Abstract
Increased tRNA abundance and amino acid coupling generally promote increased oncogenesis. By contrast, a new study shows that in breast cancer, the leucyl-tRNA synthetase LARS suppresses transformation and tumour development by increasing tRNA-LeuCAG translation of certain tumour suppressor mRNAs.
Cellular transformation and tumour development both involve rewiring the transcriptome (mRNAs expressed) and the translatome (mRNAs and specific coding regions translated). Unlike transcriptional control, translational control is less well studied in cancer. Nevertheless, translational control in cancer cells has been shown to include increased overall cancer cell protein synthesis activity and selective translation of specific mRNAs that encode proteins involved in cell proliferation, migration, survival, transformation, angiogenesis and other physiological drivers of malignant progression1,2. Both increased levels of protein synthesis and selectively increased translation of certain mRNAs typically involve the overexpression of mRNA translation factors, some common to many cancer types, and some quite specific to certain cancers. Among these factors, surprisingly, are some of the most staid and evolutionarily conserved protein synthesis factors, the aminoacyl-tRNA synthetases (ARSs) that couple amino acids to their respective tRNAs, a process known as charging. There are 20 of these synthetases, one for each type of amino acid that participates in protein synthesis. The ARSs catalyse the esterification of a tRNA to its cognate amino acid, generating an aminoacyl-tRNA. Charged tRNAs are subsequently delivered to the ribosome by elongation factors where they decode the mRNA by participating in peptide bond formation and protein synthesis. As such, the ARSs play a crucial and rate-limiting role in translation, first described more than 60 years ago3,4.
The ARS protein and gene are designated XRS and XARS, respectively, in which X denotes the single-letter amino acid code of the cognate amino acid. ARSs fall into two groups (class I and II) based on highly conserved structural differences3,4. The ARS proteins can be found in either free or complex-bound forms, the latter corresponding to a multi-tRNA synthetase complex (MSC). In recent years, it has become evident that certain ARSs possess other functions in addition to the enzymatic ligation of amino acids to tRNAs, which have important roles in promoting tumorigenesis, angiogenesis and inflammation5. This has spurred experimental development of new therapeutic agents, including small molecules, that inhibit certain ARSs for cancer therapy6.
In a seminal study, Tavazoie and colleagues extend the reach of ARSs into a new function in cancer biology7. They describe a mechanism by which the activity of a specific tRNA synthetase, the leucyl-tRNA synthetase LARS contributes to the early program of cellular transformation (Fig. 1). LARS specifically charges leucine to tRNA-LeuCAG corresponding to the leucine CUG codon. Cancers can arise, in part, by the acquisition of ‘driver’ gene mutations that confer greater neoplastic capacity, or the inactivation of ‘tumour suppressor’ genes that prevent neoplastic transformation. The authors identified a previously unknown tumour suppression program enacted by LARS that acts at the level of selective mRNA translation, and is inactivated in hormone receptor-negative and HER2-negative (triple-negative) breast cancer (TNBC) due to downregulation of LARS expression. They identified two poorly studied tumour suppressor genes: EMP3 (encoding epithelial membrane protein 3), a transmembrane signalling protein involved in cell proliferation and differentiation and implicated as a tumour suppressor8,9, and GGT5 (encoding γ-glutamyltransferase 5) that carries out extracellular cleavage of glutathione and is associated with regulation of redox potential, immune function and cell stress, but whose expression and role in human cancers remains poorly understood10,11. Tavazoie and colleagues7 found that both mRNAs were poorly translated due to loss of LARS expression in human TNBC.
Fig. 1 |. Downregulation of aminoacyl-tRNA synthetase LARS in breast cancer promotes transformation.
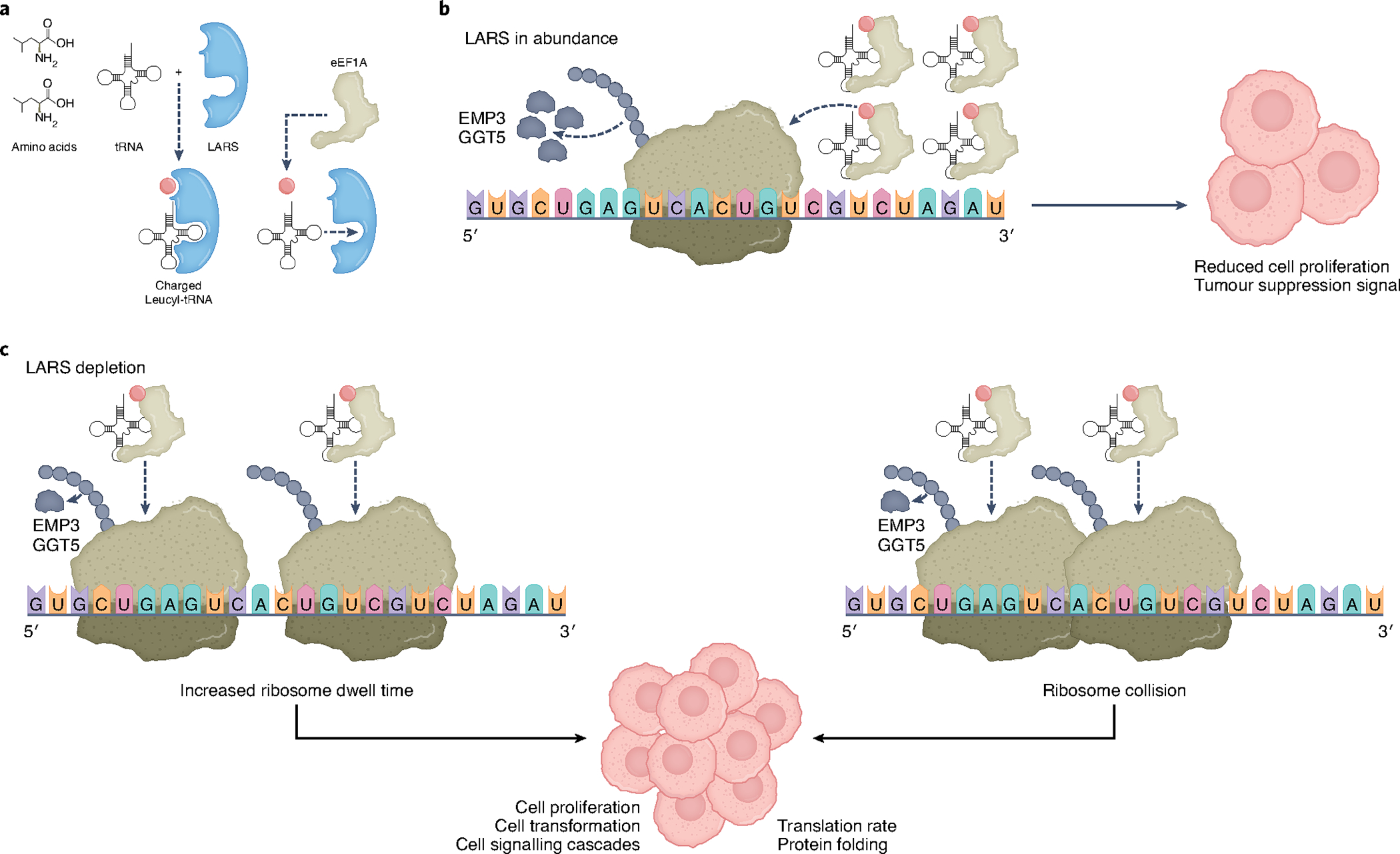
a, Leucine aminoacyl tRNA synthetase LARS covalently couples leucine (red dot) to cognate tRNAs, known as charging. Charged tRNAs are transported to elongating ribosomes by elongation factor eEF1A. b, Abundant charged tRNA-LeuCAG results in unrestricted ribosome elongation on tumour suppressor EMP3, GGT5 and other mRNAs, reducing cell proliferation and inhibiting transformation. c, Reduced levels of LARS decreases the abundance of charged tRNA-LeuCAG and translation of EMP3 and GGT5 tumour suppressor mRNAs, promoting increased cell proliferation and transformation. Two potential mechanisms are shown to account for reduced levels of EMP3 and GGT5 proteins upon limitation of tRNA-LeuCAG levels. Increased ribosome dwell times at CUG and GUC leucine codons might specifically reduce translation of encoding mRNAs, or increase ribosome collisions, provoking cell signalling responses or protein folding responses that downregulate encoded protein synthesis.
Increased expression of tRNA synthetases has been found to play an active part in promoting tumour growth in a variety of human cancers4,5. Therefore, the authors initially sought to determine the mRNA expression levels of all 20 tRNA synthetases in human breast cancers by querying the National Cancer Institute (NCI)’s The Cancer Genome Atlas (TCGA) database, comparing expression levels of normal and breast cancer mRNA, complemented with mRNA expression levels in human breast cancer cell lines. Although increased expression of certain ARSs was strongly associated with certain human cancers, the authors were surprised to find decreased expression of 4 ARSs (LARS, KARS, QARS and DARS) in TNBC specimens and cell lines, with LARS showing the most consistent reduction, whereas the expression of the remaining 16 ARSs were increased. The authors asked whether these four ARSs function as tumour suppressors.
First, the authors demonstrated that LARS but not other ARS mRNAs (that is, isoleucyl, histidyl and asparaginyl ARSs) were downregulated in human breast and murine mammary carcinoma cell lines and during tumour development. Next, to test the potential tumour suppression activity of LARS, the authors used the well characterized MMTV-PyMT mouse model of breast cancer development and metastatic progression, which they engineered to contain a monoallelic deletion of Lars in the mammary epithelium. The resulting LARS depletion resulted in a significant increase in both tumour number and burden. To determine whether the tumour suppression activity of LARS was due to its synthetase activity, the authors generated Cre-positive organoids from the LARS-depleted MMTV-PyMT mice, and then elegantly showed that wild-type LARs expression rescued the tumour suppression phenotype. Moreover, expression of LARS mutants lacking leucine-binding capacity (F50A/Y52A) or catalytic site activity (K716A/K719A) did not rescue the tumour suppression phenotype.
Given that only catalytically active LARS could repress tumour growth, the authors investigated whether LARS tumour suppression involved the alteration of specific leucyl-tRNA levels. They examined the five tRNA-Leu isoacceptors to which LARS ligates leucine. tRNA capture-sequence profiling was used to quantify charged tRNA species, which was compared to total tRNA by RNA sequencing. The results from breast cancer cell lines demonstrated that amino acid charging of four out of five leucyl-tRNAs was reduced with LARS depletion, with the most significant reduction in tRNA-LeuCAG. Using CRISPRi deletion of LARS, it was demonstrated that loss of tRNA-LeuCAG was sufficient to induce colony formation in soft agar assays.
To define the effect of reduced tRNA-LeuCAG on mRNA translation, genome-wide studies were carried out, including polysome analysis (mRNA-ribosome content), RiboTag (immunoprecipitation of ribosome-bound mRNAs) and Ribo-sequencing (ribosome exon footprint analysis). These studies demonstrated that LARS depletion mainly caused reduced translation of mRNAs that had higher densities of Leu-CUC and Leu-CUG codons. Ribo-sequencing data indicated that LARS depletion caused an increase in ribosome dwell times at Leu codons compared to other codons, suggestive of increased ribosome pausing. By carrying out tandem mass tag (TMT)-labelled proteomics, it was found that most proteins enriched in Leu-CUC and Leu-CUG codons were reduced with LARS depletion. Among these proteins were the tumour suppressors EMP3 and GGT5, the loss of which increased organoid growth. Finally, mutagenesis of the LARS-responsive codons Leu-CUC and Leu-CUG to non-LARS-responsive codons in reporter constructs of the EMP3 mRNA further confirmed that LARS depletion was responsible for reduced translation of this mRNA.
Several important questions emerge from this study. How is LARS regulated in normal and cancer cells? Although LARS is reduced in copy number, there are indications that LARS itself may be translationally controlled. Why is LARS downregulated in breast cancer but overexpressed and associated with increased tumorigenesis in renal, lung and other cancers? How does increased ribosome dwell time at cognate tRNA-LeuCAG codons impair selective mRNA translation? Studies have shown that ribosome pausing can increase ribosome collisions, which can downregulate protein synthesis and cellular transformation, and activate cell signalling cascades12–15. Whether this mechanism is involved remains to be fully determined.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Fabbri L, Chakraborty A, Robert C & Vagner S Nat. Rev. Cancer 21, 558–577 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Robichaud N, Sonenberg N, Ruggero D & Schneider RJ Cold Spring Harb. Perspect. Biol. 11, 1–16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Z, Sun B, Nie A, Yu D & Bian M Front. Cell Dev. Biol. 8, 599765 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park SG, Schimmel P & Kim S Proc. Natl Acad. Sci. USA 105, 11043–11049 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyeon DY et al. J. Biol. Chem. 294, 5340–5351 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon NH, Fox PL & Kim S Nat. Rev. Drug Discov. 18, 629–650 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Passarelli MC et al. Nat. Cell Biol. 10.1038/s41556-022-00856-5 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alaminos M et al. Cancer Res. 65, 2565–2571 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Zhou K, Sun Y, Dong D, Zhao C & Wang W Cell Death Dis. 12, 844 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W et al. Cell 165, 1092–1105 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wickham S, West MB, Cook PF & Hanigan MH Anal. Biochem. 414, 208–214 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinha NK et al. Elife 9, e58828 (2020).32744497 [Google Scholar]
- 13.Smith AM, Costello MS, Kettring AH, Wingo RJ & Moore SD Proc. Natl Acad. Sci. USA 116, 21769–21779 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu CC, Peterson A, Zinshteyn B, Regot S & Green R Cell 182, 404–416.e414 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juszkiewicz S et al. Elife 9, e60038 (2020).32657267 [Google Scholar]


