Abstract
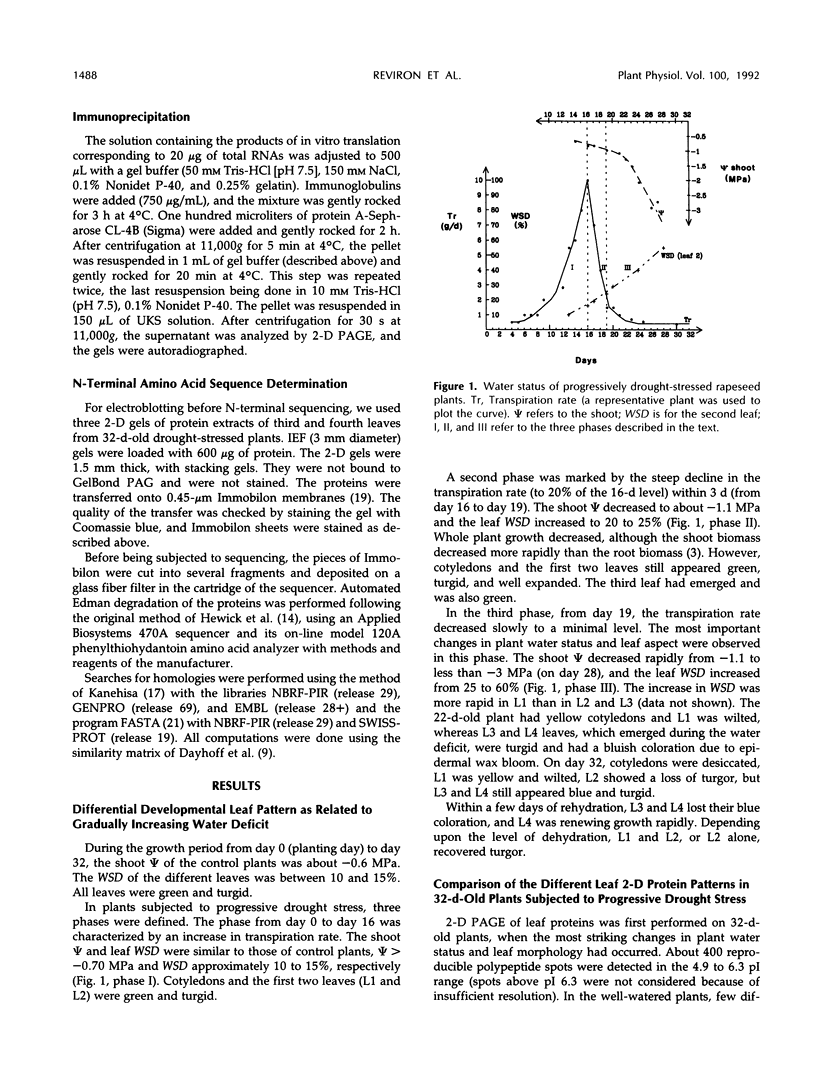
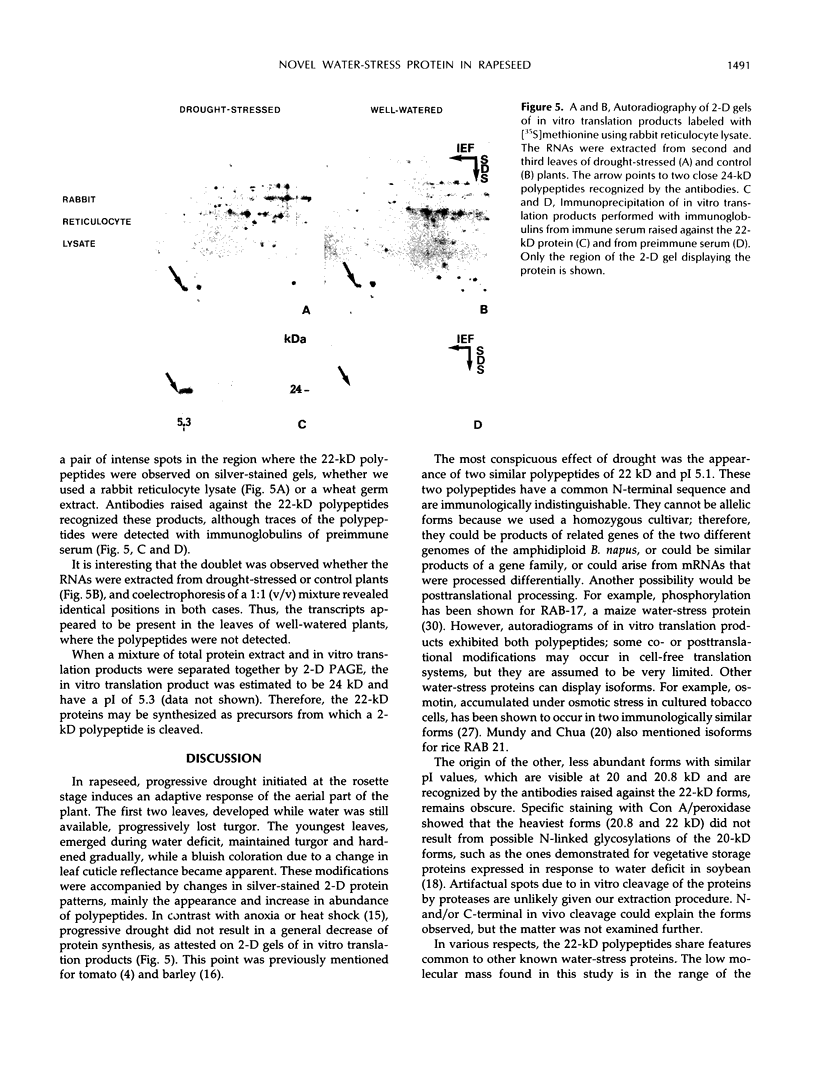
Under progressive drought stress, Brassica napus displays differential leaf modifications. The oldest leaves, developed before the onset of water deficit, wilt gradually, whereas the youngest leaves harden. Hardening was distinguished by leaf turgor and bluish wax bloom when the shoot water potential was below −3 MPa and the leaf water saturation deficit was about 60%. This adaptive change was accompanied by modifications in two-dimensional protein profiles. Ten percent of the polypeptides had altered abundance or were unique to drought-stressed plants. Two-dimensional analysis of in vitro translation products did not reveal a general decrease in mRNA population. A 22-kD double polypeptide was increased by progressive or rapid water stress and salinity and disappeared upon rehydration. These polypeptides have a common N-terminal sequence, which does not reveal homology with any known water-stress protein but which contains the signature motif of soybean Künitz trypsin inhibitors. Immunoprecipitation allowed these polypeptides to be identified on two-dimensional gels of in vitro translation products. They appeared to be synthesized as a 24-kD precursor, and their transcript was present in the control well-watered leaves, where the polypeptides were never detected, indicating a possible translational regulation. A putative function of this protein, named BnD22, in the retardation of drought-induced leaf senescence is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aspart L., Meyer Y., Laroche M., Penon P. Developmental regulation of the synthesis of proteins encoded by stored mRNA in radish embryos. Plant Physiol. 1984 Nov;76(3):664–673. doi: 10.1104/pp.76.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bairoch A. PROSITE: a dictionary of sites and patterns in proteins. Nucleic Acids Res. 1991 Apr 25;19 (Suppl):2241–2245. doi: 10.1093/nar/19.suppl.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray E. A. Drought- and ABA-Induced Changes in Polypeptide and mRNA Accumulation in Tomato Leaves. Plant Physiol. 1988 Dec;88(4):1210–1214. doi: 10.1104/pp.88.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes B., Dekeyser R., Villarroel R., Van den Bulcke M., Bauw G., Van Montagu M., Caplan A. Characterization of a rice gene showing organ-specific expression in response to salt stress and drought. Plant Cell. 1990 Jan;2(1):19–27. doi: 10.1105/tpc.2.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close T. J., Kortt A. A., Chandler P. M. A cDNA-based comparison of dehydration-induced proteins (dehydrins) in barley and corn. Plant Mol Biol. 1989 Jul;13(1):95–108. doi: 10.1007/BF00027338. [DOI] [PubMed] [Google Scholar]
- Damerval C., Vartanian N., de Vienne D. Differential Two-Dimensional Protein Patterns as Related to Tissue Specificity and Water Conditions in Brassica napus var oleifera Root System. Plant Physiol. 1988 Apr;86(4):1304–1309. doi: 10.1104/pp.86.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing W. L., Mauxion F., Fauvarque M. O., Reviron M. P., de Vienne D., Vartanian N., Giraudat J. A Brassica napus transcript encoding a protein related to the Künitz protease inhibitor family accumulates upon water stress in leaves, not in seeds. Plant J. 1992 Sep;2(5):685–693. [PubMed] [Google Scholar]
- Faye L., Chrispeels M. J. Characterization of N-linked oligosaccharides by affinoblotting with concanavalin A-peroxidase and treatment of the blots with glycosidases. Anal Biochem. 1985 Aug 15;149(1):218–224. doi: 10.1016/0003-2697(85)90498-1. [DOI] [PubMed] [Google Scholar]
- Granier F., de Vienne D. Silver staining of proteins: standardized procedure for two-dimensional gels bound to polyester sheets. Anal Biochem. 1986 May 15;155(1):45–50. doi: 10.1016/0003-2697(86)90222-8. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hurkman W. J., Tanaka C. K. Polypeptide changes induced by salt stress, water deficit, and osmotic stress in barley roots: a comparison using two-dimensional gel electrophoresis. Electrophoresis. 1988 Nov;9(11):781–787. doi: 10.1002/elps.1150091114. [DOI] [PubMed] [Google Scholar]
- Kanehisa M. Use of statistical criteria for screening potential homologies in nucleic acid sequences. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):203–213. doi: 10.1093/nar/12.1part1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason H. S., Mullet J. E. Expression of two soybean vegetative storage protein genes during development and in response to water deficit, wounding, and jasmonic acid. Plant Cell. 1990 Jun;2(6):569–579. doi: 10.1105/tpc.2.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mundy J., Chua N. H. Abscisic acid and water-stress induce the expression of a novel rice gene. EMBO J. 1988 Aug;7(8):2279–2286. doi: 10.1002/j.1460-2075.1988.tb03070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatkowski D., Schneider K., Salamini F., Bartels D. Characterization of Five Abscisic Acid-Responsive cDNA Clones Isolated from the Desiccation-Tolerant Plant Craterostigma plantagineum and Their Relationship to Other Water-Stress Genes. Plant Physiol. 1990 Dec;94(4):1682–1688. doi: 10.1104/pp.94.4.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholander P. F., Bradstreet E. D., Hemmingsen E. A., Hammel H. T. Sap Pressure in Vascular Plants: Negative hydrostatic pressure can be measured in plants. Science. 1965 Apr 16;148(3668):339–346. doi: 10.1126/science.148.3668.339. [DOI] [PubMed] [Google Scholar]
- Scopes R. K. Measurement of protein by spectrophotometry at 205 nm. Anal Biochem. 1974 May;59(1):277–282. doi: 10.1016/0003-2697(74)90034-7. [DOI] [PubMed] [Google Scholar]
- Singh N. K., Bracker C. A., Hasegawa P. M., Handa A. K., Buckel S., Hermodson M. A., Pfankoch E., Regnier F. E., Bressan R. A. Characterization of osmotin : a thaumatin-like protein associated with osmotic adaptation in plant cells. Plant Physiol. 1987 Oct;85(2):529–536. doi: 10.1104/pp.85.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skriver K., Mundy J. Gene expression in response to abscisic acid and osmotic stress. Plant Cell. 1990 Jun;2(6):503–512. doi: 10.1105/tpc.2.6.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilardell J., Goday A., Freire M. A., Torrent M., Martínez M. C., Torné J. M., Pagès M. Gene sequence, developmental expression, and protein phosphorylation of RAB-17 in maize. Plant Mol Biol. 1990 Mar;14(3):423–432. doi: 10.1007/BF00028778. [DOI] [PubMed] [Google Scholar]