Abstract
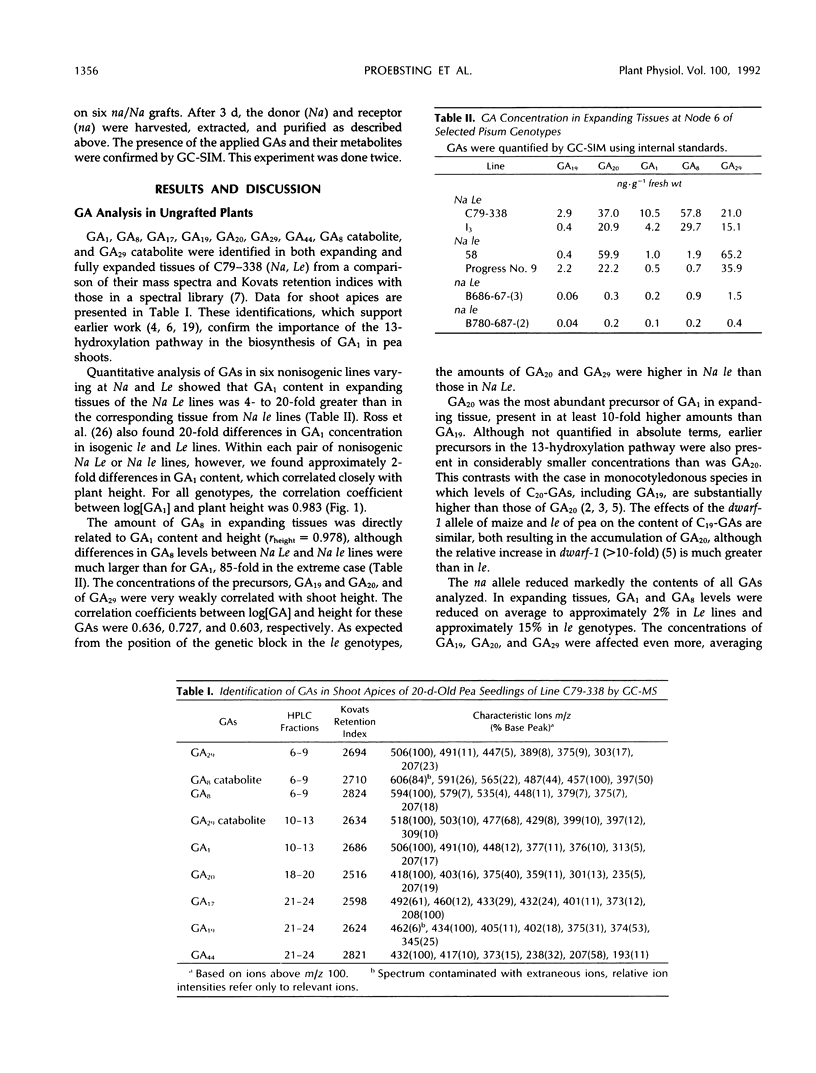
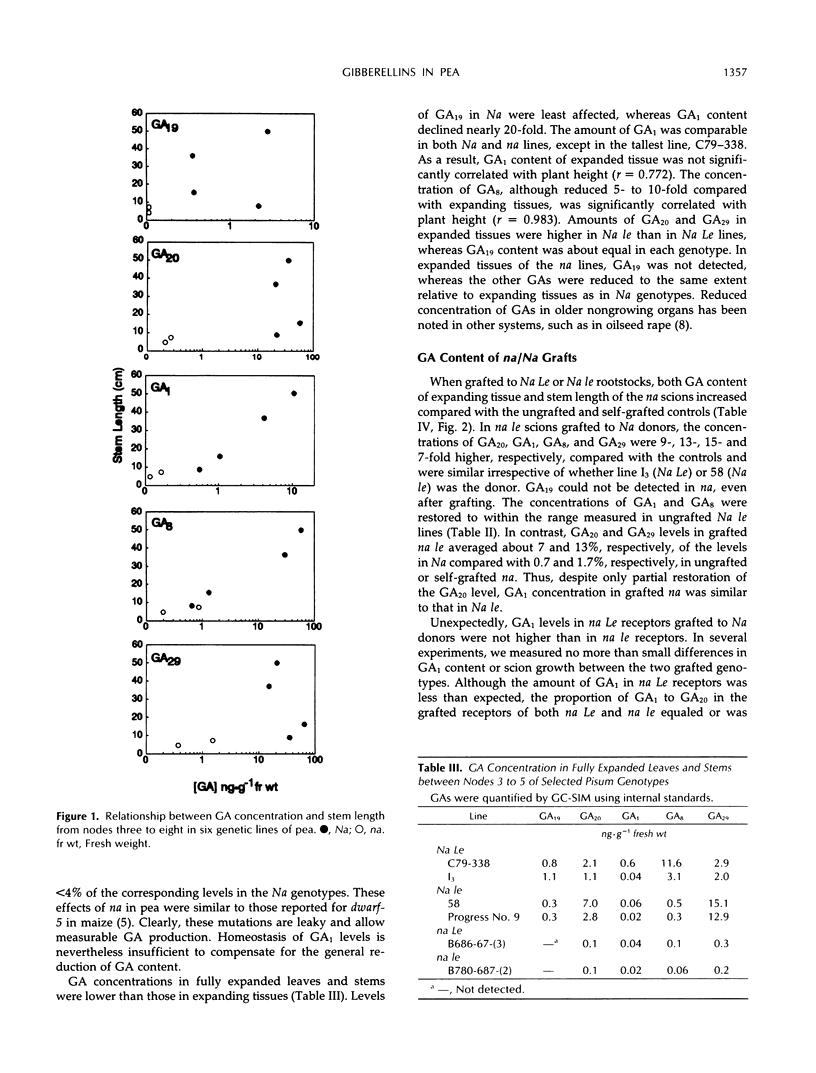
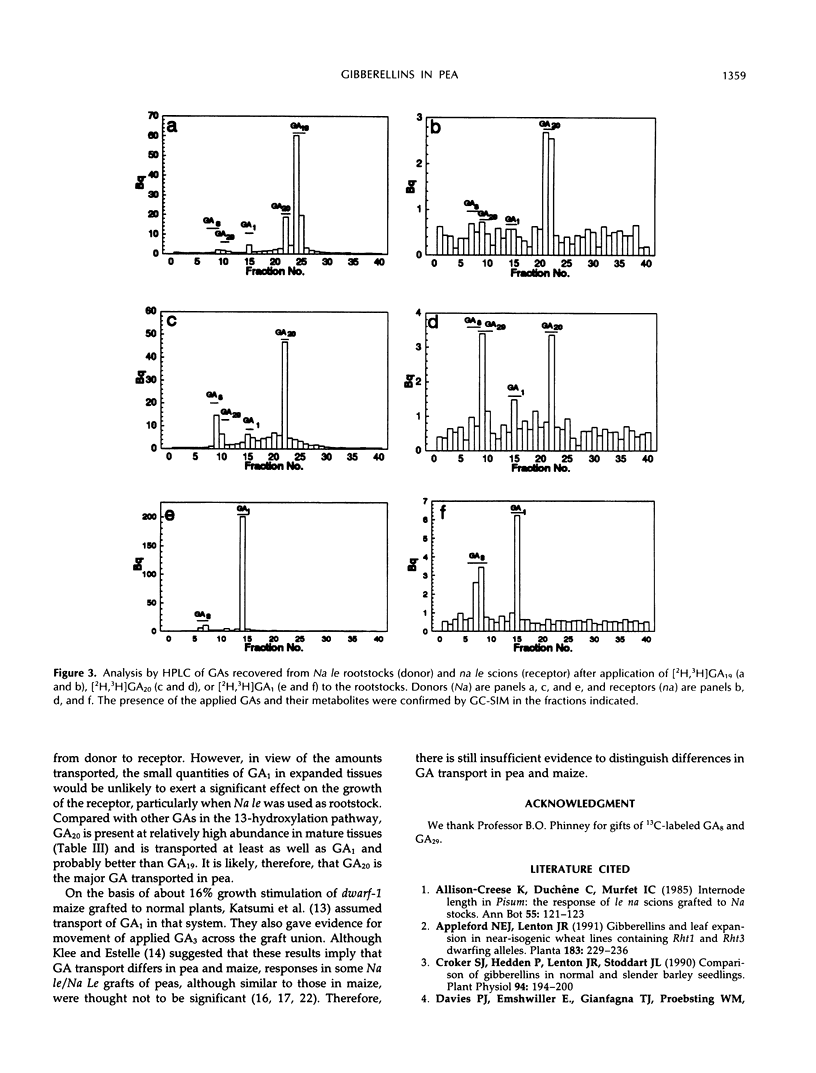
Effects of the Na and Le loci on gibberellin (GA) content and transport in pea (Pisum sativum L.) shoots were studied. GA1, GA8, GA17, GA19, GA20, GA29, GA44, GA8 catabolite, and GA29 catabolite were identified by full-scan gas chromatography-mass spectrometry in extracts of expanding and fully expanded tissues of line C79-338 (Na Le). Quantification of GAs by gas chromatography-single-ion monitoring using deuterated internal standards in lines differing at the Na and Le alleles showed that na reduced the contents of GA19, GA20, and GA29 on average to <3% and of GA1 and GA8 to <30% of those in corresponding Na lines. In expanding tissues from Na le lines, GA1 and GA8 concentrations were reduced to approximately 10 and 2%, respectively, and GA29 content increased 2- to 3-fold compared with those in Na Le plants. There was a close correlation between stem length and the concentrations of GA1 or GA8 in shoot apices in all six genotypes investigated. In na/Na grafts, internode length and GA1 concentration of nana scions were normalized, the GA20 content increased slightly, but GA19 levels were unaffected. Movement of labeled GAs applied to leaves on Na rootstocks indicated that GA19 was transported poorly to apices of na scions compared with GA20 and GA1. Our evidence suggests that GA20 is the major transported GA in peas.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Croker S. J., Hedden P., Lenton J. R., Stoddart J. L. Comparison of gibberellins in normal and slender barley seedlings. Plant Physiol. 1990 Sep;94(1):194–200. doi: 10.1104/pp.94.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S., Yamane H., Spray C. R., Gaskin P., Macmillan J., Phinney B. O., Takahashi N. Qualitative and Quantitative Analyses of Gibberellins in Vegetative Shoots of Normal, dwarf-1, dwarf-2, dwarf-3, and dwarf-5 Seedlings of Zea mays L. Plant Physiol. 1988 Dec;88(4):1367–1372. doi: 10.1104/pp.88.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram T. J., Reid J. B. Internode Length in Pisum: Gene na May Block Gibberellin Synthesis between ent-7alpha-Hydroxykaurenoic Acid and Gibberellin A(12)-Aldehyde. Plant Physiol. 1987 Apr;83(4):1048–1053. doi: 10.1104/pp.83.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockard R. G., Grunwald C. Grafting and gibberellin effects on the growth of tall and dwarf peas. Plant Physiol. 1970 Feb;45(2):160–162. doi: 10.1104/pp.45.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood S. B., Pearce D., Williams P. H., Pharis R. P. A Gibberellin-Deficient Brassica Mutant-rosette. Plant Physiol. 1989 Feb;89(2):482–487. doi: 10.1104/pp.89.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talon M., Koornneef M., Zeevaart J. A. Endogenous gibberellins in Arabidopsis thaliana and possible steps blocked in the biosynthetic pathways of the semidwarf ga4 and ga5 mutants. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7983–7987. doi: 10.1073/pnas.87.20.7983. [DOI] [PMC free article] [PubMed] [Google Scholar]



