Abstract
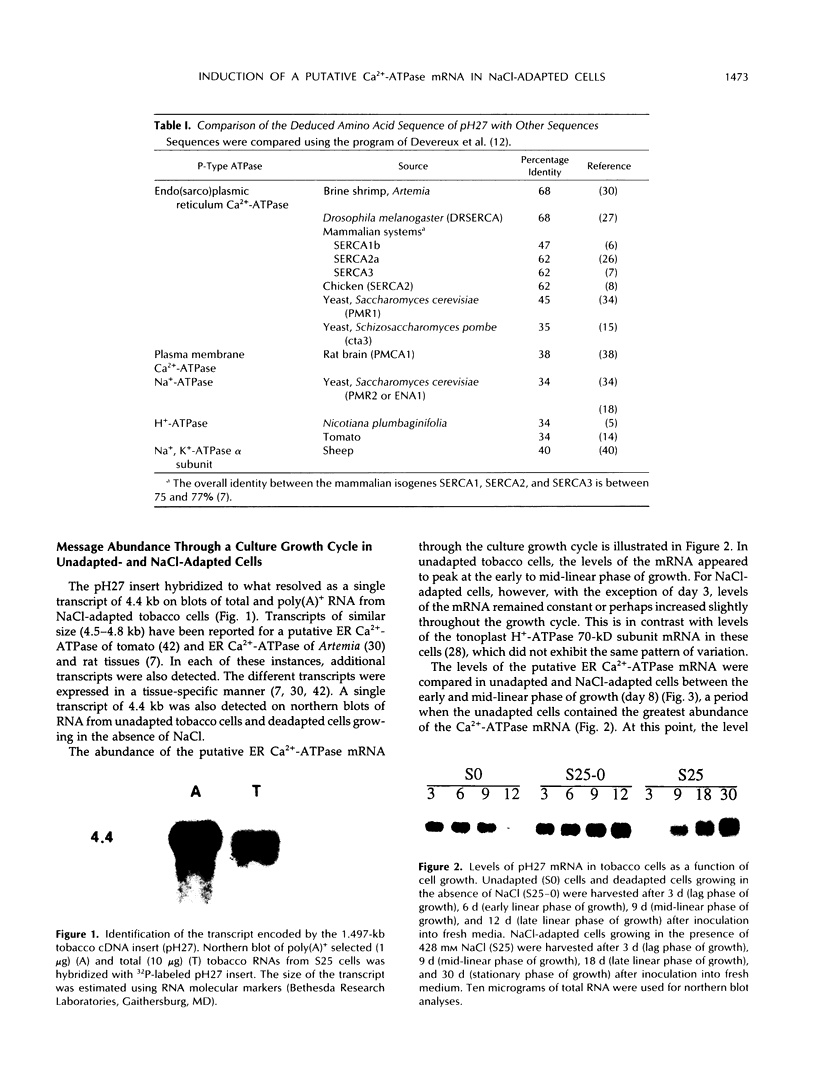
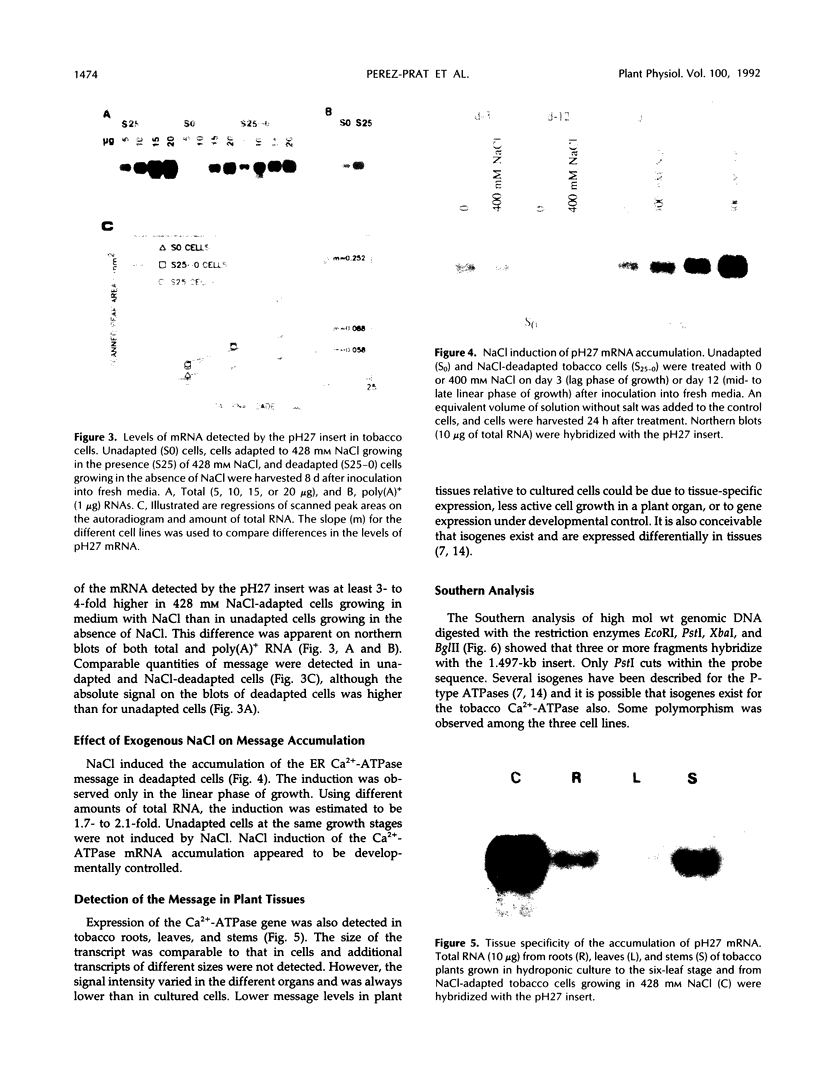
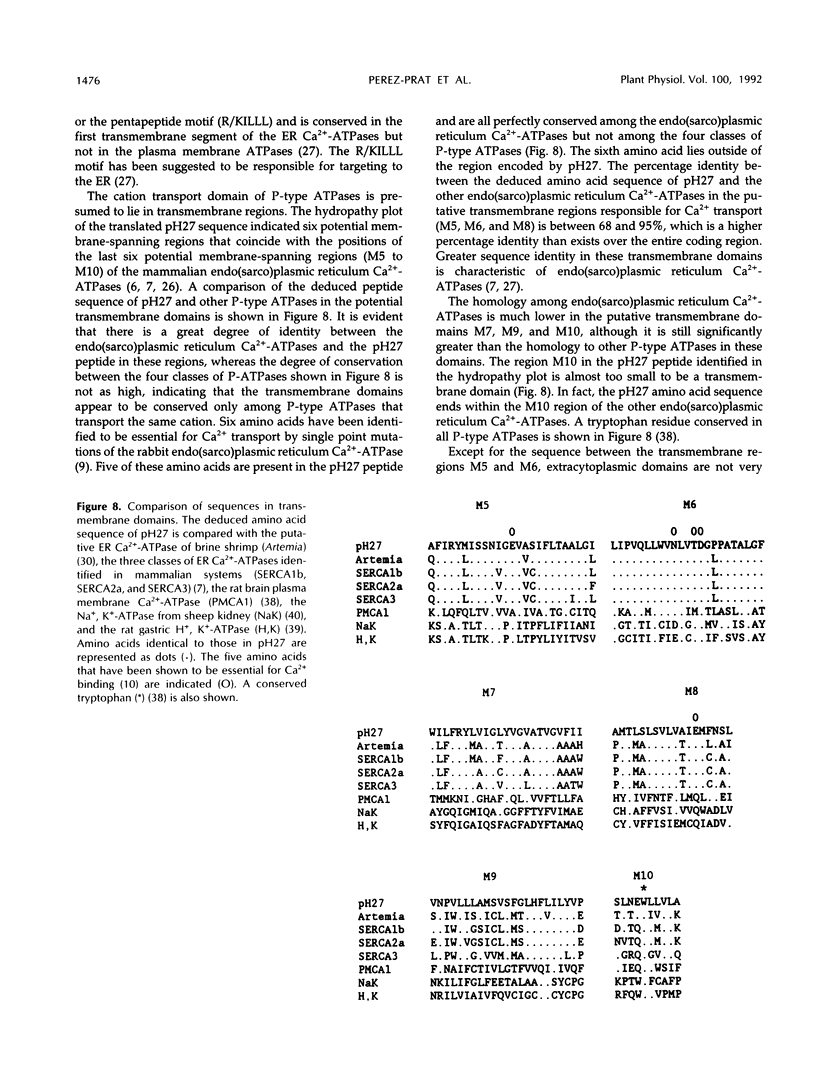
A cDNA clone was isolated that encodes the partial sequence of a putative endoplasmic reticulum Ca2+-ATPase of tobacco. The 1.497-kb insert had an open reading frame of 1.149 kb. The deduced peptide had the greatest homology to the endoplasmic reticulum Ca2+-ATPases of Drosophila and Artemia, followed by the mammalian and avian enzymes (SERCA2 and 3). The cDNA insert hybridized to a single mRNA of 4.4 kb from tobacco cultured cells or plant tissues. The level of this transcript was induced about 2-fold by NaCl shock in 428 mm NaCl-deadapted tobacco cells that were maintained in medium without salt, but not in unadapted cells. The level of this transcript was 3- to 4-fold higher in 428 mm NaCl-adapted cells growing in salt than in unadapted cells growing without salt.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binzel M. L., Hasegawa P. M., Handa A. K., Bressan R. A. Adaptation of Tobacco Cells to NaCl. Plant Physiol. 1985 Sep;79(1):118–125. doi: 10.1104/pp.79.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binzel M. L., Hess F. D., Bressan R. A., Hasegawa P. M. Intracellular compartmentation of ions in salt adapted tobacco cells. Plant Physiol. 1988 Feb;86(2):607–614. doi: 10.1104/pp.86.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Poole R. J. Kinetics of Ca/H Antiport in Isolated Tonoplast Vesicles from Storage Tissue of Beta vulgaris L. Plant Physiol. 1986 Mar;80(3):727–731. doi: 10.1104/pp.80.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutry M., Michelet B., Goffeau A. Molecular cloning of a family of plant genes encoding a protein homologous to plasma membrane H+-translocating ATPases. Biochem Biophys Res Commun. 1989 Jul 31;162(2):567–574. doi: 10.1016/0006-291x(89)92348-6. [DOI] [PubMed] [Google Scholar]
- Brandl C. J., Green N. M., Korczak B., MacLennan D. H. Two Ca2+ ATPase genes: homologies and mechanistic implications of deduced amino acid sequences. Cell. 1986 Feb 28;44(4):597–607. doi: 10.1016/0092-8674(86)90269-2. [DOI] [PubMed] [Google Scholar]
- Burk S. E., Lytton J., MacLennan D. H., Shull G. E. cDNA cloning, functional expression, and mRNA tissue distribution of a third organellar Ca2+ pump. J Biol Chem. 1989 Nov 5;264(31):18561–18568. [PubMed] [Google Scholar]
- Campbell A. M., Kessler P. D., Sagara Y., Inesi G., Fambrough D. M. Nucleotide sequences of avian cardiac and brain SR/ER Ca(2+)-ATPases and functional comparisons with fast twitch Ca(2+)-ATPase. Calcium affinities and inhibitor effects. J Biol Chem. 1991 Aug 25;266(24):16050–16055. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clarke D. M., Loo T. W., Inesi G., MacLennan D. H. Location of high affinity Ca2+-binding sites within the predicted transmembrane domain of the sarcoplasmic reticulum Ca2+-ATPase. Nature. 1989 Jun 8;339(6224):476–478. doi: 10.1038/339476a0. [DOI] [PubMed] [Google Scholar]
- Cramer G. R., Lynch J., Läuchli A., Epstein E. Influx of na, k, and ca into roots of salt-stressed cotton seedlings : effects of supplemental ca. Plant Physiol. 1987 Mar;83(3):510–516. doi: 10.1104/pp.83.3.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Bush D. S., Windle J. J., Jones R. L. Calcium and proton transport in membrane vesicles from barley roots. Plant Physiol. 1990 Sep;94(1):179–188. doi: 10.1104/pp.94.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing N. N., Wimmers L. E., Meyer D. J., Chetelat R. T., Bennett A. B. Molecular Cloning of Tomato Plasma Membrane H-ATPase. Plant Physiol. 1990 Dec;94(4):1874–1881. doi: 10.1104/pp.94.4.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghislain M., Goffeau A., Halachmi D., Eilam Y. Calcium homeostasis and transport are affected by disruption of cta3, a novel gene encoding Ca2(+)-ATPase in Schizosaccharomyces pombe. J Biol Chem. 1990 Oct 25;265(30):18400–18407. [PubMed] [Google Scholar]
- Giannini J. L., Gildensoph L. H., Reynolds-Niesman I., Briskin D. P. Calcium Transport in Sealed Vesicles from Red Beet (Beta vulgaris L.) Storage Tissue : I. Characterization of a Ca-Pumping ATPase Associated with the Endoplasmic Reticulum. Plant Physiol. 1987 Dec;85(4):1129–1136. doi: 10.1104/pp.85.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini J. L., Ruiz-Cristin J., Briskin D. P. Calcium Transport in Sealed Vesicles from Red Beet (Beta vulgaris L.) Storage Tissue : II. Characterization of Ca Uptake into Plasma Membrane Vesicles. Plant Physiol. 1987 Dec;85(4):1137–1142. doi: 10.1104/pp.85.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haro R., Garciadeblas B., Rodríguez-Navarro A. A novel P-type ATPase from yeast involved in sodium transport. FEBS Lett. 1991 Oct 21;291(2):189–191. doi: 10.1016/0014-5793(91)81280-l. [DOI] [PubMed] [Google Scholar]
- Hsieh W. L., Pierce W. S., Sze H. Calcium-pumping ATPases in vesicles from carrot cells : stimulation by calmodulin or phosphatidylserine, and formation of a 120 kilodalton phosphoenzyme. Plant Physiol. 1991 Dec;97(4):1535–1544. doi: 10.1104/pp.97.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P., Inui M., Tada M., Chiesi M., Carafoli E. Nature and site of phospholamban regulation of the Ca2+ pump of sarcoplasmic reticulum. Nature. 1989 Nov 2;342(6245):90–92. doi: 10.1038/342090a0. [DOI] [PubMed] [Google Scholar]
- Kasai M., Muto S. Ca2+ pump and Ca2+/H+ antiporter in plasma membrane vesicles isolated by aqueous two-phase partitioning from corn leaves. J Membr Biol. 1990 Mar;114(2):133–142. doi: 10.1007/BF01869094. [DOI] [PubMed] [Google Scholar]
- Ladror U. S., Zielinski R. E. Effect of Ca and Calmodulin on DeltapH Formation in Tonoplast Vesicles from Corn Roots. Plant Physiol. 1990 Mar;92(3):850–854. doi: 10.1104/pp.92.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J., Läuchli A. Salinity affects intracellular calcium in corn root protoplasts. Plant Physiol. 1988 Jun;87(2):351–356. doi: 10.1104/pp.87.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan D. H., Brandl C. J., Korczak B., Green N. M. Amino-acid sequence of a Ca2+ + Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature. 1985 Aug 22;316(6030):696–700. doi: 10.1038/316696a0. [DOI] [PubMed] [Google Scholar]
- Magyar A., Váradi A. Molecular cloning and chromosomal localization of a sarco/endoplasmic reticulum-type Ca2(+)-ATPase of Drosophila melanogaster. Biochem Biophys Res Commun. 1990 Dec 31;173(3):872–877. doi: 10.1016/s0006-291x(05)80867-8. [DOI] [PubMed] [Google Scholar]
- Narasimhan M. L., Binzel M. L., Perez-Prat E., Chen Z., Nelson D. E., Singh N. K., Bressan R. A., Hasegawa P. M. NaCl Regulation of Tonoplast ATPase 70-Kilodalton Subunit mRNA in Tobacco Cells. Plant Physiol. 1991 Oct;97(2):562–568. doi: 10.1104/pp.97.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N. Structure, Function, and Evolution of Proton-ATPases. Plant Physiol. 1988 Jan;86(1):1–3. doi: 10.1104/pp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmero I., Sastre L. Complementary DNA cloning of a protein highly homologous to mammalian sarcoplasmic reticulum Ca-ATPase from the crustacean Artemia. J Mol Biol. 1989 Dec 20;210(4):737–748. doi: 10.1016/0022-2836(89)90106-x. [DOI] [PubMed] [Google Scholar]
- Poovaiah B. W., Reddy A. S. Calcium messenger system in plants. CRC Crit Rev Plant Sci. 1987;6(1):47–103. doi: 10.1080/07352688709382247. [DOI] [PubMed] [Google Scholar]
- Rasi-Caldogno F., Pugliarello M. C., Olivari C., De Michelis M. I. Identification and Characterization of the Ca-ATPase which Drives Active Transport of Ca at the Plasma Membrane of Radish Seedlings. Plant Physiol. 1989 Aug;90(4):1429–1434. doi: 10.1104/pp.90.4.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph H. K., Antebi A., Fink G. R., Buckley C. M., Dorman T. E., LeVitre J., Davidow L. S., Mao J. I., Moir D. T. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell. 1989 Jul 14;58(1):133–145. doi: 10.1016/0092-8674(89)90410-8. [DOI] [PubMed] [Google Scholar]
- Serrano R. Structure and function of proton translocating ATPase in plasma membranes of plants and fungi. Biochim Biophys Acta. 1988 Feb 24;947(1):1–28. doi: 10.1016/0304-4157(88)90017-2. [DOI] [PubMed] [Google Scholar]
- Shull G. E., Greeb J. Molecular cloning of two isoforms of the plasma membrane Ca2+-transporting ATPase from rat brain. Structural and functional domains exhibit similarity to Na+,K+- and other cation transport ATPases. J Biol Chem. 1988 Jun 25;263(18):8646–8657. [PubMed] [Google Scholar]
- Shull G. E., Lingrel J. B. Molecular cloning of the rat stomach (H+ + K+)-ATPase. J Biol Chem. 1986 Dec 25;261(36):16788–16791. [PubMed] [Google Scholar]
- Shull G. E., Schwartz A., Lingrel J. B. Amino-acid sequence of the catalytic subunit of the (Na+ + K+)ATPase deduced from a complementary DNA. Nature. 1985 Aug 22;316(6030):691–695. doi: 10.1038/316691a0. [DOI] [PubMed] [Google Scholar]
- Singh N. K., Nelson D. E., Kuhn D., Hasegawa P. M., Bressan R. A. Molecular Cloning of Osmotin and Regulation of Its Expression by ABA and Adaptation to Low Water Potential. Plant Physiol. 1989 Jul;90(3):1096–1101. doi: 10.1104/pp.90.3.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]