Abstract
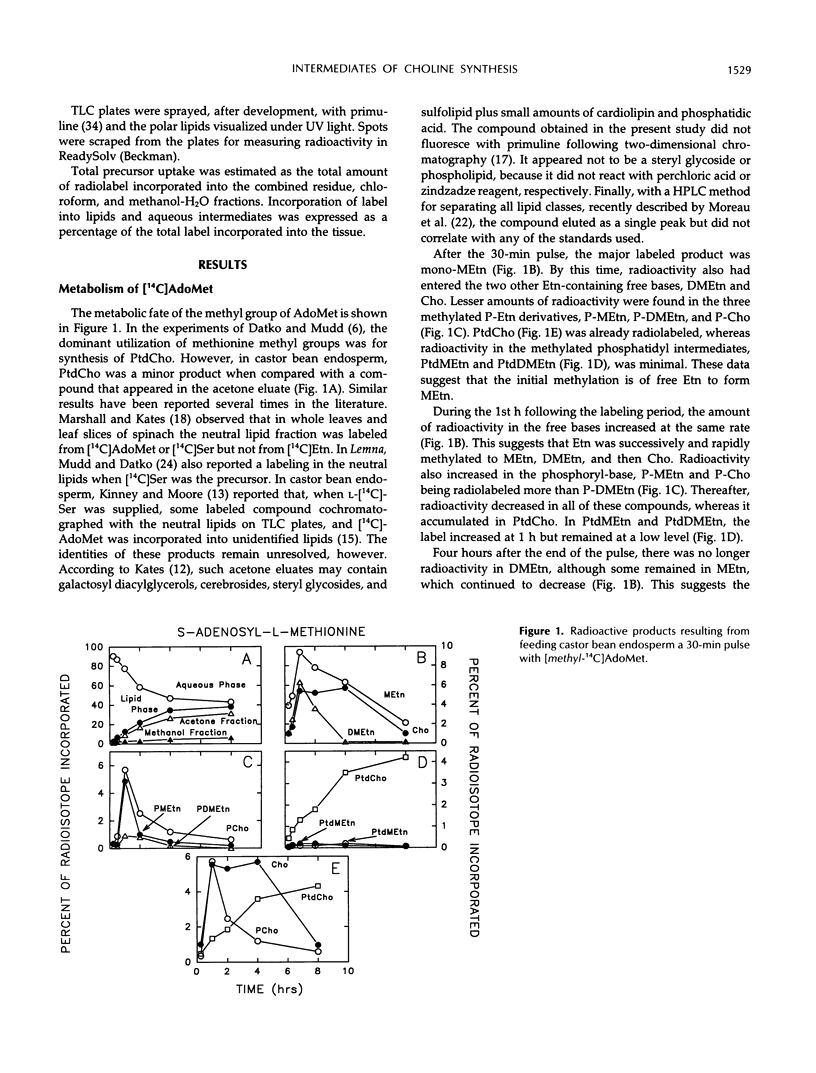
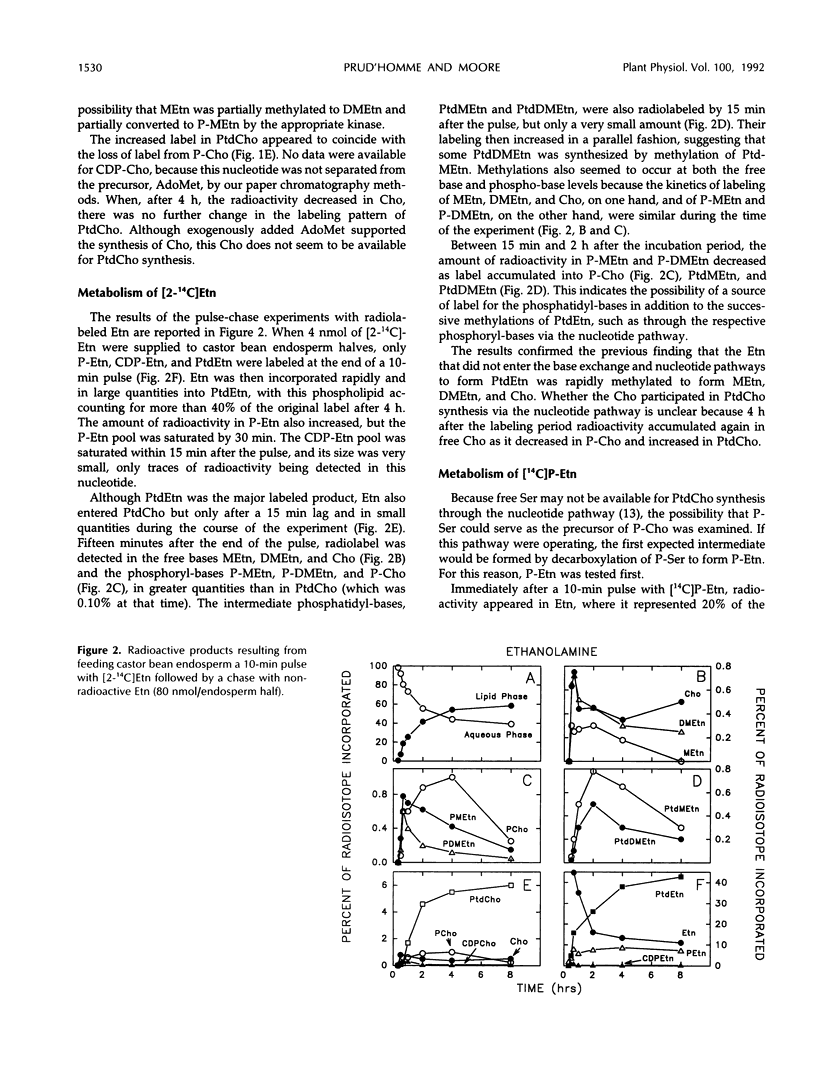
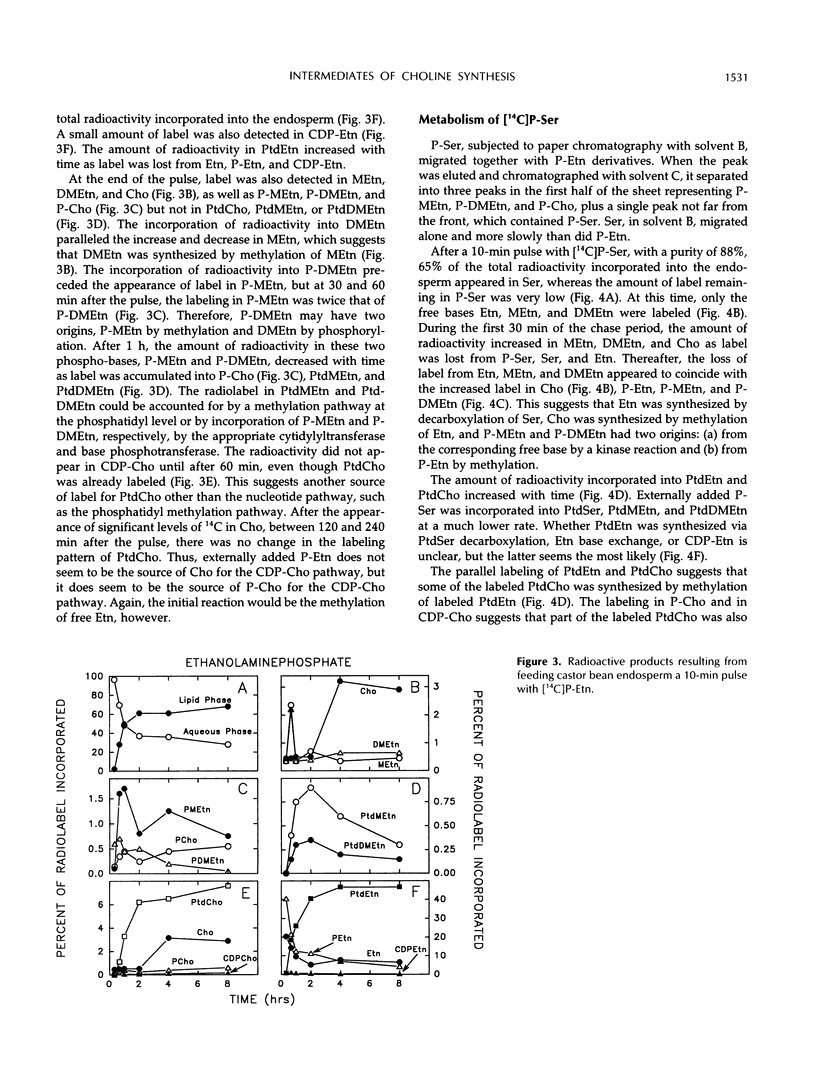
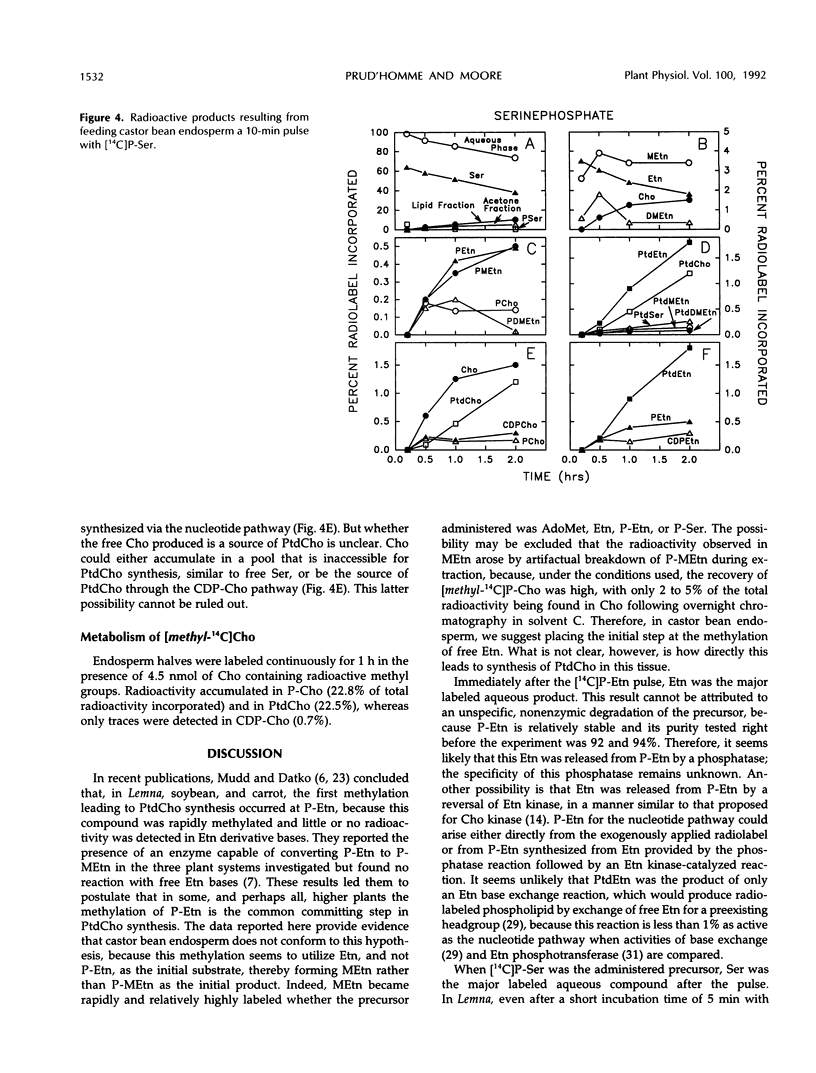
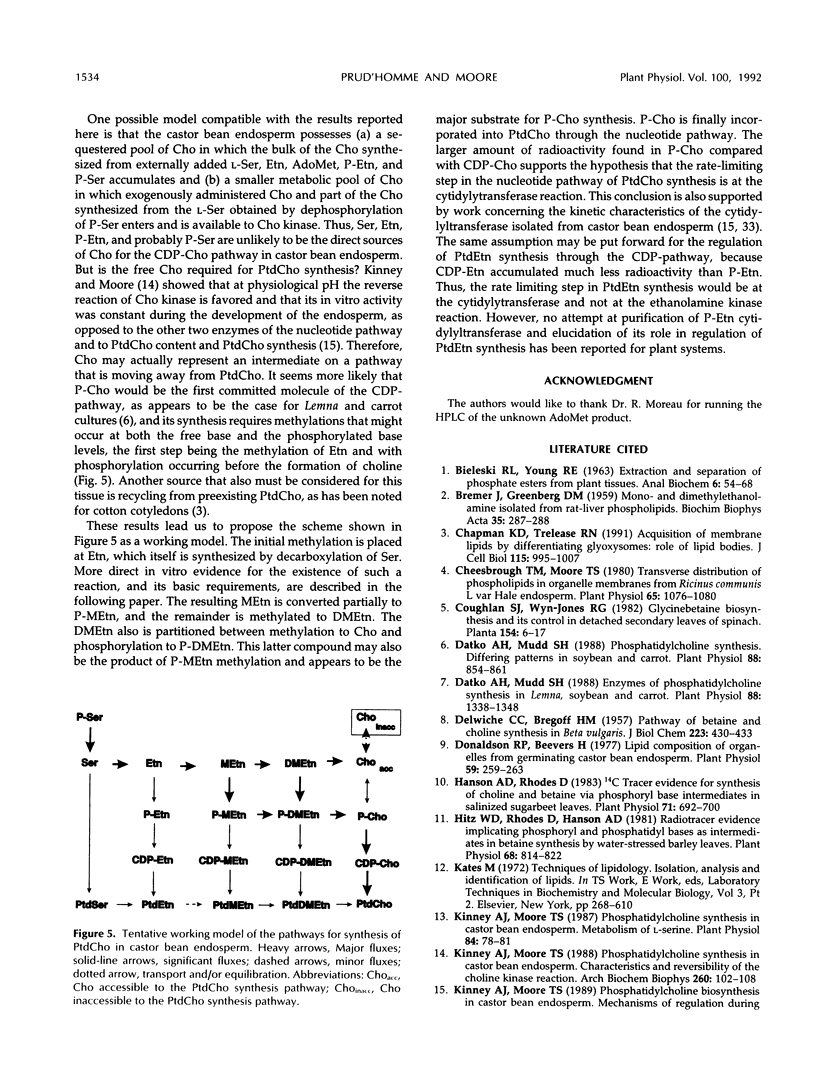
The methylation steps in the biosynthesis of phosphatidylcholine by castor bean (Ricinus communis L.) endosperm have been studied by pulse-chase labeling. Endosperm halves were incubated with [methyl-14C]S-adenosyl-l-methionine, [2-14C]ethanolamine, [14C]ethanolamine phosphate, or [14C]serine phosphate. The kinetics of appearance were followed in the free, phospho-, and phosphatidyl-bases. The initial methylation utilized ethanolamine as a substrate to form methylethanolamine, which was then converted to dimethylethanolamine, choline, and phosphomethylethanolamine. Subsequent methylations occurred at the phospho-base and, to a lesser extent, the phosphatidyl-base levels, after which the radioactivity either remained constant or decreased in these compounds and accumulated in phosphatidylcholine. Although the precursors tested did support the synthesis of choline, the kinetics of the labeling make them unlikely to be the major sources of free choline to be utilized for the nucleotide pathway. A model with two pools of choline is proposed, and the implications of these results for the pathways leading to phosphatidylcholine biosynthesis are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BREMER J., GREENBERG D. M. Mono- and dimethylethanolamine isolated from rat-liver phospholipids. Biochim Biophys Acta. 1959 Sep;35:287–288. doi: 10.1016/0006-3002(59)90375-0. [DOI] [PubMed] [Google Scholar]
- Chapman K. D., Trelease R. N. Acquisition of membrane lipids by differentiating glyoxysomes: role of lipid bodies. J Cell Biol. 1991 Nov;115(4):995–1007. doi: 10.1083/jcb.115.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheesbrough T. M., Moore T. S. Transverse Distribution of Phospholipids in Organelle Membranes from Ricinus communis L. var. Hale Endosperm: MITOCHONDRIA AND GLYOXYSOMES. Plant Physiol. 1980 Jun;65(6):1076–1080. doi: 10.1104/pp.65.6.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H. Enzymes of phosphatidylcholine synthesis in lemna, soybean, and carrot. Plant Physiol. 1988 Dec;88(4):1338–1348. doi: 10.1104/pp.88.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H. Phosphatidylcholine synthesis: differing patterns in soybean and carrot. Plant Physiol. 1988 Nov;88(3):854–861. doi: 10.1104/pp.88.3.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson R. P., Beevers H. Lipid composition of organelles from germinating castor bean endosperm. Plant Physiol. 1977 Feb;59(2):259–263. doi: 10.1104/pp.59.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett W. F., Dunn M. F. Exopolysaccharides Produced by Phytopathogenic Pseudomonas syringae Pathovars in Infected Leaves of Susceptible Hosts. Plant Physiol. 1989 Jan;89(1):5–9. doi: 10.1104/pp.89.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. D., Rhodes D. C Tracer Evidence for Synthesis of Choline and Betaine via Phosphoryl Base Intermediates in Salinized Sugarbeet Leaves. Plant Physiol. 1983 Mar;71(3):692–700. doi: 10.1104/pp.71.3.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitz W. D., Rhodes D., Hanson A. D. Radiotracer evidence implicating phosphoryl and phosphatidyl bases as intermediates in betaine synthesis by water-stressed barley leaves. Plant Physiol. 1981 Oct;68(4):814–822. doi: 10.1104/pp.68.4.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney A. J., Moore T. S., Jr Phosphatidylcholine synthesis in castor bean endosperm: characteristics and reversibility of the choline kinase reaction. Arch Biochem Biophys. 1988 Jan;260(1):102–108. doi: 10.1016/0003-9861(88)90429-8. [DOI] [PubMed] [Google Scholar]
- Kinney A. J., Moore T. S. Phosphatidylcholine Synthesis in Castor Bean Endosperm : I. Metabolism of l-Serine. Plant Physiol. 1987 May;84(1):78–81. doi: 10.1104/pp.84.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage M. Identification and composition of turnip root lipids. Lipids. 1967 May;2(3):244–250. doi: 10.1007/BF02532563. [DOI] [PubMed] [Google Scholar]
- Marshall M. O., Kates M. Biosynthesis of nitrogenous phospholipids in spinach leaves. Can J Biochem. 1974 Jun;52(6):469–482. doi: 10.1139/o74-071. [DOI] [PubMed] [Google Scholar]
- Moore T. S. Phosphatidylcholine synthesis in castor bean endosperm. Plant Physiol. 1976 Mar;57(3):382–386. doi: 10.1104/pp.57.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd S. H., Datko A. H. Phosphoethanolamine bases as intermediates in phosphatidylcholine synthesis by lemna. Plant Physiol. 1986 Sep;82(1):126–135. doi: 10.1104/pp.82.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd S. H., Datko A. H. Synthesis of Ethanolamine and Its Regulation in Lemna paucicostata. Plant Physiol. 1989 Oct;91(2):587–597. doi: 10.1104/pp.91.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S., Moore T. S. Phosphatidylethanolamine synthesis by castor bean endosperm : a base exchange reaction. Plant Physiol. 1990 May;93(1):148–153. doi: 10.1104/pp.93.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparace S. A., Wagner L. K., Moore T. S. Phosphatidylethanolamine synthesis in castor bean endosperm. Plant Physiol. 1981 May;67(5):922–925. doi: 10.1104/pp.67.5.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance D. E., Ridgway N. D. The methylation of phosphatidylethanolamine. Prog Lipid Res. 1988;27(1):61–79. doi: 10.1016/0163-7827(88)90005-7. [DOI] [PubMed] [Google Scholar]
- Wang X. M., Moore T. S., Jr Partial purification and characterization of CTP:cholinephosphate cytidylyltransferase from castor bean endosperm. Arch Biochem Biophys. 1989 Nov 1;274(2):338–347. doi: 10.1016/0003-9861(89)90447-5. [DOI] [PubMed] [Google Scholar]
- Wright R. S. A reagent for the non-destructive location of steroids and some other lipophilic materials on silica gel thin-layer chromatograms. J Chromatogr. 1971 Jul 8;59(1):220–221. doi: 10.1016/s0021-9673(01)80033-9. [DOI] [PubMed] [Google Scholar]


