Abstract
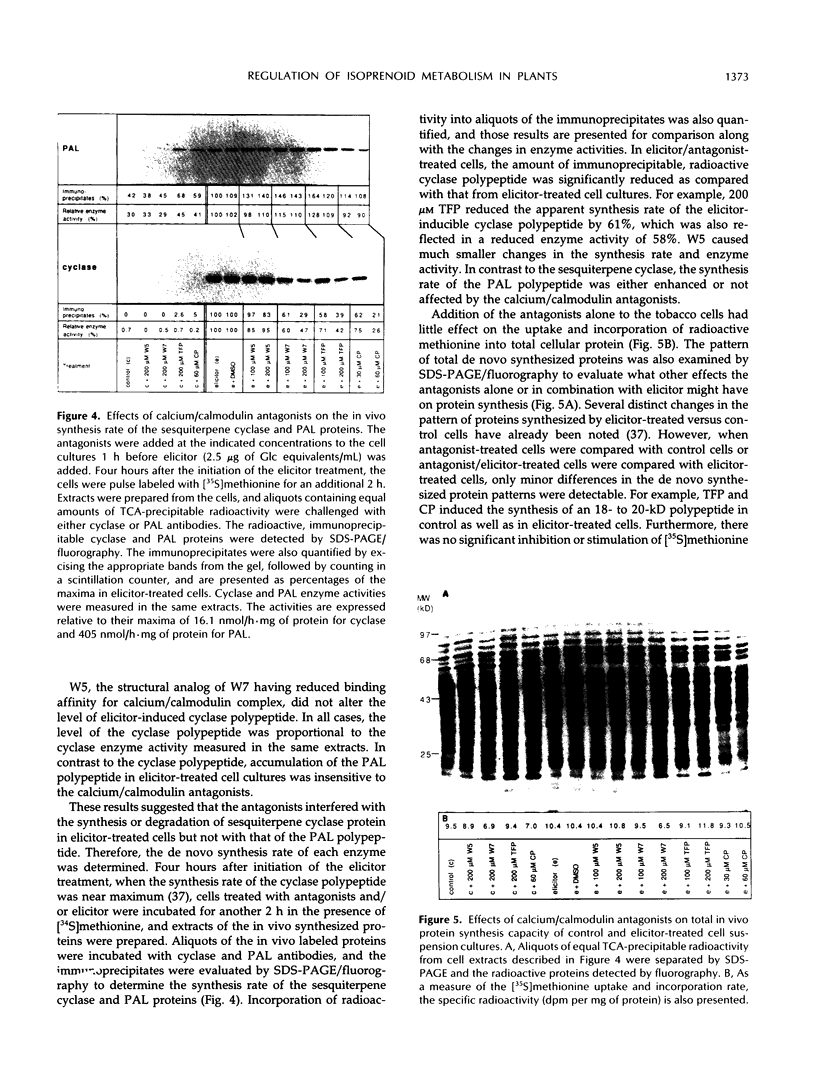
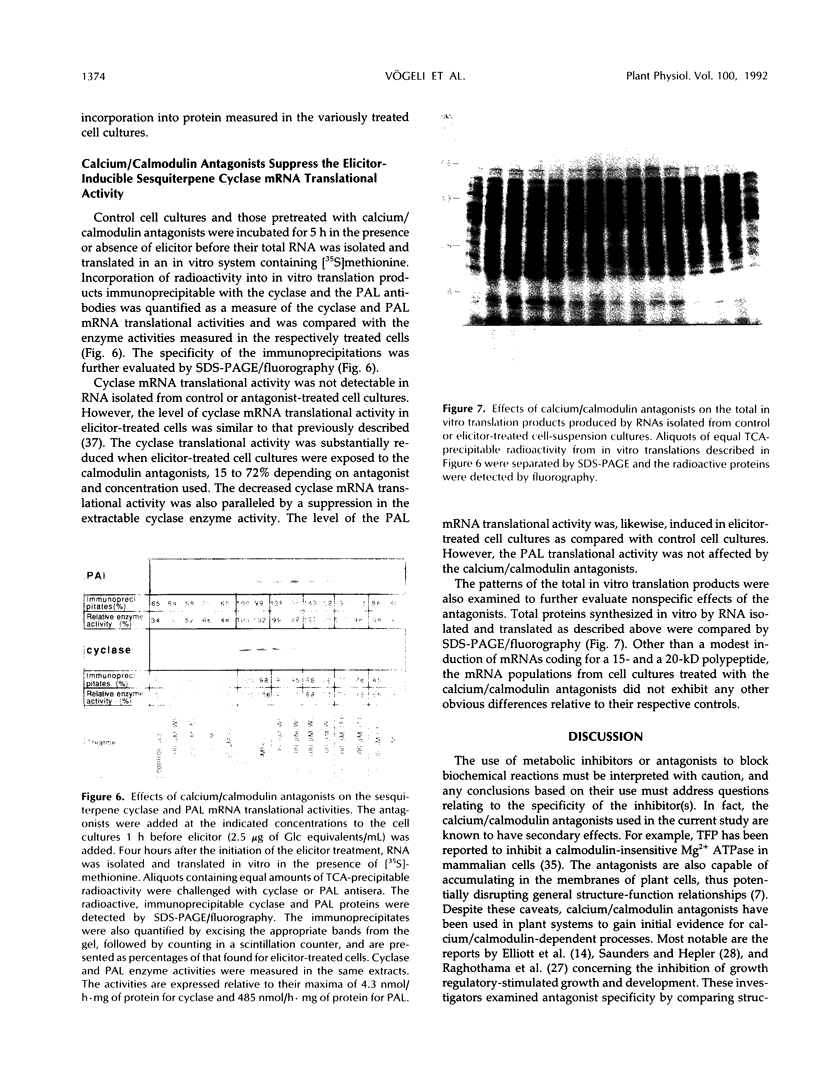
A role for calcium/calcium-binding proteins in a mechanism of signaling elicitor-inducible phytoalexin biosynthesis was investigated. Two classes of calcium/calmodulin antagonists, phenothiazines and naphthalenesulfonamides, inhibited sesquiterpene phytoalexin accumulation in tobacco (Nicotiana tabacum) cell-suspension cultures when added 1 h before elicitor. The antagonists also inhibited the induction of sesquiterpene cyclase enzyme activity, a key regulatory enzyme for sesquiterpene biosynthesis. The antagonists suppressed the induction of sesquiterpene cyclase only if added before or simultaneously with elicitor. Additionally, the antagonists inhibited (a) accumulation of the cyclase protein as measured in immunoblots; (b) the in vivo synthesis rate of the cyclase protein, measured as the incorporation of [35S]methionine into immunoprecipitable cyclase protein; and (c) the cyclase mRNA translational activity, measured as the incorporation of [35S]methionine into immunoprecipitable cyclase protein synthesized by in vitro translation of RNA isolated from antagonist-treated, elicitor-induced cells. In contrast, elicitor-inducible phenylalanine ammonia lyase enzyme activity, the level of the enzyme protein, the in vivo synthesis rate, and the mRNA translational activity were not affected by any of the antagonist treatments. Uptake and incorporation of [35S]methionine into total cellular proteins and total in vitro translation products were also not indiscriminately altered by the antagonist treatments. The current results suggest that calcium and/or calmodulin-like proteins may be elements of a signal transduction pathway mediating elicitor-induced accumulation of phytoalexins in tobacco.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayers A. R., Ebel J., Valent B., Albersheim P. Host-Pathogen Interactions: X. Fractionation and Biological Activity of an Elicitor Isolated from the Mycelial Walls of Phytophthora megasperma var. sojae. Plant Physiol. 1976 May;57(5):760–765. doi: 10.1104/pp.57.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chappell J., Nable R. Induction of sesquiterpenoid biosynthesis in tobacco cell suspension cultures by fungal elicitor. Plant Physiol. 1987 Oct;85(2):469–473. doi: 10.1104/pp.85.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell J., Vonlanken C., Vögeli U. Elicitor-inducible 3-hydroxy-3-methylglutaryl coenzyme a reductase activity is required for sesquiterpene accumulation in tobacco cell suspension cultures. Plant Physiol. 1991 Oct;97(2):693–698. doi: 10.1104/pp.97.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong J. J., Hahn M. G. A specific, high-affinity binding site for the hepta-beta-glucoside elicitor exists in soybean membranes. Plant Cell. 1991 Feb;3(2):137–147. doi: 10.1105/tpc.3.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott D. C., Batchelor S. M., Cassar R. A., Marinos N. G. Calmodulin-binding drugs affect responses to cytokinin, auxin, and gibberellic Acid. Plant Physiol. 1983 May;72(1):219–224. doi: 10.1104/pp.72.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Pearce G., Ryan C. A. In vitro phosphorylation of plant plasma membrane proteins in response to the proteinase inhibitor inducing factor. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1539–1542. doi: 10.1073/pnas.86.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G., Grosskopf D. G., Regenass M., Boller T. Rapid changes of protein phosphorylation are involved in transduction of the elicitor signal in plant cells. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8831–8834. doi: 10.1073/pnas.88.19.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. F., Sussman M. R., Schaller G. E., Putnam-Evans C., Charbonneau H., Harmon A. C. A calcium-dependent protein kinase with a regulatory domain similar to calmodulin. Science. 1991 May 17;252(5008):951–954. doi: 10.1126/science.1852075. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Tanaka T. Naphthalenesulfonamides as calmodulin antagonists. Methods Enzymol. 1983;102:185–194. doi: 10.1016/s0076-6879(83)02019-4. [DOI] [PubMed] [Google Scholar]
- Kapiloff M. S., Mathis J. M., Nelson C. A., Lin C. R., Rosenfeld M. G. Calcium/calmodulin-dependent protein kinase mediates a pathway for transcriptional regulation. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3710–3714. doi: 10.1073/pnas.88.9.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M. R., Campbell A. K., Smith S. M., Trewavas A. J. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature. 1991 Aug 8;352(6335):524–526. doi: 10.1038/352524a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li H., Dauwalder M., Roux S. J. Partial purification and characterization of a Ca(2+)-dependent protein kinase from pea nuclei. Plant Physiol. 1991;96:720–727. doi: 10.1104/pp.96.3.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POPJAK G., CORNFORTH J. W., CORNFORTH R. H., RYHAGE R., GOODMAN D. S. Studies on the biosynthesis of cholesterol. XVI. Chemical synthesis of 1-H2-3-2-C-14- and 1-D2-2-C-14-trans-trans-farnesyl pyrophosphate and their utilization in squalene biosynthesis. J Biol Chem. 1962 Jan;237:56–61. [PubMed] [Google Scholar]
- Raghothama K. G., Mizrahi Y., Poovaiah B. W. Effect of calmodulin antagonists on auxin-induced elongation. Plant Physiol. 1985 Sep;79(1):28–33. doi: 10.1104/pp.79.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders M. J., Hepler P. K. Calcium antagonists and calmodulin inhibitors block cytokinin-induced bud formation in Funaria. Dev Biol. 1983 Sep;99(1):41–49. doi: 10.1016/0012-1606(83)90252-x. [DOI] [PubMed] [Google Scholar]
- Schmidt W. E., Ebel J. Specific binding of a fungal glucan phytoalexin elicitor to membrane fractions from soybean Glycine max. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4117–4121. doi: 10.1073/pnas.84.12.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman H. The multifunctional Ca2+/calmodulin-dependent protein kinase. Adv Second Messenger Phosphoprotein Res. 1988;22:39–112. [PubMed] [Google Scholar]
- Shechter I., Bloch K. Solubilization and purification of trans-farnesyl pyrophosphate-squalene synthetase. J Biol Chem. 1971 Dec 25;246(24):7690–7696. [PubMed] [Google Scholar]
- Showalter A. M., Bell J. N., Cramer C. L., Bailey J. A., Varner J. E., Lamb C. J. Accumulation of hydroxyproline-rich glycoprotein mRNAs in response to fungal elicitor and infection. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6551–6555. doi: 10.1073/pnas.82.19.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stäb M. R., Ebel J. Effects of Ca2+ on phytoalexin induction by fungal elicitor in soybean cells. Arch Biochem Biophys. 1987 Sep;257(2):416–423. doi: 10.1016/0003-9861(87)90585-6. [DOI] [PubMed] [Google Scholar]
- Veigl M. L., Vanaman T. C., Sedwick W. D. Calcium and calmodulin in cell growth and transformation. Biochim Biophys Acta. 1984;738(1-2):21–48. doi: 10.1016/0304-419x(84)90018-0. [DOI] [PubMed] [Google Scholar]
- Vögeli U., Chappell J. Induction of sesquiterpene cyclase and suppression of squalene synthetase activities in plant cell cultures treated with fungal elicitor. Plant Physiol. 1988 Dec;88(4):1291–1296. doi: 10.1104/pp.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vögeli U., Chappell J. Regulation of a sesquiterpene cyclase in cellulase-treated tobacco cell suspension cultures. Plant Physiol. 1990 Dec;94(4):1860–1866. doi: 10.1104/pp.94.4.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S., Hahlbrock K. Light-induced changes of enzyme activities in parsley cell suspension cultures. Purification and some properties of phenylalanine ammonia-lyase (E.C.4.3.1.5). Arch Biochem Biophys. 1975 Jan;166(1):54–62. doi: 10.1016/0003-9861(75)90364-1. [DOI] [PubMed] [Google Scholar]
- Zook M. N., Rush J. S., Kuć J. A. A role for ca in the elicitation of rishitin and lubimin accumulation in potato tuber tissue. Plant Physiol. 1987 Jun;84(2):520–525. doi: 10.1104/pp.84.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]