, rats were returned to the test chamber for a second cue/drug-induced reinstatement test. The data from this test are shown in Figure 3A and 3B. These data were analyzed using a 3 × 2 × 4 mixed factorial ANOVA varying group (high heroin-taker, low heroin-taker, or saline), drug (liraglutide or vehicle), and hour (1 – 4). The results of this ANOVA revealed a significant main effect of group, (F2,16=27.02, p<0.0001), and group × time interaction (F6,48=8.56, p<0.0001). Neither the drug × time interaction (F3,48=1.77, p=0.1662) nor the group × drug × time interaction was statistically significant (F6,48=2.02, p=0.0816). Given the significant main effect of group, the data from the low takers and the high takers were analyzed separately utilizing 2 × 2 × 4 mixed factorial ANOVAs. Low drug takers. Results yielded a significant group × time interaction (F3,30=20.70, p<0.0001), but no significant drug × time (F < 1) or group × drug × time interaction (F3,30=1.39, p=0.2652). High drug takers. There was a significant group × time interaction (F3,33=11.99, p<0.0001), but again no significant drug × time (F3,33=2.65, p=0.0650) or group × drug × time interaction (F3,33=1.64, p=0.1981). Post hoc tests on the significant group × time interactions revealed greater seeking in Hour 1 and Hour 4 by rats with a history of heroin self-administration vs. the saline self-administration, regardless of drug treatment (i.e., vehicle or liraglutide), ps < 0.05. Given the effectiveness of the 0.3 mg/kg dose of liraglutide in our acute studies36,66 the same experimental subjects were challenged following a brief period of abstinence with this lower dose of liraglutide.
Figure 3: Cue-induced Seeking and Drug-induced Reinstatement After Chronic Liraglutide Titrated to 0.6 mg/kg.
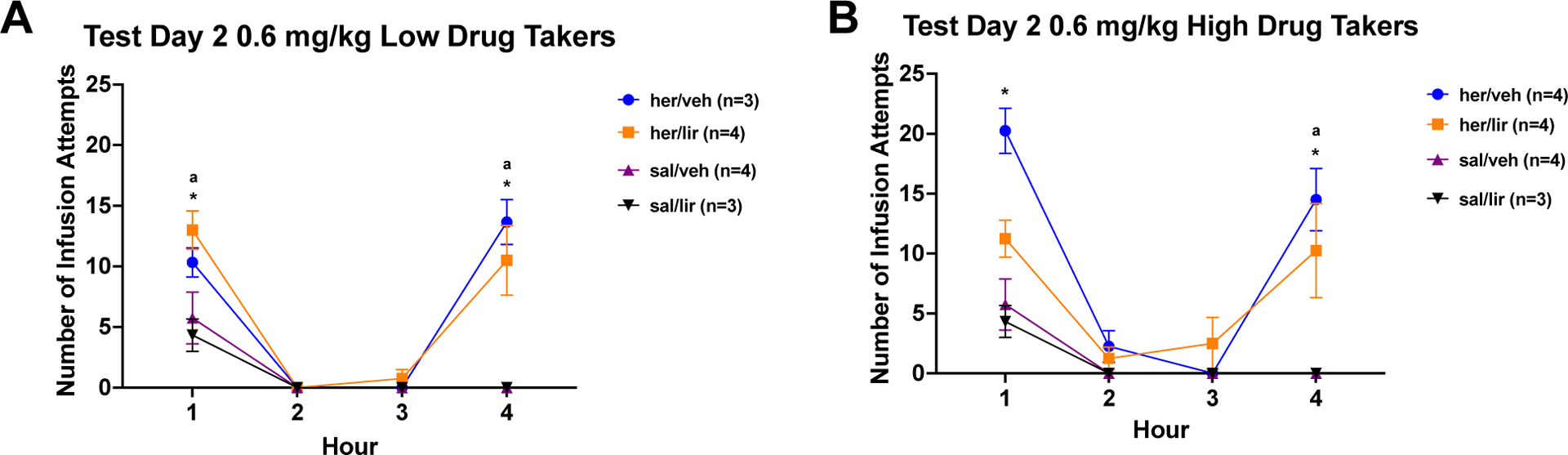
A) Low Drug Takers: Mean (+/− SEM) number of infusion attempts across Hours 1 – 4 for low heroin takers and saline self-administering controls treated with vehicle (saline) or 0.6 mg/kg liraglutide during abstinence and prior to test. B) High Drug Takers: Mean (+/− SEM) number of infusion attempts across Hours 1 – 4 for high heroin takers and saline self-administering controls treated with vehicle (saline) or 0.6 mg/kg liraglutide during abstinence and prior to test (*=p<0.05 for her/veh vs. sal/veh; ª=p<0.05 for her/lir vs. sal/lir).
3.1.5. Abstinence/Re-Test 0.3 mg/kg Liraglutide (Test 3: Experiment 1 only)
After three days of home cage abstinence and daily sc injections with vehicle or 0.3 mg/kg liraglutide, rats were injected sc with vehicle or liraglutide (0.3 mg/kg) and, 6 h later, placed back into the test chamber for Cue/Drug-Induced Reinstatement Test 3. The data for this test are shown in Figure 4A and 4B.
Figure 4: Cue-induced Seeking and Drug-induced Reinstatement after 3 days of Vehicle or 0.3 mg/kg Liraglutide Treatment.
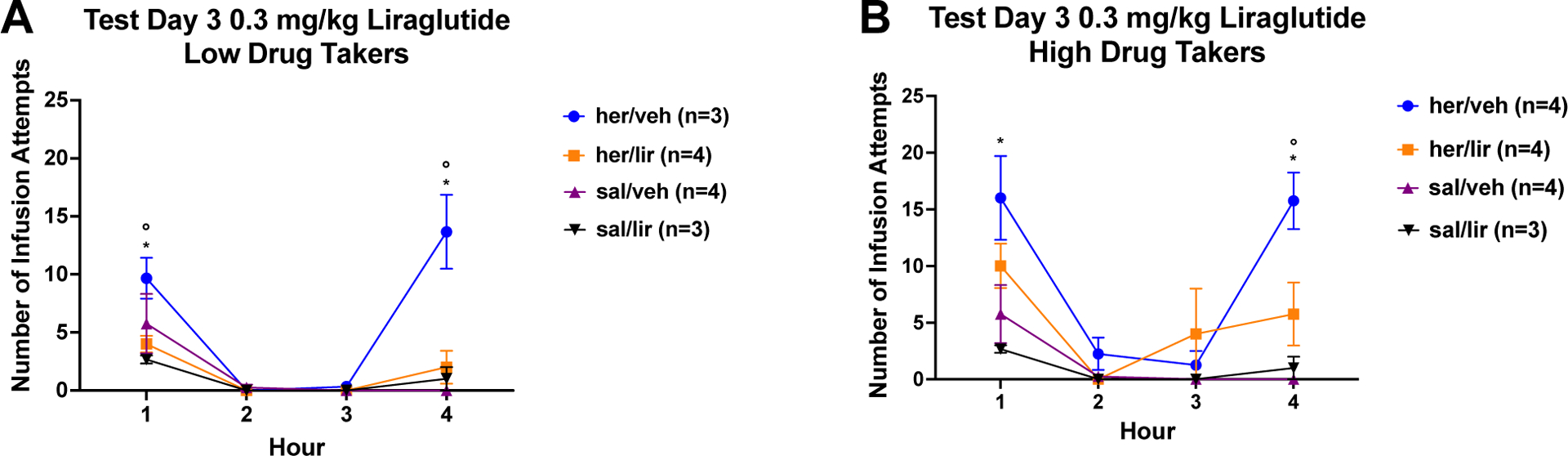
A) Low Drug Takers: Mean (+/− SEM) number of infusion attempts across Hours 1 – 4 for low heroin takers and saline self-administering controls treated with vehicle (saline) or 0.3 mg/kg liraglutide during 3 days of abstinence and 6 h prior to test. B) High Drug Takers: Mean (+/− SEM) number of infusion attempts across Hours 1 – 4 for high heroin takers and saline self-administering controls treated with vehicle (saline) or 0.3 mg/kg liraglutide during 3 days of abstinence and 6 h prior to test. Symbols denote significance between groups (*=p<0.05 for her/veh vs. sal/veh; °=p<0.05 for her/veh high (or low) vs. her/lir high (or low)).
Once again, the data were analyzed using a 3 × 2 × 4 ANOVA varying group, drug, and time. The results revealed a significant group × time interaction (F6,48=4.52, p=0.0011) and significant drug × time interaction (F3,48=6.90, p=0.0006), but only trending significance with group × drug × time interaction (F6,48= 2.20, p=0.0591). Given a significant main effect of group, (F2,16=11.51, p=0.0008), the data from the low takers and the high takers were once again analyzed separately utilizing 2 × 2 × 4 mixed factorial ANOVAs. Low drug takers. There was a significant group × time (F3,30=9.62, p=0.0001), drug × time (F3,30=6.09, p=0.0023), and group × drug × time interaction (F3,30=7.30, p=0.0008). Post hoc Newman-Keuls tests of this significant 3-way ANOVA confirmed higher cue-induced seeking and drug induced reinstatement in her/veh (heroin rats treated with vehicle) compared to sal/veh (saline rats treated with vehicle) rats, p < 0.05. There also was reduced cue-induced seeking in Hour 1 and drug-induced reinstatement in her/lir (heroin rats treated with liraglutide) in Hour 4 compared to her/veh treated rats, p < 0.05. High drug takers. For high drug takers, the group × time interaction attained statistical significance (F3,33=6.12, p=0.0020), but this was not the case for either the drug × time (F3,33=2.42, p=0.0835) or group × drug × time (F3,33=2.42, p=0.0837) interaction. Overall, high drug takers had higher cue-induced seeking and drug-induced reinstatement of heroin seeking compared to rats with a history of saline self-administration, p < 0.05. When the data from Hour 1 (cue-seeking) was analyzed alone, there was a significant main effect of group (F1,11=10.85, p=0.0072), but not drug (F1,11=2.89, p=0.1169) nor group × drug interaction (F<1). Thus, there was greater cue-induced seeking and drug-induced reinstatement for group her/veh compared with group sal/veh. An analysis of the data from Hour 4 showed a significant group × drug interaction (F1,11=7.08, p=0.0221). Post hoc Newman-Keuls tests on the group × drug interaction demonstrated reduced heroin seeking in group her/lir vs. group her/veh, p < 0.05.
3.2. Experiment 2
As discussed, in a separate set of studies, the acute administration of 0.3 mg/kg liraglutide robustly reduced cue-induced heroin seeking and drug- and stress-induced reinstatement of heroin seeking.36 Similar findings were reported with fentanyl (Urbanik et al., this issue).66 Based on the results from Experiment 1, and the now completed series of studies investigating the effectiveness of the lower 0.3 mg/kg dose of liraglutide,36 we replicated the above study, but titrated the dose only to 0.3 mg/kg liraglutide. The data from one high heroin taker treated with liraglutide was removed due to equipment failure.
3.2.1. Acquisition and Division of High and Low Heroin Takers
To identify high and low heroin takers, the number of infusions self-administered during terminal Trials 10 and 11 were averaged for all rats in the heroin condition (n=15). The median number of terminal infusions of heroin was 16.5. Thus, high drug-takers were identified as rats that self-administered more than the median (n=7) and low drug-takers, rats that self-administered less than the median (n=8).
3.2.2. Acquisition
Figure 5A shows the mean number of infusions per trial for high drug takers, low drug takers, and saline controls. Conduct of a 3 × 11 ANOVA varying group and trial found a significant main effect of group (F2,19=22.03, p<0.0001) and a significant group × trial interaction (F20,190=4.05, p<0.0001). Post hoc Newman-Keuls tests revealed a significant split between high and low drug takers on acquisition Trial 10, ps < 0.05, with high drug takers self-administering a higher number of infusions. High drug takers also took significantly more infusions of heroin than saline self-administering controls across Trials 2 through 11, ps < 0.05. Post hoc tests did not find any significant differences in infusions between low drug takers and saline self-administering controls on a trial-by-trial basis.
Figure 5: A. Heroin or Saline Acquisition Over 11 Trials; B. Cue-induced Seeking and Drug-induced Reinstatement Test 1 for Experiment 2.
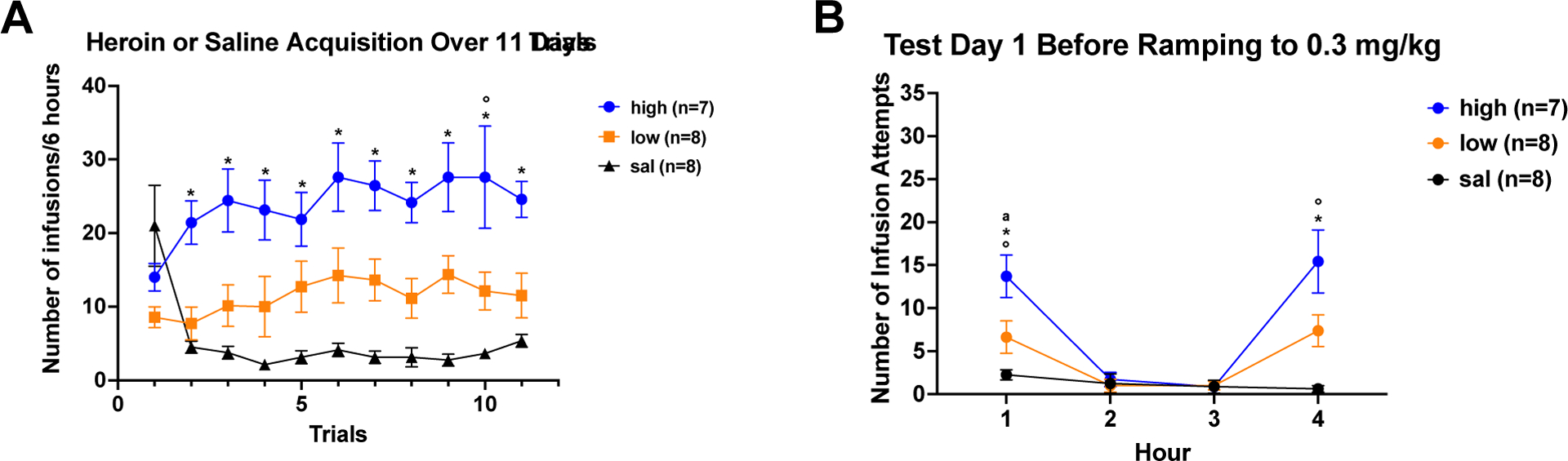
A) Acquisition: Mean (+/− SEM) number of infusions/6 hours across 11 daily trials for heroin high drug takers (blue), heroin low drug takers (orange), and saline controls. B) Test Day 1: Mean (+/− SEM) number of infusion attempts across Hours 1 – 4 of extinction for heroin high takers (blue), heroin low takers (orange) and saline controls (black). A single iv infusion of heroin was administered at the end of Hour 3. Cue-induced heroin seeking was assessed in Hour 1; Drug-induced reinstatement of heroin seeking was assessed in Hour 4. Symbols denote significance between groups (*=p<0.05 for her/high vs. sal; a= p<0.05 for her/low vs. sal; °=p<0.05 for her/high vs. her/low).
3.2.3. Cue/Drug-Induced Reinstatement Test 1
Figure 5B shows the results of Test Day 1. Infusion attempts were analyzed using 3 × 4 ANOVA which showed a significant group × time interaction (F6,60=6.17, p<0.0001). For cue-induced seeking in Hour 1, there were significantly more infusion attempts emitted by high drug takers vs. low drug takers, high drug takers vs. saline controls, and low drug takers vs. saline controls, ps < 0.05. For drug-induced reinstatement in Hour 4, there was a significantly higher number of infusion attempts made by high drug takers vs. saline controls and high drug takers vs. low drug takers, ps< 0.05.
3.2.4. Cue/Drug-Induced Reinstatement Test 2
Figure 6A and 6B show the results of Cue/Drug-Induced Reinstatement Test 2, i.e., the extinction test that followed 2 weeks of home cage abstinence and daily treatment with saline or increasing doses of liraglutide up to 0.3 mg/kg. Again, the data were analyzed using a 3 × 2 × 4 mixed factorial ANOVA varying group, drug, and time and results found a significant group × drug × time interaction (F6,51=3.44, p=0.0062). For the low drug takers, post hoc Newman-Keuls tests confirmed that in Hour 1, rats in the her/veh group exhibited significantly more infusion attempts than rats in the sal/veh group, p < 0.05. Liraglutide was not effective in reducing cue-induced heroin seeking in the low drug takers. During Hour 4, drug-induced reinstatement of heroin seeking was greater in the her/veh group compared with the sal/veh group and when compared with the her/lir group, ps < 0.05. For high drug takers, post hoc tests of the significant 3-way ANOVA found significantly more infusion attempts in the her/veh group compared with the sal/veh group for cue-induced seeking (Hour 1) and for drug-induced reinstatement of heroin seeking (Hour 4), ps < 0.05. For high drug takers, treatment with the titrated dose of liraglutide during abstinence and prior to test significantly reduced both cue-induced heroin seeking in Hour 1 and drug-induced reinstatement of heroin seeking in Hour 4 compared to the her/veh treated controls, ps < 0.05. These results demonstrate that titrating the dose of liraglutide to 0.3 mg/kg significantly attenuates cue-induced heroin seeking in high drug takers and drug-induced reinstatement of heroin seeking in both high and low drug takers.
Figure 6: Cue-induced Seeking and Drug-induced Reinstatement After Chronic Liraglutide Titrated to 0.3 mg/kg.
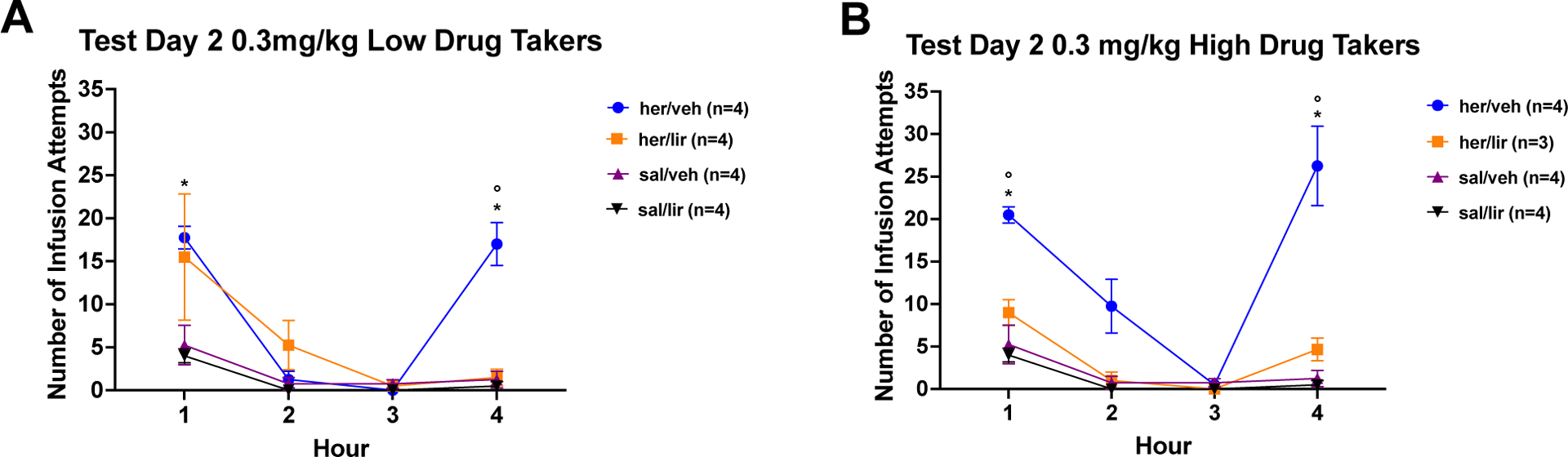
A) Low Drug Takers: Mean (+/− SEM) number of infusion attempts across Hours 1 – 4 for low heroin takers and saline self-administering controls treated with vehicle (saline) or 0.3 mg/kg liraglutide during abstinence and prior to test. B) High Drug Takers: Mean (+/− SEM) number of infusion attempts across Hours 1 – 4 for high heroin takers and saline self-administering controls treated with vehicle (saline) or 0.3 mg/kg liraglutide during 3 days of abstinence and prior to test (*=p<0.05 for her/veh vs. sal/veh; °=p<0.05 for her/veh high (or low) vs. her/lir high (or low); ª=p<0.05 for her/lir vs. sal/lir).
3.3. Experiment 3
Liraglutide, across a range of doses, does not significantly alter levels of circulating glucose or insulin when measured as change from baseline using a within-subjects design. This conclusion was supported by a non-significant two-way repeated measures ANOVA varying dose and time, F<1, for plasma glucose levels (Figure 7A) and a non-significant two-way repeated measures ANOVA, F < 1, for insulin (Figure 7B). When evaluated for the area under the curve, one-way ANOVAs again failed to find any effect of liraglutide, across a range of doses, on either plasma glucose (F4,12=1.30, p=0.32) or plasma insulin (F4,12=1.03, p=0.43) Figure 7C and 7D. Taken together, these findings show that, liraglutide, at these doses does not cause hypoglycemia in rats, which is consistent with that previously reported.41
Figure 7: Glucose and Insulin Levels After Liraglutide Treatment.
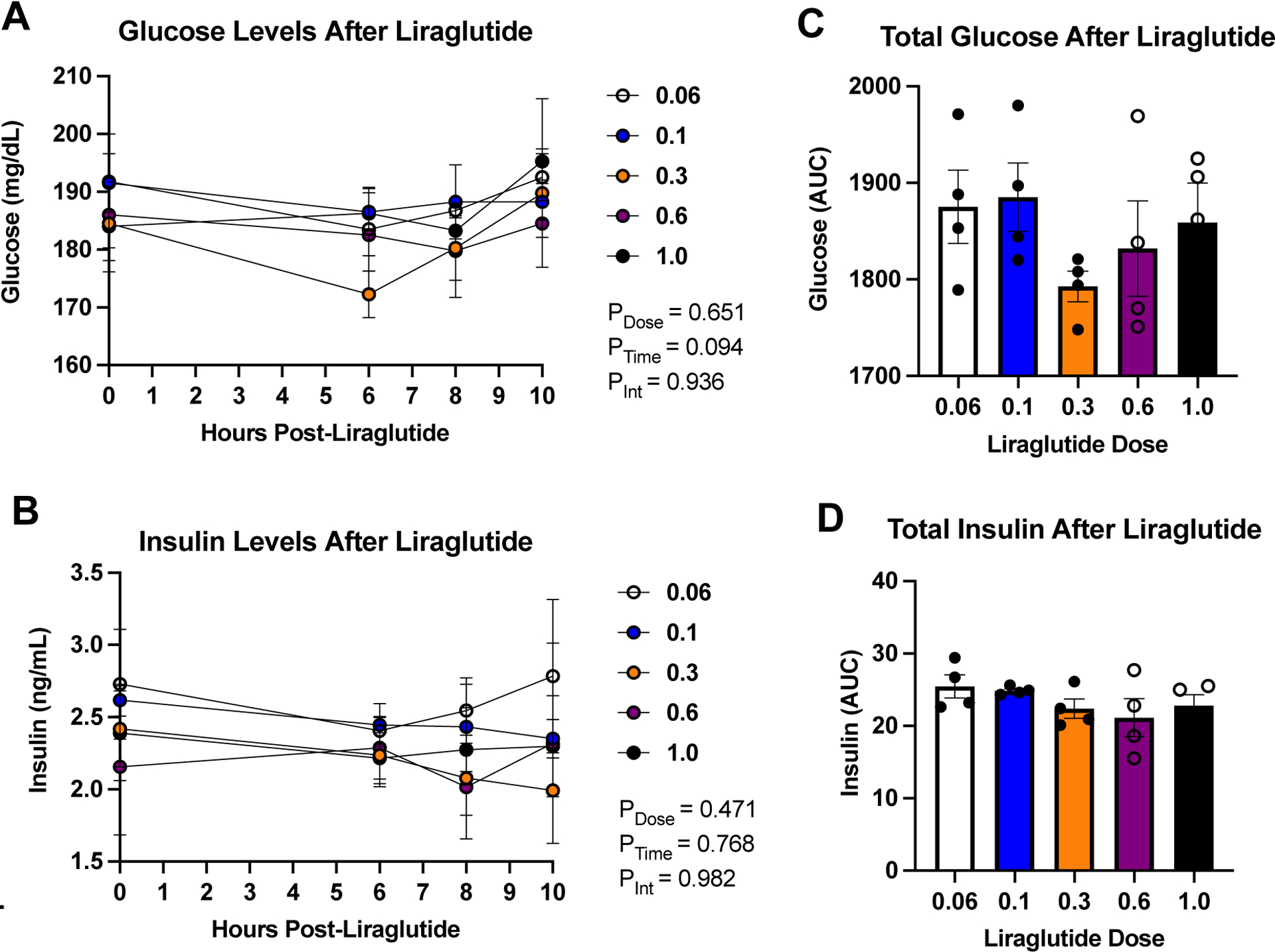
There is no significant decrease or increase in (A) glucose, (B) insulin, (C) total glucose (p=0.324 one-way repeated measures ANOVA), and (D) total insulin levels (p=0.431 one-way repeated measures ANOVA) following the administration of a range of doses of liraglutide (0.06, 0.1, 0.3, 0.6, and 1.0 mg/kg). AUC = Area Under the Curve.
4. Discussion
The results demonstrate that chronic treatment with increasing doses of liraglutide across a 2-week abstinence period and prior to test in rats reduces cue-induced heroin seeking for high drug takers and drug-induced reinstatement of heroin seeking for both high and low drug takers. Thus, titrating to the 0.3 mg/kg dose of liraglutide was effective in reducing cue-induced heroin seeking in high drug takers and drug-induced reinstatement of heroin seeking in high and low drug takers; while titrating to the 0.6 mg/kg dose of liraglutide was not. Further, the protective effect of liraglutide treatment was greater in high vs. low drug takers. Finally, across a wide range of doses of liraglutide, there was no significant effect on plasma glucose or insulin levels in rats. Taken together, these data suggest that the GLP-1RA, liraglutide, can effectively reduce both cue-induced heroin seeking and drug-induced reinstatement of heroin seeking in rats - even when the dose is gradually titrated to the 0.3 mg/kg dose of the drug.
The present data add to a now growing literature suggesting that GLP-1RAs show promise as a new treatment for substance use disorders. As alluded to, a large number of reports suggest that GLP-1RAs may be useful in the treatment of other SUDs,14–15,17,19–21 while our lab and others have provided evidence that GLP-1RAs also are effective in animal models of OUD.31–33,36,41 In the present paper we found that titrating the dose to 0.3 mg/kg liraglutide was most effective in reducing heroin-seeking behaviors, particularly in rats with a history of high heroin self-administration. This is consistent with our findings that the acute injection of 0.3 mg/kg of liraglutide significantly reduced cue-induced heroin seeking and drug- and stress-induced reinstatement of heroin seeking in rats,36 and with similar findings with cue-induced fentanyl seeking and drug-induced reinstatement of fentanyl seeking.66 As discussed, the present data also are consistent with a previously published report showing protective effects of chronic daily treatment with 0.1 mg/kg liraglutide. In that case, however, chronic daily treatment with 0.1 mg/kg liraglutide reduced drug self-administration and drug-induced reinstatement of heroin seeking behavior, but not cue-induced heroin seeking.41 The failure of the 0.1 mg/kg dose of liraglutide to reduce cue-induced heroin seeking in Douton et al. likely was due to the use of a short 1 h pretreatment time. Interestingly, titrating to the higher 0.6 mg/kg dose of liraglutide, even with the 6 h pretreatment time, was not more effective than was titrating to the 0.3 mg/kg dose. Such a finding suggests the development of tolerance following chronic administration of higher doses of the drug. Depending on the cell or tissue type, GLP-1Rs can be desensitized and internalized with chronic exposure of GLP-1RA.61 Thus, with higher doses there may be a higher likelihood for desensitization or internalization – i.e., tolerance (see below for further discussion).
Cues associated with drug taking can lead to onset of withdrawal symptoms in humans45,46 and rats.47,48 Cue-induced seeking likely is an index of cue-induced withdrawal since rats are placed into an environment with drug-associated cues (e.g., the self-administration chambers, the light cue, white noise, sounds of spouts advancing). Further, a fairly recent report showed that greater withdrawal in humans with an OUD was associated with greater activation of the NAc to drug-paired cues.71 Because GLP-1RAs are able to reduce cue-induced seeking in rats, as demonstrated in our previous papers,31–33,36,41 and in Figure 6 of this report (see high drug takers), we speculate that GLP-1RAs are able to reduce cue-induced withdrawal. In accordance, in our hands, treatment with Ex-4 also greatly reduces the conditioned aversive taste reactivity behavior (i.e., gapes) associated with naloxone induced withdrawal.67 Like exposure to drug-related cues, re-exposure to the drug itself also can prompt withdrawal symptoms in people with a history of fentanyl taking that are treated with buprenorphine, for example,65 and drug-induced reinstatement can sensitize an individual to the rewarding properties of the drug.52 Thus, because GLP-1RAs also are able to reduce drug-induced reinstatement as demonstrated in our previous papers,31–33,36,41 and in Figures 4 and 6 of this manuscript, we speculate that GLP-1RAs also may reduce sensitization to the rewarding properties of the drug. Finally, our data showing that liraglutide does not alter glucose homeostasis is consistent with published preclinical and clinical data indicating that GLP-1RAs do not induce hypoglycemia in the absence of resting hyperglycemia.49,50
In several studies, we focused on acute treatment of liraglutide and its effect on opioid seeking and taking behavior. While we have found that acute treatment of liraglutide is able to reduce cue-induced heroin seeking, drug- and stress-induced reinstatement of heroin seeking41, cue-induced fentanyl seeking, and drug-induced reinstatement of fentanyl seeking66, long term treatment of OUD will require chronic, rather than acute, administration of the drug. As such, for the present report, we modeled the titration regimen used to treat obesity and T2DM in humans where the dose of the drug is titrated over time to prevent side effects (e.g., nausea).35 Here, we found that chronic treatment with liraglutide titrated to the 0.3 mg/kg dose across a 2-week abstinence period and prior to test was able to reduce cue-induced heroin seeking in high drug takers and drug-induced reinstatement of heroin seeking. While we cannot be certain if liraglutide-reduced seeking at test is due to chronic or acute administration of the drug (as liraglutide was administered chronically, but also 6 h prior to test), it appears to the best of our knowledge, that the drug needs to be administered daily – i.e., the drug needs to be on board for the drug to be effective. Further, higher doses of the drug may not be more effective, as higher doses may be more likely to support the development of tolerance. Here, titrating to the 0.6 mg/kg dose of liraglutide was not effective in reducing drug seeking; and this effect of apparent tolerance was moderated when the dose of the drug was reduced by half to 0.3 mg/kg sc. Of course, this 0.3 mg/kg dose was most effective in Experiment 2, when the rats had no experience with the higher 0.6 mg/kg dose of the drug. In rodents, tolerance develops to the glucose lowering effects and to the gastric emptying effect of liraglutide.72,74 However, there may not be tolerance development to glucose lowering effects in humans.73
4.1. Mechanism of Action
As alluded to, in 2008, we hypothesized that withdrawal is a need state like starvation or dehydration.8 Thus, withdrawal would drive individuals to seek drug like one would pursue food when starved or water when severely dehydrated. In line with this hypothesis, we demonstrated that treatment with a GLP-1RA, a known satiety agent, reduced evidence of this ‘need’ state (i.e., reduced cue- and drug-induced heroin seeking). That being said, while we have obtained evidence that treatment with GLP-1RAs reduces heroin taking and seeking behaviors in rats, the underlying mechanism of action remains unknown. There are GLP-1Rs in brain areas associated with reward including the ventral tegmental area (VTA) and the NAc.53 There also are GLP-1 producing neurons that project directly from the NST to the VTA and NAc10,54 and activation of GLP-1Rs in the NTS leads to a reduction in the expression of dopamine-related genes in the VTA.55 As such, it is possible that GLP-1R activation modulates dopamine production and release in mesolimbic reward areas, thereby blunting the rewarding effects of the drug. This conclusion is consistent with the hypothesis that GLP-1RAs may have a general inhibitory effect on motivation, per se. While this may be so, and GLP-1RAs do reduce a great deal of motivated behavior and NAc dopamine as discussed,14–16,19–20 it is interesting to note that the GLP-1RA in the present report reduced responding more in the high heroin takers than in the low heroin takers – a dissociation that is not necessarily consistent with a general motivation deficit. Further, humans treated with GLP-1RAs for T2DM report a decrease, rather than an increase, in depression.75 Finally, GLP-1 also is implicated in stress. It is possible that GLP-1RAs are able to modulate the stress system and reduce withdrawal symptoms associated with drug-seeking.17 For example, there is evidence that stimulation of GLP-1Rs increases stress hormones when in a fed state.56 Thus, when one is sated, GLP-1R activation increases stress to prevent the organism from taking risks to search for food. When an organism is in a fasted state, decreased activation of GLP-1 receptors attenuates the stress response.62 Accordingly, when starved, there is decreased GLP-1R activation and a decrease in perceived stress, allowing for engagement in higher-risk behaviors in an effort to successfully satisfy one’s needs.
4.2. Individual differences
There is evidence that only 20% of people that take heroin develop an OUD.42 This demonstrates that some people are more susceptible than others to develop OUD or other SUDs. There also is evidence that OUD has a hereditary component,43–44 which further demonstrates that genes are important for developing OUD. Through our previous research, and as shown in Figures 2 and 5, we have discovered that about half of the rats become high drug takers and the other half become low drug takers.34,37–38,40 In this paradigm, we do find a higher percentage (50%) of rats that we categorize as high drug takers compared to humans who take heroin and develop OUD (20%). This simply may be due to the use of the median split where, by definition, 50% of the subjects are denoted as high drug takers. A second consideration is that our rats, unlike many humans, live in an unenriched environment. In our hands, rats with environmental enrichment self-administer less cocaine than their non-enriched counterparts,63 work less for heroin on a progressive ratio schedule of reinforcement, exhibit less cue-induced seeking for heroin, and demonstrate less drug-induced reinstatement of heroin seeking behavior.57 Thus, while biology is important to consider for risks of developing OUD, social factors also play a prominent role.58 That being said, and regardless of the percentages, treatment with the GLP-1RA throughout abstinence and prior to test clearly reduced heroin seeking, and this effect was most robust in the most vulnerable high drug-taking/seeking rats.
4.3. Glucose and Insulin (and GLP-1RA Side Effects)
Glucose and insulin levels were examined across a range of doses of liraglutide. While GLP-1RAs are good for treating diabetes and obesity due to the fact that they help increase insulin levels and decrease glucose levels, there is a risk of hypoglycemia in those receiving GLP-1RA treatment for OUD.51 The results shown in Figure 7, however, demonstrate that liraglutide treatment at 0.06, 0.1, 0.3, 0.6, and 1.0 mg/kg did not lead to any significant changes in glucose and insulin levels. While there was a numerical reduction in plasma glucose at the critical 0.3 mg/kg dose of liraglutide, this trend did not approach statistical significance, it did not lead to a plasma glucose level below 170 mg/dL, which remains high, and the results of a power analysis indicated that 10 – 12 subjects would be needed to attain statistical significance at the 6 h time point and, even then, would result in only a 6% reduction in plasma glucose relative to time zero (i.e., BL). It is well established that there is a circadian rhythm of insulin secretion and blood glucose concentrations in rats76, with these hormones peaking at night during the active phase and during feeding behavior.77 Despite this, recent studies using continuous blood glucose monitoring show very small differences in mean night versus day blood glucose concentrations (<10 mg/dL) in healthy rats in the absence of obesity or diabetes.78 Further, for our experiments, rats were fasted throughout blood sample collection, which would have minimized circadian fluctuation of glucose and insulin levels.79 Taken together, the data suggest that, while a saline control should be included in the future, treatment with liraglutide likely did not block the naturally occurring circadian rhythm of these hormones and it did not result in hypoglycemia or abnormal insulin levels when assessed using a within subjects design. Finally, baseline glucose levels (time point zero) in these rats were high. This likely reflects the fact that glucose was measured from arterial plasma. It is known that whole blood arterial glucose is ~5% higher than whole blood venous glucose, and glucose measured from plasma can be 10–20% higher than when measured in whole blood.64 This may explain why resting glucose values were ~180 mg/dL in these rats, which would correspond to 140–150 mg/dL in whole blood. Thus, in addition to our previous research showing that various doses of liraglutide did not lead to nausea,41 we conclude at this juncture that liraglutide, at doses effective in treating OUD in the rodent model, does not precipitate the adverse side effects of either nausea or hypoglycemia in the rat. This finding expands the null effect on plasma glucose as previously reported.41 Together, these findings suggest that the GLP-1RA may be not only effective, but also safe for the treatment of OUD in humans.
4.4. Limitations/promise
Whereas this paper further demonstrates the promise of GLP-1RAs for the treatment of OUD, and provides important information on its use, there are limitations. This study was conducted in male rats only. Future studies will need to repeat these experiments in female rats. Additionally, differences have been found between rats and mice, where a study of GLP-1RA treatment for OUD in mice did not demonstrate reduction of opioid seeking behaviors as has been found in rat studies.30 Future studies will need to more thoroughly test the effectiveness of GLP-1RA treatment in mice. That being said, and despite these limitations, the present data combine with a growing number of published reports to suggest that treatment with a GLP-1RA can reduce cue-induced heroin seeking and drug-induced reinstatement of heroin seeking in rats.
Figure 1: Outline for Experiments 1 and 2.
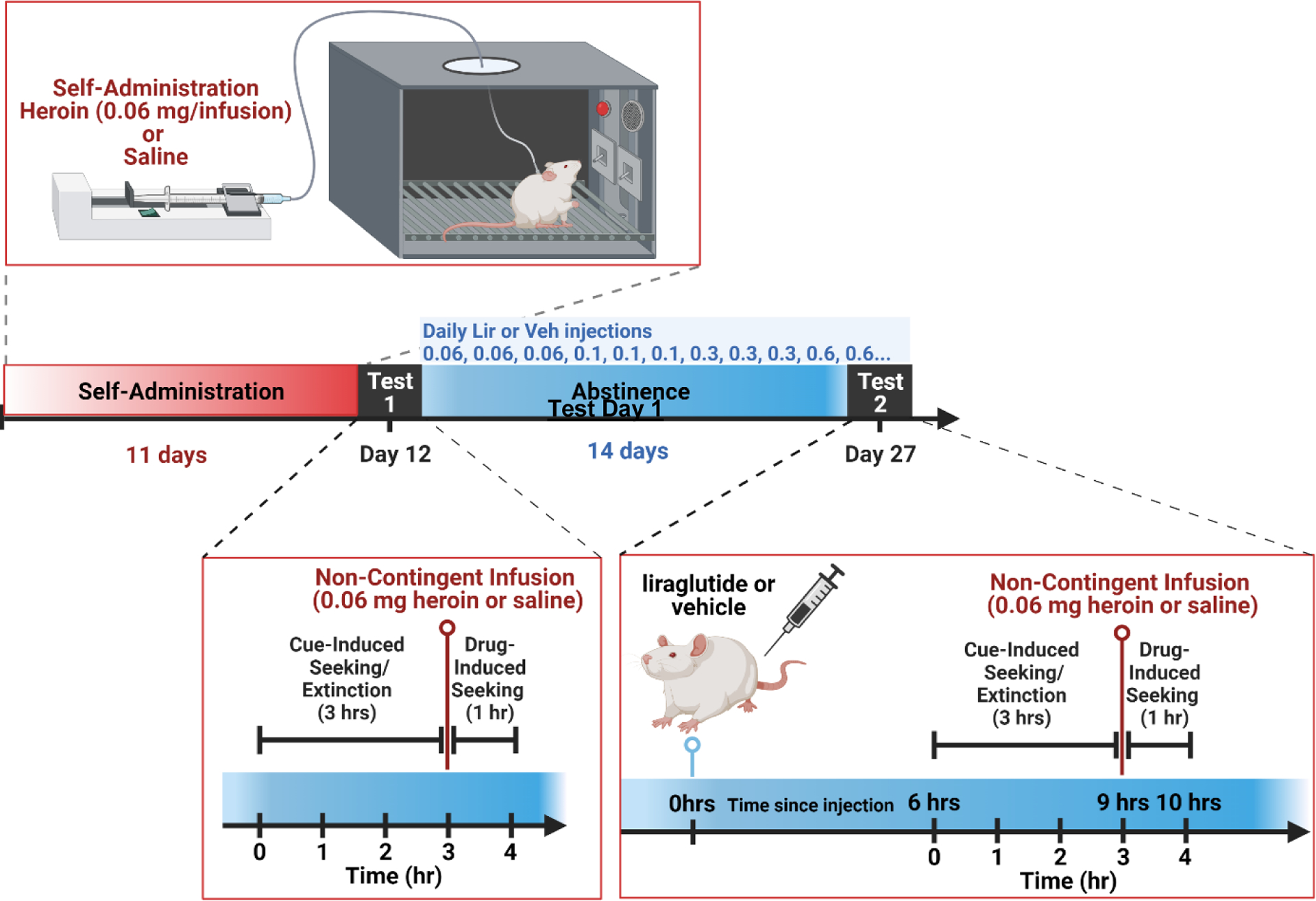
After jugular catheter implantation surgery, recovery, and habituation, the rats had 11 days of saline or heroin self-administration (SA). On the 12th day, the rats had a first test day (Test Day 1) to assess cue-induced seeking during extinction and drug-induced reinstatement. After Test Day 1, the rats had 2 weeks of abstinence in their home cage and were treated with the titrated doses of liraglutide or saline. The dose starts at 0.06 mg/kg and goes up every three days until it reaches the maximum (0.6 mg/kg for Experiment 1 and 0.3 mg/kg for Experiment 2). Thereafter, that dose is given for the remaining days of abstinence. After two weeks of abstinence and titrated dosing with liraglutide or saline, the rats received a second test day (Test Day 2) to test the effect of the titrated dose of liraglutide on cue-induced seeking and drug-induced reinstatement of heroin seeking. For Experiment 1, the rats had an additional three days of abstinence and daily saline or 0.3 mg/kg of liraglutide treatment before going through a similarly conducted Test Day 3. Figure created with BioRender.com.
Acknowledgements
This work was supported by the National Institutes of Health grant UG3 DA050325 (PSG/SB) and a Catalyst Award from the Penn State University Social Science Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.Pandemic and Policy Options to Move Forward. To the Point: Commonwealth Fund; 2021. Available from: 10.26099/gyf5-3z49. [DOI] [Google Scholar]
- 2.Hser YI, Saxon AJ, Huang D, Hasson A, Thomas C, Hillhouse M, et al. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction. 2014;109(1):79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pierce M, Bird SM, Hickman M, Marsden J, Dunn G, Jones A, et al. Impact of treatment for opioid dependence on fatal drug-related poisoning: a national cohort study in England. Addiction. 2016;111(2):298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JD, Nunes EV Jr., Novo P, Bachrach K, Bailey GL, Bhatt S, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatia G, Sarkar S. Sublingual buprenorphine-naloxone precipitated withdrawal-A case report with review of literature and clinical considerations. Asian J Psychiatr. 2020;53:102121. [DOI] [PubMed] [Google Scholar]
- 6.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242(4879):715–23. [DOI] [PubMed] [Google Scholar]
- 7.Bejerot N [Addiction as an artificially induced instinct. A theory]. Nord Med. 1971;85(1):20–7. [PubMed] [Google Scholar]
- 8.Grigson PS. Reward Comparison: The Achilles’ heel and hope for addiction. Drug Discov Today Dis Models. 2008;5(4):227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prasad-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context. 2015;4:212283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012;153(2):647–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. The Journal of comparative neurology. 1999;403(2):261–80. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Ferreras L, Richard JE, Noble EE, Eerola K, Anderberg RH, Olandersson K, et al. Lateral hypothalamic GLP-1 receptors are critical for the control of food reinforcement, ingestive behavior and body weight. Molecular psychiatry. 2018;23(5):1157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379(6560):69–72. [DOI] [PubMed] [Google Scholar]
- 14.Egecioglu E, Engel JA, Jerlhag E. The glucagon-like peptide 1 analogue, exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice. PLoS One. 2013;8(7):e69010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorensen G, Reddy IA, Weikop P, Graham DL, Stanwood GD, Wortwein G, et al. The glucagon-like peptide 1 (GLP-1) receptor agonist exendin-4 reduces cocaine self-administration in mice. Physiology & Behavior. 2015;149:262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy IA, Pino JA, Weikop P, Osses N, Sorensen G, Bering T, et al. Glucagon-like peptide 1 receptor activation regulates cocaine actions and dopamine homeostasis in the lateral septum by decreasing arachidonic acid levels. Transl Psychiatry. 2016;6:e809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt HD, Mietlicki-Baase EG, Ige KY, Maurer JJ, Reiner DJ, Zimmer DJ, et al. Glucagon-Like Peptide-1 Receptor Activation in the Ventral Tegmental Area Decreases the Reinforcing Efficacy of Cocaine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016;41(7):1917–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKay NJ, Daniels D. Glucagon-like peptide-1 receptor agonist administration suppresses both water and saline intake in rats. J Neuroendocrinol. 2013;25(10):929–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham DL, Erreger K, Galli A, Stanwood GD. GLP-1 analog attenuates cocaine reward. Molecular psychiatry. 2013;18(9):961–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egecioglu E, Engel JA, Jerlhag E. The glucagon-like peptide 1 analogue Exendin-4 attenuates the nicotine-induced locomotor stimulation, accumbal dopamine release, conditioned place preference as well as the expression of locomotor sensitization in mice. PLoS One. 2013;8(10):e77284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernandez NS, Ige KY, Mietlicki-Baase EG, Molina-Castro GC, Turner CA, Hayes MR, et al. Glucagon-like peptide-1 receptor activation in the ventral tegmental area attenuates cocaine seeking in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2018;43(10):2000–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blonde L, Rosenstock J, Triplitt C. What are incretins, and how will they influence the management of type 2 diabetes? J Manag Care Pharm. 2006;12(7 Suppl A):S2–12; quiz S4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fink-Jensen A Does treatment with GLP-1 reduce alcohol intake in patients with alcohol depence? (EXALT) NCT03232112. ClinicalTrials.gov: National Institutes of Health: U.S. National Library of Medicine; 2017. Available from: https://clinicaltrials.gov/ct2/show/NCT03232112. [Google Scholar]
- 24.Boston Medical Center. The effects of exenatide, a GLP-1agonist, on alcohol self-administration in heavy drinkers. NCT03645408. ClinicalTrials.gov: National Institutes of Health: U.S. National Library of Medicine; 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT03645408. [Google Scholar]
- 25.Imperial College of London. Gut hormones in obesity, nicotine and alcohol dependence (GHADD). NCT02690987. ClinicalTrials.gov: National Insitutes of Health: U.S. National Library of Medicine; 2015. Available from: https://clinicaltrials.gov/ct2/show/NCT02690987. [Google Scholar]
- 26.Ashare R Daily liraglutide for nicotine dependence (DAL). NCT03712098. ClinicalTrials.gov: National Institutes of Health: U.S. National Library of Medicine; 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT03712098. [Google Scholar]
- 27.University Hospital B, Switzerland,. Smoking cessation facilitated by glucagon-like peptide-1 (GLP-1) Analogues (SKIP). NCT03204396. ClinicalTrials.gov: National Institutes of Health: U.S. National Library of Medicine; 2017. Available from: https://clinicaltrials.gov/ct2/show/NCT03204396. [Google Scholar]
- 28.The University of Texas Health Science Center Houston. Exenatide once weekly for smoking cessation. NCT02975297. ClinicalTrials.gov: National Institutes of Health: U.S. National Library of Medicine; 2016. Available from: https://clinicaltrials.gov/ct2/show/NCT02975297. [Google Scholar]
- 29.Bunce S Use of a GLP-1R Agonist to Treat Opioid Use Disorder. 2021. Available from: https://clinicaltrials.gov/ct2/show/NCT04199728?term=Bunce&draw=2&rank=1. NLM identifier: NCT04199728
- 30.Bornebusch AB, Fink-Jensen A, Wortwein G, Seeley RJ, Thomsen M. Glucagon-Like Peptide-1 Receptor Agonist Treatment Does Not Reduce Abuse-Related Effects of Opioid Drugs. eNeuro. 2019;6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Kahng MW, Elkind JA, Weir VR, Hernandez NS, Stein LM, et al. Activation of GLP-1 receptors attenuates oxycodone taking and seeking without compromising the antinociceptive effects of oxycodone in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2020;45(3):451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Douton JE, Augusto C, Stoltzfus B, Carkaci-Salli N, Vrana KE, Grigson PS. Glucagon-like peptide-1 receptor agonist, exendin-4, reduces reinstatement of heroin-seeking behavior in rats. Behavioural pharmacology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Rahematpura S, Ragnini KH, Moreno A, Stecyk KS, Kahng MW, Milliken BT, Hayes MR, Doyle RP, Schmidt HD. A novel dual agonist of glucagon-like peptide-1 receptors and neuropeptide Y2 receptors attenuates fentanyl taking and seeking in male rats. Neuropharmacology. 2021. Jul 1;192:108599. doi: 10.1016/j.neuropharm.2021.108599. Epub 2021 May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colechio EM, Imperio CG, Grigson PS. Once is too much: conditioned aversion develops immediately and predicts future cocaine self-administration behavior in rats. Behav Neurosci. 2014. Apr;128(2):207–16. doi: 10.1037/a0036264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lundgren JR, Janus C, Jensen SBK, Juhl CR, Olsen LM, Christensen RM, Svane MS, Bandholm T, Bojsen-Møller KN, Blond MB, Jensen JB, Stallknecht BM, Holst JJ, Madsbad S, Torekov SS. Healthy Weight Loss Maintenance with Exercise, Liraglutide, or Both Combined. N Engl J Med. 2021. May 6;384(18):1719–1730. doi: 10.1056/NEJMoa2028198. [DOI] [PubMed] [Google Scholar]
- 36.Douton JE, Acharya NK, Stoltzfus B, Sun D, Grigson PS, & Nyland JE (2021). Acute Glucagon-Like Peptide-1 Receptor Agonist Liraglutide Prevents Drug-Induced Heroin Seeking in Rats. Behav Pharmacol. 2022. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Twining RC, Bolan M, Grigson PS. Yoked delivery of cocaine is aversive and protects against the motivation for drug in rats. Behav Neurosci. 2009. Aug;123(4):913–25. doi: 10.1037/a0016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuntz KL, Twining RC, Baldwin AE, Vrana KE, Grigson PS. Heroin self-administration: I. Incubation of goal-directed behavior in rats. Pharmacol Biochem Behav. 2008. Sep;90(3):344–8. doi: 10.1016/j.pbb.2008.03.018. Epub 2008 Apr 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puhl MD, Boisvert M, Guan Z, Fang J, Grigson PS. A novel model of chronic sleep restriction reveals an increase in the perceived incentive reward value of cocaine in high drug-taking rats. Pharmacol Biochem Behav. 2013;109:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: a model of drug- induced devaluation of natural rewards. Behav Neurosci. 2002;116(2):321–333. [PubMed] [Google Scholar]
- 41.Douton JE, Horvath N, Mills-Huffnagle S, Nyland JE, Hajnal A, Grigson PS. Glucagon-like peptide-1 receptor agonist, liraglutide, reduces heroin self-administration and drug-induced reinstatement of heroin-seeking behaviour in rats. Addict Biol. 2022. Mar;27(2):e13117. doi: 10.1111/adb.13117. Epub 2021 Nov 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Institute on Drug Abuse. (2014). Drug Facts: Heroin. Bethesda, MD: National Institute on Drug Abuse. Available at http://www.drugabuse.gov/publications/drugfacts/heroin. [Google Scholar]
- 43.Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, True W, Lin N, Toomey R, Eaves L. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry. 1998. Nov;55(11):967–72. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- 44.Wilens TE, Biederman J, Bredin E, Hahesy AL, Abrantes A, Neft D, Millstein R, Spencer TJ. A family study of the high-risk children of opioid- and alcohol-dependent parents. Am J Addict. 2002. Winter;11(1):41–51. doi: 10.1080/10550490252801620. [DOI] [PubMed] [Google Scholar]
- 45.Childress AR, McLellan AT, O’Brien CP. Measurement and extinction of conditioned withdrawal-like responses in opiate-dependent patients. NIDA Res Monogr. 1984. Mar;49:212–9. [PubMed] [Google Scholar]
- 46.O’Brien CP, Testa T, O’Brien TJ, Brady JP, Wells B. Conditioned narcotic withdrawal in humans. Science. 1977. Mar 11;195(4282):1000–2. doi: 10.1126/science.841320. [DOI] [PubMed] [Google Scholar]
- 47.Zhou W, Zhang F, Liu H, Tang S, Lai M, Zhu H, Kalivas PW. Effects of training and withdrawal periods on heroin seeking induced by conditioned cue in an animal of model of relapse. Psychopharmacology (Berl). 2009. May;203(4):677–84. doi: 10.1007/s00213-008-1414-2. Epub 2008 Nov 29. [DOI] [PubMed] [Google Scholar]
- 48.Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000. Apr;22(4):413–21. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- 49.Ja’arah D, Al Zoubi MS, Abdelhady G, Rabi F, Tambuwala MM. Role of Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists in Hypoglycemia. Clin Med Insights Endocrinol Diabetes. 2021. Oct 17;14:11795514211051697. doi: 10.1177/11795514211051697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang P, Liu Y, Ren Y, Bai J, Zhang G, Cui Y. The efficacy and safety of liraglutide in the obese, non-diabetic individuals: a systematic review and meta-analysis. Afr Health Sci. 2019. Sep;19(3):2591–2599. doi: 10.4314/ahs.v19i3.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edwards CM, Stanley SA, Davis R, Brynes AE, Frost GS, Seal LJ, Ghatei MA, Bloom SR. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab. 2001. Jul;281(1):E155–61. doi: 10.1152/ajpendo.2001.281.1.E155. [DOI] [PubMed] [Google Scholar]
- 52.De Vries TJ, Schoffelmeer AN, Binnekade R, Mulder AH, Vanderschuren LJ. Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. Eur J Neurosci. 1998. Nov;10(11):3565–71. doi: 10.1046/j.1460-9568.1998.00368.x. [DOI] [PubMed] [Google Scholar]
- 53.van Bloemendaal L, Ten Kulve JS, la Fleur SE, Ijzerman RG, Diamant M. Effects of glucagon-like peptide 1 on appetite and body weight: focus on the CNS. J Endocrinol. 2014. Mar 7;221(1):T1–16. doi: 10.1530/JOE-13-0414. [DOI] [PubMed] [Google Scholar]
- 54.Rinaman L Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010. Sep 2;1350:18–34. doi: 10.1016/j.brainres.2010.03.059. Epub 2010 Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richard JE, Anderberg RH, Göteson A, Gribble FM, Reimann F, & Skibicka KP (2015). Activation of the GLP-1 receptors in the nucleusof the solitary tract reduces food reward behavior and targets themesolimbic system. PLoS ONE,10(3), 1–21. 10.1371/journal.pone.0119034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kinzig KP, D’Alessio DA, Herman JP, Sakai RR, Vahl TP, Figueiredo HF, Murphy EK, Seeley RJ. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J Neurosci. 2003. Jul 16;23(15):6163–70. doi: 10.1523/JNEUROSCI.23-15-06163.2003. Erratum in: J Neurosci. 2003 Sep 3;23(22):following 8158. Figueredo, HF [corrected to Figueiredo, HF]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imperio CG, McFalls AJ, Hadad N, Blanco-Berdugo L, Masser DR, Colechio EM, Coffey AA, Bixler GV, Stanford DR, Vrana KE, Grigson PS, Freeman WM. Exposure to environmental enrichment attenuates addiction-like behavior and alters molecular effects of heroin self-administration in rats. Neuropharmacology. 2018. Sep 1;139:26–40. doi: 10.1016/j.neuropharm.2018.06.037. Epub 2018 Jun 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Webster LR. Risk Factors for Opioid-Use Disorder and Overdose. Anesth Analg. 2017. Nov;125(5):1741–1748. doi: 10.1213/ANE.0000000000002496. [DOI] [PubMed] [Google Scholar]
- 59.Agersø H, Jensen LB, Elbrønd B, Rolan P, Zdravkovic M. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia. 2002. Feb;45(2):195–202. doi: 10.1007/s00125-001-0719-z. [DOI] [PubMed] [Google Scholar]
- 60.Liu J, Pang ZP. Glucagon-like peptide-1 drives energy metabolism on the synaptic highway. FEBS J. 2016. Dec;283(24):4413–4423. doi: 10.1111/febs.13785. Epub 2016 Jul 1. [DOI] [PubMed] [Google Scholar]
- 61.Fletcher MM, Halls ML, Christopoulos A, Sexton PM, Wootten D. The complexity of signalling mediated by the glucagon-like peptide-1 receptor. Biochem Soc Trans. 2016. Apr 15;44(2):582–8. doi: 10.1042/BST20150244. [DOI] [PubMed] [Google Scholar]
- 62.Maniscalco JW, Zheng H, Gordon PJ, Rinaman L. Negative Energy Balance Blocks Neural and Behavioral Responses to Acute Stress by “Silencing” Central Glucagon-Like Peptide 1 Signaling in Rats. J Neurosci. 2015. Jul 29;35(30):10701–14. doi: 10.1523/JNEUROSCI.3464-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Puhl MD, Blum JS, Acosta-Torres S, Grigson PS. Environmental enrichment protects against the acquisition of cocaine self-administration in adult male rats, but does not eliminate avoidance of a drug-associated saccharin cue. Behav Pharmacol. 2012. Feb;23(1):43–53. doi: 10.1097/FBP.0b013e32834eb060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim HS. Blood Glucose Measurement: Is Serum Equal to Plasma? Diabetes Metab J. 2016. Oct;40(5):365–366. doi: 10.4093/dmj.2016.40.5.365. Epub 2016 Oct 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Varshneya NB, Thakrar AP, Hobelmann JG, Dunn KE, Huhn AS. Evidence of Buprenorphine-precipitated Withdrawal in Persons Who Use Fentanyl. J Addict Med. 2021. Nov 23. doi: 10.1097/ADM.0000000000000922. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Urbanik LA, Acharya N, and Grigson PS. Acute treatment with the glucagon-like peptide-1 receptor agonist, liraglutide, reduces cue- and drug-induced fentanyl seeking in rats. Submitted to Brain Research Bulletin on May 2, 2022. [DOI] [PubMed]
- 67.Olsen MN, Fields C, Grigson PS. Glucagon-like peptide-1 receptor agonist, exendin-4, pretreatment decreases aversive taste reactivity to naloxone-induced opioid withdrawal in rats. In preparation.
- 68.Perreault L Obesity in adults: Drug therapy. Post TW, ed. UpToDate. Waltham, MA: UpToDate Inc. http://www.uptodate.com. Accessed March 12, 2020. [Google Scholar]
- 69.Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci. 2012. Apr 4;32(14):4812–20. doi: 10.1523/JNEUROSCI.6326-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, Olivos DR, Alhadeff AL, Pierce RC, Hayes MR. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am J Physiol Endocrinol Metab. 2013. Dec 1;305(11):E1367–74. doi: 10.1152/ajpendo.00413.2013. Epub 2013 Oct 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shi Z, Jagannathan K, Padley JH, Wang AL, Fairchild VP, O’Brien CP, Childress AR, Langleben DD. The role of withdrawal in mesocorticolimbic drug cue reactivity in opioid use disorder. Addict Biol. 2021. Jul;26(4):e12977. doi: 10.1111/adb.12977. Epub 2020 Oct 23. [DOI] [PubMed] [Google Scholar]
- 72.Sedman T, Krass M, Rünkorg K, Vasar E, Volke V. Tolerance develops toward GLP-1 receptor agonists’ glucose-lowering effect in mice. Eur J Pharmacol. 2020. Oct 15;885:173443. doi: 10.1016/j.ejphar.2020.173443. Epub 2020 Aug 1. [DOI] [PubMed] [Google Scholar]
- 73.Sedman T, Vasar E, Volke V. Tolerance Does Not Develop Toward Liraglutide’s Glucose-Lowering Effect. J Clin Endocrinol Metab. 2017. Jul 1;102(7):2335–2339. doi: 10.1210/jc.2017-00199. [DOI] [PubMed] [Google Scholar]
- 74.Jelsing J, Vrang N, Hansen G, Raun K, Tang-Christensen M, Knudsen LB. Liraglutide: short-lived effect on gastric emptying -- long lasting effects on body weight. Diabetes Obes Metab. 2012. Jun;14(6):531–8. doi: 10.1111/j.1463-1326.2012.01557.x. Epub 2012 Feb 8. [DOI] [PubMed] [Google Scholar]
- 75.Wium-Andersen IK, Osler M, Jørgensen MB, Rungby J, Wium-Andersen MK. Diabetes, antidiabetic medications and risk of depression - A population-based cohort and nested case-control study. Psychoneuroendocrinology. 2022. Jun;140:105715. doi: 10.1016/j.psyneuen.2022.105715. Epub 2022 Mar 15. [DOI] [PubMed] [Google Scholar]
- 76.Kalsbeek A, Strubbe JH. Circadian control of insulin secretion is independent of the temporal distribution of feeding. Physiol Behav. 1998. Feb 15;63(4):553–8. doi: 10.1016/s0031-9384(97)00493-9. [DOI] [PubMed] [Google Scholar]
- 77.Qian J, Block GD, Colwell CS, Matveyenko AV. Consequences of exposure to light at night on the pancreatic islet circadian clock and function in rats. Diabetes. 2013. Oct;62(10):3469–78. doi: 10.2337/db12-1543. Epub 2013 Jun 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.King AJ, Austin AL, Nandi M, Bowe JE. Diabetes in Rats Is Cured by Islet Transplantation…But Only During Daytime. Cell Transplant. 2017. Jan 24;26(1):171–172. doi: 10.3727/096368916X692258. Epub 2016 Aug 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bizot-Espiard JG, Doublé A, Guardiola-Lemaitre B, Delagrange P, Ktorza A, Pénicaud L. Diurnal rhythms in plasma glucose, insulin, growth hormone and melatonin levels in fasted and hyperglycaemic rats. Diabetes Metab. 1998. Jun;24(3):235–40. [PubMed] [Google Scholar]


