Abstract
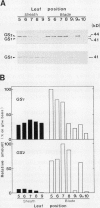
To further explore the function of NADH-dependent glutamate synthase (GOGAT), the tissue distribution of NADH-GOGAT protein and activity was investigated in rice (Oryza sativa L.) leaves. The distributions of ferredoxin (Fd)-dependent GOGAT, plastidic glutamine synthetase, and cytosolic glutamine synthetase proteins were also determined in the same tissues. High levels of NADH-GOGAT protein (33.1 μg protein/g fresh weight) and activity were detected in the 10th leaf blade before emergence. The unexpanded, nongreen portion of the 9th leaf blade contained more than 50% of the NADH-GOGAT protein and activity per gram fresh weight when compared with the 10th leaf. The expanding, green portion of the 9th leaf blade outside of the sheath contained a slightly lower abundance of NADH-GOGAT protein than the nongreen portion of the 9th blade on a fresh weight basis. The fully expanded leaf blades at positions lower than the 9th leaf had decreased NADH-GOGAT levels as a function of increasing age, and the oldest, 5th blade contained only 4% of the NADH-GOGAT protein compared with the youngest 10th leaf blade. Fd-GOGAT protein, on the other hand, was the major form of GOGAT in the green tissues, and the highest amount of Fd-GOGAT protein (111 μg protein/g fresh weight) was detected in the 7th leaf blade. In the nongreen 10th leaf blade, the content of Fd-GOGAT protein was approximately 7% of that found in the 7th leaf blade. In addition, the content of NADH-GOGAT protein in the 10th leaf blade was about 4 times higher than that of Fd-GOGAT protein. The content of plastidic glutamine synthetase polypeptide was also the highest in the 7th leaf blade (429 μg/g fresh weight) and lowest in nongreen blades and sheaths. On the other hand, the relative abundance of the cytosolic glutamine synthetase polypeptide was the highest in the oldest leaf blade, decreasing to 10 to 20% of that value in young, nongreen leaves. These results suggest that NADH-GOGAT is important for the synthesis of glutamate from the glutamine that is transported from senescing source tissues through the phloem in the nongreen sink tissues in rice leaves.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. P., Vance C. P., Heichel G. H., Miller S. S. Purification and Characterization of NADH-Glutamate Synthase from Alfalfa Root Nodules. Plant Physiol. 1989 May;90(1):351–358. doi: 10.1104/pp.90.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botella J. R., Verbelen J. P., Valpuesta V. Immunocytolocalization of Ferredoxin-GOGAT in the Cells of Green Leaves and Cotyledons of Lycopersicon esculentum. Plant Physiol. 1988 May;87(1):255–257. doi: 10.1104/pp.87.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen F. L., Cullimore J. V. Two Isoenzymes of NADH-dependent Glutamate Synthase in Root Nodules of Phaseolus vulgaris L: Purification, Properties and Activity Changes during Nodule Development. Plant Physiol. 1988 Dec;88(4):1411–1417. doi: 10.1104/pp.88.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. W., Coruzzi G. M. Photorespiration and light act in concert to regulate the expression of the nuclear gene for chloroplast glutamine synthetase. Plant Cell. 1989 Feb;1(2):241–248. doi: 10.1105/tpc.1.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. W., Walker E. L., Coruzzi G. M. Cell-specific expression in transgenic plants reveals nonoverlapping roles for chloroplast and cytosolic glutamine synthetase. Proc Natl Acad Sci U S A. 1990 May;87(9):3459–3463. doi: 10.1073/pnas.87.9.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T., Yamaya T., Kamachi K., Ojima K. Purification, Characterization, and Immunological Properties of NADH-Dependent Glutamate Synthase from Rice Cell Cultures. Plant Physiol. 1992 Apr;98(4):1317–1322. doi: 10.1104/pp.98.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamachi K., Yamaya T., Hayakawa T., Mae T., Ojima K. Changes in Cytosolic Glutamine Synthetase Polypeptide and its mRNA in a Leaf Blade of Rice Plants during Natural Senescence. Plant Physiol. 1992 Apr;98(4):1323–1329. doi: 10.1104/pp.98.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamachi K., Yamaya T., Hayakawa T., Mae T., Ojima K. Vascular bundle-specific localization of cytosolic glutamine synthetase in rice leaves. Plant Physiol. 1992 Aug;99(4):1481–1486. doi: 10.1104/pp.99.4.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamachi K., Yamaya T., Mae T., Ojima K. A Role for Glutamine Synthetase in the Remobilization of Leaf Nitrogen during Natural Senescence in Rice Leaves. Plant Physiol. 1991 Jun;96(2):411–417. doi: 10.1104/pp.96.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. L., Greenleaf A. L., Lehman I. R. Isolation of the nuclear gene encoding a subunit of the yeast mitochondrial RNA polymerase. J Biol Chem. 1986 Aug 5;261(22):10348–10351. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morris P. F., Layzell D. B., Canvin D. T. Ammonia Production and Assimilation in Glutamate Synthase Mutants of Arabidopsis thaliana. Plant Physiol. 1988 May;87(1):148–154. doi: 10.1104/pp.87.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara H., Watanabe M., Hase T., Sugiyama T. Molecular cloning and characterization of complementary DNA encoding for ferredoxin-dependent glutamate synthase in maize leaf. J Biol Chem. 1991 Feb 5;266(4):2028–2035. [PubMed] [Google Scholar]
- Suzuki A., Audet C., Oaks A. Influence of light on the ferredoxin-dependent glutamate synthase in maize leaves. Plant Physiol. 1987 Jul;84(3):578–581. doi: 10.1104/pp.84.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Gadal P. Glutamate synthase from rice leaves. Plant Physiol. 1982 Apr;69(4):848–852. doi: 10.1104/pp.69.4.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Vidal J., Gadal P. Glutamate synthase isoforms in rice: immunological studies of enzymes in green leaf, etiolated leaf, and root tissues. Plant Physiol. 1982 Sep;70(3):827–832. doi: 10.1104/pp.70.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallsgrove R. M., Turner J. C., Hall N. P., Kendall A. C., Bright S. W. Barley mutants lacking chloroplast glutamine synthetase-biochemical and genetic analysis. Plant Physiol. 1987 Jan;83(1):155–158. doi: 10.1104/pp.83.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]