Abstract
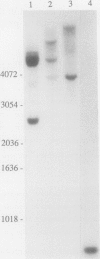
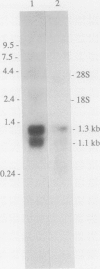
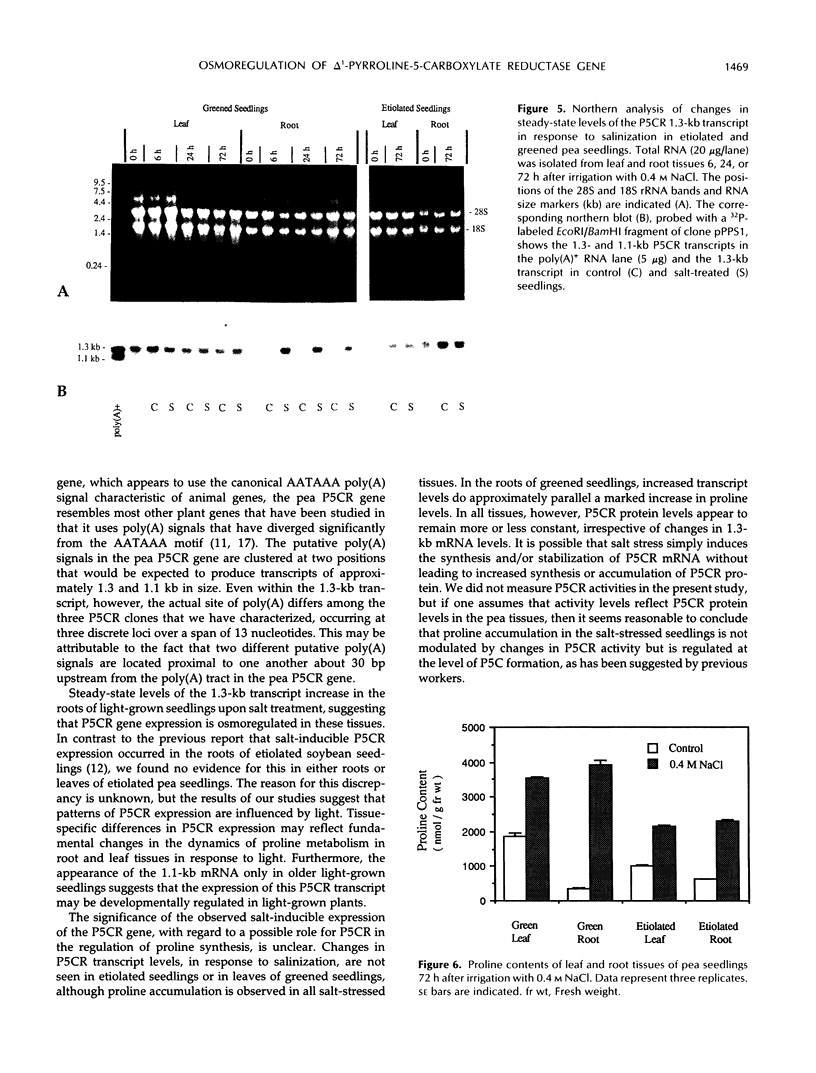
Several cDNA clones encoding Δ1-pyrroline-5-carboxylate reductase (P5CR, l-proline:NAD[P]+ 5-oxidoreductase, EC 1.5.1.2), which catalyzes the terminal step in proline biosynthesis, were isolated from a pea leaf library screened with a 32P-labeled Aval fragment of a soybean nodule P5CR cDNA (A.J. Delauney, D.P.S. Verma [1990] Mol Gen Genet 221: 299-305). DNA sequence analysis of one full-length 1.3-kb clone (pPPS3) indicated that the pea P5CR gene contains a single major open reading frame encoding a polypeptide of 28,242 Da. Genomic analysis suggested that two to three copies of the P5CR gene are present per haploid genome in pea. The primary structure of pea P5CR is 85% identical with that of soybean and exhibits significant homology to human, yeast, and Escherichia coli P5CR. The sequence of one of four highly conserved domains found in all prokaryotic and eukaryotic P5CRs is similar to the consensus sequence for the NAD(P)H-binding site of other enzymes. The pea P5CR cDNA hybridized to two transcripts, 1.3 and 1.1 kb in size, in polyadenylated RNA purified from leaf tissues of mature, light-grown plants (4 weeks old). Only the 1.3-kb transcript was detected in younger (1 week old) greened seedlings or in etiolated seedlings. In greened seedlings, steady-state levels of this 1.3-kb mRNA increased approximately 5-fold in root tissues within 6 h after plants were irrigated with 0.4 m NaCl, suggesting that expression of the P5CR gene is osmoregulated.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boggess S. F., Stewart C. R. Effect of water stress on proline synthesis from radioactive precursors. Plant Physiol. 1976 Sep;58(3):398–401. doi: 10.1104/pp.58.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brandriss M. C., Magasanik B. Subcellular compartmentation in control of converging pathways for proline and arginine metabolism in Saccharomyces cerevisiae. J Bacteriol. 1981 Mar;145(3):1359–1364. doi: 10.1128/jb.145.3.1359-1364.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Chilson O. P., Kelly-Chilson A. E., Siegel N. R. Pyrroline-5-carboxylate reductase in soybean nodules: isolation/partial primary structure/evidence for isozymes. Arch Biochem Biophys. 1991 Aug 1;288(2):350–357. doi: 10.1016/0003-9861(91)90206-x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Csonka L. N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989 Mar;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., Tamaki S., Dunsmuir P., Favreau M., Katayama C., Dooner H., Bedbrook J. mRNA transcripts of several plant genes are polyadenylated at multiple sites in vivo. Nucleic Acids Res. 1986 Mar 11;14(5):2229–2240. doi: 10.1093/nar/14.5.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delauney A. J., Verma D. P. A soybean gene encoding delta 1-pyrroline-5-carboxylate reductase was isolated by functional complementation in Escherichia coli and is found to be osmoregulated. Mol Gen Genet. 1990 May;221(3):299–305. doi: 10.1007/BF00259392. [DOI] [PubMed] [Google Scholar]
- Deutch A. H., Smith C. J., Rushlow K. E., Kretschmer P. J. Escherichia coli delta 1-pyrroline-5-carboxylate reductase: gene sequence, protein overproduction and purification. Nucleic Acids Res. 1982 Dec 11;10(23):7701–7714. doi: 10.1093/nar/10.23.7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty K. M., Brandriss M. C., Valle D. Cloning human pyrroline-5-carboxylate reductase cDNA by complementation in Saccharomyces cerevisiae. J Biol Chem. 1992 Jan 15;267(2):871–875. [PubMed] [Google Scholar]
- Hamilton P. T., Reeve J. N. Structure of genes and an insertion element in the methane producing archaebacterium Methanobrevibacter smithii. Mol Gen Genet. 1985;200(1):47–59. doi: 10.1007/BF00383311. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Joshi C. P. Putative polyadenylation signals in nuclear genes of higher plants: a compilation and analysis. Nucleic Acids Res. 1987 Dec 10;15(23):9627–9640. doi: 10.1093/nar/15.23.9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl D. H., Schubert K. R., Carter M. B., Hagedorn C. H., Shearer G. Proline metabolism in N2-fixing root nodules: energy transfer and regulation of purine synthesis. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2036–2040. doi: 10.1073/pnas.85.7.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger R., Jäger H. J., Hintz M., Pahlich E. Purification to homogeneity of pyrroline-5-carboxylate reductase of barley. Plant Physiol. 1986 Jan;80(1):142–144. doi: 10.1104/pp.80.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laliberté G., Hellebust J. A. Pyrroline-5-Carboxylate Reductase in Chlorella autotrophica and Chlorella saccharophila in Relation to Osmoregulation. Plant Physiol. 1989 Nov;91(3):917–923. doi: 10.1104/pp.91.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larosa P. C., Rhodes D., Rhodes J. C., Bressan R. A., Csonka L. N. Elevated Accumulation of Proline in NaCl-Adapted Tobacco Cells Is Not Due to Altered Delta-Pyrroline-5-Carboxylate Reductase. Plant Physiol. 1991 May;96(1):245–250. doi: 10.1104/pp.96.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Rudulier D., Strom A. R., Dandekar A. M., Smith L. T., Valentine R. C. Molecular biology of osmoregulation. Science. 1984 Jun 8;224(4653):1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- Mogen B. D., MacDonald M. H., Graybosch R., Hunt A. G. Upstream sequences other than AAUAAA are required for efficient messenger RNA 3'-end formation in plants. Plant Cell. 1990 Dec;2(12):1261–1272. doi: 10.1105/tpc.2.12.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E. E., Lotzer J., Eberle M. Codon usage in plant genes. Nucleic Acids Res. 1989 Jan 25;17(2):477–498. doi: 10.1093/nar/17.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G., Thompson W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayapati P. J., Stewart C. R., Hack E. Pyrroline-5-Carboxylate Reductase Is in Pea (Pisum sativum L.) Leaf Chloroplasts. Plant Physiol. 1989 Oct;91(2):581–586. doi: 10.1104/pp.91.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D., Handa S., Bressan R. A. Metabolic changes associated with adaptation of plant cells to water stress. Plant Physiol. 1986 Dec;82(4):890–903. doi: 10.1104/pp.82.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savioz A., Jeenes D. J., Kocher H. P., Haas D. Comparison of proC and other housekeeping genes of Pseudomonas aeruginosa with their counterparts in Escherichia coli. Gene. 1990 Jan 31;86(1):107–111. doi: 10.1016/0378-1119(90)90121-7. [DOI] [PubMed] [Google Scholar]
- Slocum R. D., Williamson C. L., Poggenburg C. A., Lynes M. A. Immunological characterization of plant ornithine transcarbamylases. Plant Physiol. 1990;92:1205–1210. doi: 10.1104/pp.92.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. R. Inhibition of proline oxidation by water stress. Plant Physiol. 1977 May;59(5):930–932. doi: 10.1104/pp.59.5.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga R. K., Terpstra P., Hol W. G. Prediction of the occurrence of the ADP-binding beta alpha beta-fold in proteins, using an amino acid sequence fingerprint. J Mol Biol. 1986 Jan 5;187(1):101–107. doi: 10.1016/0022-2836(86)90409-2. [DOI] [PubMed] [Google Scholar]