Abstract
The leading cause of heart disease in developed countries is coronary atherosclerosis, which is not simply a result of ageing but a chronic inflammatory process that can lead to acute clinical events upon atherosclerotic plaque rupture or erosion and arterial thrombus formation. The composition and location of atherosclerotic plaques determine the phenotype of the lesion and whether it is more likely to rupture or to erode. Although plaque rupture and erosion both initiate platelet activation on the exposed vascular surface, the contribution of platelets to thrombus formation differs between the two phenotypes. In this review, plaque phenotype is discussed in relation to thrombus composition, and an overview of important mediators (haemodynamics, matrix components, and soluble factors) in plaque-induced platelet activation is given. As thrombus formation on disrupted plaques does not necessarily result in complete vessel occlusion, plaque healing can occur. Therefore, the latest findings on plaque healing and the potential role of platelets in this process are summarized. Finally, the clinical need for more effective antithrombotic agents is highlighted.
Keywords: Plaque rupture, Plaque erosion, Thrombus formation, Platelet activation
Graphical Abstract
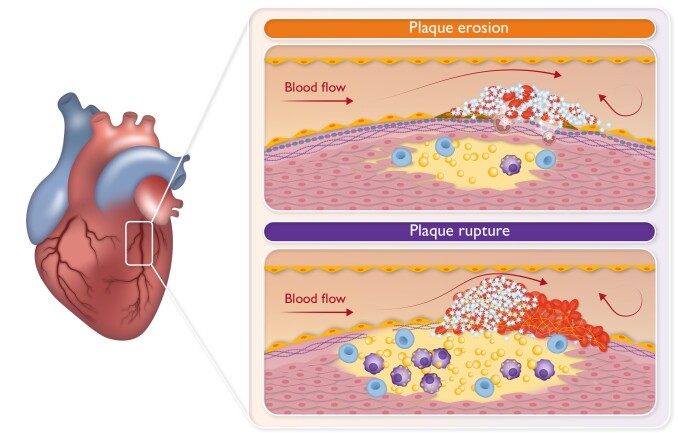
Graphical Abstract.
Schematic representation of the differences in thrombus formation induced by either erosion or rupture of coronary atherosclerotic plaques.
Introduction
Following vascular injury, matrix components and vascular smooth muscle cells (VSMCs) that are typically inaccessible to blood are exposed to the circulation, leading to the activation of the haemostatic system to prevent further blood loss. Within this process, platelets are the first to respond by recognizing and binding to von Willebrand factor (vWF), exposed collagen, and other matrix components. Upon binding, platelets become activated and release autocrine and paracrine mediators from their granules, which will further enhance platelet incorporation into the growing blood clot or thrombus.1 In addition, platelet activation is augmented by exposure to the endocrine mediators norepinephrine and epinephrine, which are highly increased during myocardial infarction (MI).2 Different platelet populations with specialized functions can be recognized within a thrombus as a consequence of environmental factors as well as intrinsic platelet factors (inherited from the megakaryocyte).1 While megakaryocytes residing in the lungs display a more inflammatory phenotype compared to bone marrow megakaryocytes,3,4 it is unknown whether platelets derived from these megakaryocytes also differ in their contribution to haemostatic and atherothrombotic processes.
Also, thrombin generation takes place as a consequence of the activation of the extrinsic and intrinsic coagulation pathways resulting in fibrin formation and further platelet activation. These two processes, platelet activation and coagulation, are required for the formation of a stable haemostatic plug.1,5 In atherothrombotic pathologies, the haemostatic system is triggered by the atherosclerotic lesion, i.e. in a situation of endothelial injury but not blood loss. The result is the formation of a thrombus within the vessel, culminating in the development of acute coronary artery disease (CAD).
In this review, we discuss plaque phenotypes and its implications for thrombus composition. Further, we provide an overview of important mediators in plaque-induced platelet activation and subsequent thrombus formation. Lastly, we consider plaque healing and the potential role of platelets herein.
Plaque composition and phenotype
Atherosclerotic lesions develop over time starting with adaptive thickening of the arterial vessel wall that gradually advances into atheromatous, fibrous, and fibroatheromatous plaques.6 For a detailed overview on plaque development and the role of platelets therein, we refer to detailed reviews.7–10
Advanced plaques can erode or rupture, leading to arterial thrombosis and tissue ischaemia10–12 (Graphical Abstract). The composition of an atherosclerotic plaque will determine whether it is prone to rupture or to erode. Ruptured plaques are typically thin-cap fibroatheroma with a large lipid core that can show signs of recent intra-plaque haemorrhage.13,14 Upon rupture, the fissure extends into the plaque exposing the highly thrombogenic lipid rich, necrotic core to the circulation. In ruptured plaques, macrophages and foam cells are abundantly present and are typically present at the margins of the rupture.13–15 Also, T cells are commonly detected at the luminal surface near the fission, although in smaller numbers than macrophages.13,15 Interestingly, at the site of macrophage and T-cell accumulation, generally no VSMCs are detected, and rupture usually does not occur in VSMC-rich areas.13 Furthermore, extensive inflammatory reactions contribute to the degradation of the extracellular matrix.16 In contrast, eroded plaques consist for a major part of VSMCs and are rich in proteoglycans (e.g. versican) and hyaluronan at the site of erosion.14,17 Erosion of the endothelial layer will leave the underlying VSMCs and extracellular matrix (e.g. proteoglycans, collagen) exposed to the circulation. In eroded plaques, inflammation is limited with a sparse distribution of macrophages and T cells,15,18 while accumulation of neutrophil extracellular traps (NETs) has been reported. Neutrophils and NETs locate in plaques with superficial erosion within the atheroma and at the luminal surface at the site of endothelial denudation or the thrombus.19,20 However, recent work has shown that they can also be found in ruptured plaques.21 Peptidyl arginine deiminase 4 (PAD4)-mediated NET formation is reported to activate endothelial cells (ECs) and induce tissue factor (TF) production,19,22 via interleukin (IL)-1α and cathepsin G. Further, neutrophils and NETs colocalize with Toll-like receptor 2 (TLR2) expression in plaques rich in VSMCs. In combination with in vitro findings showing potentiation of TLR2-induced endothelial stress by neutrophils, these findings suggest an important role for TLR2 and neutrophils in superficial plaque erosion.20 Further, calcification of eroded plaques tends to be less than for ruptured plaques14 and is restricted to areas with speckled calcifications in contrast to ruptured plaques that have areas with diffuse or fragmented calcifications.18 In general, the atherosclerotic burden is smaller for eroded plaques compared to ruptured plaques.14,18
It is estimated that in ∼40% of patients, the culprit lesion responsible for acute coronary syndrome (ACS) is eroded rather than ruptured.23 Advances in intravascular imaging techniques have improved plaque phenotyping, resulting in a higher prevalence of eroded plaques than reported in autopsy studies. Nonetheless, the prevalence of plaque erosion has increased independent of the improvements made in diagnostic modalities. Better and earlier primary prevention of traditional risk factors (e.g. LDL, smoking, hypertension) has contributed to a gradual shift in plaque phenotype towards superficial erosion.23 Plaque erosion is typically associated with a non-ST-elevation MI (NSTEMI) presentation of ACS, while plaque rupture is more common in ST-elevation MI (STEMI) patients.23 Additionally, patients with erosion of the culprit plaque are more likely to have a favourable prognosis than those with plaque rupture.24 In a small cohort of ACS patients, patients with plaques with an intact fibrous cap had a lower overall occurrence of major adverse cardiovascular events (MACEs) during follow-up and a better MACE-free survival than patients with ruptured plaques.25 It is suggested that the more favourable prognosis of patients with eroded plaques is in part due to a lower complexity and smaller overall atherosclerotic burden of the eroded lesions; lower plasma levels of LDL cholesterol, triglyceride, and C-reactive protein; and a lower prevalence of traditional risk factors.23,24 Although most studies only categorize coronary plaques as either eroded or ruptured, a recent transcriptomics study demonstrated five distinct plaque subsets associated with clinical outcomes. The expression levels of genes associated with extracellular matrix organization, glycolysis, and transforming growth factor (TGF)-β signalling were higher in the cluster containing the highest rate of ischaemic samples.26 Interestingly, this cluster showed high similarity to pathways found in carotid plaques associated with clinical symptoms.26 The same subset exhibited a higher prevalence of pathways and cell types traditionally associated with two distinct plaque phenotypes, the ‘stabilizing’ phenotype characterized by the abundance of smooth muscle cell-specific genes and the ‘destabilizing’ phenotype characterized by the abundance of macrophage specific genes.26 Novel studies using single-cell mass spectrometry and RNA sequencing provided further insight into the immune cell content of the plaque microenvironments and their relation to plaque stability.27–30 Depuydt et al.29 showed that plaques contain various T-cell populations, pro-inflammatory macrophages, and foam cell-like macrophages. Plaque stability was mainly affected by pro-inflammatory macrophages, although functional B cells contributed as well.28 In plaques from symptomatic patients, abundant CD4+ T cells, activated and differentiated T cells, and exhausted T cells were observed,27 although activated T cells and IL-1β-positive macrophages were present in plaques from asymptomatic patients too.27 These results indicate the presence of multiple, partially overlapping plaque phenotypes with distinct cellular signatures; however, novel studies are warranted for further characterization.
While atherosclerotic plaque formation can take place throughout the vessels, branching points of the vascular tree are the most susceptible locations. These regional differences can be explained by the differences in rheological conditions as atherosclerosis tends to develop in regions with flow disturbances, lower shear, and oscillatory flow.31 Studies showed different prevalence of plaques and their subsequent phenotypes within the coronary circuit (Table 1).32–36
Table 1.
General overview of the differences in plaque, patients, and thrombus characteristics between eroded and ruptured plaques
| Plaque erosion | Plaque rupture | References | |
|---|---|---|---|
| Plaque composition | Little or no lipid core | Large lipid pool | 13–17 |
| Endothelial denudation | Greater necrotic core | ||
| Smooth muscle cells rich | Thin fibrous cap | ||
| Matrix rich (proteoglycan, glycosaminoglycan, hyaluronic acid) | Abundant monocytes, macrophages, and foam cells | ||
| Neutrophil predominance | Greater plaque burden | ||
| Plaque location | |||
| LAD | 40%–66% | 40%–46% | 32–36 |
| RCA | 22%–30.6% | 35%–59% | 32–36 |
| LCX | 8.1%–14.9% | 9.2%–15.6% | 32–36 |
| Sex and age differences a | |||
| Female | <50 years: | <50 years: | 37–39 |
| 74.0%–77.1% | 22.9%–26.0% | ||
| >50 years: | >50 years: | ||
| 30.3%–44.1% | 55.9%–69.7% | ||
| Male | <50 years: | <50 years: | 37–39 |
| 45.6%–65.2% | 34.8%–53.4% | ||
| >50 years: | >50 years: | ||
| 18.1%–43.4% | 56.5%–81.9% | ||
| Clinical presentation | NSTEMI | STEMI | 23 |
| Thrombus composition | Non-occlusive | Occlusive | 40–43 |
| Platelet rich | Platelet and fibrin rich | ||
| RBCs poor | Abundant RBCs | ||
| NETs present | |||
LAD, left anterior descending artery; LCX, left circumflex artery; NETs, neutrophil extracellular traps; NSTEMI, non-ST elevation myocardial infarction; RBC, red blood cells; RCA, right coronary artery; STEMI, ST elevation myocardial infarction.
aOnly post-mortem studies.
Whether sex influences plaque phenotype is not straightforward. Post-mortem studies reported a prevalence of around 75% for plaque erosion in women younger than 50 years who suffered sudden coronary death, while for men in the same age category numbers range from 35% to 65%,37–39 demonstrating an increased prevalence of plaque erosion in women compared with men (Table 1). This was further confirmed using optical coherence tomography (OCT) imaging in patients with stable CAD or ACS.44 In contrast, the study by Kim et al.45 showed no significant sex difference in plaque phenotype analysed by OCT in ACS patients, although their study demonstrated higher frequency of plaque erosion in younger (<50 years) vs. older (>70 years) women. This relative higher frequency of plaque rupture in older female patients may be partially explained by the loss of anti-atherosclerotic effects of oestrogen during and post-menopause. Interestingly, oestrogen does not appear to affect plaque erosion.46Therefore, insight into oestrogen levels as well as potential hormone replacement therapy can be informative when assessing the relation among age, sex, and plaque phenotype.
Platelet response to plaque phenotype and consequences for clot composition
The different phenotypes of eroded and ruptured plaques also affect the structure and composition of the thrombus formed at the culprit lesion. Thrombi associated with ruptured plaques tend to be rich in fibrin and erythrocytes and to a lesser extent in platelets, giving them a typical ‘red’ appearance.40 On the other hand, eroded plaques provoke the formation of white thrombi, predominantly rich in platelets, but poor in erythrocytes.40 Insights into the composition of thrombi are crucial, as this will determine the mechanical properties of the clot in terms of stability and embolization, as well as resistance to lysis.47 Here, an increased platelet and erythrocyte content and denser fibrin network are related to a higher resistance to lysis.47 The heterogeneous composition of plaque-associated thrombi is well illustrated by thrombi obtained by aspiration thrombectomy from STEMI patients, where relatively more fibrin and platelets are present in the outer layer, while erythrocytes and polyhedrocytes are relatively more abundant in the inner core of the thrombus.41 Moreover, thrombus composition changes over time. Studies on (aspirated) thrombi from culprit arteries from patients suffering from STEMI or sudden cardiac death showed that a longer ischaemic period correlates with increased fibrin and decreased platelet content.42,43 Erythrocyte and leukocyte contents were unaffected by ischaemic time.43
Differences in thrombus composition in response to plaque rupture or erosion occur at multiple levels. In the following paragraphs, the influence of local haemodynamics, matrix composition, soluble factors, and platelet interactions with other cell types on thrombus composition is discussed, as illustrated in Figures 1 and 2.
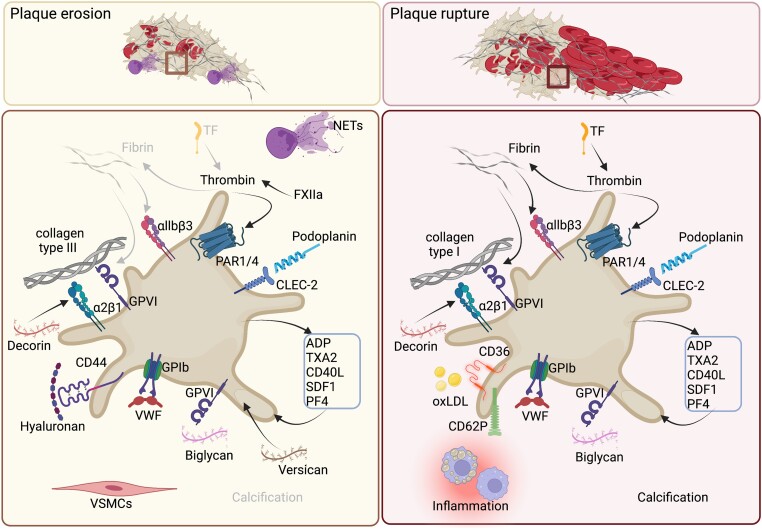
Figure 1.
Platelet response towards prominent matrix components and soluble factors in either plaque erosion or rupture. Overview of mechanisms responsible for platelet activation and consequent thrombus formation. For more information, see Matrix components and Soluble factors. ADP, adenosine diphosphate; CD36, cluster of differentiation 36; CD40L, cluster of differentiation 40 ligand; CD44, cluster of differentiation 44; CD62P, P-selectin; CLEC-2, C-type lectin-like type II; FXIIa, activated coagulation factor XII; GPIb, glycoprotein Ib; GPVI, glycoprotein VI; integrin α2β1; integrin αIIbβ3; NETs, neutrophils extracellular traps; oxLDL, oxidized low density lipoprotein; PAR1/4, protease activated receptor 1/4; PF4, platelet factor 4; SDF-1, stromal cell derived factor 1; TF, tissue factor; TXA2, thromboxane A2; VSMCs, vascular smooth muscle cells; VWF, von Willebrand factor. Figure was created with Biorender.com
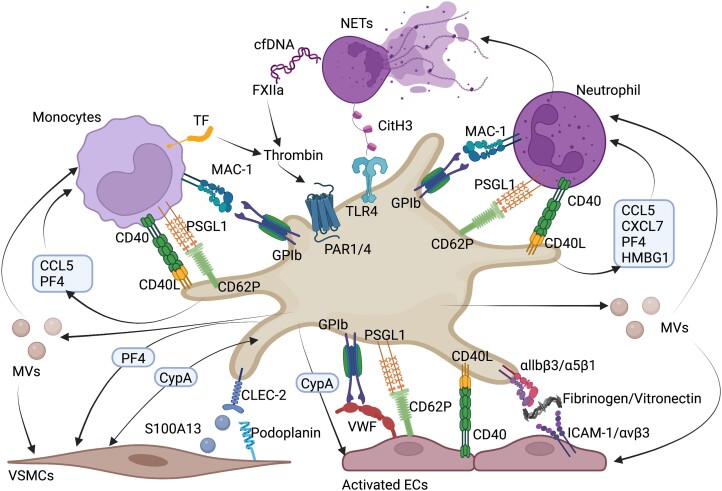
Figure 2.
Interactions of platelets with leukocytes, endothelial cells, and vascular smooth muscle cells in atherosclerosis. Overview of ligand–receptor interactions and soluble important factors in the crosstalk between platelets and leukocytes (neutrophils, monocytes, and T cells), endothelial cells, and vascular smooth muscle cells.48 CCL5, chemokine (C-C motif) ligand 5, CD40, cluster of differentiation 40, CD40L, cluster of differentiation 40 ligand, CD62P, P-selectin, CitH3, citrullinated histone H3, cfDNA, cell free DNA, CLEC-2, C-type lectin-like type II, CypA, cyclophilin A, CXCL7, chemokine (C-X-C motif) ligand 7, ECs, endothelial cells, FXIIa, activated coagulation factor XII, GPIb, glycoprotein Ib, HMGB1, high mobility group box 1 protein, ICAM-1, intracellular adhesion molecule 1, integrin αIIbβ3, integrin α5β1, integrin αvβ3, MAC-1, macrophage-1 antigen, MVs, microvesicles, NETs, neutrophil extracellular traps, PAR1/4, protease activated receptor 1/4, PF4, platelet factor 4, S100A13, S100 calcium binding protein 13, TLR4, toll-like receptor 4, VSMCs, vascular smooth muscle cells, VWF, von Willebrand factor. Figure was created with Biorender.com
Haemodynamic alterations
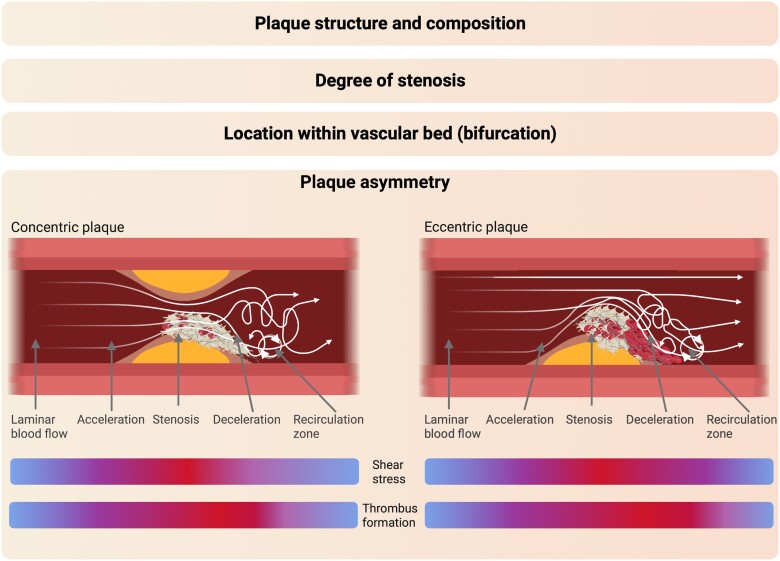
By causing endothelial dysfunction, haemodynamic disturbances drive atherosclerotic plaque development, but also in later stages, haemodynamic alterations contribute to plaque erosion49 and rupture.50 The rate of these flow disturbances depends on the asymmetry of the plaque (i.e. concentric vs. eccentric). Concentric plaques exhibit minimal alterations during the cardiac cycle, while eccentric plaques can undergo significant changes as their shoulder region (i.e. the junction between the plaque and the adjacent plaque-free vessel wall) bends, increasing the risk for plaque disruption/rupture.51 The majority (80%) of plaques observed in patients with coronary atherosclerosis exhibited eccentricity.52,53 Previous studies demonstrated that during ACS, regions of plaque rupture often coincide with elevated levels of endothelial shear stress and that the circumferential stress is centred at the shoulder region in coronary atherosclerosis.54–56
Also, platelet adhesion and aggregation are affected by local shear microgradients.57,58 Thereby, local disturbances in haemodynamics shape plaque-induced thrombus formation (Figure 3). In silico modelling has shown that—using a straight vessel model—platelets adhered and aggregated to the injured site in a homogeneous fashion, leading to an even coverage.59 With increasing stenosis, thrombus formation gradually became more skewed towards the distal part of the injured stenotic site.59 At 70% stenosis, platelet aggregation was predominantly initiated at the apex of the stenosis, and the thrombus was located at the distal and downstream parts of the injured site, leaving most of the upstream injured site uncovered.59 In both in vitro and in silico models of vessel stenosis, the highest wall shear stress can be found at the apex of the stenosis, which is also the initiation and anchor point of platelet aggregation.58,60 Depending on the level of turbulence, the thrombus either grows along the post-stenosis channel wall or lines up along the post-stenotic recirculation zone.58,60 The rate at which thrombi grow at these pathological shear rates is strongly accelerated compared to physiological arterial shear rates,60,61 with post-stenotic deceleration amplifying the rate of platelet aggregation.58 Also stenosis length and surface roughness affect platelet adhesion and activation.62 An increasing stenosis length results in enhanced priming of platelets for activation in vitro.63
Figure 3.
Factors influencing local haemodynamics and contributing to plaque-induced thrombus formation. Schematic representation of factors that have a local effect on haemodynamics and shape thrombus formation. For more information, see Haemodynamic alterations. Figure was created with Biorender.com
Matrix components
As demonstrated by in vitro experiments, the thrombogenic surface strongly determines the type of thrombus formed.64 Whereas the basement membrane of healthy vessels consists for a major part of collagen Type IV,65 atherosclerotic lesions consist predominantly of collagen Types I and III.17 Importantly, significant heterogeneity in collagen composition exists between the different plaque phenotypes. Stable lesions contain a mixture of collagen Types I and III with an accumulation of Type I in the fibrous cap.17 In contrast, in regions of plaque erosion Type III predominates, while in ruptured plaques at the site of cap thinning, attenuated strands of Type I can be found.17 In terms of thrombogenic properties, the different types of collagen support platelet adhesion and activation to a different extent depending on the differential contribution of the platelet collagen receptors glycoprotein VI (GPVI) and α2β1.66 However, in vitro whole blood perfusion experiments over human atherosclerotic plaque homogenate showed a great dependency on GPVI as blockage of GPVI effectively inhibited thrombus formation. Inhibition of α2β1, however, was without effects.67–70 Notably, plaque composition and structure are not taken into account in these experimental set-ups.
In contrast to stable or ruptured plaques, eroded plaques are typically rich in hyaluronan and proteoglycans like versican.17 Interestingly, in eroded plaques, the receptor for hyaluronan cluster of differentiation 44 (CD44) is highly expressed by VSMCs at the plaque/thrombus interface, whereas weakly expressed in platelets.17 Although hyaluronan has anti-inflammatory and antithrombotic properties when incorporated into the glycocalyx, the opposite is true for hyaluronan within atherosclerotic plaques.71 Platelet binding to hyaluronan has been reported;72 however, to which extent hyaluronan actively supports platelet adhesion and activation is unknown. Alternatively, platelets express hyaluronidase 2 and thereby contribute to degradation of hyaluronan into fragments which are pro-inflammatory and angiogenic.73 A higher (platelet) hyaluronidase 2 expression has also been observed in patients with ACS compared to patients with stable angina, with a significantly higher expression in patients with ruptured vs. intact plaques.74,75 In case of versican, it has been shown that versican isolated from human aortic tissue can support platelet adhesion, especially at low shear, in a GPIbα- and integrin αIIbβ3-independent manner.76 Moreover, co-coating of versican with collagen Types I and III significantly enhanced thrombus formation.76 The proteoglycans, decorin and biglycan, can also support platelet adhesion and activation,77,78 although their non-differential expression17 does not argue for a relatively larger contribution to thrombus formation on eroded vs. ruptured plaques.
Within advanced atherosclerotic lesions, an up-regulated expression of C-type lectin-like type II (CLEC-2) ligands has been detected,79,80 with podoplanin expression in human atherosclerotic lesions79 and S100A13 in murine plaques.80 Whether expression of these ligands is differentially regulated in eroded vs. ruptured plaques is unknown. In a rat model of vascular occlusion, gene transfer of human podoplanin resulted in the formation of occlusive thrombi at the site of endothelial overexpression.81 However, thrombus formation on coated human plaque material was not affected by blockage of CLEC-2, suggesting that other matrix interactions are more important in humans.82
In healthy arteries, TF expression is predominantly restricted to the adventitial fibroblasts.83 However, atherosclerotic lesions exhibit a significant up-regulation of procoagulant TF, expressed and released by activated macrophages and VSMCs.83,84 This up-regulation can be triggered by exposure to modified LDLs or pro-inflammatory cytokines, such as IL-1, interferon (IFN)-γ, and tumour necrosis factor (TNF)-α.85 This strong up-regulation in activity of the extrinsic coagulation pathway results in thrombin-mediated platelet activation and fibrin formation, where the expression level of TF is positively associated with thrombus size.86 Although TF has a dominant role in plaque-induced coagulation, factor XIIa ensures thrombus stability as shown by an enhanced embolization rate upon plaque rupture in mice injected with a Factor XIIa inhibitor as well as an impaired plaque-induced thrombus formation in vitro using blood from Factor XII deficient mice and patients.87
That TF contributes more to thrombus formation in plaque rupture than in plaque erosion is reflected by the relatively higher fibrin content of thrombi formed on ruptured plaques than on eroded plaques.40 Moreover, in ruptured plaques, fibrin deposition as a result of intra-plaque haemorrhage has been observed in regions rich in macrophages and cholesterol clefts as well as in the necrotic core.88 With fibrin supporting platelet adhesion and aggregation under flow,89 fibrin deposits within plaques could thereby serve as scaffold for thrombus formation.88 Furthermore, GPVI–fibrin interactions contribute to platelet procoagulant activity and GPVI modulates clot structure, resulting in the formation of a denser and less permeable fibrin network.90
Soluble factors
Plaque-induced thrombus formation is highly dependent on platelet autocrine and paracrine feed-forward signalling. This is for example well-illustrated in an in vitro stenosis model where inhibition of adenosine diphosphate (ADP) and thromboxane A2 (TXA2) signalling significantly reduced platelet aggregation post-stenosis.91 Also, the stabilizing role of platelet P2Y12 receptors in thrombus formation was shown in an in vivo mouse model of plaque rupture.92 P2Y12 inhibition or deficiency resulted in the formation of smaller thrombi that more easily embolized.92 In healthy blood vessels, the endothelium contributes to the continuous conversion of adenosine triphosphate and ADP into adenosine by adenosine diphosphatase (ADPase). Typically, ADPase activity reduces with increasing severity of atherosclerosis.93 The importance of vascular ADPase activity in limiting thrombus formation has been shown in a rat model of thrombosis, where over-expression of human E-NTPDase in VSMCs significantly inhibited photochemically induced thrombus formation.94
Circulating vWF, supporting GPIbα-mediated platelet rolling, is also a key in plaque-induced thrombus formation. von Willebrand factor is synthesized and released by ECs as ultralarge multimers (ultra-large von Willebrand factor [ULVWF]), but upon inflammatory triggers its expression is significantly up-regulated.95 Also in stenotic regions, vWF expression is increased, and thrombus formation post-stenosis is highly vWF dependent.91 In physiological conditions, the protease ADAMTS13 cleaves ULVWF into smaller, less thrombogenic fragments.96 Pro-inflammatory cytokines like TNFα, IFNγ, and cluster of differentiation 40 ligand (CD40L), important in atherosclerosis, have been shown to reduce ADAMTS13 expression in ECs in vitro.97 Moreover, it has been reported that CAD patients had significantly lower plasma ADAMTS13 levels and activity.96 Consequently, cultured ECs exposed to plasma obtained from CAD patients showed significantly increased platelet tethering to ULVWF as well as an up-regulation of ULVWF, demonstrating the functional impact of the reduction in plasma ADAMTS13 in CAD patients.96 Along the same line, it has been shown that MMP13, a matrix metalloproteinase significantly up-regulated in atherosclerotic plaques,98 can cleave vWF resulting in the generation of fragments that potently support platelet adhesion.99 Furthermore, these vWF fragments induced the formation of larger thrombi on a collagen surface, suggesting that by cleaving vWF, MMP13 can augment the thrombotic response of the atherosclerotic plaque.99
Upon activation, platelets are a major source of plasma CD40L.100In vivo mouse models of atherosclerosis have shown that CD40L plays an instrumental role in the development of atherosclerotic plaques as it accelerates plaque development and progression.101 Interestingly, platelet CD40L does not appear to be involved in atherogenesis but rather contributes to atherothrombosis.102 Here, CD40L has a stimulatory effect on collagen and plaque-induced thrombus formation, illustrated by the reduction in thrombus size in CD40L−/− mice103 as well as the increased aggregate size upon the supplementation of CD40L.104 Another chemokine highly up-regulated in smooth muscle cells, ECs, and macrophages within atherosclerotic plaques is stromal cell-derived factor-1 (SDF-1).105 Also platelet expression levels of SDF-1 are up-regulated in ACS patients, although no difference was observed between NSTEMI and STEMI patients.106 SDF-1 can activate platelets as well as enhance collagen- and plaque-induced platelet activation and thrombus formation in a CXCR4-dependent manner, resulting in increased TXA2 generation and granule release.105,107,108 In line with in vitro findings, SDF-1−/− mice exhibited a prolonged time to vessel occlusion in an arterial thrombosis model as thrombus growth and stability were reduced without impacting haemostasis.108 Interestingly, systemic treatment of mice with SDF-1 resulted in the stabilization of atherosclerotic plaques as the accumulation of smooth muscle progenitor cells was enhanced.109 However, in a cohort of patients with stable CAD or ACS, a high platelet surface expression of SDF-1 was significantly associated with the composite of all-cause death, MI, and/or stroke,110 suggesting that the negative effects outrule the plaque stabilizing effects of SDF-1.
Oxidized LDL (oxLDL) is a key in the development of atherosclerotic lesions, but besides its activating effects on the endothelium and immune system, oxLDL affects platelet reactivity too.111 Platelet activation upon oxLDL exposure is triggered via cluster of differentiation 36 (CD36) signalling, illustrated by a reduction in platelet aggregation and granule secretion in hyperlipidaemic CD36−/− mice.112,113 Furthermore, genetic knockout of CD36 corrected the prothrombotic phenotype of hyperlipidaemic ApoE−/− mice as time to vessel occlusion and tail bleeding times were comparable with those of wild-type mice.112 Given the high degree of oxLDL accumulation within atherosclerotic plaques, especially upon plaque rupture where the circulation is in direct contact with the lipid core, platelets can be exposed locally to high concentrations of oxLDL. Moreover, platelets can contribute to oxidation of LDL, thereby fuelling oxLDL-induced activation.114 Further, cholesterol deposited within the atherosclerotic plaque can crystallize, leading to the expansion of the plaque and potential disruption of the cap by sharp-edged crystals.115,116 Upon contact with the circulation, these cholesterol crystals can further amplify the platelet response117,118 and support coagulation in a complement-dependent manner.119 In human carotid and coronary plaques, a significant association between thrombus formation and crystal content was observed, with a higher crystal content resulting in an increased risk of atherothrombosis.116
Platelet interactions with other cell types
In arterial thrombi of patients with MI, neutrophils are the most abundant leukocyte subset. Through direct interactions, platelets and neutrophils interact and activate each other (Figure 2). Cytokines and chemokines held within platelet granules can recruit and activate neutrophils, but also neutrophils can lead to platelet activation through, e.g., NET formation.120 Studies have found the resulting platelet-neutrophil aggregates to independently predict atherothrombotic events.121
Within both eroded and ruptured plaques, neutrophils and NETs were mostly observed within the thrombus and at the thrombus–plaque interface. Moreover, neutrophils and NETs were scarce in intact lesions, suggesting that, in humans, their role is limited in stages of atherosclerosis in which thrombus formation has not yet occurred.21 Given their direct and indirect prothrombotic effects, NETs can augment the thrombotic response once incorporated into the plaque and growing thrombus. Vice versa, activated platelets can also elicit NET formation (Figure 2).122 The central role of NETs and its mediator PAD4 in atherothrombosis was shown in a mouse model that mimics aspects of plaque erosion, where bone marrow PAD4 deficiency was associated with a decrease in endothelial injury, smaller thrombi, and lower luminal TF levels.19 Loss of (endothelial) nuclease activity could potentially also contribute to neutrophil activation and subsequent NET formation, in addition to platelet activation.123 Whether neutrophils and NETs have a differential contribution to thrombus formation driven by plaque rupture or erosion in humans requires more investigation.
Although not as abundant as neutrophils, other leukocyte subtypes in plaques can interact with platelets and affect platelet activation. Monocytes and T cells bind to platelets, mostly through similar mechanisms as for platelet–neutrophil interactions (Figure 2).124,125 Given the subtle differences in abundance of the various leukocyte subtypes associated with plaque vulnerability,28,29 platelet activation and subsequent thrombus formation may be differentially influenced.
Whereas the interaction between healthy ECs and platelets is actively prevented, activated ECs in atherosclerotic plaques support platelet adhesion and activation through a plethora of mechanisms. Also here, activated platelets can enhance endothelial dysfunction by secretion of granular cargo (Figure 2). In addition to direct cell–cell contact or soluble mediators, extracellular vesicles derived from platelets effectuate another way of intercellular communication. Extracellular vesicle generated from platelets with specific phenotypes may have either protective or detrimental effects on endothelial integrity.126,127 A recent study showed that platelets trigger endothelial activation through platelet matrix metalloproteinase 2 (MMP-2)-mediated activation of endothelial PAR1, indicating that platelet MMP-2 is involved in the early stages of atherogenesis. Moreover, in a cohort of CAD patients, it was observed a positive correlation between platelet MMP-2 expression and the degree of carotid artery stenosis at ultrasound imaging.128 As MMP-2 activity has been linked to plaque instability and rupture,16,28 platelet MMP-2 activity may contribute to the progression of stable atherosclerotic plaques towards ruptured plaques.
VSMCs within atherosclerotic lesions switch phenotype and thereby alter plaque characteristics.129 As their phenotype changes, the thrombogenic potential of the VSMC may also change as recently shown for calcified VSMCs.130 Which mediators are involved are largely unknown, although a role for CLEC-2–S100A13 interactions has been shown in mice (Figure 2).80
Plaque and thrombus healing
Thrombus formation on disrupted plaques is a critical process in the onset of acute cardiovascular events, but it does not always lead to complete vessel occlusion with subsequent acute symptomatic events. In ACS, a non-occlusive or transiently occlusive thrombus most often results in NSTEMI, while STEMI patients more commonly present with a more stable and occlusive thrombus.131 This can be seen in the different prevalence of healed plaques in ACS patients which is indicative for previous non-occlusive thrombotic events.
Healed plaques are identified by multi-layers of different optical characteristics observed at sites previously associated with thrombi formed through an acute event and which have undergone a so-called healing process. The first matrix layer consists of a platelet- and fibrin-rich thrombus, infiltrating VSMCs, and macrophages, and eventually the plaque becomes fully re-endothelialized upon complete healing.132,133 The healed plaque may undergo further destabilization and eventual healing without manifesting in clinical symptoms as shown by autopsy studies.134,135 Clinical manifestation of this process (i.e. ACS) is believed to be the result of disruption of the delicate balance between atherosclerotic plaque activation and passivation.133
Autopsy studies revealed that 32%–61% of sudden coronary death was associated with a healed plaque phenotype contributing to narrowing of the lumen.134,135 In patients with coronary syndrome, the rate of healed plaques varies between 3% and 90% depending mainly on the detection method and clinical presentation.136–141 In the last decades, healed plaques have been associated with higher luminal stenosis,138,139,142 higher vulnerability,142,143 but not with thrombus burden.142 Interestingly, newly formed healed plaques have been shown to have a larger thrombus burden and lower luminal stenosis which gradually increases during the healing process.136
The concept of the dynamic nature of the process behind plaque rupture and healing occurring in ACS has been supported by the presence of differently matured thrombi in STEMI patients. This thrombus maturation process allows the thrombus to evolve gradually over a period of days or weeks before fully obstructing the lumen.134,144 During this maturation/healing process, clot composition changes from a platelet and fibrin rich fresh thrombus into a lytic and eventually organized thrombus with infiltrated VSMCs and/or ECs. The occurrence of these aged thrombi was first recognized in an autopsy study of young adults who died from sudden coronary occlusion.144 Histopathological analyses on aspirated thrombi from STEMI patients showed a prevalence of aged (i.e. >1 day old) thrombi of 7%–63%, whereas fresh thrombi (i.e. <1 day old) had a prevalence of 34%–76%.36,134,145–151 In a more in-depth analysis, it was shown that lytic thrombi (thrombi aged 1–5 days) and organized thrombi (thrombi aged >5 days) displayed comparable occurrence rates (17%–35% vs. 7%–36%, respectively), indicating that acute coronary occlusion frequently marks the final stage of thrombotic events that took place in the preceding days or weeks.36,145–147,150 To reinforce the notion of an active process, thrombi consisting of both freshly formed and lytic or organized components were observed in 7% of STEMI patients.146,151
Platelets play a significant role in both the development of arterial plaques and the process of wound healing, suggesting their potential involvement in plaque healing. However, the mechanism behind this process is not yet well understood. Studies have demonstrated that plaque healing occurs as a result of VSMC proliferation, triggered by growth factors such as platelet-derived growth factor BB and platelet TGF-β, as well as mitogens (e.g. thrombin) that are released during acute thrombosis.12,152In vivo studies in mice have shown that activated platelets adhering to an injured arterial wall can facilitate the recruitment of bone marrow-derived VSMC progenitor cells from circulating blood through the expression of P-selectin and SDF-1.153–155
Therapeutic implications and novel antiplatelet targets
Dual antiplatelet therapy consisting of aspirin with a P2Y₁₂ inhibitor is the cornerstone of initial management of ACS, whether or not combined with anticoagulant therapy and followed by an invasive approach.156 Especially in the first month after an ACS event, the benefit of antithrombotic therapy outweighs the risk of bleeding. However, over time the bleeding risk can increase relative to the thrombotic risk.156 In addition, antithrombotic treatment, in particular anticoagulant treatment, has been shown in a meta-analysis to be associated with a higher risk of intra-plaque haemorrhage in carotid atherosclerotic plaques,157 a characteristic of unstable plaques.158 For patients with chronic coronary syndromes (CCS), including stable CAD or an ACS event >1 year ago, current guidelines for those in sinus rhythm recommend single antiplatelet therapy, while dual antiplatelet therapy (DAPT) or low-dose dual antithrombotic therapy (aspirin and rivaroxaban) should be considered in patients with a high ischaemic risk but low bleeding risk.159 Active and forthcoming clinical trials evaluate more potent P2Y12 inhibitors, combinations of antiplatelet agents and direct oral anticoagulants in addition to antiplatelet therapy in ACS and CCS patients (Table 2).
Table 2.
Ongoing and forthcoming clinical trials in acute coronary syndrome and chronic coronary syndrome patients
| Trial number | Trial name | Study population | Interventions | Primary outcome measures | Phase |
|---|---|---|---|---|---|
| NCT03331484 | CAPITAL PCI AF | ACS patients with AF (n = 40) | Ticagrelor plus rivaroxaban | Composite of TIMI bleeds | 3 |
| NCT03357874 | TROUPER | ACS patients with CKD (n = 514) | Clopidogrel vs. ticagrelor | Rate of MACE | 3 |
| NCT04718025 | ELECTRA-SIRIO | ACS patients (n = 4500) | Low-dose ticagrelor plus aspirin vs. low-dose ticagrelor plus placebo vs. standard dose ticagrelor plus aspirin | Bleeding, death from any cause, non-fatal MI, or non-fatal stroke | 3 |
| NCT05162053 | PK/PD Study of Vicagrel and Clopidogrel in healthy subjects with different CYP2C19 metabolizers | Healthy subjects with different CYP2C19 metabolizers (n = 128) | Cross-dosing of vicagrel and clopidogrel in different metabolizer groups | Inhibition of platelet aggregation, platelet reactivity, maximum plasma concentration of vicagrel and clopidogrel, AUC over a dosing interval | 1 |
| NCT05233124 | OVER-TIME | ACS patients with coronary artery ectasia (n = 60) | DAPT of aspirin plus clopidogrel vs. clopidogrel monotherapy plus low-dose rivaroxaban | Composite of cardiovascular death, recurrent MI, and repeated vascularization, composite of minor and major bleeding events | 2 |
| NCT05577988 | ADEN | ACS patients (n = 2468) | Stop aspirin for ticagrelor or prasugrel vs. aspirin or clopidogrel guided by genetic testing | Rate of combined major and minor bleeding events | 3 |
| NCT05638867 | NOAC therapy guided by PARIS Risk Score and D-dimer in patients with ACS after PCI | ACS patients with high ischaemic risk (n = 3944) | Aspirin plus clopidogrel plus rivaroxaban for 3 months followed by DAPT vs. aspirin plus clopidogrel after PCI | MACCE | 3 |
| NCT05779059 | PROTEUS | ACS patients (NSTEMI, unstable angina) (n = 50) | Initial ticagrelor and switch to prasugrel at Day 45 or initial prasugrel and switch to ticagrelor at Day 45 | Platelet reactivity | 3 |
| NCT05825573 | ARGONAUT | Patients with intra-cardiac thrombus (n = 340) | VKA vs. DOAC | Net clinical benefit | 3 |
| NCT05093790 | A study to evaluate BMS-986141 added on to aspirin or ticagrelor or the combination, on thrombus formation in a thrombosis chamber model in participants with stable coronary artery disease and healthy participants | Stable CAD (n = 55) | Ticagrelor plus BMS-986141 vs. aspirin plus BMS-986141 vs. ticagrelor plus aspirin plus BMS-986141 vs. BMS-986141 | Change from baseline in thrombus area post-treatment BMS-986141 | 2 |
| NCT05122455 | Effects of edoxaban on platelet aggregation in patients with stable CAD | Stable CAD (n = 70) | Aspirin vs. aspirin plus edoxaban, followed by clopidogrel monotherapy vs. clopidogrel plus edoxaban, followed by edoxaban monotherapy | Platelet aggregability | 2/3 |
Includes clinical trials (Phase 1–3) based on ClinicalTrials.gov for platelet aggregation inhibitors and anticoagulants. Selection criteria disease: acute coronary syndrome ACS, (stable) coronary artery disease (CAD), chronic coronary syndrome (CCS).
AF, atrial fibrillation; CKD, chronic kidney disease; CYP2C19, cytochrome P450 2C19; DAPT, dual antiplatelet therapy; DOAC, direct oral anticoagulant; MACE, major adverse cardiovascular event; MACCE, major adverse cardiac and cerebrovascular event; MI, myocardial infarction; NOAC, novel oral anticoagulants; NSTEM, non-ST-elevation myocardial infarction; PCI, percutaneous coronary intervention; PK/PD, pharmacokinetic/pharmacodynamic; STEMI, ST-elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction; VKA, vitamin-K antagonist.
Despite antithrombotic therapy, recurrent thrombotic events are reported in patients with CAD underscoring the clinical need for more effective drugs that prevent thrombosis without compromising haemostasis. Especially upon plaque rupture, TF/FXIIa-induced thrombin generation will contribute to platelet activation. The findings that thrombin generation remains high after ACS160 and thrombin signalling via platelet PAR1/4 is preserved in patients on DAPT161 indicate that antagonism of these receptors is of interest. Since PAR1 inhibition is associated with significant bleeding, PAR4 is seen as a promising new therapeutic target.162 The small molecule BMS-986141 blocks PAR4 and is currently assessed in a Phase 2 clinical trial (NCT05093790) in CCS patients (Table 2).
GPVI plays an important role in platelet activation via the interaction with collagen, present in both eroded and ruptured plaques, as well as in enhancing thrombus growth via binding to fibrin. The recombinant GPVI-Fc complex revacept and the humanized Fab fragment ACT017 have been designed to block GPVI. In a Phase 2 trial (ISAR-PLASTER), revacept did not reduce the incidence of MI in patients with stable ischaemic heart disease undergoing PCI.163 ACT017 is now assessed in a Phase 2/3 trial (NCT05070260) on top of standard therapy in patients with acute ischaemic stroke, awaiting further investigation in patients with CAD. In ACS patients, a higher fraction of procoagulant platelets (exposing phosphatidylserine) was reported.164 This procoagulant response is typically induced by combined stimulation of GPVI and thrombin receptors, meaning that inhibition of GPVI signalling might be beneficial in lowering the procoagulant activity of platelets and subsequent thrombin generation. Furthermore, ACS patients in the acute phase present with a higher fraction of highly reactive young platelets,165 and GPVI expression is increased in young platelets.166
Other promising antiplatelet targets include vWF, GPIbα, 5-hydroxytryptamine receptor subtype 2A, protein disulfide isomerase, P-selectin, phosphoinositide 3-kinase β, and phosphodiesterase-3, as reviewed elsewhere.162
Future perspectives
In patients with ACS, the phenotype and location of the plaque in the coronary circuit determine the extent of platelet activation and structural composition of the thrombus. Advancements in technology gradually enable characterization of multiple plaque phenotypes beyond eroded and ruptured plaques, and these phenotypes can be mechanistically linked to the process of thrombus formation and the specific platelet populations involved. Future research focused on in-depth characterization of thrombus architectures is essential for targeting the main pathophysiological factors and thereby optimizing the efficacy of antithrombotic therapy in ACS patients. In our opinion, the development and application of atherothrombosis models (in vivo, ex vivo, in silico) based on different plaque phenotypes is crucial. These models allow the testing of novel platelet inhibitors (e.g. GPVI inhibitors) and/or anticoagulants either alone or on top of standard therapy.
Since patients with ACS are often of advanced age and present with comorbidities, the complexity of antithrombotic management has increased and should be more tailored to individual characteristics of patients. Considering the importance of inflammatory processes in the pathophysiology of the main processes of atherothrombosis (plaque formation, progression, and rupture), it has also been suggested to include anti-inflammatory drugs in the prevention of cardiovascular atherothrombotic events. This combination of antithrombotic and anti-inflammatory approaches might be a promising treatment strategy for patients with ACS.
Supplementary data
Supplementary data are not available at European Heart Journal online.
Contributor Information
Constance C F M J Baaten, Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Universiteitssingel 50, 6229 ER, Maastricht, the Netherlands; Institute for Molecular Cardiovascular Research (IMCAR), University Hospital RWTH Aachen, Aachen, Germany.
Magdolna Nagy, Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Universiteitssingel 50, 6229 ER, Maastricht, the Netherlands.
Wolfgang Bergmeier, Department of Biochemistry and Biophysics, School of Medicine, University of North Caroline at Chapel Hill, Chapel Hill, NC, USA; Blood Research Center, School of Medicine, University of North Caroline at Chapel Hill, Chapel Hill, NC, USA.
Henri M H Spronk, Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Universiteitssingel 50, 6229 ER, Maastricht, the Netherlands; Department of Internal Medicine, Maastricht University Medical Center+, Maastricht, the Netherlands; Thrombosis Expertise Center, Heart+ Vascular Center, Maastricht University Medical Center+, P. Debeyelaan 25, Maastricht, the Netherlands.
Paola E J van der Meijden, Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Universiteitssingel 50, 6229 ER, Maastricht, the Netherlands; Thrombosis Expertise Center, Heart+ Vascular Center, Maastricht University Medical Center+, P. Debeyelaan 25, Maastricht, the Netherlands.
Declarations
Disclosure of Interest
H.S. is a shareholder of Coagulation Profile.
Data Availability
No data were generated or analysed for or in support of this paper.
Funding
This work was supported by the Dutch Heart Foundation (2020T020 to C.C.F.M.J.B.) and by a National Institutes of Health grant (R35HL144976 to W.B.).
References
- 1. van der Meijden PEJ, Heemskerk JWM. Platelet biology and functions: new concepts and clinical perspectives. Nat Rev Cardiol 2019;16:166–79. 10.1038/s41569-018-0110-0 [DOI] [PubMed] [Google Scholar]
- 2. Anfossi G, Trovati M. Role of catecholamines in platelet function: pathophysiological and clinical significance. Eur J Clin Invest 1996;26:353–70. 10.1046/j.1365-2362.1996.150293.x [DOI] [PubMed] [Google Scholar]
- 3. Yeung AK, Villacorta-Martin C, Hon S, Rock JR, Murphy GJ. Lung megakaryocytes display distinct transcriptional and phenotypic properties. Blood Adv 2020;4:6204–17. 10.1182/bloodadvances.2020002843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pariser DN, Hilt ZT, Ture SK, Blick-Nitko SK, Looney MR, Cleary SJ, et al. . Lung megakaryocytes are immune modulatory cells. J Clin Invest 2021;131:e137377. doi : 10.1172/JCI137377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Versteeg HH, Heemskerk JWM, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev 2013;93:327–58. 10.1152/physrev.00016.2011 [DOI] [PubMed] [Google Scholar]
- 6. Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr, et al. . A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1995;92:1355–74. 10.1161/01.CIR.92.5.1355 [DOI] [PubMed] [Google Scholar]
- 7. Lievens D, von Hundelshausen P. Platelets in atherosclerosis. Thromb Haemost 2011;106:827–38. 10.1160/TH11-08-0592 [DOI] [PubMed] [Google Scholar]
- 8. Huilcaman R, Venturini W, Fuenzalida L, Cayo A, Segovia R, Valenzuela C, et al. . Platelets, a key cell in inflammation and atherosclerosis progression. Cells 2022;11:1014. 10.3390/cells11061014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mancuso ME, Santagostino E. Platelets: much more than bricks in a breached wall. Br J Haematol 2017;178:209–19. 10.1111/bjh.14653 [DOI] [PubMed] [Google Scholar]
- 10. Libby P. The changing landscape of atherosclerosis. Nature 2021;592:524–33. 10.1038/s41586-021-03392-8 [DOI] [PubMed] [Google Scholar]
- 11. Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res 2014;114:1852–66. 10.1161/CIRCRESAHA.114.302721 [DOI] [PubMed] [Google Scholar]
- 12. Libby P. Inflammation in atherosclerosis. Nature 2002;420:868–74. 10.1038/nature01323 [DOI] [PubMed] [Google Scholar]
- 13. van der Wal AC, Becker AE, van der Loos CM, Das PK. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 1994;89:36–44. 10.1161/01.CIR.89.1.36 [DOI] [PubMed] [Google Scholar]
- 14. White SJ, Newby AC, Johnson TW. Endothelial erosion of plaques as a substrate for coronary thrombosis. Thromb Haemost 2016;115:509–19. 10.1160/th15-09-0765 [DOI] [PubMed] [Google Scholar]
- 15. Farb A, Burke AP, Tang AL, Liang TY, Mannan P, Smialek J, et al. . Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation 1996;93:1354–63. 10.1161/01.CIR.93.7.1354 [DOI] [PubMed] [Google Scholar]
- 16. Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 1994;94:2493–503. 10.1172/JCI117619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kolodgie FD, Burke AP, Farb A, Weber DK, Kutys R, Wight TN, et al. . Differential accumulation of proteoglycans and hyaluronan in culprit lesions: insights into plaque erosion. Arterioscler Thromb Vasc Biol 2002;22:1642–8. 10.1161/01.ATV.0000034021.92658.4C [DOI] [PubMed] [Google Scholar]
- 18. Kolodgie FD, Burke AP, Wight TN, Virmani R. The accumulation of specific types of proteoglycans in eroded plaques: a role in coronary thrombosis in the absence of rupture. Curr Opin Lipidol 2004;15:575–82. 10.1097/00041433-200410000-00012 [DOI] [PubMed] [Google Scholar]
- 19. Franck G, Mawson TL, Folco EJ, Molinaro R, Ruvkun V, Engelbertsen D, et al. . Roles of PAD4 and NETosis in experimental atherosclerosis and arterial injury: implications for superficial erosion. Circ Res 2018;123:33–42. 10.1161/CIRCRESAHA.117.312494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quillard T, Araújo HA, Franck G, Shvartz E, Sukhova G, Libby P. TLR2 And neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion. Eur Heart J 2015;36:1394–404. 10.1093/eurheartj/ehv044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pertiwi KR, van der Wal AC, Pabittei DR, Mackaaij C, van Leeuwen MB, Li X, et al. . Neutrophil extracellular traps participate in all different types of thrombotic and haemorrhagic complications of coronary atherosclerosis. Thromb Haemost 2018;118:1078–87. 10.1055/s-0038-1641749 [DOI] [PubMed] [Google Scholar]
- 22. Folco EJ, Mawson TL, Vromman A, Bernardes-Souza B, Franck G, Persson O, et al. . Neutrophil extracellular traps induce endothelial cell activation and tissue factor production through interleukin-1α and cathepsin G. Arterioscler Thromb Vasc Biol 2018;38:1901–12. 10.1161/ATVBAHA.118.311150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fahed AC, Jang IK. Plaque erosion and acute coronary syndromes: phenotype, molecular characteristics and future directions. Nat Rev Cardiol 2021;18:724–34. 10.1038/s41569-021-00542-3 [DOI] [PubMed] [Google Scholar]
- 24. Vergallo R, Jang IK, Crea F. New prediction tools and treatment for ACS patients with plaque erosion. Atherosclerosis 2021;318:45–51. 10.1016/j.atherosclerosis.2020.10.016 [DOI] [PubMed] [Google Scholar]
- 25. Niccoli G, Montone RA, Di Vito L, Gramegna M, Refaat H, Scalone G, et al. . Plaque rupture and intact fibrous cap assessed by optical coherence tomography portend different outcomes in patients with acute coronary syndrome. Eur Heart J 2015;36:1377–84. 10.1093/eurheartj/ehv029 [DOI] [PubMed] [Google Scholar]
- 26. Mokry M, Boltjes A, Slenders L, Bel-Bordes G, Cui K, Brouwer E, et al. . Transcriptomic-based clustering of human atherosclerotic plaques identifies subgroups with different underlying biology and clinical presentation. Nat Cardiovasc Res 2022;1:1140–55. 10.1038/s44161-022-00171-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fernandez DM, Rahman AH, Fernandez NF, Chudnovskiy A, Amir EAD, Amadori L, et al. . Single-cell immune landscape of human atherosclerotic plaques. Nat Med 2019;25:1576–88. 10.1038/s41591-019-0590-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ge P, Li H, Ya X, Xu Y, Ma L, He Q, et al. . Single-cell atlas reveals different immune environments between stable and vulnerable atherosclerotic plaques. Front Immunol 2022;13:1085468. 10.3389/fimmu.2022.1085468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Depuydt MAC, Prange KHM, Slenders L, Örd T, Elbersen D, Boltjes A, et al. . Microanatomy of the human atherosclerotic plaque by single-cell transcriptomics. Circ Res 2020;127:1437–55. 10.1161/CIRCRESAHA.120.316770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Edsfeldt A, Swart M, Singh P, Dib L, Sun J, Cole JE, et al. . Interferon regulatory factor-5-dependent CD11c+ macrophages contribute to the formation of rupture-prone atherosclerotic plaques. Eur Heart J 2022;43:1864–77. 10.1093/eurheartj/ehab920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Caro CG, Fitz-Gerald JM, Schroter RC. Arterial wall shear and distribution of early atheroma in man. Nature 1969;223:1159–60. 10.1038/2231159a0 [DOI] [PubMed] [Google Scholar]
- 32. Yamamoto E, Yonetsu T, Kakuta T, Soeda T, Saito Y, Yan BP, et al. . Clinical and laboratory predictors for plaque erosion in patients with acute coronary syndromes. J Am Heart Assoc 2019;8:e012322. 10.1161/JAHA.119.012322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dai J, Xing L, Jia H, Zhu Y, Zhang S, Hu S, et al. . In vivo predictors of plaque erosion in patients with ST-segment elevation myocardial infarction: a clinical, angiographical, and intravascular optical coherence tomography study. Eur Heart J 2018;39:2077–85. 10.1093/eurheartj/ehy101 [DOI] [PubMed] [Google Scholar]
- 34. Wang J, Fang C, Zhang S, Li L, Lu J, Wang Y, et al. . Systemic and local factors associated with reduced thrombolysis in myocardial infarction flow in ST-segment elevation myocardial infarction patients with plaque erosion detected by intravascular optical coherence tomography. Int J Cardiovasc Imaging 2021;37:399–409. 10.1007/s10554-020-02021-1 [DOI] [PubMed] [Google Scholar]
- 35. Costopoulos C, Huang Y, Brown AJ, Calvert PA, Hoole SP, West NEJ, et al. . Plaque rupture in coronary atherosclerosis is associated with increased plaque structural stress. JACC Cardiovasc Imaging 2017;10:1472–83. 10.1016/j.jcmg.2017.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kramer MCA, Rittersma SZH, de Winter RJ, Ladich ER, Fowler DR, Liang YH, et al. . Relationship of thrombus healing to underlying plaque morphology in sudden coronary death. J Am Coll Cardiol 2010;55:122–32. 10.1016/j.jacc.2009.09.007 [DOI] [PubMed] [Google Scholar]
- 37. Yahagi K, Davis HR, Arbustini E, Virmani R. Sex differences in coronary artery disease: pathological observations. Atherosclerosis 2015;239:260–7. 10.1016/j.atherosclerosis.2015.01.017 [DOI] [PubMed] [Google Scholar]
- 38. Sato Y, Kawakami R, Sakamoto A, Cornelissen A, Mori M, Kawai K, et al. . Sex differences in coronary atherosclerosis. Curr Atheroscler Rep 2022;24:23–32. 10.1007/s11883-022-00980-5 [DOI] [PubMed] [Google Scholar]
- 39. Seegers LM, Araki M, Nakajima A, Yonetsu T, Minami Y, Ako J, et al. . Sex differences in culprit plaque characteristics among different age groups in patients with acute coronary syndromes. Circ Cardiovasc Interv 2022;15:e011612. 10.1161/CIRCINTERVENTIONS.121.011612 [DOI] [PubMed] [Google Scholar]
- 40. Sato Y, Hatakeyama K, Yamashita A, Marutsuka K, Sumiyoshi A, Asada Y. Proportion of fibrin and platelets differs in thrombi on ruptured and eroded coronary atherosclerotic plaques in humans. Heart 2005;91:526–30. 10.1136/hrt.2004.034058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zalewski J, Bogaert J, Sadowski M, Woznicka O, Doulaptsis K, Ntoumpanaki M, et al. . Plasma fibrin clot phenotype independently affects intracoronary thrombus ultrastructure in patients with acute myocardial infarction. Thromb Haemost 2015;113:1258–69. 10.1160/TH14-09-0801 [DOI] [PubMed] [Google Scholar]
- 42. Silvain J, Collet JP, Nagaswami C, Beygui F, Edmondson KE, Bellemain-Appaix A, et al. . Composition of coronary thrombus in acute myocardial infarction. J Am Coll Cardiol 2011;57:1359–67. 10.1016/j.jacc.2010.09.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Silvain J, Collet JP, Guedeney P, Varenne O, Nagaswami C, Maupain C, et al. . Thrombus composition in sudden cardiac death from acute myocardial infarction. Resuscitation 2017;113:108–14. 10.1016/j.resuscitation.2017.01.030 [DOI] [PubMed] [Google Scholar]
- 44. Kataoka Y, Puri R, Hammadah M, Duggal B, Uno K, Kapadia SR, et al. . Sex differences in nonculprit coronary plaque microstructures on frequency-domain optical coherence tomography in acute coronary syndromes and stable coronary artery disease. Circ Cardiovasc Imaging 2016;9:e004506. 10.1161/CIRCIMAGING.116.004506 [DOI] [PubMed] [Google Scholar]
- 45. Kim HO, Kim CJ, Kim W, Cho JM, Soeda T, Takano M, et al. . Relative risk of plaque erosion among different age and sex groups in patients with acute coronary syndrome. J Thromb Thrombolysis 2020;49:352–9. 10.1007/s11239-019-01969-9 [DOI] [PubMed] [Google Scholar]
- 46. Burke AP, Farb A, Malcom G, Virmani R. Effect of menopause on plaque morphologic characteristics in coronary atherosclerosis. Am Heart J 2001;141:S58–62. 10.1067/mhj.2001.109946 [DOI] [PubMed] [Google Scholar]
- 47. Alkarithi G, Duval C, Shi Y, Macrae FL, Ariëns RAS. Thrombus structural composition in cardiovascular disease. Arterioscler Thromb Vasc Biol 2021;41:2370–83. 10.1161/ATVBAHA.120.315754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nagy M, van der Meijden PEJ, Glunz J, Schurgers L, Lutgens E, Ten Cate H, et al. . Integrating mechanisms in thrombotic peripheral arterial disease. Pharmaceuticals [Internet] 2022;15:1428:. 10.3390/ph15111428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sumi T, Yamashita A, Matsuda S, Goto S, Nishihira K, Furukoji E, et al. . Disturbed blood flow induces erosive injury to smooth muscle cell-rich neointima and promotes thrombus formation in rabbit femoral arteries. J Thromb Haemost 2010;8:1394–402. 10.1111/j.1538-7836.2010.03843.x [DOI] [PubMed] [Google Scholar]
- 50. Thondapu V, Mamon C, Poon EKW, Kurihara O, Kim HO, Russo M, et al. . High spatial endothelial shear stress gradient independently predicts site of acute coronary plaque rupture and erosion. Cardiovasc Res 2021;117:1974–85. 10.1093/cvr/cvaa251 [DOI] [PubMed] [Google Scholar]
- 51. Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation 1995;92:657–71. 10.1161/01.CIR.92.3.657 [DOI] [PubMed] [Google Scholar]
- 52. Yamagishi M, Terashima M, Awano K, Kijima M, Nakatani S, Daikoku S, et al. . Morphology of vulnerable coronary plaque: insights from follow-up of patients examined by intravascular ultrasound before an acute coronary syndrome. J Am Coll Cardiol 2000;35:106–11. 10.1016/S0735-1097(99)00533-1 [DOI] [PubMed] [Google Scholar]
- 53. Enrico B, Suranyi P, Thilo C, Bonomo L, Costello P, Schoepf UJ. Coronary artery plaque formation at coronary CT angiography: morphological analysis and relationship to hemodynamics. Eur Radiol 2009;19:837–44. 10.1007/s00330-008-1223-3 [DOI] [PubMed] [Google Scholar]
- 54. Fukumoto Y, Hiro T, Fujii T, Hashimoto G, Fujimura T, Yamada J, et al. . Localized elevation of shear stress is related to coronary plaque rupture: a 3-dimensional intravascular ultrasound study with in-vivo color mapping of shear stress distribution. J Am Coll Cardiol 2008;51:645–50. 10.1016/j.jacc.2007.10.030 [DOI] [PubMed] [Google Scholar]
- 55. Bourantas CV, Papafaklis MI, Naka KK, Tsakanikas VD, Lysitsas DN, Alamgir FM, et al. . Fusion of optical coherence tomography and coronary angiography—in vivo assessment of shear stress in plaque rupture. Int J Cardiol 2012;155:e24-6. 10.1016/j.ijcard.2011.07.005 [DOI] [PubMed] [Google Scholar]
- 56. Richardson PD, Davies MJ, Born GV. Influence of plaque configuration and stress distribution on fissuring of coronary atherosclerotic plaques. Lancet 1989;2:941–4. 10.1016/S0140-6736(89)90953-7 [DOI] [PubMed] [Google Scholar]
- 57. Miyazaki Y, Nomura S, Miyake T, Kagawa H, Kitada C, Taniguchi H, et al. . High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood 1996;88:3456–64. 10.1182/blood.V88.9.3456.bloodjournal8893456 [DOI] [PubMed] [Google Scholar]
- 58. Nesbitt WS, Westein E, Tovar-Lopez FJ, Tolouei E, Mitchell A, Fu J, et al. . A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med 2009;15:665–73. 10.1038/nm.1955 [DOI] [PubMed] [Google Scholar]
- 59. Kamada H, Tsubota KI, Nakamura M, Wada S, Ishikawa T, Yamaguchi T. Computational study on effect of stenosis on primary thrombus formation. Biorheology 2011;48:99–114. 10.3233/BIR-2011-0585 [DOI] [PubMed] [Google Scholar]
- 60. Ha H, Lee SJ. Hemodynamic features and platelet aggregation in a stenosed microchannel. Microvasc Res 2013;90:96–105. 10.1016/j.mvr.2013.08.008 [DOI] [PubMed] [Google Scholar]
- 61. Bark DL Jr, Para AN, Ku DN. Correlation of thrombosis growth rate to pathological wall shear rate during platelet accumulation. Biotechnol Bioeng 2012;109:2642–50. 10.1002/bit.24537 [DOI] [PubMed] [Google Scholar]
- 62. Bark DL Jr, Ku DN. Wall shear over high degree stenoses pertinent to atherothrombosis. J Biomech 2010;43:2970–7. 10.1016/j.jbiomech.2010.07.011 [DOI] [PubMed] [Google Scholar]
- 63. Rahman SM, Eichinger CD, Hlady V. Effects of upstream shear forces on priming of platelets for downstream adhesion and activation. Acta Biomater 2018;73:228–35. 10.1016/j.actbio.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. de Witt SM, Swieringa F, Cavill R, Lamers MME, van Kruchten R, Mastenbroek T, et al. . Identification of platelet function defects by multi-parameter assessment of thrombus formation. Nat Commun 2014;5:4257. 10.1038/ncomms5257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Manon-Jensen T, Kjeld NG, Karsdal MA. Collagen-mediated hemostasis. J Thromb Haemost 2016;14:438–48. 10.1111/jth.13249 [DOI] [PubMed] [Google Scholar]
- 66. Jooss NJ, Henskens YMC, Watson SP, Farndale RW, Gawaz MP, Jandrot-Perrus M, et al. . Pharmacological inhibition of glycoprotein VI- and integrin α2β1-induced thrombus formation modulated by the collagen type. Thromb Haemost [Internet] 2023;123:597–612.. 10.1055/s-0043-1761463 [DOI] [PubMed] [Google Scholar]
- 67. Jooss NJ, Smith CW, Slater A, Montague SJ, Di Y, O’Shea C, et al. . Anti-GPVI nanobody blocks collagen- and atherosclerotic plaque-induced GPVI clustering, signaling, and thrombus formation. J Thromb Haemost 2022;20:2617–31. 10.1111/jth.15836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Penz S, Reininger AJ, Brandl R, Goyal P, Rabie T, Bernlochner I, et al. . Human atheromatous plaques stimulate thrombus formation by activating platelet glycoprotein VI. FASEB J 2005;19:898–909. 10.1096/fj.04-2748com [DOI] [PubMed] [Google Scholar]
- 69. Cosemans JMEM, Kuijpers MJE, Lecut C, Loubele STBG, Heeneman S, Jandrot-Perrus M, et al. . Contribution of platelet glycoprotein VI to the thrombogenic effect of collagens in fibrous atherosclerotic lesions. Atherosclerosis 2005;181:19–27. 10.1016/j.atherosclerosis.2004.12.037 [DOI] [PubMed] [Google Scholar]
- 70. Schulz C, Penz S, Hoffmann C, Langer H, Gillitzer A, Schneider S, et al. . Platelet GPVI binds to collagenous structures in the core region of human atheromatous plaque and is critical for atheroprogression in vivo. Basic Res Cardiol 2008;103:356–67. 10.1007/s00395-008-0722-3 [DOI] [PubMed] [Google Scholar]
- 71. Fischer JW. Role of hyaluronan in atherosclerosis: current knowledge and open questions. Matrix Biol 2019;78–79:324–36. 10.1016/j.matbio.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 72. Koshiishi I, Shizari M, Underhill CB. CD44 can mediate the adhesion of platelets to hyaluronan. Blood 1994;84:390–6. 10.1182/blood.V84.2.390.390 [DOI] [PubMed] [Google Scholar]
- 73. de la Motte C, Nigro J, Vasanji A, Rho H, Kessler S, Bandyopadhyay S, et al. . Platelet-derived hyaluronidase 2 cleaves hyaluronan into fragments that trigger monocyte-mediated production of proinflammatory cytokines. Am J Pathol 2009;174:2254–64. 10.2353/ajpath.2009.080831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vinci R, Pedicino D, D’Aiello A, Ciampi P, Ponzo M, Bonanni A, et al. . Platelet hyaluronidase 2 enrichment in acute coronary syndromes: a conceivable role in monocyte-platelet aggregate formation. J Enzyme Inhib Med Chem 2021;36:785–9. 10.1080/14756366.2021.1900159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pedicino D, Vinci R, Giglio AF, Pisano E, Porto I, Vergallo R, et al. . Alterations of hyaluronan metabolism in acute coronary syndrome: implications for plaque erosion. J Am Coll Cardiol 2018;72:1490–503. 10.1016/j.jacc.2018.06.072 [DOI] [PubMed] [Google Scholar]
- 76. Mazzucato M, Cozzi MR, Pradella P, Perissinotto D, Malmstrom A, Morgelin M, et al. . Vascular PG-M/versican variants promote platelet adhesion at low shear rates and cooperate with collagens to induce aggregation. FASEB J 2002;16:1903–16. 10.1096/fj.02-0382com [DOI] [PubMed] [Google Scholar]
- 77. Guidetti G, Bertoni A, Viola M, Tira E, Balduini C, Torti M. The small proteoglycan decorin supports adhesion and activation of human platelets. Blood 2002;100:1707–14. 10.1182/blood.V100.5.1707.h81702001707_1707_1714 [DOI] [PubMed] [Google Scholar]
- 78. Hoermann H, Krueger I, Maurus N, Reusswig F, Sun Y, Kohlmorgen C, et al. . The proteoglycan biglycan modulates platelet adhesion and thrombus formation in a GPVI-dependent manner. Int J Mol Sci [Internet] 2021;22:12168. 10.3390/ijms222212168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hatakeyama K, Kaneko MK, Kato Y, Ishikawa T, Nishihira K, Tsujimoto Y, et al. . Podoplanin expression in advanced atherosclerotic lesions of human aortas. Thromb Res 2012;129:e70–6. 10.1016/j.thromres.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 80. Inoue O, Hokamura K, Shirai T, Osada M, Tsukiji N, Hatakeyama K, et al. . Vascular smooth muscle cells stimulate platelets and facilitate thrombus formation through platelet CLEC-2: implications in atherothrombosis. PLoS One 2015;10:e0139357. 10.1371/journal.pone.0139357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Furukoji E, Yamashita A, Nakamura K, Hirai T, Asada Y. Podoplanin expression on endothelial cells promotes superficial erosive injury and thrombus formation in rat carotid artery: implications for plaque erosion. Thromb Res 2019;183:76–9. 10.1016/j.thromres.2019.10.015 [DOI] [PubMed] [Google Scholar]
- 82. Bourne JH, Smith CW, Jooss NJ, Di Y, Brown HC, Montague SJ, et al. . CLEC-2 Supports platelet aggregation in mouse but not human blood at arterial shear. Thromb Haemost 2022;122:1988–2000. 10.1055/a-1896-6992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wilcox JN, Smith KM, Schwartz SM, Gordon D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc Natl Acad Sci U S A 1989;86:2839–43. 10.1073/pnas.86.8.2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hatakeyama K, Asada Y, Marutsuka K, Sato Y, Kamikubo Y, Sumiyoshi A. Localization and activity of tissue factor in human aortic atherosclerotic lesions. Atherosclerosis 1997;133:213–9. 10.1016/S0021-9150(97)00132-9 [DOI] [PubMed] [Google Scholar]
- 85. Asada Y, Yamashita A, Sato Y, Hatakeyama K. Pathophysiology of atherothrombosis: mechanisms of thrombus formation on disrupted atherosclerotic plaques. Pathol Int 2020;70:309–22. 10.1111/pin.12921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Okuyama N, Matsuda S, Yamashita A, Moriguchi-Goto S, Sameshima N, Iwakiri T, et al. . Human coronary thrombus formation is associated with degree of plaque disruption and expression of tissue factor and hexokinase II. Circ J 2015;79:2430–8. 10.1253/circj.CJ-15-0394 [DOI] [PubMed] [Google Scholar]
- 87. Kuijpers MJE, van der Meijden PEJ, Feijge MAH, Mattheij NJA, May F, Govers-Riemslag J, et al. . Factor XII regulates the pathological process of thrombus formation on ruptured plaques. Arterioscler Thromb Vasc Biol 2014;34:1674–80. 10.1161/ATVBAHA.114.303315 [DOI] [PubMed] [Google Scholar]
- 88. Yamashita A, Nishihira K, Gi T, Maekawa K, Hatakeyama K, Horiuchi S, et al. . Pathological features of ruptured coronary plaque and thrombus interfaces: fibrin and von Willebrand factor as platelet scaffolds on rupture sites. Thromb Haemost 2021;121:234–41. 10.1055/s-0040-1716539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhang D, Ebrahim M, Adler K, Blanchet X, Jamasbi J, Megens RTA, et al. . Glycoprotein VI is not a functional platelet receptor for fibrin formed in plasma or blood. Thromb Haemost 2020;120:977–93. 10.1055/s-0040-1710012 [DOI] [PubMed] [Google Scholar]
- 90. Gauer JS, Duval C, Xu RG, Macrae FL, McPherson HR, Tiede C, et al. . Fibrin-glycoprotein VI interaction increases platelet procoagulant activity and impacts clot structure. J Thromb Haemost [Internet] 2022;21:667–681. 10.1016/j.jtha.2022.09.004 [DOI] [PubMed] [Google Scholar]
- 91. Westein E, van der Meer AD, Kuijpers MJE, Frimat JP, van den Berg A, Heemskerk JWM. Atherosclerotic geometries exacerbate pathological thrombus formation poststenosis in a von Willebrand factor-dependent manner. Proc Natl Acad Sci U S A 2013;110:1357–62. 10.1073/pnas.1209905110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Nergiz-Unal R, Cosemans JMEM, Feijge MAH, van der Meijden PEJ, Storey RF, van Giezen JJJ, et al. . Stabilizing role of platelet P2Y(12) receptors in shear-dependent thrombus formation on ruptured plaques. PLoS One 2010;5:e10130. 10.1371/journal.pone.0010130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. du p Heyns A, Badenhorst CJ, Retief FP. ADPase activity of normal and atherosclerotic human aorta intima. Thromb Haemost 1977;37:429–35. 10.1055/s-0038-1649251 [DOI] [PubMed] [Google Scholar]
- 94. Furukoji E, Matsumoto M, Yamashita A, Yagi H, Sakurai Y, Marutsuka K, et al. . Adenovirus-mediated transfer of human placental ectonucleoside triphosphate diphosphohydrolase to vascular smooth muscle cells suppresses platelet aggregation in vitro and arterial thrombus formation in vivo. Circulation 2005;111:808–15. 10.1161/01.CIR.0000155239.46511.79 [DOI] [PubMed] [Google Scholar]
- 95. Manz XD, Bogaard HJ, Aman J. Regulation of VWF (von Willebrand factor) in inflammatory thrombosis. Arterioscler Thromb Vasc Biol 2022;42:1307–20. 10.1161/ATVBAHA.122.318179 [DOI] [PubMed] [Google Scholar]
- 96. Popa M, Tahir S, Elrod J, Kim SH, Leuschner F, Kessler T, et al. . Role of CD40 and ADAMTS13 in von Willebrand factor-mediated endothelial cell-platelet-monocyte interaction. Proc Natl Acad Sci U S A 2018;115:E5556-65. 10.1073/pnas.1801366115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Popa M, Hecker M, Wagner AH. Inverse regulation of confluence-dependent ADAMTS13 and von Willebrand factor expression in human endothelial cells. Thromb Haemost 2022;122:611–22. 10.1055/s-0041-1733800 [DOI] [PubMed] [Google Scholar]
- 98. Jeng AY, Chou M, Sawyer WK, Caplan SL, Von Linden-Reed J, Jeune M, et al. . Enhanced expression of matrix metalloproteinase-3, -12, and -13 mRNAs in the aortas of apolipoprotein E-deficient mice with advanced atherosclerosis. Ann N Y Acad Sci 1999;878:555–8. 10.1111/j.1749-6632.1999.tb07725.x [DOI] [PubMed] [Google Scholar]
- 99. Howes JM, Knäuper V, Malcor JD, Farndale RW. Cleavage by MMP-13 renders VWF unable to bind to collagen but increases its platelet reactivity. J Thromb Haemost 2020;18:942–54. 10.1111/jth.14729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Henn V, Slupsky JR, Gräfe M, Anagnostopoulos I, Förster R, Müller-Berghaus G, et al. . CD40 Ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 1998;391:591–4. 10.1038/35393 [DOI] [PubMed] [Google Scholar]
- 101. Michel NA, Zirlik A, Wolf D. CD40L and its receptors in atherothrombosis-an update. Front Cardiovasc Med 2017;4:40. 10.3389/fcvm.2017.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lacy M, Bürger C, Shami A, Ahmadsei M, Winkels H, Nitz K, et al. . Cell-specific and divergent roles of the CD40L-CD40 axis in atherosclerotic vascular disease. Nat Commun 2021;12:3754. 10.1038/s41467-021-23909-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lievens D, Zernecke A, Seijkens T, Soehnlein O, Beckers L, Munnix ICA, et al. . Platelet CD40L mediates thrombotic and inflammatory processes in atherosclerosis. Blood 2010;116:4317–27. 10.1182/blood-2010-01-261206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kuijpers MJE, Mattheij NJA, Cipolla L, van Geffen JP, Lawrence T, Donners MMPC, et al. . Platelet CD40L modulates thrombus growth via phosphatidylinositol 3-kinase β, and not via CD40 and IκB kinase α. Arterioscler Thromb Vasc Biol 2015;35:1374–81. 10.1161/ATVBAHA.114.305127 [DOI] [PubMed] [Google Scholar]
- 105. Abi-Younes S, Sauty A, Mach F, Sukhova GK, Libby P, Luster AD. The stromal cell-derived factor-1 chemokine is a potent platelet agonist highly expressed in atherosclerotic plaques. Circ Res 2000;86:131–8. 10.1161/01.RES.86.2.131 [DOI] [PubMed] [Google Scholar]
- 106. Stellos K, Bigalke B, Langer H, Geisler T, Schad A, Kögel A, et al. . Expression of stromal-cell-derived factor-1 on circulating platelets is increased in patients with acute coronary syndrome and correlates with the number of CD34 + progenitor cells. Eur Heart J 2009;30:584–93. 10.1093/eurheartj/ehn566 [DOI] [PubMed] [Google Scholar]
- 107. Walsh TG, Harper MT, Poole AW. SDF-1α is a novel autocrine activator of platelets operating through its receptor CXCR4. Cell Signal 2015;27:37–46. 10.1016/j.cellsig.2014.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Leberzammer J, Agten SM, Blanchet X, Duan R, Ippel H, Megens RTA, et al. . Targeting platelet-derived CXCL12 impedes arterial thrombosis. Blood 2022;139:2691–705. 10.1182/blood.2020010140 [DOI] [PubMed] [Google Scholar]
- 109. Akhtar S, Gremse F, Kiessling F, Weber C, Schober A. CXCL12 Promotes the stabilization of atherosclerotic lesions mediated by smooth muscle progenitor cells in Apoe-deficient mice. Arterioscler Thromb Vasc Biol 2013;33:679–86. 10.1161/ATVBAHA.112.301162 [DOI] [PubMed] [Google Scholar]
- 110. Rath D, Chatterjee M, Bongartz A, Müller K, Droppa M, Stimpfle F, et al. . Platelet surface expression of SDF-1 is associated with clinical outcomes in the patients with cardiovascular disease. Platelets 2017;28:34–9. 10.1080/09537104.2016.1203399 [DOI] [PubMed] [Google Scholar]
- 111. Obermayer G, Afonyushkin T, Binder CJ. Oxidized low-density lipoprotein in inflammation-driven thrombosis. J Thromb Haemost 2018;16:418–28. 10.1111/jth.13925 [DOI] [PubMed] [Google Scholar]
- 112. Podrez EA, Byzova TV, Febbraio M, Salomon RG, Ma Y, Valiyaveettil M, et al. . Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat Med 2007;13:1086–95. 10.1038/nm1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Chen K, Febbraio M, Li W, Silverstein RL. A specific CD36-dependent signaling pathway is required for platelet activation by oxidized low-density lipoprotein. Circ Res 2008;102:1512–9. 10.1161/CIRCRESAHA.108.172064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Carnevale R, Bartimoccia S, Nocella C, Di Santo S, Loffredo L, Illuminati G, et al. . LDL Oxidation by platelets propagates platelet activation via an oxidative stress-mediated mechanism. Atherosclerosis 2014;237:108–16. 10.1016/j.atherosclerosis.2014.08.041 [DOI] [PubMed] [Google Scholar]
- 115. Abela GS, Aziz K. Cholesterol crystals rupture biological membranes and human plaques during acute cardiovascular events–a novel insight into plaque rupture by scanning electron microscopy. Scanning 2006;28:1–10. 10.1002/sca.4950280101 [DOI] [PubMed] [Google Scholar]
- 116. Abela GS, Aziz K, Vedre A, Pathak DR, Talbott JD, Dejong J. Effect of cholesterol crystals on plaques and intima in arteries of patients with acute coronary and cerebrovascular syndromes. Am J Cardiol 2009;103:959–68. 10.1016/j.amjcard.2008.12.019 [DOI] [PubMed] [Google Scholar]
- 117. Fernández-Ortiz A, Badimon JJ, Falk E, Fuster V, Meyer B, Mailhac A, et al. . Characterization of the relative thrombogenicity of atherosclerotic plaque components: implications for consequences of plaque rupture. J Am Coll Cardiol 1994;23:1562–9. 10.1016/0735-1097(94)90657-2 [DOI] [PubMed] [Google Scholar]
- 118. Shi C, Kim T, Steiger S, Mulay SR, Klinkhammer BM, Bäuerle T, et al. . Crystal clots as therapeutic target in cholesterol crystal embolism. Circ Res 2020;126:e37-52. 10.1161/CIRCRESAHA.119.315625 [DOI] [PubMed] [Google Scholar]
- 119. Gravastrand CS, Steinkjer B, Halvorsen B, Landsem A, Skjelland M, Jacobsen EA, et al. . Cholesterol crystals induce coagulation activation through complement-dependent expression of monocytic tissue factor. J Immunol 2019;203:853–63. 10.4049/jimmunol.1900503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Colicchia M, Perrella G, Gant P, Rayes J. Novel mechanisms of thrombo-inflammation during infection: spotlight on neutrophil extracellular trap-mediated platelet activation. Res Pract Thromb Haemost 2023;7:100116. 10.1016/j.rpth.2023.100116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Pircher J, Engelmann B, Massberg S, Schulz C. Platelet-neutrophil crosstalk in atherothrombosis. Thromb Haemost 2019;119:1274–82. 10.1055/s-0039-1692983 [DOI] [PubMed] [Google Scholar]
- 122. Döring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res 2017;120:736–43. 10.1161/CIRCRESAHA.116.309692 [DOI] [PubMed] [Google Scholar]
- 123. Carminita E, Crescence L, Brouilly N, Altié A, Panicot-Dubois L, Dubois C. DNAse-dependent, NET-independent pathway of thrombus formation in vivo. Proc Natl Acad Sci U S A [Internet] 2021;118:e2100561118. 10.1073/pnas.2100561118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wang Y, Gao H, Shi C, Erhardt PW, Pavlovsky A, Soloviev D A, et al. . Leukocyte integrin Mac-1 regulates thrombosis via interaction with platelet GPIbα. Nat Commun 2017;8:15559. 10.1038/ncomms15559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sreeramkumar V, Adrover JM, Ballesteros I, Cuartero MI, Rossaint J, Bilbao I, et al. . Neutrophils scan for activated platelets to initiate inflammation. Science 2014;346:1234–8. 10.1126/science.1256478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Chatterjee V, Yang X, Ma Y, Wu MH, Yuan SY. Extracellular vesicles: new players in regulating vascular barrier function. Am J Physiol Heart Circ Physiol 2020;319:H1181–96. 10.1152/ajpheart.00579.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ferreira PM, Bozbas E, Tannetta SD, Alroqaiba N, Zhou R, Crawley JTB, et al. . Mode of induction of platelet-derived extracellular vesicles is a critical determinant of their phenotype and function. Sci Rep 2020;10:18061. 10.1038/s41598-020-73005-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Momi S, Falcinelli E, Petito E, Ciarrocca Taranta G, Ossoli A, Gresele P. Matrix metalloproteinase-2 on activated platelets triggers endothelial PAR-1 initiating atherosclerosis. Eur Heart J 2022;43:504–14. 10.1093/eurheartj/ehab631 [DOI] [PubMed] [Google Scholar]
- 129. Grootaert MOJ, Bennett MR. Vascular smooth muscle cells in atherosclerosis: time for a re-assessment. Cardiovasc Res 2021;117:2326–39. 10.1093/cvr/cvab046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. van Gorp RH, Baaten CCFMJ, Habibi A, Jaminon AMG, Peeters FECM, Leenders P, et al. . Vitamin K antagonist use induces calcification and atherosclerotic plaque progression resulting in increased hypercoagulability. Eur Heart J Open 2021;1:oeab017. 10.1093/ehjopen/oeab017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists’ view. Eur Heart J 2013;34:719–28. 10.1093/eurheartj/ehs411 [DOI] [PubMed] [Google Scholar]
- 132. Otsuka F, Yasuda S, Noguchi T, Ishibashi-Ueda H. Pathology of coronary atherosclerosis and thrombosis. Cardiovasc Diagn Ther 2016;6:396–408. 10.21037/cdt.2016.06.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Vergallo R, Crea F. Atherosclerotic plaque healing. N Engl J Med 2020;383:846–57. 10.1056/NEJMra2000317 [DOI] [PubMed] [Google Scholar]
- 134. Burke AP, Kolodgie FD, Farb A, Weber DK, Malcom GT, Smialek J, et al. . Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation 2001;103:934–40. 10.1161/01.CIR.103.7.934 [DOI] [PubMed] [Google Scholar]
- 135. Mann J, Davies MJ. Mechanisms of progression in native coronary artery disease: role of healed plaque disruption. Heart 1999;82:265–8. 10.1136/hrt.82.3.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Yin Y, Fang C, Jiang S, Wang J, Wang Y, Guo J, et al. . In vivo evidence of atherosclerotic plaque erosion and healing in patients with acute coronary syndrome using serial optical coherence tomography imaging. Am Heart J 2022;243:66–76. 10.1016/j.ahj.2021.09.007 [DOI] [PubMed] [Google Scholar]
- 137. Hughey M. Time, money and the housestaff officer. IMJ Ill Med J 1975;148:534–57. 10.1186/1532-429X-13-64 [DOI] [PubMed] [Google Scholar]
- 138. Wang C, Hu S, Wu J, Yu H, Pan W, Qin Y, et al. . Characteristics and significance of healed plaques in patients with acute coronary syndrome and stable angina: an in vivo OCT and IVUS study. EuroIntervention 2019;15:e771-8. 10.4244/EIJ-D-18-01175 [DOI] [PubMed] [Google Scholar]
- 139. Yin WJ, Jing J, Zhang YQ, Tian F, Zhang T, Zhou SS, et al. . Association between non-culprit healed plaque and plaque progression in acute coronary syndrome patients: an optical coherence tomography study. J Geriatr Cardiol 2021;18:631–44. 10.11909/j.issn.1671-5411.2021.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Vergallo R, Porto I, D’Amario D, Annibali G, Galli M, Benenati S, et al. . Coronary atherosclerotic phenotype and plaque healing in patients with recurrent acute coronary syndromes compared with patients with long-term clinical stability: an in vivo optical coherence tomography study. JAMA Cardiol 2019;4:321–9. 10.1001/jamacardio.2019.0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Shimokado A, Matsuo Y, Kubo T, Nishiguchi T, Taruya A, Teraguchi I, et al. . In vivo optical coherence tomography imaging and histopathology of healed coronary plaques. Atherosclerosis 2018;275:35–42. 10.1016/j.atherosclerosis.2018.05.025 [DOI] [PubMed] [Google Scholar]
- 142. Fracassi F, Crea F, Sugiyama T, Yamamoto E, Uemura S, Vergallo R, et al. . Healed culprit plaques in patients with acute coronary syndromes. J Am Coll Cardiol 2019;73:2253–63. 10.1016/j.jacc.2018.10.093 [DOI] [PubMed] [Google Scholar]
- 143. Russo M, Kim HO, Kurihara O, Araki M, Shinohara H, Thondapu V, et al. . Characteristics of non-culprit plaques in acute coronary syndrome patients with layered culprit plaque. Eur Heart J Cardiovasc Imaging 2020;21:1421–30. 10.1093/ehjci/jez308 [DOI] [PubMed] [Google Scholar]
- 144. Henriques de Gouveia R, van der Wal AC, van der Loos CM, Becker AE. Sudden unexpected death in young adults. Discrepancies between initiation of acute plaque complications and the onset of acute coronary death. Eur Heart J 2002;23:1433–40. 10.1053/euhj.2002.3159 [DOI] [PubMed] [Google Scholar]
- 145. Li X, Kramer MC, Van Der Loos CM, Ploegmakers HJP, De Boer OJ, Koch KT, et al. . Early onset of endothelial cell proliferation in coronary thrombi of patients with an acute myocardial infarction: implications for plaque healing. J Thromb Haemost 2012;10:466–73. 10.1111/j.1538-7836.2012.04620.x [DOI] [PubMed] [Google Scholar]
- 146. Rittersma SZH, van der Wal AC, Koch KT, Piek JJ, Henriques JPS, Mulder KJ, et al. . Plaque instability frequently occurs days or weeks before occlusive coronary thrombosis: a pathological thrombectomy study in primary percutaneous coronary intervention. Circulation 2005;111:1160–5. 10.1161/01.CIR.0000157141.00778.AC [DOI] [PubMed] [Google Scholar]
- 147. Carol A, Bernet M, Curós A, Rodríguez-Leor O, Serra J, Fernández-Nofrerías E, et al. . Thrombus age, clinical presentation, and reperfusion grade in myocardial infarction. Cardiovasc Pathol 2014;23:126–30. 10.1016/j.carpath.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 148. Kramer MCA, van der Wal AC, Koch KT, Ploegmakers JPHM, van der Schaaf RJ, Henriques JPS, et al. . Presence of older thrombus is an independent predictor of long-term mortality in patients with ST-elevation myocardial infarction treated with thrombus aspiration during primary percutaneous coronary intervention. Circulation 2008;118:1810–6. 10.1161/CIRCULATIONAHA.108.780734 [DOI] [PubMed] [Google Scholar]
- 149. Eriksen E, Herstad J, Pertiwi KR, Tuseth V, Nordrehaug JE, Bleie Ø, et al. . Thrombus characteristics evaluated by acute optical coherence tomography in ST elevation myocardial infarction. PLoS One 2022;17:e0266634. 10.1371/journal.pone.0266634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Nishihira K, Shibata Y, Yamashita A, Kuriyama N, Asada Y. Relationship between thrombus age in aspirated coronary material and mid-term major adverse cardiac and cerebrovascular events in patients with acute myocardial infarction. Atherosclerosis 2018;268:138–44. 10.1016/j.atherosclerosis.2017.12.001 [DOI] [PubMed] [Google Scholar]
- 151. Kramer MC, van der Wal AC, Koch KT, Rittersma SZ, Li X, Ploegmakers HP, et al. . Histopathological features of aspirated thrombi after primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction. PLoS One 2009;4:e5817. 10.1371/journal.pone.0005817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Amento EP, Ehsani N, Palmer H, Libby P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler Thromb 1991;11:1223–30. 10.1161/01.ATV.11.5.1223 [DOI] [PubMed] [Google Scholar]
- 153. Massberg S, Konrad I, Schürzinger K, Lorenz M, Schneider S, Zohlnhoefer D, et al. . Platelets secrete stromal cell-derived factor 1alpha and recruit bone marrow-derived progenitor cells to arterial thrombi in vivo. J Exp Med 2006;203:1221–33. 10.1084/jem.20051772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Schäfer K, Schroeter MR, Dellas C, Puls M, Nitsche M, Weiss E, et al. . Plasminogen activator inhibitor-1 from bone marrow-derived cells suppresses neointimal formation after vascular injury in mice. Arterioscler Thromb Vasc Biol 2006;26:1254–9. 10.1161/01.ATV.0000215982.14003.b7 [DOI] [PubMed] [Google Scholar]
- 155. Tanaka K, Sata M, Hirata Y, Nagai R. Diverse contribution of bone marrow cells to neointimal hyperplasia after mechanical vascular injuries. Circ Res 2003;93:783–90. 10.1161/01.RES.0000096651.13001.B4 [DOI] [PubMed] [Google Scholar]
- 156. Rodriguez F, Harrington RA. Management of antithrombotic therapy after acute coronary syndromes. N Engl J Med 2021;384:452–60. 10.1056/NEJMra1607714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Cao X, Zhang J, Geng D. Use of oral anticoagulant drugs is associated with carotid intraplaque hemorrhage in atherosclerosis patients: a meta-analysis. J Thromb Thrombolysis 2019;48:68–76. 10.1007/s11239-019-01865-2 [DOI] [PubMed] [Google Scholar]
- 158. Sedding DG, Boyle EC, Demandt JAF, Sluimer JC, Dutzmann J, Haverich A, et al. . Vasa vasorum angiogenesis: key player in the initiation and progression of atherosclerosis and potential target for the treatment of cardiovascular disease. Front Immunol 2018;9:706. 10.3389/fimmu.2018.00706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Parker WA, Storey RF. Antithrombotic therapy for patients with chronic coronary syndromes. Heart 2021;107:925–33. 10.1136/heartjnl-2020-316914 [DOI] [PubMed] [Google Scholar]
- 160. Réganon E, Vila V, Martínez-Sales V, Vaya A, Aznar J. Inflammation, fibrinogen and thrombin generation in patients with previous myocardial infarction. Haematologica 2002;87:740–5; discussion 745. 10.3324/%x [DOI] [PubMed] [Google Scholar]
- 161. Wadowski PP, Pultar J, Weikert C, Eichelberger B, Panzer B, Huber K, et al. . Protease-activated receptor-mediated platelet aggregation in acute coronary syndrome patients on potent P2Y12 inhibitors. Res Pract Thromb Haemost 2019;3:383–90. 10.1002/rth2.12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Alenazy FO, Thomas MR. Novel antiplatelet targets in the treatment of acute coronary syndromes. Platelets 2021;32:15–28. 10.1080/09537104.2020.1763731 [DOI] [PubMed] [Google Scholar]
- 163. Mayer K, Hein-Rothweiler R, Schüpke S, Janisch M, Bernlochner I, Ndrepepa G, et al. . Efficacy and safety of revacept, a novel lesion-directed competitive antagonist to platelet glycoprotein VI, in patients undergoing elective percutaneous coronary intervention for stable ischemic heart disease: the randomized, double-blind, placebo-controlled ISAR-PLASTER phase 2 trial. JAMA Cardiol 2021;6:753–61. 10.1001/jamacardio.2021.0475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Pasalic L, Wing-Lun E, Lau JK, Campbell H, Pennings GJ, Lau E, et al. . Novel assay demonstrates that coronary artery disease patients have heightened procoagulant platelet response. J Thromb Haemost 2018;16:1198–210. 10.1111/jth.14008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Grove EL, Hvas AM, Kristensen SD. Immature platelets in patients with acute coronary syndromes. Thromb Haemost 2009;101:151–6. 10.1160/TH08-03-0186 [DOI] [PubMed] [Google Scholar]
- 166. Veninga A, Baaten CCFMJ, De Simone I, Tullemans BME, Kuijpers MJE, Heemskerk JWM, et al. . Effects of platelet agonists and priming on the formation of platelet populations. Thromb Haemost 2022;122:726–38. 10.1055/s-0041-1735972 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were generated or analysed for or in support of this paper.