Abstract
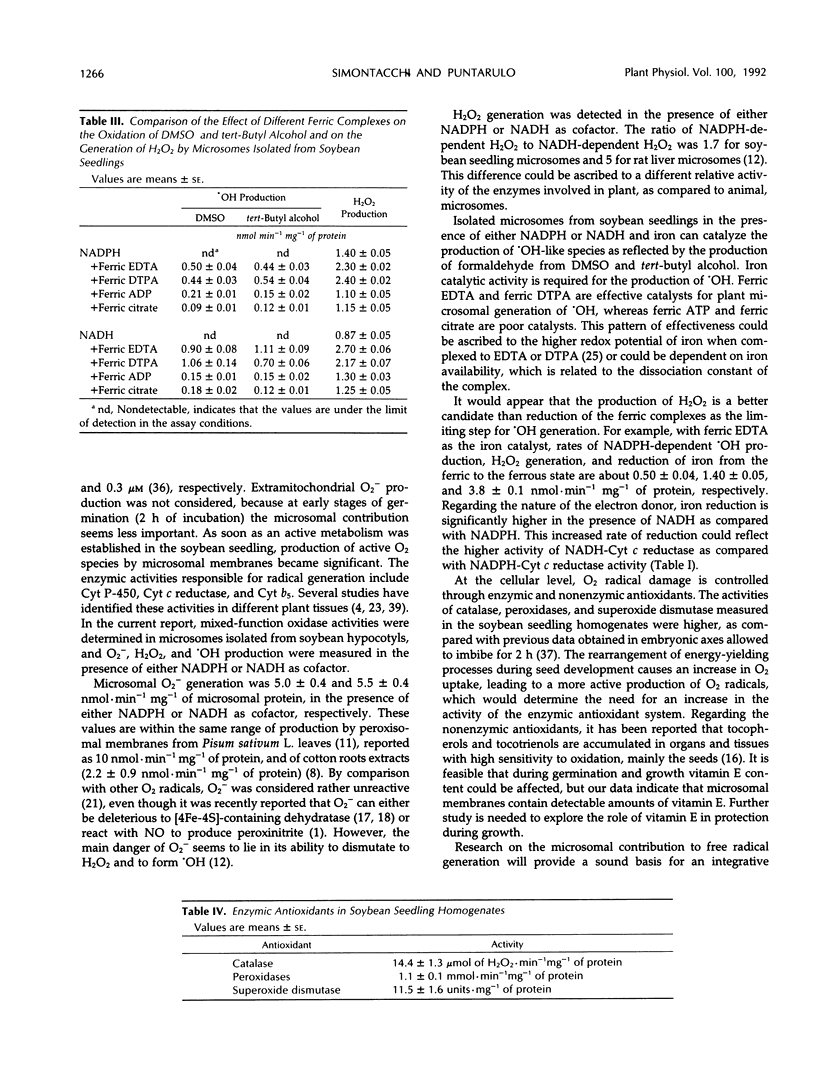
The generation of active oxygen species by microsomes isolated from soybean seedlings was studied. NADPH-dependent superoxide anion production was 5.0 ± 0.4 nmol · min−1 mg−1 of microsomal protein. Hydrogen peroxide generation by microsomes was 1.40 ± 0.05 nmol · min−1 mg−1 of protein. Hydroxyl radical production, in the presence of ferric EDTA, evaluated through the generation of formaldehyde from dimethyl sulfoxide or tert-butyl alcohol was 0.50 ± 0.04 and 0.44 ± 0.03 nmol · min−1 mg−1, respectively. NADH proved to be suitable as cofactor for oxygen radical generation by microsomes from soybean seedlings. Because transition metals are implicated in radical generation by biological systems, the ability of microsomal membranes to reduce iron complexes was studied. Ferric ATP, ferric citrate, ferric ADP, ferric diethylenetriamine pentaacetic acid, and ferric EDTA were efficiently reduced in the presence of either NADPH or NADH as cofactor. The pattern of effectiveness of the different ferric complexes, on superoxide anion, hydrogen peroxide, and hydroxyl radical production, was similar to that found with animal microsomes. The data presented here indicate that microsomal ability to catalyze oxygen radical generation must be considered as an important contribution to cellular radical steady-state concentrations in cells from soybean seedlings.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste I., Lesot A., Hasenfratz M. P., Durst F. Immunochemical characterization of NADPH-cytochrome P-450 reductase from Jerusalem artichoke and other higher plants. Biochem J. 1989 May 1;259(3):847–853. doi: 10.1042/bj2590847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste I., Salaün J. P., Simon A., Reichhart D., Durst F. Cytochrome P-450-Dependent omega-Hydroxylation of Lauric Acid by Microsomes from Pea Seedlings. Plant Physiol. 1982 Jul;70(1):122–126. doi: 10.1104/pp.70.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlakoglu J. T., John P. Cytochrome P-450-dependent metabolism of xenobiotics. A comparative study of rat hepatic and plant microsomal metabolism. Comp Biochem Physiol C. 1989;94(2):613–617. doi: 10.1016/0742-8413(89)90121-7. [DOI] [PubMed] [Google Scholar]
- Boveris A. Determination of the production of superoxide radicals and hydrogen peroxide in mitochondria. Methods Enzymol. 1984;105:429–435. doi: 10.1016/s0076-6879(84)05060-6. [DOI] [PubMed] [Google Scholar]
- Braughler J. M., Duncan L. A., Chase R. L. The involvement of iron in lipid peroxidation. Importance of ferric to ferrous ratios in initiation. J Biol Chem. 1986 Aug 5;261(22):10282–10289. [PubMed] [Google Scholar]
- Buege J. A., Aust S. D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Cederbaum A. I., Cohen G. Microsomal oxidant radical production and ethanol oxidation. Methods Enzymol. 1984;105:516–522. doi: 10.1016/s0076-6879(84)05071-0. [DOI] [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Del Río L. A., Fernández V. M., Rupérez F. L., Sandalio L. M., Palma J. M. NADH Induces the Generation of Superoxide Radicals in Leaf Peroxisomes. Plant Physiol. 1989 Mar;89(3):728–731. doi: 10.1104/pp.89.3.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker E., Cederbaum A. I. NADH-dependent generation of reactive oxygen species by microsomes in the presence of iron and redox cycling agents. Biochem Pharmacol. 1991 Jul 15;42(3):529–535. doi: 10.1016/0006-2952(91)90315-v. [DOI] [PubMed] [Google Scholar]
- Farber J. L., Kyle M. E., Coleman J. B. Mechanisms of cell injury by activated oxygen species. Lab Invest. 1990 Jun;62(6):670–679. [PubMed] [Google Scholar]
- Gardner P. R., Fridovich I. Superoxide sensitivity of the Escherichia coli 6-phosphogluconate dehydratase. J Biol Chem. 1991 Jan 25;266(3):1478–1483. [PubMed] [Google Scholar]
- Gardner P. R., Fridovich I. Superoxide sensitivity of the Escherichia coli aconitase. J Biol Chem. 1991 Oct 15;266(29):19328–19333. [PubMed] [Google Scholar]
- Hagmann M. L., Heller W., Grisebach H. Induction and characterization of a microsomal flavonoid 3'-hydroxylase from parsley cell cultures. Eur J Biochem. 1983 Aug 15;134(3):547–554. doi: 10.1111/j.1432-1033.1983.tb07601.x. [DOI] [PubMed] [Google Scholar]
- Hildebrandt A. G., Roots I., Tjoe M., Heinemeyer G. Hydrogen peroxide in hepatic microsomes. Methods Enzymol. 1978;52:342–350. doi: 10.1016/s0076-6879(78)52037-5. [DOI] [PubMed] [Google Scholar]
- Jansson I., Schenkman J. B. Studies on three microsomal electron transfer enzyme systems. Specificity of electron flow pathways. Arch Biochem Biophys. 1977 Jan 15;178(1):89–107. doi: 10.1016/0003-9861(77)90174-6. [DOI] [PubMed] [Google Scholar]
- Kochs G., Grisebach H. Phytoalexin synthesis in soybean: purification and reconstitution of cytochrome P450 3,9-dihydroxypterocarpan 6a-hydroxylase and separation from cytochrome P450 cinnamate 4-hydroxylase. Arch Biochem Biophys. 1989 Sep;273(2):543–553. doi: 10.1016/0003-9861(89)90514-6. [DOI] [PubMed] [Google Scholar]
- Madyastha K. M., Meehan T. D., Coscia C. J. Characterization of a cytochrome P-450 dependent monoterpene hydroxylase from the higher plant Vinca rosea. Biochemistry. 1976 Mar 9;15(5):1097–1102. doi: 10.1021/bi00650a023. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- NASH T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 1953 Oct;55(3):416–421. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Potts J. R., Weklych R., Conn E. E., Rowell J. The 4-hydroxylation of cinnamic acid by sorghum microsomes and the requirement for cytochrome P-450. J Biol Chem. 1974 Aug 25;249(16):5019–5026. [PubMed] [Google Scholar]
- Puntarulo S., Cederbaum A. I. Comparison of the ability of ferric complexes to catalyze microsomal chemiluminescence, lipid peroxidation, and hydroxyl radical generation. Arch Biochem Biophys. 1988 Aug 1;264(2):482–491. doi: 10.1016/0003-9861(88)90313-x. [DOI] [PubMed] [Google Scholar]
- Puntarulo S., Cederbaum A. I. Increased NADPH-dependent chemiluminescence by microsomes after chronic ethanol consumption. Arch Biochem Biophys. 1988 Nov 1;266(2):435–445. doi: 10.1016/0003-9861(88)90275-5. [DOI] [PubMed] [Google Scholar]
- Puntarulo S., Galleano M., Sanchez R. A., Boveris A. Superoxide anion and hydrogen peroxide metabolism in soybean embryonic axes during germination. Biochim Biophys Acta. 1991 Jul 8;1074(2):277–283. doi: 10.1016/0304-4165(91)90164-c. [DOI] [PubMed] [Google Scholar]
- Puntarulo S., Sánchez R. A., Boveris A. Hydrogen peroxide metabolism in soybean embryonic axes at the onset of germination. Plant Physiol. 1988 Feb;86(2):626–630. doi: 10.1104/pp.86.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahier A., Taton M. The 14 alpha-demethylation of obtusifoliol by a cytochrome P-450 monooxygenase from higher plants' microsomes. Biochem Biophys Res Commun. 1986 Nov 14;140(3):1064–1072. doi: 10.1016/0006-291x(86)90743-6. [DOI] [PubMed] [Google Scholar]
- Rich P. R., Bendall D. S. Cytochrome components of plant microsomes. Eur J Biochem. 1975 Jul 1;55(2):333–341. doi: 10.1111/j.1432-1033.1975.tb02167.x. [DOI] [PubMed] [Google Scholar]
- Salaün J. P., Benveniste I., Reichhart D., Durst F. A microsomal (cytochrome P-450)-linked lauric-acid-monooxygenase from aged Jerusalem-artichoke-tuber tissues. Eur J Biochem. 1978 Sep 15;90(1):155–159. doi: 10.1111/j.1432-1033.1978.tb12586.x. [DOI] [PubMed] [Google Scholar]
- Soliday C. L., Kolattukudy P. E. Biosynthesis of Cutin omega-Hydroxylation of Fatty Acids by a Microsomal Preparation from Germinating Vicia faba. Plant Physiol. 1977 Jun;59(6):1116–1121. doi: 10.1104/pp.59.6.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianello A., Macrì F. NAD(P)H oxidation elicits anion superoxide formation in radish plasmalemma vesicles. Biochim Biophys Acta. 1989 Apr 14;980(2):202–208. doi: 10.1016/0005-2736(89)90400-8. [DOI] [PubMed] [Google Scholar]


