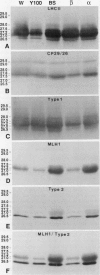
Abstract
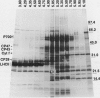
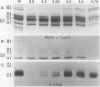
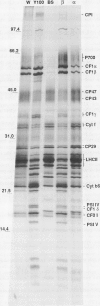
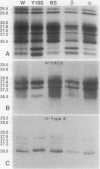
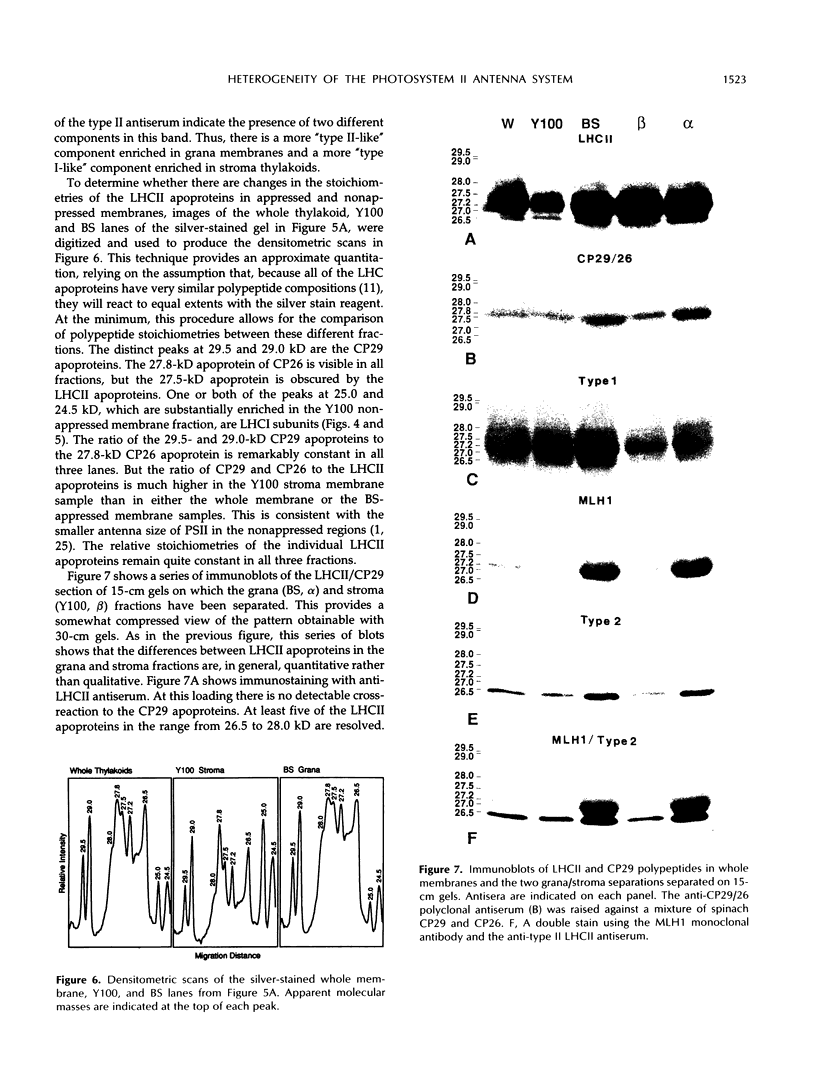
The photosystem (PS) II antenna system comprises several biochemically and spectroscopically distinct complexes, including light-harvesting complex II (LHCII), chlorophyll-protein complex (CP) 29, CP26, and CP24. LHCII, the most abundant of these, is both structurally and functionally diverse. The photosynthetic apparatus is laterally segregated within the thylakoid membrane into PSI-rich and PSII-rich domains, and the distribution of antenna complexes between these domains has implications for antenna function. We report a detailed analysis of the differences in the polypeptide composition of LHCII, CP29, and CP26 complexes associated with grana and stroma thylakoid fractions from spinach (Spinacia oleracea L.), making use of a very high-resolution denaturing gel system, coupled with immunoblots using monospecific antibodies to identify specific antenna components. We first show that the polypeptide composition of the PSII antenna system is more complex than previously thought. We resolved at least five type I LHCII apoproteins and two to three type II LHCII apoproteins. We also resolved at least two apoproteins each for CP29 and CP26. In state 1-adapted grana and stroma thylakoid membranes, the spectrum of LHCII apoproteins is surprisingly similar. However, in addition to overall quantitative differences, we saw subtle but reproducible qualitative differences in the spectrum of LHCII apoproteins in grana and stroma membrane domains, including two forms of the major type II apoprotein. The implications of these findings for models of PSII antenna function in spinach are discussed.
Full text
PDF


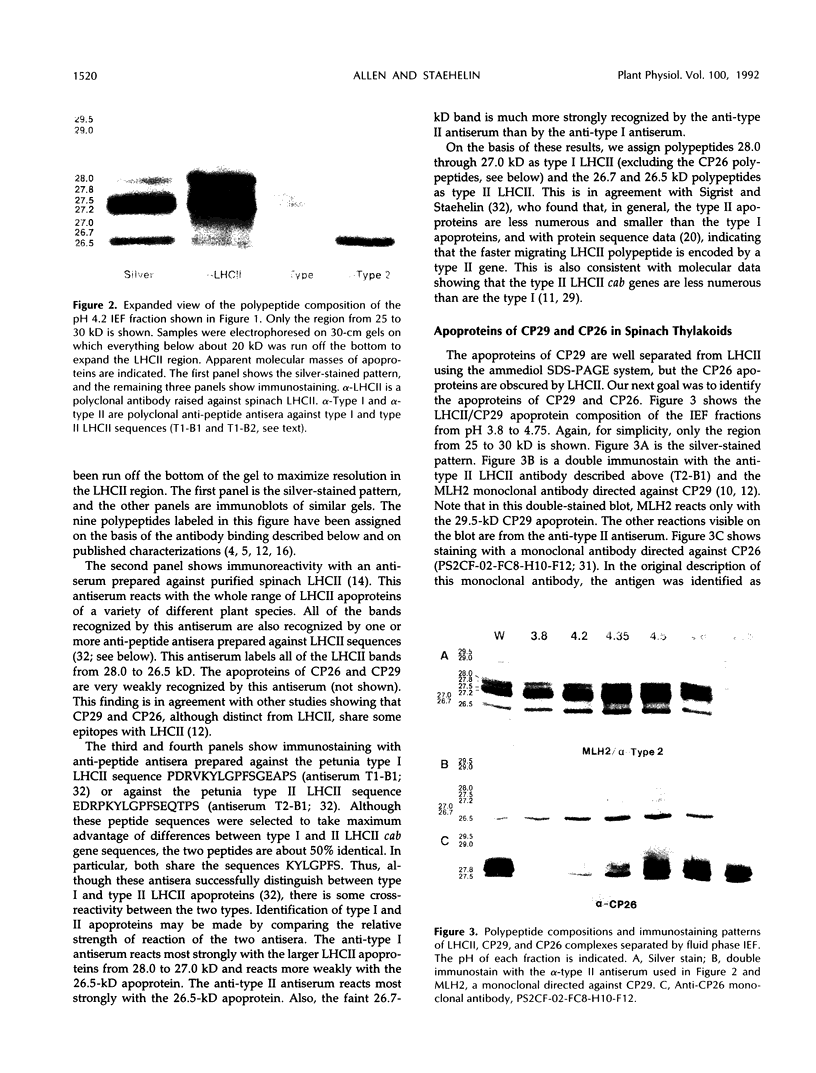
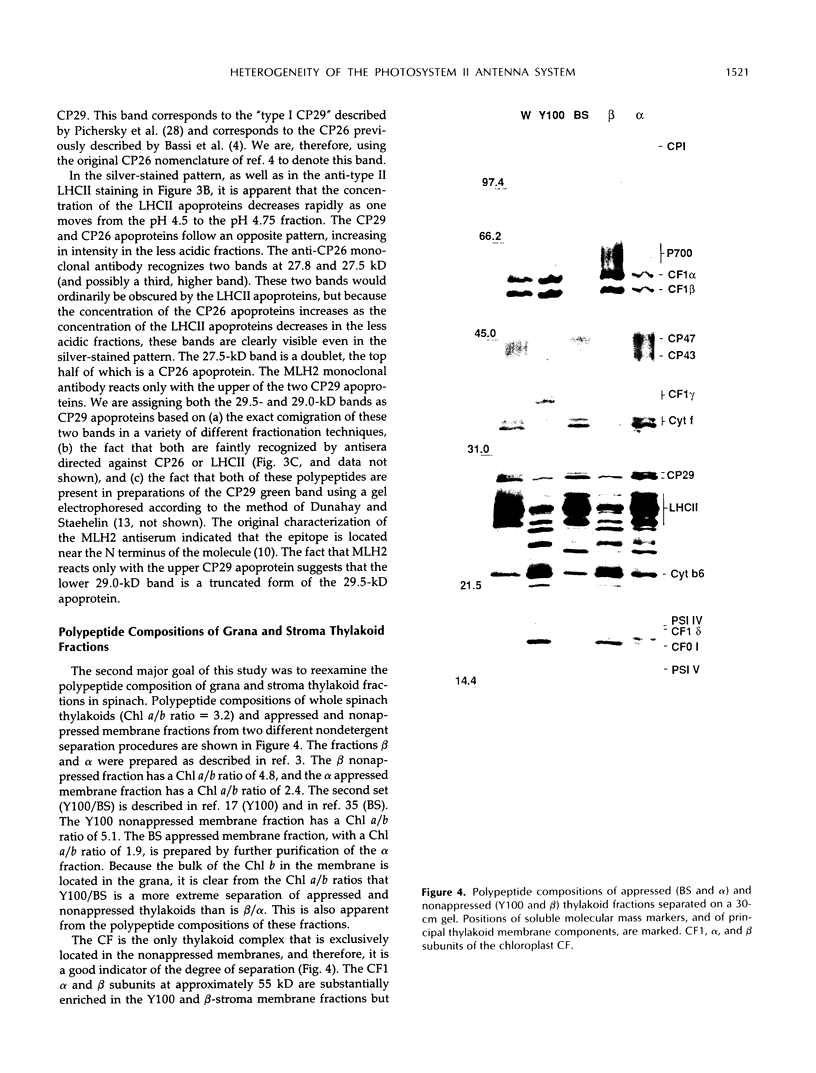
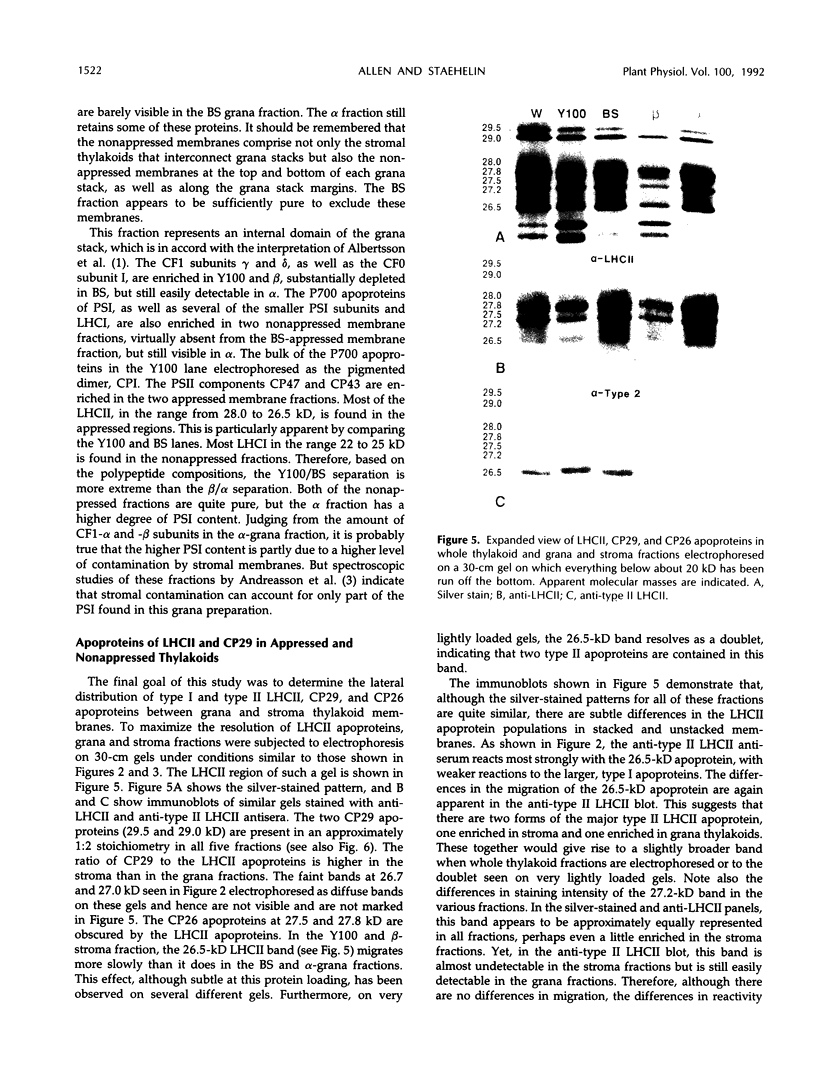



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertsson P. A., Andreasson E., Svensson P. The domain organization of the plant thylakoid membrane. FEBS Lett. 1990 Oct 29;273(1-2):36–40. doi: 10.1016/0014-5793(90)81045-p. [DOI] [PubMed] [Google Scholar]
- Allen K. D., Staehelin L. A. Resolution of 16 to 20 chlorophyll-protein complexes using a low ionic strength native green gel system. Anal Biochem. 1991 Apr;194(1):214–222. doi: 10.1016/0003-2697(91)90170-x. [DOI] [PubMed] [Google Scholar]
- Bassi R., Høyer-Hansen G., Barbato R., Giacometti G. M., Simpson D. J. Chlorophyll-proteins of the photosystem II antenna system. J Biol Chem. 1987 Sep 25;262(27):13333–13341. [PubMed] [Google Scholar]
- Darr S. C., Somerville S. C., Arntzen C. J. Monoclonal antibodies to the light-harvesting chlorophyll a/b protein complex of photosystem II. J Cell Biol. 1986 Sep;103(3):733–740. doi: 10.1083/jcb.103.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmin D. S., Stockinger E. J., Chang Y. C., Walling L. L. Phylogenetic relationships between the chlorophyll a/b binding protein (CAB) multigene family: an intra- and interspecies study. J Mol Evol. 1989 Sep;29(3):266–279. doi: 10.1007/BF02100210. [DOI] [PubMed] [Google Scholar]
- Dunahay T. G., Staehelin L. A. Isolation and Characterization of a New Minor Chlorophyll a/b-Protein Complex (CP24) from Spinach. Plant Physiol. 1986 Feb;80(2):429–434. doi: 10.1104/pp.80.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B. R., Pichersky E., Kloppstech K. Chlorophyll a/b-binding proteins: an extended family. Trends Biochem Sci. 1991 May;16(5):181–186. doi: 10.1016/0968-0004(91)90072-4. [DOI] [PubMed] [Google Scholar]
- Jansson S., Selstam E., Gustafsson P. The rapidly phosphorylated 25 kDa polypeptide of the light- harvesting complex of photosystem II is encoded by the type 2 cab-II genes. Biochim Biophys Acta. 1990 Aug 30;1019(2):110–114. doi: 10.1016/0005-2728(90)90130-v. [DOI] [PubMed] [Google Scholar]
- Kühlbrandt W., Wang D. N. Three-dimensional structure of plant light-harvesting complex determined by electron crystallography. Nature. 1991 Mar 14;350(6314):130–134. doi: 10.1038/350130a0. [DOI] [PubMed] [Google Scholar]
- Mason J. G. Nucleotide sequence of a cDNA encoding the light-harvesting chlorophyll a/b binding protein from spinach. Nucleic Acids Res. 1989 Jul 11;17(13):5387–5387. doi: 10.1093/nar/17.13.5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishige D. T., Thornber J. P. Identification and Analysis of a Barley cDNA Clone Encoding the 31-Kilodalton LHC IIa (CP29) Apoprotein of the Light-Harvesting Antenna Complex of Photosystem II. Plant Physiol. 1992 Jan;98(1):238–245. doi: 10.1104/pp.98.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter G. F., Thornber J. P. Biochemical composition and organization of higher plant photosystem II light-harvesting pigment-proteins. J Biol Chem. 1991 Sep 5;266(25):16745–16754. [PubMed] [Google Scholar]
- Pichersky E., Subramaniam R., White M. J., Reid J., Aebersold R., Green B. R. Chlorophyll a/b binding (CAB) polypeptides of CP29, the internal chlorophyll a/b complex of PSII: characterization of the tomato gene encoding the 26 kDa (type I) polypeptide, and evidence for a second CP29 polypeptide. Mol Gen Genet. 1991 Jun;227(2):277–284. doi: 10.1007/BF00259681. [DOI] [PubMed] [Google Scholar]
- Piechulla B., Kellmann J. W., Pichersky E., Schwartz E., Förster H. H. Determination of steady-state mRNA levels of individual chlorophyll a/b binding protein genes of the tomato cab gene family. Mol Gen Genet. 1991 Dec;230(3):413–422. doi: 10.1007/BF00280298. [DOI] [PubMed] [Google Scholar]
- Sigrist M., Staehelin L. A. Identification of type 1 and type 2 light-harvesting chlorophyll a/b-binding proteins using monospecific antibodies. Biochim Biophys Acta. 1992 Jan 16;1098(2):191–200. doi: 10.1016/s0005-2728(05)80336-6. [DOI] [PubMed] [Google Scholar]
- Staehelin L. A., Arntzen C. J. Regulation of chloroplast membrane function: protein phosphorylation changes the spatial organization of membrane components. J Cell Biol. 1983 Nov;97(5 Pt 1):1327–1337. doi: 10.1083/jcb.97.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]