Abstract
Background:
Recent studies have shown an increasing number of patients with cutaneous leishmaniasis (CL) who do not respond to pentavalent antimonials as the first line of treatment for CL. Nanocarriers such as extracellular vesicles (EVs) are efficient vehicles that might be used as drug delivery systems for the treatment of diseases. Therefore, we aimed to isolate and characterize the EVs of Leishmania major, load them with Amphotericin B (AmB), and investigate the toxicity and efficacy of the prepared drug form.
Methods:
The EVs of L. major were isolated, characterized, and loaded with amphotericin B (AmB), and the EVs-Amphotericin B (EVs-AmB) form was synthesized. Relevant in vitro and in vivo methods were performed to evaluate the toxicity and efficacy of EVs-AmB compared to the control.
Results:
The anti-leishmanial activity of the EVs-AmB showed a higher percentage inhibition (PI%) (P = 0.023) compared to the AmB at different concentrations and time points. Obtained data showed a significant increase in the lesion size and parasite load in the lesion, PBS, and EVs mice groups in comparison with EVs-AmB, AmB, and Glucantime groups (P < 0.05), EVs-AmB had a significant decrease in lesion sizes in comparison with AmB (P < 0.05). Results showed that EVs-AmB decreased its toxicity to the kidneys and liver (P < 0.05).
Conclusion:
EVs-AmB improved the efficacy of AmB in mouse skin lesions and reduced hepatorenal toxicity. Furthermore, EVs could be a promising nanoplatform for the delivery of AmB in CL caused by L. major.
Keywords: Leishmania major, Extracellular vesicles, Drug delivery, Amphotericin B
Introduction
Leishmaniasis is a neglected tropical disease (NTD) caused by over 20 species of protozoan Leishmania parasites and is known to be endemic in 98 out of 197 countries. Cutaneous leishmaniasis is the most common form of this disease in the Middle East, mainly caused by L. major and L. tropica, identified by ulcerative skin lesions on exposed parts of the body resulting in life-long scar (1–7).
The causative agents of cutaneous leishmaniasis (CL) in Iran are L. major and L. tropical, which respectively are responsible for 67.3% and 32.1% of cases of CL. Every year about 20,000 new cases of CL are reported by the disease control centers in 25 of the 31 provinces in Iran (8–10).
Pentavalent antimonials have been applied for many years and are widely administered for leishmaniasis treatment. However, these agents are highly toxic, with severe side effects, which can be life-threatening (11, 12). As expected, the emergence of drug resistance due to non-standard drug regimens is another concern (13). In recent years, due to the emergence of antimonial resistance in Iran, amphotericin B (AmB), paromomycin, miltefosine, and sitamaquine are applied alone or in combination with antimonials to replace the old therapeutics (14, 15).
Nanoplatforms can effectively push the barriers of drug resistance and adverse reactions by improving drug delivery and absorption (16, 17). In this regard, liposomal AmB has been using as a new treatment for leishmaniasis. It was the less toxic and therefore better tolerated, more bioavailable, and with a short half-life in blood circulation (18).
Extracellular vesicles (EVs) are small cell-derived vesicles that naturally circulate in body fluids as an intracellular communication mechanism (19). Their content is believed to mediate the immunomodulatory effect (20). Recently, EVs have been introduced as nano-delivery systems with superior properties to synthetic nanocarriers. They have longer circulation half-life and an innate immunomodulatory effect. (21, 22).
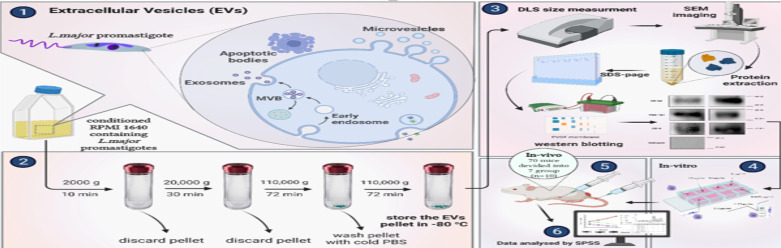
In this study, an EV-based formulation of the deoxycholate form of AmB (EVs-AmB) was prepared and evaluated for efficacy and toxicity in in vitro and in vivo studies. In brief, EVs have been purified and characterized from L. major culture media. AmB was incorporated into EVs by simple incubation, and the prepared formulation and free drug were compared for efficacy, toxicity, and half-maximal inhibitory concentration (IC50) in macrophage cell culture. Subsequently, inoculated animals were treated with EVs-AmB, AmB, EVs, and positive and negative controls and the efficacy of this therapeutic strategy was evaluated by relevant in vivo methods (Fig. 1).
Fig. 1:
The schematic illustration of the study (Re-shaped after www.biorender.com)
Material & Methods
Cell culture
This experimental study was conducted at Tehran University of Medical Sciences in 2019–2022. L. major promastigotes (MRHO/IR/75/ER strain) were cultured at 25±1 °C in RPMI 1640 medium (Gibco, Germany) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco, Germany), and penicillin-streptomycin (penstrep; Merck KGaA, Germany). The medium was refreshed every five days, and promastigotes were isolated in the stationary phase.
The murine macrophage cell line (RAW 264.7) was cultured in FBS, and pen/strep supplemented high-glucose Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Germany) at 37 °C with 5% CO2 according to the relevant guidelines (15).
Purification of EVs
EVs were purified from the culture media of promastigotes in the stationary phase. The isolation process was performed according to an ultracentrifugation-based method (23). In brief, the media were harvested and centrifuged at 2000 g for 10 min to remove the cellular debris. The supernatant was centrifuged at 20,000 g for 30 min. The supernatant was transferred to ultracentrifuge tubes and ultra-centrifuged at 110,000 g for 70 min (L5–65 Ultracentrifuge, Beckman Coulter, Type 60 Ti Rotor, USA). The pellet was washed once with cold PBS and ultracentrifuged again under the same prementioned condition. All procedures were carried out at 4 °C. Finally, the pellet was resuspended in PBS, aliquoted, and stored at −80 °C.
Characterization of EVs
According to the relevant guidelines, EVs were characterized regarding the presence of specific protein markers (CD6, CD63, TSG1021, and Calnexin), size, and morphology (24). All tests repeat in duplicate and triplicate.
Identification
Bicinchoninic acid (BCA) protein assay
Protein concentration was estimated by the BCA method using bovine serum albumin (BSA) as a standard. In Brief, 5 μL of the sample was added in triplicate to a 96-well microplate containing 100 μL of BCA reagent (DNAbiotech, Iran). The plate was incubated at 37 °C for 30 min and the results were read at 560 nm (25).
Western blotting
Briefly, a 20 μg EV sample was dissolved in sample buffer, sonicated and boiled at 95 °C for 5 min, and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using Mini-Protein II electrophoresis cells (Bio-Rad, USA), as described earlier (24, 26). The proteins were visualized using the Coomassie brilliant blue. For western blotting, separated protein bands were transferred (25 V, 150 min) into a polyvinylidene fluoride (PVDF) membrane (GE Healthcare, USA). The membrane was blocked with 5% BSA for an hour, then washed and incubated overnight at 4 °C with a primary antibody. Primary antibodies include Calnexin (1:500, Santa Cruz, USA), CD63 (1:500, Santa Cruz, USA), CD9 (1:500, Santa Cruz, USA), and TSG 101 (1:500, Santa Cruz, USA). Then, the blot was washed and treated with a secondary anti-mouse (1:50000, Sigma-Aldrich, Germany) for two hours. The band conjugates were detected by enhanced chemiluminescence (ECL) detection reagent (Amersham, GE Healthcare, Buckinghamshire, UK) visualized by the Uvitec documentation system (UK).
Size distribution
Size distribution and polydispersity index (PDI) of EVs were determined (in triplicate) by dynamic light scattering (DLS) on a Malvern Zetasizer (UK) at room temperature. To avoid aggregation, the sample was sonicated for 5 seconds before assay (27).
Morphology
Briefly, 20 μl of EVs was placed on a carbon-coated grid and air-dried for 20 min, washed three times with PBS, fixed with 2.5% glutaraldehyde for 10 min, and then imaged by KYKY-EM3200 (China) for scanning electron microscopy (SEM) (27).
Drug Loading into EVs
Several loading studies were performed with different loading parameters (in duplicate) to achieve an optimized formulation. The loading methods were based on a two-hour incubation with or without prior sonication. Further details of drug loading methods were summarized in Table 1.
Table 1:
Drug loading strategies. The details of different loading studies of EVs-AmB based on 2-hour incubation.
| Formulation | EV:Drug ratio (μg:μg) | Sonication | Sonication time (s) | Incubation temperature | |
|---|---|---|---|---|---|
|
| |||||
| Bath | Probe | ||||
| 1 | 70:50 | - | - | - | RT |
| 2 | 70:500 | - | - | - | RT |
| 3 | 70:50 | □ | - | 30 | RT |
| 4 | 70:500 | □ | - | 30 | RT |
| 5 | 70:50 | □ | - | 60 | RT |
| 6 | 70:500 | □ | - | 60 | RT |
| 7 | 70:500 | - | - | - | BT |
| 8 | 70:500 | - | □ | 30 | RT |
BT: body temperature; RT: room temperature
After the encapsulation process, EVs-AmB were isolated by ultracentrifugation. The free AmB (Excitation: 326 nm, Emission: 473 nm) concentration of the supernatant was measured by a fluorometer (Cary, Eclipse, Varian, USA). The entrapment efficiency (EE%) was calculated by the following formula: EE% = (added drug (μg) − unloaded drug (μg))/added drug (μg) × 100
In vitro study
Macrophages were cultured in FBS-supplemented high-glucose DMEM. Then, unattached macrophages were washed off with a pre-warmed medium. L. major promastigotes were then added to infect adherent macrophages and incubated at 37 °C for 24 h. Wells were rewashed with a warm medium to remove non-phagocytosed promastigotes and then treated for 24, 48, and 72 hours with a fresh medium containing AmB or EVs-AmB with concentrations of 15, 10, 5, 2.5, 1.25, and 0.625 μg/mL. Uninfected macrophages and infected macrophages were considered as controls. The wells were emptied at the time points, air-dried, fixed, and stained by Giemsa (15).
Anti-leishmanial activity of EVs-AmB
The anti-leishmanial activity was assessed as follows: (28).
Percentage inhibition (PI%) = 100 − ((number of amastigotes in 100 macrophages (test)/number of amastigotes in 100 macrophages (control)) × 100)
Macrophage cytotoxicity
Cytotoxicity was performed in different time points and 100 microscopic fields and the macrophages were directly counted and further assessed by the following formula: (15).
Cytotoxicity = Mean of macrophages (test)/mean of macrophages (control) × 100
Determination of IC50
IC50 was evaluated in 24, 48, and 72 hours, and calculated by GraphPad Prism software (V8, USA) (29).
Animal Studies
Animals
Seventy inbred female Balb/c mice (5–7 weeks old, 20 ± 3 g) were placed in standard cages kept at 25 °C, 55% humidity, a 12-h/day/night cycle, and permanent access to water and food (30).
All experiments were performed according to the Medical Ethics Committee of Tehran University of Medical Sciences guidelines, No; IR.TUMS.SPH.REC.1400.240.
Parasite inoculation
Briefly, 2×106 L. major promastigotes in the stationary phase were injected subcutaneously into the base of the mice’s tails. 2–3 weeks after injection, a lesion developed at the injection site. Animals were included in the study based on the lesion size (3–4 mm lesion), and eligible mice were randomly divided into seven groups (n = 10), as presented in Table 2.
Table 2:
Groups design. A summary of different study groups and their relevant interventions.
| Variable | Control | Lesion | PBS | EVs | AmB | EVs-AmB | Glucantime |
|---|---|---|---|---|---|---|---|
| Lesion | - | + | + | + | + | + | + |
| Treatment | - | - | PBS | EVs | AmB | EVs-AmB | Glucantime |
| Injection site | - | IV | IV | IV | IV | IV | IP |
| Number | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
IV: Intravenous, IP: Intraperitoneal
Drug administration
As shown in Table 2, Negative control and lesion groups do not receive any treatment. PBS group was administered with 0.2 ml of PBS. EVs, AmB, and EVs-AmB groups were administered intravenously for five consecutive days, followed by a sixth dose on day 10th with 1 mg/kg of respectively; crude isolated Leishmania extracellular vesicle, Amphotericin B and EVs-AmB. Glucantime (200 mg/kg) was administered intraperitoneally for 14 consecutive days as the positive control in the glucantime group. (31, 32).
Efficacy evaluation
The efficacy of the treatments was assessed based on the alteration in lesion size and parasite load. The mean diameter of the lesions was measured horizontally and vertically with a caliper tool (Mitutoyo, Taiwan). The parasite load was evaluated by preparing a Giemsa-stained smear of the lesion margins samples once a week for four weeks. The load of L. major amastigotes was analyzed as follows: +6 (more than 100 amastigotes/1 field), +5 (1–100 amastigotes/1 field), +4 (1–10 amastigotes/1 field), +3 (1–10 amastigotes/10 field), +2 (1–10 amastigotes/100 field), and +1 (1–10 amastigotes/1000 field) (33).
Toxicity study
Renal and hepatic toxicity was evaluated to monitor probable drug-induced toxicity. The concentration of serum urea, creatinine, glutamic oxaloacetic transaminase (SGOT), and glutamic pyruvic transaminase (SGPT) was measured at the end of the 4th week of the in vivo study by an automated biochemistry analyzer (Hitachi, Japan) and relevant kits (Audit, Iran) (34).
Statistical analysis
Statistical analysis studies were performed by GraphPad Prism software (V8, USA), and a P < 0.05 was considered statistically significant.
Results
Characterization of EVs
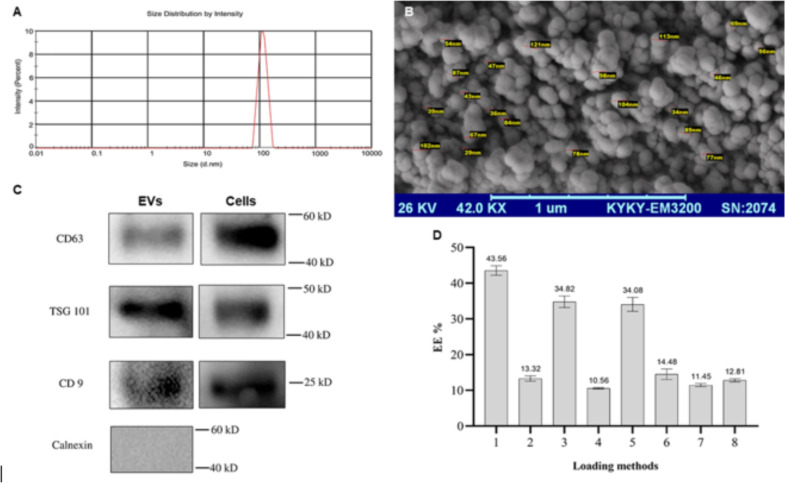
DLS data showed a mean diameter of 92.6 nm in an 89–124 nm distribution that is a sharp peak at 107±17.6 nm, with a PDI of 0.218±0.18 (Fig. 2A). Also, SEM exhibited spherical nanostructures with the expected size (Fig. 2B). Western blot analysis confirmed the presence of EV markers (CD9, CD63, and TSG101) and the absence of Calnexin in the purified EV sample (Fig. 2C).
Fig. 2:
Characterization studies on EVs. (A) Size distribution result from DLS method; (B) SEM image of purified EVs; (C) Western blot images of obtained protein bands; (D) EE% data of loading studies on EVs-AmB (n=3; mean±SD)
Drug loading studies
According to Table 1, eight formulations were prepared to achieve an optimal AmB nano-drug. As shown in Figs. 2D and F1 exhibited the highest EE% (43.56 ± 1.33) by the simple incubation at RT for two hous without sonication. Further studies and method modifications showed no superiority, and sonication could not elevate the method’s efficiency (Fig. 2D)
Anti-leishmanial activity, macrophage toxicity, and IC50 of EVs-AmB and AmB
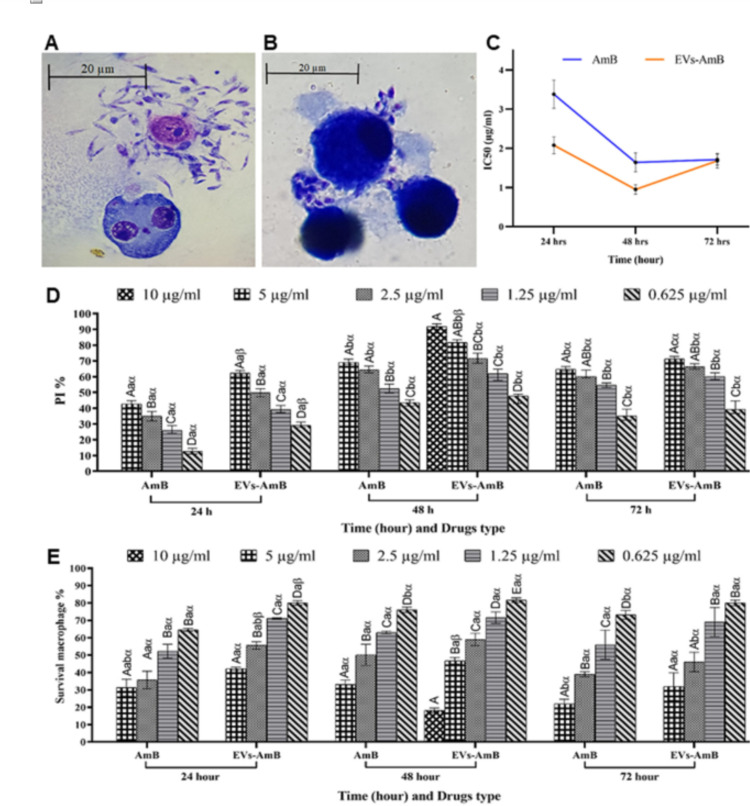
The process of promastigote’s invasion into macrophages and release of amastigotes are shown in Figs. 3A and 3B. The IC50 of EVs-AmB and AmB was evaluated by GraphPad Prism software. It was observed that EVs-AmB had a lower IC50 compared to the free AmB (Fig. 3C). The PI% formula determine the anti-Leishmania activity of the drug and its EV-based formulation and EVs-AmB showed higher PI% compared to AmB at different concentrations and time points (Fig. 3D). The macrophage toxicity of drug forms was evaluated and the macrophages survival rate is shown in Fig. 3E. Two drug forms exhibited similar macrophage toxicity, which was to be slightly higher for AmB. At concentrations of 10 μg / ml of both drugs, only macrophages at the 48-hour time point for EVs-AmB could be able to survive and at other time points and drugs, all macrophages were killed (Fig. 3E).
Fig. 3:
In vitro studies for comparison of the efficacy of EVs-AmB compared to free AmB. (A) The invasion of promastigotes into macrophages (at 1000X magnification); (B) The release of amastigotes from macrophages (at 1000X magnification); (C) Calculated IC50 for EVs-AmB and AmB; (D) The PI study results at 24, 48, and 72 hours; (E) The survival rate of macrophages incubated with different concentrations of AmB and EVs-AmB at different time points (n=3; Mean ± SD; uppercase letters are used to check the significance between drug concentrations in the same time frames for the same drug.
Lowercase letters are used to check the significance between drug concentrations in the different time frames for the same drug, and α and β letters are used to check the significance between two drug forms in the same time frames; different letters indicate significant differences (P < 0.05))
In vivo evaluation of EVs-AmB as a treatment strategy for cutaneous leishmaniasis
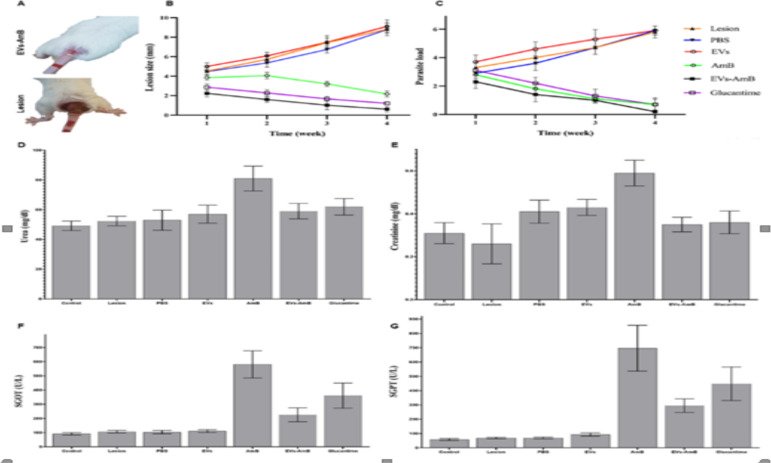
Eligible mice were treated and divided into seven groups (n = 10). Animals were evaluated weekly for lesion size and parasite load. In Fig. 4A, shows the lesion improvement in the group of EVs-AmB-treated mice presented, compared to a lesion control animal. Obtained data showed a significant increase in lesion size and parasite load in the lesion, PBS, and EVs control groups, compared with EVs-AmB, AmB, and Glucantime groups (P < 0.05). EVs-AmB had a significant decrease in lesion sizes in comparison with AmB (P < 0.05) (Figs. 4B and 4C). According to the results of the kidney and liver toxicity studies, the encapsulation of AmB into EVs decreased its toxicity (P < 0.05) (Figs. 4D–4G).
Fig. 4:
In vivo studies on the efficacy and safety of EVs-AmB. (A) The wound improvement in a mouse who received EVs-AmB, compared to an untreated mouse at the end of 4th week of the treatment; (B) The mean size of lesions at the end of each week of the study; (C) The parasite load of the lesions during four weeks of the study; The amounts of (D) urea, (E) creatinine, (F) SGOT, and (G) SGPT in different groups at the end of the experiment (n=10; mean ± SD)
Discussion
Leishmaniasis is a complex neglected tropical disease in the world caused by the Leishmania genus (13). Antimonials and miltefosine are considered first-line chemotherapeutic agents for cutaneous leishmaniasis, but they have life-threatening side effects (11). In addition, the emergence of drug resistance led to the consideration of the demand for new anti-leishmanial agents with bearable adverse effects (35). In this regard, using of nanocarriers for efficient and targeted delivery of available options is a promising approach to maximize efficacy and minimize side effects and resistance risk. EVs are nanovesicles secreted by most cell types in the body and medium culture and many studies reveal that can be used as vehicles for drug delivery purposes (36). EVs mediate some signaling pathways of innate immune responses in leishmaniasis (37). With this approach, liposomal AmB was introduced to the market and showed higher bioavailability and fewer adverse reactions compared to the conventional form of AmB. However, its blood circulation half-life was not favorable (18).
In the present study, AmB was loaded into Leishmania EVs to provide a nanoplatform of AmB with an optimal circulation half-life. In this regard, EVs were purified from promastigotes culture media and characterized by DLS, western blot analysis, and SEM microscopy. The results of morphological characterization studies confirmed the vesicular structure of EVs with acceptable size distribution. Furthermore, western blot analysis identified the nanovesicles as EVs (Figs. 2A–2C). The data is in agreement with other studies and MISEV2018 guideline (1, 38).
After that, EVs were loaded with AmB in different loading methods varying in loading parameters. Two EV: drug ratios were investigated in two incubation conditions of room and body temperatures for a fixed duration (2 hours). As expected, a higher EV: drug ratio exhibited more EE% due to the higher loading space of nanostructures. In addition, probe and bath sonication with different sonication durations did not significantly improve EE% compared to the simple incubation (Fig. 2D). This can be due to the saturation of EV structures by the drug molecules. Eventually, the formulation prepared by incubation at room temperature and a higher EV. Drug ratio was selected for further studies because of its higher EE% (43.56 ± 1.33) and this rate of EE% was almost similar to Kanchanapally study (42.5%) (39), but was higher than Ebrahimian study (20.8%) (40).
Our cellular assessments (Fig. 3) suggest the superior potential of this new formulation compared to the currently used AmB drug. Importantly when considering the clinical effects of the nephrotoxic, hepatotoxic, and cardiotoxic administration of the AmB, the lowest IC50 = 0.95 μg/ml was associated with EVs-AmB at 48 hours (Fig. 3C). This may be due to the reduced toxicity of the drug in the encapsulated form, but macrophage survival was almost similar between the two drug forms.
The serum levels of urea and creatinine in Mishra’s study of the amphiphilic formulation of amphotericin B (KalsomeTM10) compared to the pharmaceutical form of Fungizon were 51.5±1.92 and 0.04±0.40 respectively, which is somewhat similar to our study (59.1±5.29 and 0.35±0.3 respectively) (41).
In addition, EVs-AmB showed a higher PI% compared to AmB (Fig. 3D), which may mean that EVs facilitate the internalization of AmB. Drug encapsulation in nanocarriers may lead to higher bioavailability at lower drug concentrations and less toxicity and this condition reported in same study (28).
Our in vivo studies also confirmed the comparable efficacy of EVs-AmB to the conventional form of the drug (Fig. 4). This result may be an indication that amphotericin B is safe when it is loaded into EVs as similar to Parvez et al report (28).
Based on our knowledge, our study is the first one to report the therapeutic efficiency of Amphotericin B-loaded EVs derived from Leishmania. However, more studies including the Optimization of this formulation, further characterization of drug-loaded EVs, and studies that are more animal are required to pave the road to the administration of this formulation in clinical settings.
Conclusion
EVs-AmB improved the efficacy of AmB in mouse skin lesions and lowered its systemic toxicity for macrophages and hepatorenal systems. It can be proposed that EVs can be a promising nanoplatform for delivering AmB in CL caused by L. major. Future studies should consider the collection of complementary evidence for introducing this introduction of this formulation into clinical trials.
Acknowledgements
This work was approved and financially supported by the Tehran University of Medical Sciences (TUMS) (Project No: 37237) and the Center Zoonosis Disease (TUMS) with Ethical Code No: IR.TUMS.SPH.REC.1400.240).
We are grateful to all of those with whom we have had the pleasure to work during this Project. We thank Abdoreza Nazari for cooperation on the Western blot technique.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Thery C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvar J, Vélez ID, Bern C, et al. Leishmaniasis worldwide and global estimates of its incidence. PloS One. 2012;7(5):e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63(12):e202–e64. [DOI] [PubMed] [Google Scholar]
- 4.Nemati S, Fazaeli A, Hajjaran H, et al. Genetic diversity and phylogenetic analysis of the Iranian Leishmania parasites based on HSP70 gene PCR-RFLP and sequence analysis. Korean J Parasitol. 2017; 55(4):367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rostamian M, Bashiri H, Yousefinejad V, et al. Prevalence of human visceral leishmaniasis in Iran: A systematic review and meta-analysis. Comp Immunol Microbiol Infect Dis. 2021; 75:101604. [DOI] [PubMed] [Google Scholar]
- 6.Madusanka RK, Silva H, Karunaweera ND. Treatment of cutaneous leishmaniasis and insights into species-specific responses: a narrative review. Infect Dis Therapy. 2022;11(2):695–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. Leishmaniasis, Neglected tropical diseases. 2022. https://www.who.int/data/gho/data/themes/topics/gho-ntd-leishmaniasis. (accessed 2021).
- 8.Hajjaran H, Saberi R, Borjian A, et al. The geographical distribution of human cutaneous and visceral Leishmania species identified by molecular methods in Iran: a systematic review with meta-analysis. Front Public Health. 2021;9: 661674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Razavi MR, Shirzadi MR, Mohebali M, et al. Human Cutaneous Leishmaniosis in Iran, Up to Date-2019. J Arthropod Borne Dis. 2021;15(2):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabzevari S, Teshnizi SH, Shokri A, et al. Cutaneous leishmaniasis in Iran: A systematic review and meta-analysis. Microb Pathog. 2021;152:104721. [DOI] [PubMed] [Google Scholar]
- 11.López L, Robayo M, Vargas M. Thermotherapy. An alternative for the treatment of American cutaneous leishmaniasis. Trials. 2012;13:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohammad N, Sagheer F, Paracha MM, et al. Comparison Of Efficacy Of Miltefosine Versus Meglumine Antimonate In The Treatment Of Cutaneous Leishmaniasis. J Ayub Med Coll Abbottabad. 2022;34(4):849–53. [DOI] [PubMed] [Google Scholar]
- 13.Ponte-Sucre A, Gamarro F, Dujardin J-C, et al. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl Trop Dis. 2017;11(12):e0006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eskandari SE, Khamesipour A, Jaafari MR, et al. Combination of topical liposomal amphotericin B and Glucantime in comparison with glucantime alone for the treatment of anthroponotic cutaneous leishmaniasis (ACL). Iran J Microbiol. 2021;13(5):718–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohtasebi S, Mohebali M, Elikaee S, et al. In vitro and in vivo anti-parasitic activity of biogenic antimony sulfide nanoparticles on Leishmania major (MRHO/IR/75/ER). Parasitol Res. 2019;118(9):2669–78. [DOI] [PubMed] [Google Scholar]
- 16.Bayat F, Hosseinpour R, Mehryab F, et al. Potential application of liposomal nanodevices for non-cancer diseases: an update on design, characterization and biopharmaceutical evaluation. Adv Colloid Interface Sci. 2020;277:102121. [DOI] [PubMed] [Google Scholar]
- 17.Ghorbani M, Farhoudi R. Leishmaniasis in humans: drug or vaccine therapy? Drug Des Devel Ther. 2017; 12:25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akbari M, Oryan A, Hatam G. Application of nanotechnology in treatment of leishmaniasis: a review. Acta Trop. 2017;172:86–90. [DOI] [PubMed] [Google Scholar]
- 19.Coakley G, Maizels RM, Buck AH. Exosomes and other extracellular vesicles: the new communicators in parasite infections. Trend Parasitol. 2015;31(10):477–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong G, Filho AL, Olivier M. Modulation of host-pathogen communication by extracellular vesicles (EVs) of the protozoan parasite Leishmania. Front Cell Infect Microbiol. 2019;9:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehryab F, Rabbani S, Shahhosseini S, et al. Exosomes as a next-generation drug delivery system: an update on drug loading approaches, characterization, and clinical application challenges. Acta Biomater. 2020;113:42–62. [DOI] [PubMed] [Google Scholar]
- 22.Soleymani M, Saberi S, Shekari F. Extracellular vesicles in regenerative medicine, a brief review. Modern Med Lab J. 2019;2(2):118–26. [Google Scholar]
- 23.Rai A, Fang H, Fatmous M, et al. A protocol for isolation, purification, characterization, and functional dissection of exosomes. Methods Mol Biol. 2021:2261:105–149. [DOI] [PubMed] [Google Scholar]
- 24.Brennan K, Martin K, FitzGerald S.P, et al. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci Rep. 2020;10(1):1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heidari S, Hajjaran H, Kazemi B, et al. Identification of immunodominant proteins of Leishmania infantum by immunoproteomics to evaluate a recombinant multi-epitope designed antigen for serodiagnosis of human visceral leishmaniasis. Exp Parasitol. 2021;222:108065. [DOI] [PubMed] [Google Scholar]
- 26.Mardpour S, Ghanian MH, Sadeghi-Abandansari H, et al. Hydrogel-mediated sustained systemic delivery of mesenchymal stem cell-derived extracellular vesicles improves hepatic regeneration in chronic liver failure. ACS Appl Mater Interfaces. 2019;11(41):37421–33. [DOI] [PubMed] [Google Scholar]
- 27.Seyedrazizadeh S-Z, Poosti S, Nazari A, et al. Extracellular vesicles derived from human ES-MSCs protect retinal ganglion cells and preserve retinal function in a rodent model of optic nerve injury. Stem Cell Res Ther. 2020;11(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parvez S, Yadagiri G, Gedda MR, et al. Modified solid lipid nanoparticles encapsulated with Amphotericin B and Paromomycin: an effective oral combination against experimental murine visceral leishmaniasis. Sci Rep. 2020;10(1): 12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abu Ammar A, Nasereddin A, Ereqat S, et al. Amphotericin B-loaded nanoparticles for local treatment of cutaneous leishmaniasis. Drug Deliv Transl Res. 2019;9(1):76–84. [DOI] [PubMed] [Google Scholar]
- 30.Salehi-Sangani G, Mohebali M, Jajarmi V, et al. Immunization against Leishmania major infection in BALB/c mice using a subunit-based DNA vaccine derived from TSA, LmSTI1, KMP11, and LACK predominant antigens. Iran J Basic Med Sci. 2019;22(12):1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehrizi TZ, Ardestani MS, Molla Hoseini MH, et al. Novel nano-sized chitosan amphotericin B formulation with considerable improvement against Leishmania major. Nanomedicine. 2018;13(24):3129–47. [DOI] [PubMed] [Google Scholar]
- 32.Shirzadi MR. Lipsosomal amphotericin B: a review of its properties, function, and use for treatment of cutaneous leishmaniasis. Res Rep Trop Med. 2019; 10:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO. Regional Office for the Eastern Mediterranean. Manual for case management of cutaneous leishmaniasis in the WHO Eastern Mediterranean Region. https://apps.who.int/iris/handle/10665/120002. (accessed 2014).
- 34.Asad M, Bhattacharya P, Banerjee A. Therapeutic and immunomodulatory activities of short-course treatment of murine visceral leishmaniasis with KALSOME™ 10, a new liposomal amphotericin B. BMC Infect Dis. 2015;15:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sundar S, Chakravarty J, Meena LP. Leishmaniasis: treatment, drug resistance and emerging therapies. Expert Opin Orphan Drug. 2019;7(1):1–10. [Google Scholar]
- 36.Bunggulawa EJ, Wang W, Yin T, et al. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnology. 2018;16(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carrera-Bravo C, Koh EY, Tan KS. The roles of parasite-derived extracellular vesicles in disease and host-parasite communication. Parasitol Int. 2021;83:102373. [DOI] [PubMed] [Google Scholar]
- 38.Pérez-Cabezas B, Santarém N, Cecílio P, et al. More than just exosomes: distinct Leishmania infantum extracellular products potentiate the establishment of infection. J Extracell Vesicles. 2018;8(1):1541708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanchanapally R, Deshmukh SK, Chavva SR, et al. Drug-loaded exosomal preparations from different cell types exhibit distinctive loading capability, yield, and antitumor efficacies: a comparative analysis. Int J Nanomedicine. 2019;14:531–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebrahimian M, Hashemi M, Etemad L, et al. Thymoquinone-loaded mesenchymal stem cell-derived exosome as an efficient nano-system against breast cancer cells. Iran J Basic Med Sci. 2022;25(6):723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mishra J, Dey A, Singh N, Somvanshi R, et al. Evaluation of toxicity & therapeutic efficacy of a new liposomal formulation of amphotericin B in a mouse model. Indian J Med Res. 2013;137(4):767–76. [PMC free article] [PubMed] [Google Scholar]