Abstract
Relapsed/refractory (R/R) Acute Myeloid Leukemia (AML) is a genetically complex and heterogeneous disease with a poor prognosis and limited treatment options. Thus, there is an urgent need to develop therapeutic combinations to overcome drug resistance in AML. This open-label, multicenter, international, phase 1b study evaluated the safety, efficacy, and pharmacokinetics of venetoclax in combination with alvocidib in patients with R/R AML. Patients were treated with escalating doses of venetoclax (400, 600, and 800 mg QD, orally, days 1-28) and alvocidib (45 and 60 mg/m2, intravenously, days 1-3) in 28-day cycles. The combination was found to be safe and tolerable, with no maximum tolerated dose reached. Drug-related Grade ≥3 adverse events were reported in 23 (65.7%) for venetoclax and 24 (68.6%) for alvocidib. No drug-related AEs were fatal. Gastrointestinal toxicities, including diarrhea, nausea, and vomiting were notable and frequent; otherwise, the toxicities reported were consistent with the safety profile of both agents. The response rate was modest (complete remission [CR] + incomplete CR [CRi], 11.4%; CR+CRi+partial response rate+morphologic leukemia-free state, 20%). There was no change in alvocidib pharmacokinetics with increasing doses of venetoclax. However, when venetoclax was administered with alvocidib, AUC24 and Cmax decreased by 18% and 19%, respectively. A recommended phase 2 dose was not established due to lack of meaningful increase in efficacy across all cohorts compared to what was previously observed with each agent alone. Future studies could consider the role of the sequence, dosing, and the use of a more selective MCL1 inhibitor for the R/R AML population.
Keywords: Acute Myeloid Leukemia, Drug Resistance, BCL-2, MCL-1, Venetoclax, Alvocidib
Introduction
Relapsed/refractory (R/R) acute myeloid leukemia (AML) is a genetically complex and heterogeneous disease.1, 2 Despite advances in therapy, treatment of patients with R/R AML remains challenging due to poor response rates.3, 4 No standard of care is available for R/R AML; hence, there is an urgent need to develop newer therapies and combinations to overcome treatment resistance and dismal outcomes.5
Venetoclax is an orally bioavailable, selective inhibitor of BCL-2 currently being investigated in several hematologic malignancies.6,7 In R/R AML, venetoclax showed modest clinical activity as a single agent.8 In combination with hypomethylating agents (HMAs) or cytarabine, venetoclax showed promise at overcoming the potential resistance mechanisms and enhancing the clinical benefits in patients with R/R AML.9–13
Due to genetic and molecular heterogeneity, AML cells can be co-dependent on other pro-survival proteins. Studies suggest that the related anti-apoptotic BCL-2 family member MCL-1 is important for sensitivity to cytotoxic agents and drives treatment resistance to BCL-2 and BCL-xL targeting compounds.14–16 Alvocidib is a potent CDK9 inhibitor that impacts a variety of short-lived mRNA transcripts and proteins critical for the growth and survival of tumor cells, including MCL-1.17 Alvocidib induces cancer cell apoptosis through MCL-1 downregulation.18 However, this effect can be resisted by increased activity of BCL-2. Alvocidib has shown a synergistic effect with venetoclax in vitro and in vivo, both in venetoclax-resistant and venetoclax-sensitive AML cells by decreasing MCL-1.19 Thus, a combination of both agents was hypothesized to overcome drug resistance.2, 20 However, both alvocidib and venetoclax have known hematological and gastrointestinal toxicities that could be additive.20–22
This phase 1b study evaluated the safety, pharmacokinetics, and preliminary efficacy of venetoclax combined with alvocidib in adults with R/R AML as a potential combination therapy for treating patients with R/R AML.
Methods
Patients
Enrolled patients were ≥ 18 years old with a confirmed diagnosis of relapsed/refractory AML (excluding acute promyelocytic leukemia with PML::RARA) by World Health Organization criteria and an Eastern Cooperative Oncology Group (ECOG) performance status of ≤ 2.
Patients were excluded if they had active or prior central nervous system leukemia, severe chronic obstructive pulmonary disease with hypoxemia, history of any malignancy within the last 6 months, allogeneic stem cell transplant within the last 6 months, or history of tumor lysis syndrome (TLS) due to previous exposure to venetoclax.
Study Design
This was an open-label, international, multicenter Phase 1b dose-escalation study (NCT03441555) conducted at 11 sites. The primary objectives were to determine the toxicity, pharmacokinetic (PK) profiles, and the recommended Phase 2 dose of intravenous (IV) alvocidib combined with daily oral venetoclax in patients with R/R AML. The secondary objective was to evaluate the efficacy of venetoclax and alvocidib, and the exploratory objective was to evaluate correlative efficacy biomarkers. The study was conducted per the International Conference on Harmonization, Good Clinical Practice Guidelines, and the Declaration of Helsinki. Local ethics committee approval was obtained, and patients provided written informed consent.
Treatments
Patients received IV alvocidib on days 1, 2, and 3 and daily oral venetoclax according to dose levels (cohorts 1—5) in 28-day treatment cycles. All venetoclax dose levels used a 3- or 4-day dose ramp-up. To mitigate the risk of TLS when initiating alvocidib with venetoclax, alvocidib dosing for cycle 1 used a 3-day dose ramp-up except for cohort 1. Concomitant use of strong CYP3A inducers was prohibited throughout the study. Dose escalation was guided by a Bayesian optimal interval design.23 A dose-limiting toxicity (DLT) review occurred after each dose-escalation cohort. Considerations for dose escalation are provided in the Supplementary Methods.
Study assessments
Adverse events (AEs) were investigator-assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.24 DLTs were assessed during the first and second cycles of venetoclax and alvocidib. A complete definition of a DLT is provided in the Supplementary Methods.
Disease assessments were performed by bone marrow aspiration with morphological and flow cytometry analysis. Disease assessments were performed at Day 28 ± 7 days in the first treatment cycle, and subsequent assessments were performed at Day 28 ± 5 days in every 3rd cycle. Patient responses were evaluated by the investigator per institutional practices and reported according to modified International Working Group (IWG) criteria for AML and European LeukemiaNet (ELN) recommendations.25, 26 Efficacy endpoints assessed were complete remission (CR) rate, composite CR rate (CRc; CR + CRi [CR with incomplete blood count recovery]), objective response rate (ORR; CR + CRi + PR [partial response]), and the leukemia response rate (CR + CRi + PR + MLFS [morphologic leukemia-free state]). The time to first response and duration for patients who achieved CR+CRi was evaluated per IWG criteria for AML.25 Duration of response (DoR) was defined as the number of days from the date of first response (CR or CRi) to the earliest evidence of confirmed morphologic relapse, disease progression, or death due to disease progression. Methods for pharmacokinetic and biomarker assessments and statistical analyses are described in the Supplementary Methods.
Results
Patient characteristics and treatment groups
The final data cut-off date was January 25, 2021. The study enrolled a total of 35 patients. The median age was 66 (range, 22—80) years. Twenty-two (62.9%) patients had de novo AML and 13 (37.1%) had secondary AML, of which 11 (84.6%) were post MDS/CMML and 2 (5.7%) were therapy-related AML. All patients had received at least 2 prior systemic therapies, with 11 (31.4%) receiving ≥ 6 prior regimens for their AML. Eleven (31.4%) patients had previously received venetoclax. Eight (22.9%) patients had received a prior transplant (6 allogeneic, 1 autologous, 1 cord blood). The demographic and baseline characteristics were similar across dose-escalation cohorts (Table 1).
Table 1.
Patient demographics and baseline characteristics
| Cohort | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Ven 400 mg | Ven 600 mg | Ven 800 mg | |||
| Alvo 45 mg/m2: “no ramp-up” (N = 8) | Alvo 45 mg/m2 (N = 6) | Alvo 60 mg/m2 (N = 11) | Alvo 60 mg/m2 (N = 5) | Alvo 60 mg/m2 (N = 5) | Total (N = 35) | |
| Median age, years (range) | 69.0 (36 – 80) | 69.0 (33 – 79) | 51.0 (22 – 76) | 66.0 (62– 79) | 73.0 (59 – 78) | 66.0 (22 – 80) |
| Gender, n (%) | ||||||
| Female | 1 (12.5) | 1 (16.7) | 5 (45.5) | 1 (20.0) | 3 (60.0) | 11 (31.4) |
| Male | 7 (87.5) | 5 (83.3) | 6 (54.5) | 4 (80.0) | 2 (40.0) | 24 (68.6) |
| AML status, n (%) | ||||||
| de novo | 7 (87.5) | 3 (50.0) | 7 (63.6) | 2 (40.0) | 3 (60.0) | 22 (62.9) |
| Secondary | 1 (12.5) | 3 (50.0) | 4 (36.4) | 3 (60.0) | 2 (40.0) | 13 (37.1) |
| ECOG performance status, n (%) | ||||||
| 0 | 4 (50.0) | 2 (33.3) | 0 | 0 | 1 (20.0) | 7 (20.0) |
| 1 | 4 (50.0) | 3 (50.0) | 10 (90.9) | 4 (80.0) | 4 (80.0) | 25 (71.4) |
| 2 | 0 | 1 (16.7) | 1 (9.1) | 1 (20.0) | 0 | 3 (8.6) |
| Cytogenetics | ||||||
| Favorable | 0 | 0 | 0 | 0 | 1 (20.0) | 1 (2.9) |
| Intermediate | 2 (25.0) | 4 (66.7) | 5 (45.5) | 4 (80.0) | 3 (60.0) | 18 (51.4) |
| Poor | 5 (62.5) | 2 (33.3) | 6 (54.5) | 1 (20.0) | 1 (20.0) | 15 (42.9) |
| No Mitoses | 1 (12.5) | 0 | 0 | 0 | 0 | 1 (2.9) |
| No. of prior systemic therapies, n (%) | ||||||
| 2-5 | 5 (62.5) | 5 (83.3) | 7 (63.6) | 3 (60.0) | 4 (80.0) | 24 (68.6) |
| ≥6 | 3 (37.5) | 1 (16.7) | 4 (36.4) | 2 (40.0) | 1 (20.0) | 11 (31.4) |
| Prior venetoclax therapy, n (%) | 4 (50.0) | 2 (33.3) | 3 (27.3) | 1 (20.0) | 1 (20.0) | 11 (31.4) |
| Prior radiation therapy, n (%) | 0 | 1 (16.7) | 1 (9.1) | 0 | 0 | 2 (5.7) |
| Prior allogeneic transplant, n (%) | 1 (12.5) | 3 (50.0) | 1 (9.1) | 0 | 1 (20.0) | 6 (17.1) |
Alvo, alvocidib, AML; acute myeloid leukemia; ECOG, Eastern Cooperative Oncology Group; Ven, venetoclax
The maximum dose combination administered was 800 mg of venetoclax (Ven 800) with 60 mg/m2 of alvocidib (Alvo 60). The maximum tolerated dose (MTD) for venetoclax was not determined. The median number of treatment cycles across all cohorts was 1.0 (range, 1.0—6.0). The median duration of exposure to venetoclax and alvocidib was 30 days (range, 1—454) and 11 days (range, 1—443), respectively. The median number of doses per cycle was 23 for venetoclax and 3 for alvocidib.
Safety
All 35 (100%) patients experienced at least 1 treatment-emergent adverse event (TEAE), and 33 (94.3%) patients had at least one Grade 3 or 4 TEAE (Table 2). The most common TEAEs of any grade (occurring in >40% of patients) were diarrhea (88.6%), nausea (77.1%), vomiting (62.9%), hypokalemia (54.3%), febrile neutropenia (45.7%), fatigue (40.0%), and hypomagnesemia (40.0%). Grade ≥3 TEAEs occurring in >20% of patients were febrile neutropenia (45.7%), diarrhea (31.4%), and hypokalemia (28.6%). Among the grade 3 or 4 TEAEs, 23 (65.7%) were considered possibly related to venetoclax, and 24 (68.6%) were considered possibly related to alvocidib.
Table 2.
Summary of treatment-emergent adverse events
| Venetoclax + Alvocidib (N = 35) | ||
|---|---|---|
| Any Grade n (%) | Grade ≥ 3 n (%) | |
| Any adverse event occurring in ≥ 20% of patients | 35 (100.0) | 33 (94.3) |
| Diarrhea | 31 (88.6) | 11 (31.4) |
| Nausea | 27 (77.1) | 5 (14.3) |
| Vomiting | 22 (62.9) | 4 (11.4) |
| Hypokalemia | 19 (54.3) | 10 (28.6) |
| Febrile neutropenia | 16 (45.7) | 16 (45.7) |
| Fatigue | 14 (40.0) | 0 |
| Hypomagnesaemia | 14 (40.0) | 0 |
| Decreased appetite | 13 (37.1) | 0 |
| Pyrexia | 13 (37.1) | 0 |
| Abdominal pain | 11 (31.4) | 2 (5.7) |
| Hyperphosphatemia | 9 (25.7) | 0 |
| Thrombocytopenia | 9 (25.7) | 8 (22.9) |
| Constipation | 8 (22.9) | 0 |
| Hypophosphatemia | 8 (22.9) | 6 (17.1) |
| Hypotension | 8 (22.9) | 1 (2.9) |
| Odema peripheral | 8 (22.9) | 0 |
| Serious adverse events occurring in ≥ 10% of patients | 32 (91.4) | |
| Febrile neutropenia | 12 (34.3) | |
| Diarrhea | 6 (17.1) | |
| Vomiting | 4 (11.4) | |
| Sepsis | 4 (11.4) | |
Thirty-two (91.4%) patients experienced at least 1 serious adverse event (SAE) (Table 2). The most common SAEs (occurring in > 10% of patients) were febrile neutropenia (34.3%), diarrhea (17.1%), vomiting (11.4%), and sepsis (11.4%). Twenty (57.1%) and 22 (62.9%) patients had SAEs considered possibly related to venetoclax and alvocidib, respectively. Among these SAEs, 5.2% of patients experienced drug-related febrile neutropenia, 17.1% of patients experienced drug-related diarrhea, 11.4% of patients experienced drug-related vomiting, and 5.7% of patients experienced drug-related sepsis. The incidence of febrile neutropenia (n = 5; 83.3%) was higher in the Ven 400 Alvo 45 dose-escalation cohort compared with the other dose cohorts (range, 0%—40.0%); otherwise, the incidence of any SAEs varied across cohorts, with no clear dose-related trends.
Thirteen (37.1%) patients had TEAEs leading to venetoclax and alvocidib discontinuation, diarrhea (n = 3 [8.6%]) and nausea (n = 2 [5.7%]) being the most common. Two patients experienced DLTs: One patient in the Ven 400 Alvo 45 “no ramp-up” cohort experienced tumor lysis syndrome [TLS], and 1 patient in the Ven 400 Alvo 60 cohort experienced a respiratory tract fungal infection. Treatment-emergent clinical TLS was reported in 3 (8.6%) patients, including the DLT.
Twenty-eight (80.0%) deaths occurred; 22 (62.9%) were due to disease progression. Thirteen deaths (37.0%) occurred ≥ 30 days after the last dose of the study drug. Six (17.1%) patients experienced fatal TEAEs (neutropenic sepsis [n = 1], pneumonia [n = 2], sepsis [n = 2], multiple organ failure [n = 1]), acute kidney injury [n = 1]); 1 patient had two primary TEAEs of sepsis and multiple organ failure. No fatal TEAEs were considered to be related to either venetoclax or alvocidib. All patients discontinued the study; the primary reasons were death (n = 28), AEs (n = 7), withdrew consent (n = 5), allogeneic stem cell transplant (n = 1), and other (n = 1). The reasons for discontinuation were similar across the dose-escalation cohorts.
Efficacy
We observed a leukemia response (CR, CRi, PR, or MLFS) in 7 (20.0%) patients. Four (11.4%) patients achieved a CRc response, including 1 (2.9%) CR and 3 (8.6%) CRi (Table 3). MLFS was observed in 3 (8.6%) patients. The median time to first response for CRc was 0.9 months (range, 0.8—1.2), and the median duration of response was 8.4 months (95% CI, 5.1—not evaluable [NE]; Figure 1). One patient was reported as having an allogeneic transplant post-study. Of the 7 responding patients, 4 were “prolonged responders,” with at least 2 disease assessments and a response of CR, CRi, MLFS, or PR for 3 months or longer. All 4 prolonged responders had no prior venetoclax exposure (Figure 1). We observed an interesting non-responder that had over 16 cycles of therapy, a 79-year-old man with de novo AML. Before study entry, they had 2 lines of venetoclax-based therapies (Ven/Aza and Ven/Decitabine) with a best response of refractory disease (RD). The response assessment at the end of his first cycle was RD which remained RD for over a year before progression.
Table 3.
Summary of response rates
| Cohort | ||||||
|---|---|---|---|---|---|---|
| Ven 400 mg | Ven 600 mg | Ven 800 mg | ||||
| Response, n(%) | Alvo 45 mg/m2: “no ramp-up” (N = 8) | Alvo 45 mg/m2 (N = 6) | Alvo 60 mg/m2 (N = 11) | Alvo 60 mg/m2 (N = 5) | Alvo 60 mg/m2 (N = 5) | Total (N = 35) |
| CR | 1 (12.5) | 0 | 0 | 0 | 0 | 1 (2.9) |
| CRi | 0 | 0 | 2 (18.2) | 1 (20.0) | 0 | 3 (8.6) |
| CR + CRi | 1 (12.5) | 0 | 2 (18.2) | 1 (20.0) | 0 | 4 (11.4) |
| MLFS | 0 | 0 | 0 | 2 (40.0) | 1 (20.0) | 3 (8.6) |
| Median Time to best response, months (range) | ||||||
| CR | 1.2 (1.2–1.2) | NA | NA | NA | NA | 1.2 (1.2-1.2) |
| CRi | NA | NA | 0.9 (0.8–0.9) | 1.0 (1.0–1.0) | NA | 0.9 (0.8–1.0) |
| CR + CRi | 1.2 (1.2–1.2) | NA | 0.9 (0.8–0.9) | 1.0 (1.0–1.0) | NA | 0.9 (0.8–1.2) |
| Objective Response Rate (CR + CRi + PR), n (%) | 1 (12.5) | 0 | 2 (18.2) | 1 (20.0) | 0 | 4 (11.4) |
| Leukemia Response Rate (CR + CRi + PR + MLFS), n (%) | 1 (12.5) | 0 | 2 (18.2) | 3 (60.0) | 1 (20.0) | 7 (20.0) |
| Median DoR for CR + CRi, months (95%CI) | 6.8 (5.1–NE) | 8.3 (NE–NE) | 8.4 (5.1–NE) | |||
Alvo, alvocidib; CR, complete remission; CRi, CR+incomplete blood count recovery; DoR, duration of response; MLFS, morphologic leukemia-free state; PR, partial remission; Ven, venetoclax
Figure 1.
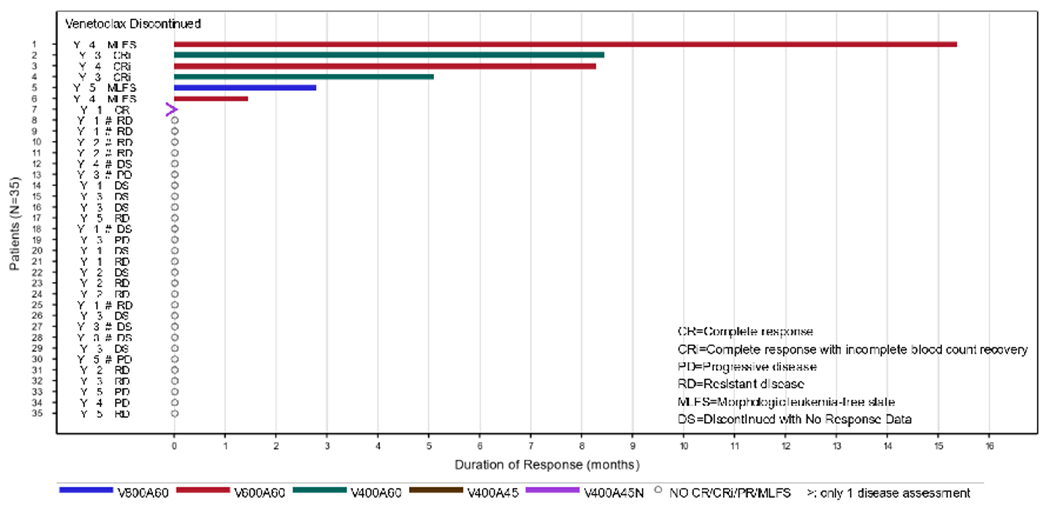
Swimmer plot of best treatment response and survival for all patients since the first dose of study drug. Each lane represents one patient in the study, with the colored bars indicating the drug exposure. The numbers on the left indicate the treatment cohort each patient was a part of: 1, Ven 400 Alvo 45 “no ramp-up”; 2, Ven 400 Alvo 45; 3, Ven 400 Alvo 60. 4, Ven 600 Alvo 60. 5, Ven 800 Alvo 60. Note: #, prior venetoclax.
Pharmacokinetics
The PK parameters for venetoclax are summarized in Table S2. Compared with venetoclax alone, co-administration with alvocidib resulted in 19% and 18% lower venetoclax Cmax and AUC24, respectively (Figure 2, Table S3). There was no apparent change in alvocidib PK with increasing doses of venetoclax (Table S4).
Figure 2.
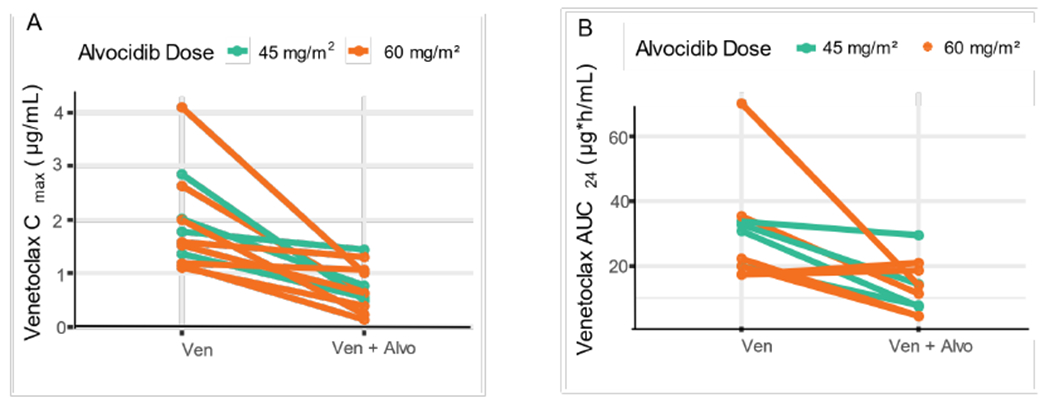
Co-administration with alvocidib reduced venetoclax exposure. A) Cmax or B) AUC24 of venetoclax by each patient when given alone, or in combination with alvocidib.
Biomarker Correlates
Twenty-seven patients were evaluable for mutational profiles at baseline. The most frequently observed mutations were TP53 (8/27), RUNX1 (7/27), DNMT3A (6/27), and ETV6 (6/27; Figure 3). Mutations associated with venetoclax sensitivity, SRSF2 (3/27), IDH2 (2/27), and IDH1 (1/27), were uncommon. Two of the 4 patients who achieved CR/CRi had IDH2 mutations, and 1 of the 8 patients with a TP53 mutation achieved MLFS. The non-responder who received over 16 cycles of therapy had an NF1 gene mutation at baseline. Comparative biomarker analysis showed that their NF1 gene had no changes from baseline to post-therapy.
Figure 3.
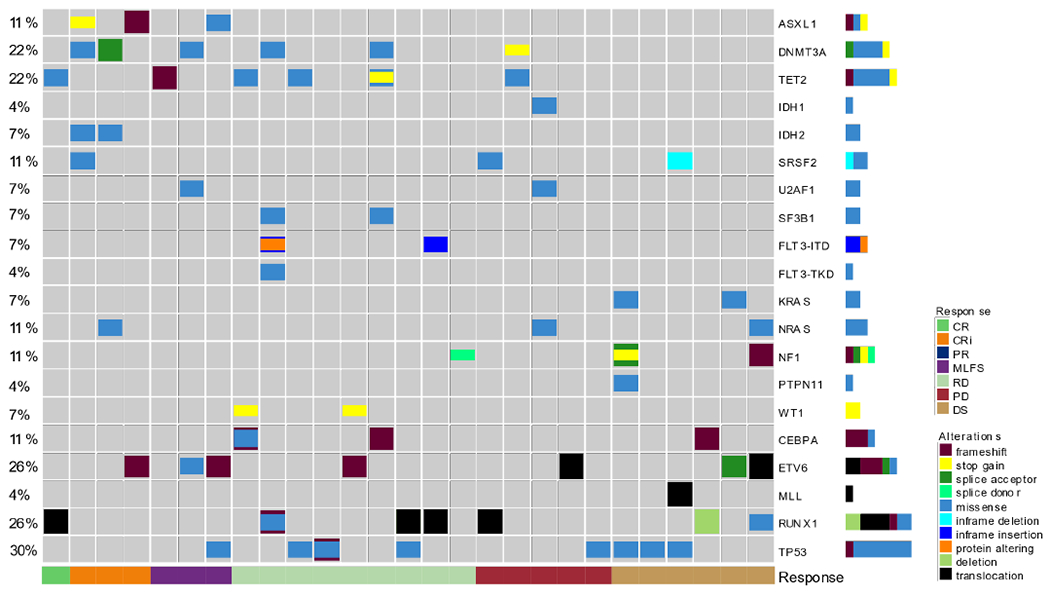
Molecular patterns of response. Heatmap showing the frequency of molecular markers detected at baseline for each patient as it relates to best response on study, which is color coded on the bottom. The molecular marker is on the right and the frequency is on the left, with the colors indicating the type of alteration. CR, complete response; CRi, CR with incomplete hematologic recovery; PR, partial response; MLFS, morphologic leukemia-free state; RD, resistant disease; PD, progressive disease; DS, discontinued with no response data.
Fourteen patients with ≥ 30% blasts were evaluable for gene expression of BCL2 and BCL2L1. The median BCL2 mRNA expression (2−ΔCt) in bone marrow blasts was 0.48 (95% CI, 0.34—0.70), whereas the median expression of BCL2L1 was considerably higher at 17.5 (95% CI, 8.0—38.0). The median gene expression of BCL2 was similar in patients regardless of prior lines of therapy (median 0.45 vs 0.52, for patients with < 4 prior lines of therapy vs patients with ≤ 4 prior lines of therapy). In contrast, the median BCL2xL gene expression was nearly 2 times greater in patients with ≤ 4 prior lines of therapy (25.0 vs 12.2) vs. those patients with < 4 (Table S5).
Twenty-seven patients were assessed by BH3 profiling to determine the apoptotic potential of BCL2, BCL-xL, MCL-1 and/or BCL-w, of which 21 were evaluable (Table S6). There were 3 patients whose tumor cells were resistant to all 4 BH3 mimetics; 1 had a best response of MLFS. Nine patients showed dependency on a single BH3 family member; 4 were dependent on BCL-xL, with 1 patient achieving a CRi; 4 were dependent on BCL-w. One patient with BCL-2 dependency discontinued the study before evaluation for clinical response. Of the 8 patients with dual dependency, 6 were dependent upon MCL-1 and BCL-xL; 2 of whom achieved MLFS as the best response. The other 2 patients were non-responders. One was dependent on BCL-2 and BCL-xL, and the prolonged non-responder showed dependency on MCL-1 and BCL-w at baseline. Notably, the last visit sample for the prolonged non-responder revealed dependency on MCL-1, BCL-2, and BCL-xL. The remaining patient was dependent upon 3 BH3 family members (BCL-2, MCL-1, and BCL-xL) at baseline, and achieved a CRi. No clear correlation of MCL-1 dependency and alvocidib exposure was observed.
Discussion
The combination of venetoclax and alvocidib was tested in patients with R/R AML, including at the highest planned dose levels, and no MTD was determined. The types and severity of AEs were expected based on the prior clinical experiences of the two agents and primarily included cytopenia and gastrointestinal disorders such as diarrhea and nausea.6, 27 Two events of clinical TLS were considered SAEs; these patients were deemed at high risk for TLS at baseline. Diarrhea and nausea were the most reported grade ≥3 adverse events leading to discontinuation of venetoclax and alvocidib and occurred in three and two patients, respectively. The TLS and other toxicities were managed with standard of care, or the patient’s treatment was interrupted. The safety data from this study were consistent with the previously demonstrated safety profile of venetoclax and alvocidib monotherapy.8, 22 Hence, the study met its safety endpoint for the dose escalation phase. However, the combination did not result in a meaningful increase in efficacy compared to what was previously observed with each agent alone. Therefore, the study was terminated without cohort expansion to confirm safety, explore efficacy, and confirm suitability of a recommended Phase 2 dose.
Previous in vitro and in vivo studies reported that venetoclax in combination with alvocidib was synergistic in venetoclax-sensitive and -resistant AML models, providing a rationale for clinically testing this combination. Limited efficacy at various dose levels was observed, and only seven patients (20%) achieved a response with a median duration of response of 8.4 months. These outcomes are comparable to previously reported studies in R/R AML where venetoclax combination therapy resulted in responses of 31% and a median duration of response of 7.8 months.9 All patients who obtained a response in this study had adverse risk features, and none had prior exposure to venetoclax. In another study, single-agent alvocidib produced a transient CRi in 1 out of 9 patients.9, 28 Here, some patients had initial signs of activity when receiving venetoclax and alvocidib together but then progressed on venetoclax alone. Given that an MTD was not reached with 60% of patients receiving ≥ 4 prior lines of therapy, it is likely that the dose levels or schedule of alvocidib used in this study may have been sub-optimal. Future studies could explore different treatment schedules to achieve more consistent MCL1 inhibition.
The PK of venetoclax has been described alone or in combination with other agents.29, 30 In this study, there was no apparent change in alvocidib PK with increasing doses of venetoclax, yet the co-administration with alvocidib decreased the AUC24 and Cmax of venetoclax. There was also no apparent relationship between increasing venetoclax dose and the overall response observed in the present study. Since alvocidib was only co-administered for a few days, the slight reduction in exposure was probably limited and unlikely to explain a significant decline in efficacy.
Although patient numbers were too small to make definitive conclusions, our exploratory analysis found 2 of 4 patients who achieved a CR or CRi response had IDH2 mutations and no previous exposure to venetoclax, consistent with previous observations that IDH1/2 mutations were associated with sensitivity to venetoclax mono- or combination therapy with HMAs.31, 32 Notably, the RD patient that stayed on study treatment for over a year with previous exposure to venetoclax-based regimens did not have an IDH1/2 mutation. TP53 mutations were the most frequently observed aberrations at baseline, and have been reported to be associated with inferior outcomes with venetoclax combination therapies in treatment-naïve AML.31, 33, 34 Given that in vitro disruption of TP53 also reduces sensitivity to MCL-1 inhibition, this subset of R/R AML patients may not benefit from co-targeting BCL-2 and MCL-1.35 Interestingly, it was observed that 3 of the 6 responders with DNMT3A mutations had previous exposure to HMA; typically, such patients are refractory to subsequent therapy and have poor survival.36, 37 Preclinical evidence suggests that mutant DNMT3A can promote expression of BCL2 and MCL1, which could explain sensitivity in these patients.38 Emerging clinical data show that DNMT3A mutations may predict response to venetoclax-containing combinations that can be further explored in more extensive studies.9
Study patients were heavily pre-treated and had a heterogenous disposition at baseline, which, while typical of R/R disease, prevents a strong correlation of molecular markers to outcomes. It is possible that indirectly targeting MCL-1 through CDK9 inhibition with alvocidib is not ideal, particularly in the R/R setting. MCL-1 has historically been a challenging drug target. Thus, transcriptional repression of MCL-1 was a potentially more accessible mechanism than direct inhibitors.39 A previous report evaluating a combination regimen of alvocidib and vorinostat in patients with AML produced inconsistent shifts in MCL-1 protein levels, suggesting that there are unknown factors involved that regulate the activity of alvocidib in vivo.40 Direct targeting of both BCL-2 and MCL-1 could be explored in future studies as more selective MCL-1 inhibitors are available.41
In summary, the MTD and recommended Phase 2 dose for the combination of venetoclax and alvocidib were not determined due to a lack of efficacy in the dose-escalation portion. For this reason, the study was stopped before opening the safety expansion cohort. While the phase 1b study design and the small sample size across dose cohorts limited the interpretations of the findings, the biomarker data presented here may help generate new hypotheses regarding the R/R AML patients who may or may not benefit from venetoclax combination therapies. These results highlight that this novel combination of active drugs in AML did not prove to be synergistic. However, this was a very high-risk, heavily pre-treated population, and many patients had received prior treatment with venetoclax. While there are no plans to proceed with this regimen in further studies, preclinical investigations of potential independent resistance mechanisms could be undertaken to guide the development of new combination strategies.
Supplementary Material
Acknowledgements
Medical writing support was provided by Dalia Majumdar, PhD and Liza Selwan-Lewis, PhD of AbbVie Inc, and funded by AbbVie Inc. Editorial support was provided by Angela Hadsell, of AbbVie Inc, and funded by AbbVie Inc.
Funding:
AbbVie and Sumitomo Pharma Oncology, Inc. funded this study.
Conflict of Interest Statement
Brian A Jonas: Served as a consultant/advisor for AbbVie, BMS, Genentech, Gilead, GlycoMimetics, Jazz Pharmaceuticals, Pfizer, Servier, Takeda, Tolero, and Treadwell; protocol steering committee for GlycoMimetics; data monitoring committee for Gilead; received travel reimbursement from AbbVie; received research funding to his institution from Forty Seven, AbbVie, Accelerated Medical Diagnostics, Amgen, Aptose, AROG, BMS, Celgene, Daiichi Sankyo, F. Hoffmann-La Roche, Forma, Genentech/Roche, Gilead, GlycoMimetics, Hanmi, Immune-Onc, Incyte, Jazz Pharmaceuticals, Loxo, LP Therapeutics, Pfizer, Pharmacyclics, Sigma Tau, and Treadwell
Jing-Zhou Hou: Investigator on an AbbVie-funded trial
Gail J Roboz: Served as a consultant/advisor or on Data and Safety Monitoring Committee: AbbVie, Agios, Amgen, Astellas, AstraZeneca, Bristol Myers Squibb, Blueprint Medicines, Bluebird Bio, Celgene, Glaxo SmithKline, Janssen, Jasper Therapeutics, Jazz, MEI Pharma (IDMC Chair), Mesoblast, Novartis, Pfizer, Syndax, Takeda (IRC Chair); Research Support: Janssen
Caroline L Alvares: Served as a consultant/advisor for AbbVie. Received research funding to her institution at Cardiff University from Celgene and Jazz Pharmaceuticals.
Deepa Jeyakumar: Research funding from Jazz and Pfizer
John R Edwards: Served as a consultant/advisor for Jazz, Astellas, Roche; served on steering committee for Roche
Harry P Erba: Received grants/research support from AbbVie, Agios, ALX Oncology, Amgen, Daiichi Sankyo, Forma, Forty Seven, Gilead, Glycomimetics, ImmunoGen, Jazz, MacroGenics, Novartis, PTC
Consultant: AbbVie, Agios, Astellas, Celgene/BMS, Daiichi Sankyo, Genentech, Glycomimetics, Incyte, Jazz, Kura Oncology, Novartis, Syros, Takeda, Trillium Speakers’ Bureau: AbbVie, Agios, Celgene/BMS, Incyte, Jazz, Novartis Other: Celgene/BMS, Chair, scientific steering committee of registry study AbbVie, Chair, IRC.
Richard J Kelly: Served as a consultant/advisor for Abbvie, Alexion, Amgen, Jazz, Pfizer, Sobi. Received lecture fees for Alexion, Astellas, Biologix, Jazz, Pfizer, Sobi
Christoph Röllig: Served as a consultant/advisor for AbbVie, Amgen, Astellas, Bristol-Myers Squibb, Celgene, Daiichi Sankyo, Janssen, Jazz, Novartis, Pfizer, Roche, Servier; Received institutional research funding from AbbVie, Novartis, Pfizer
Walter Fiedler: Advisory role: Amgen, ARIAD/Incyte, Novartis, Pfizer; Celgene, Morphosys, Abbvie, Jazz Pharmaceuticals, Stemline, Clinigene, Servier; Royalties: Amgen; Received support for meeting attendance from Amgen, Gilead, Jazz Pharmaceuticals, Daiichi Sankyo, Servier
Justin Watts: Served as a consultant/advisor for Takeda, Bristol-Myers Squibb, Reven Pharma, Rafael Pharma. Received research support from Takeda, Immune System Key, Ltd.
Satya R Siddani, Brenda Chyla and Jacqueline Hilger-Rolfe: Employee of AbbVie and may hold stocks
Deanna Brackman: former employee of AbbVie, currently employed by Amunix, a Sanofi company, and may hold AbbVie stock.
Venetoclax is being developed in a collaboration between AbbVie and Genentech.
AbbVie and Sumitomo Pharma Oncology, Inc. funded this study (NCT03441555) and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this manuscript. No honoraria or payments were made for authorship.
References
- 1.Döhner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. N Engl J Med. 2015;373(12):1136–1152. [DOI] [PubMed] [Google Scholar]
- 2.Rapaport F, Neelamraju Y, Baslan T, et al. Genomic and evolutionary portraits of disease relapse in acute myeloid leukemia. Leukemia. 2021;35(9):2688–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Caner Institute. SEER Cancer Stat Facts: Acute Myeloid Leukemia. 2022. [Google Scholar]
- 4.Walter RB, Othus M, Burnett AK, et al. Resistance prediction in AML: analysis of 4601 patients from MRC/NCRI, HOVON/SAKK, SWOG and MD Anderson Cancer Center. Leukemia. 2015;29(2):312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thol F, Heuser M. Treatment for Relapsed/Refractory Acute Myeloid Leukemia. Hemasphere. 2021;5(6):e572–e572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Eng J Med. 2020;383(7):617–629. [DOI] [PubMed] [Google Scholar]
- 7.Jain N, Keating M, Thompson P, et al. Ibrutinib and Venetoclax for First-Line Treatment of CLL. N Eng J Med. 2019;380(22):2095–2103. [DOI] [PubMed] [Google Scholar]
- 8.Konopleva M, Pollyea DA, Potluri J, et al. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer Discov. 2016;6(10):1106–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stahl M, Menghrajani K, Derkach A, et al. Clinical and molecular predictors of response and survival following venetoclax therapy in relapsed/refractory AML. Blood Adv. 2021;5(5):1552–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leverson JD, Sampath D, Souers AJ, et al. Found in Translation: How Preclinical Research Is Guiding the Clinical Development of the BCL2-Selective Inhibitor Venetoclax. Cancer Discov. 2017;7(12):1376–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202–8. [DOI] [PubMed] [Google Scholar]
- 12.Tenold ME, Moskoff BN, Benjamin DJ, et al. Outcomes of Adults With Relapsed/Refractory Acute Myeloid Leukemia Treated With Venetoclax Plus Hypomethylating Agents at a Comprehensive Cancer Center. Front Oncol. 2021;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jan Philipp B, Smith G, Rong W, et al. Venetoclax as monotherapy and in combination with hypomethylating agents or low dose cytarabine in relapsed and treatment refractory acute myeloid leukemia: a systematic review and meta-analysis. Haematologica. 2020;105(11):2659–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niu X, Zhao J, Ma J, et al. Binding of Released Bim to Mcl-1 is a Mechanism of Intrinsic Resistance to ABT-199 which can be Overcome by Combination with Daunorubicin or Cytarabine in AML Cells. Clin Cancer Res. 2016;22(17):4440–4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan R, Hogdal LJ, Benito JM, et al. Selective BCL-2 Inhibition by ABT-199 Causes On-Target Cell Death in Acute Myeloid Leukemia. Cancer Discov. 2014;4(3):362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bogenberger JM, Kornblau SM, Pierceall WE, et al. BCL-2 family proteins as 5-Azacytidine-sensitizing targets and determinants of response in myeloid malignancies. Leukemia. 2014;28(8):1657–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morales F, Giordano A. Overview of CDK9 as a target in cancer research. Cell Cycle. 2016;15(4):519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahendra Kadia T, Kantarjian HM, Konopleva M. Myeloid cell leukemia-1 dependence in acute myeloid leukemia: a novel approach to patient therapy. Oncotarget. 2019;10(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bogenberger J, Whatcott C, Hansen N, et al. Combined venetoclax and alvocidib in acute myeloid leukemia. Oncotarget. 2017;8(63). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishi R, Shigemi H, Negoro E, et al. Venetoclax and alvocidib are both cytotoxic to acute myeloid leukemia cells resistant to cytarabine and clofarabine. BMC Cancer. 2020;20(1):984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin TS, Ruppert AS, Johnson AJ, et al. Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. Journal of Clinical Oncology 2009;27(35):6012–6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blum W, Phelps MA, Klisovic RB, et al. Phase I clinical and pharmacokinetic study of a novel schedule of flavopiridol in relapsed or refractory acute leukemias. Haematologica. 2010;95(7):1098–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan Y, Hess KR, Hilsenbeck SG, Gilbert MR. Bayesian Optimal Interval Design: A Simple and Well-Performing Design for Phase I Oncology Trials. Clin Cancer Res. 2016;22(17):4291–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), version 4.03. [Google Scholar]
- 25.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–9. [DOI] [PubMed] [Google Scholar]
- 26.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeidner JF, Lin TL, Vigil CE, et al. A prospective biomarker analysis of alvocidib followed by cytarabine and mitoxantrone in MCL-1-dependent relapsed/refractory acute myeloid leukemia. Blood Cancer J. 2021;11(10):175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.William B, Mitch AP, Rebecca BK, et al. Phase I clinical and pharmacokinetic study of a novel schedule of flavopiridol in relapsed or refractory acute leukemias. Haematologica. 2010;95(7):1098–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H, Michmerhuizen MJ, Lao Y, et al. Metabolism and Disposition of a Novel B-Cell Lymphoma-2 Inhibitor Venetoclax in Humans and Characterization of Its Unusual Metabolites. Drug Metab Dispos. 2017;45(3):294–305. [DOI] [PubMed] [Google Scholar]
- 30.Brackman D, Eckert D, Menon R, et al. Venetoclax exposure-efficacy and exposure-safety relationships in patients with treatment-naïve acute myeloid leukemia who are ineligible for intensive chemotherapy. Hematol Oncol. 2022;40(2):269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pollyea DA, DiNardo CD, Arellano ML, et al. Impact of Venetoclax and Azacitidine in Treatment-Naïve Patients with Acute Myeloid Leukemia and IDH1/2 Mutations. Clin Cancer Res. 2022;28(13):2753–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chyla B, Daver N, Doyle K, et al. Genetic biomarkers of sensitivity and resistance to venetoclax monotherapy in patients with relapsed acute myeloid leukemia. Am J Hematol. 2018;93(8):E202–E205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DiNardo CD, Tiong IS, Quaglieri A, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood. 2020;135(11):791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim K, Maiti A, Loghavi S, et al. Outcomes of TP53-mutant acute myeloid leukemia with decitabine and venetoclax. Cancer. 2021;127(20):3772–3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nechiporuk T, Kurtz SE, Nikolova O, et al. The TP53 Apoptotic Network Is a Primary Mediator of Resistance to BCL2 Inhibition in AML Cells. Cancer Discov. 2019;9(7):910–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ball BJ, Famulare CA, Stein EM, et al. Venetoclax and hypomethylating agents (HMAs) induce high response rates in MDS, including patients after HMA therapy failure. Blood Adv. 2020;4(13):2866–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prébet T, Gore SD, Thépot S, et al. Outcome of acute myeloid leukaemia following myelodysplastic syndrome after azacitidine treatment failure. Br J Haematol. 2012;157(6):764–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bera R, Chiu MC, Huang YJ, et al. DNMT3A mutants provide proliferating advantage with augmentation of self-renewal activity in the pathogenesis of AML in KMT2A-PTD-positive leukemic cells. Oncogenesis. 2020. Feb 3;9(2):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tibes R, Bogenberger JM. Transcriptional Silencing of MCL-1 Through Cyclin-Dependent Kinase Inhibition in Acute Myeloid Leukemia. Front Oncol. 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holkova B, Supko JG, Ames MM, et al. A Phase I Trial of Vorinostat and Alvocidib in Patients with Relapsed, Refractory, or Poor Prognosis Acute Leukemia, or Refractory Anemia with Excess Blasts-2. Clin Cancer Res. 2013;19(7):1873–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bolomsky A, Vogler M, Köse MC, et al. MCL-1 inhibitors, fast-lane development of a new class of anti-cancer agents. J Hematol Oncol. 2020;13(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


