Abstract
Background: Cross-sectional evidence has suggested a high prevalence of atopic diseases in patients with hidradenitis suppurativa (HS). However, there is a lack of evidence based on longitudinal studies. This study aimed to assess the risk of different atopic diseases, including asthma, atopic dermatitis, and allergic rhinitis, in patients with HS.
Methods: In this retrospective cohort study, data from the TriNetX research network were obtained. Patients with HS were enrolled, and a 1:1 propensity score matching was performed to select a non-HS control group. Matching covariates included age, sex, race, comorbidities, comedications, socioeconomic status, lab data, and medical utilization status. Hazard ratios (HR) for atopic diseases were assessed.
Results: Over a 15-year follow-up period, patients with HS were found to be at a higher risk for atopic dermatitis (HR = 1.65; 95% CI, 1.44-1.90), asthma (HR = 1.41; 95% CI, 1.33-1.49), and allergic rhinitis (HR = 1.08; 95% CI, 1.03-1.13). A similar trend was observed in shorter follow-up periods. The association between HS, atopic dermatitis, and asthma was consistent across different age and sex subgroups.
Conclusion: Atopic diseases including atopic dermatitis, asthma and allergic rhinitis are associated with HS. Further investigation is needed to assess the necessity of early screening for atopic diseases in patients with HS.
Keywords: hidradenitis suppurativa, atopic dermatitis, cohort, epidemiology, electronic medical records
Introduction
As a chronic inflammatory skin condition, hidradenitis suppurativa (HS) typically affects areas rich in sweat glands, including the groin, breasts, and perineum 1. Despite its relatively low prevalence, ranging from 0.00033% to 4.10%, HS substantially affects people's quality of life due to its chronicity and potential systemic implications 2. In particular, HS exhibits gender and racial disparities, disproportionately affecting young women, particularly those of African-American descent 3.
Concomitantly, atopic diseases, including allergic rhinitis, allergic dermatitis, and asthma, globally pose a health burden for 30-40% of the worldwide population 4. These conditions manifest themselves through a dominant immune response of type 2 T (Th2), orchestrated by cytokines such as IL-4 and IL-135. Although HS has historically been associated with distinct inflammatory pathways characterized by cytokines like IL-1β, TNFα, IFN-γ, and IL-17/22, a recent meta-analysis provided evidence regarding the association between HS and atopic dermatitis and asthma, challenging the conventional separation of these conditions 1, 6.
A recent large-scale cohort study shed light on the intricate interplay between HS and atopic dermatitis, suggesting a bidirectional connection. It indicated that individuals with HS had a twofold higher likelihood of developing atopic dermatitis 7. Moreover, another recent study proposed a causal link between the two conditions, implicating the potential role of HS in the development of atopic dermatitis 8. These revelations accentuate the complex nature of immune interactions underlying these disorders. In addition, a study highlighted a plausible correlation between heightened apoptosis of type 1 T-helper cells (TH1), responsible for producing elevated levels of IFN-γ, and the prevailing TH2 predominance characteristic of atopic diseases 9. This mechanistic insight introduces an additional dimension to the immune interplay, suggesting the existence of shared pathways between these entities.
To the best of our knowledge, the real-world association between HS and atopic diseases has not been fully explored. Furthermore, the available evidence is predominantly derived from cross-sectional studies, which do not offer insights into the risk of developing atopic diseases in HS patients within a longitudinal study design. To address this gap, we conducted a multi-center, propensity score-matched cohort study.
Methods and Materials
We obtained data from the global TriNetX research network, which compiles electronic medical records from collaborative healthcare organizations (HCOs) worldwide. TriNetX has been previously utilized for investigating the relationships between exposures and outcomes in prior studies 10-12. Specifically, we employed the US collaborative network, a subset within the TriNetX research network, which sources its data from American healthcare organizations. This dataset includes records from 60 healthcare organizations across the United States. Our definitions for exposure, outcome, and covariates were established using administrative codes, such as ICD-10-CM codes for diseases, all of which are available within the TriNetX research network. Additional algorithm details are provided in the supplementary file. In the TriNetX research network, missing data for variables such as sex or race were categorized as 'unknown sex and 'unknown race' within the system. These missing data were not excluded from subsequent analyses, and imputation was not conducted. The information in the current dataset was properly de-identified in compliance with the regulations outlined in Section §164.514(a) of the HIPAA Privacy Rule. All processes and ethical considerations were reviewed by a qualified expert, as defined in Section §164.514(b)(1) of the HIPAA Privacy Rule, and this formal assessment negated the necessity for IRB permission or a waiver in studies conducted using the TriNetX research network 13, 14. This study adhered to the STROBE guideline and the TriNetX publication guideline. All analyses were executed through the analytical system within the TriNetX research network on November 11th, 2023. No direct interaction or intervention with human participants was undertaken in this study.
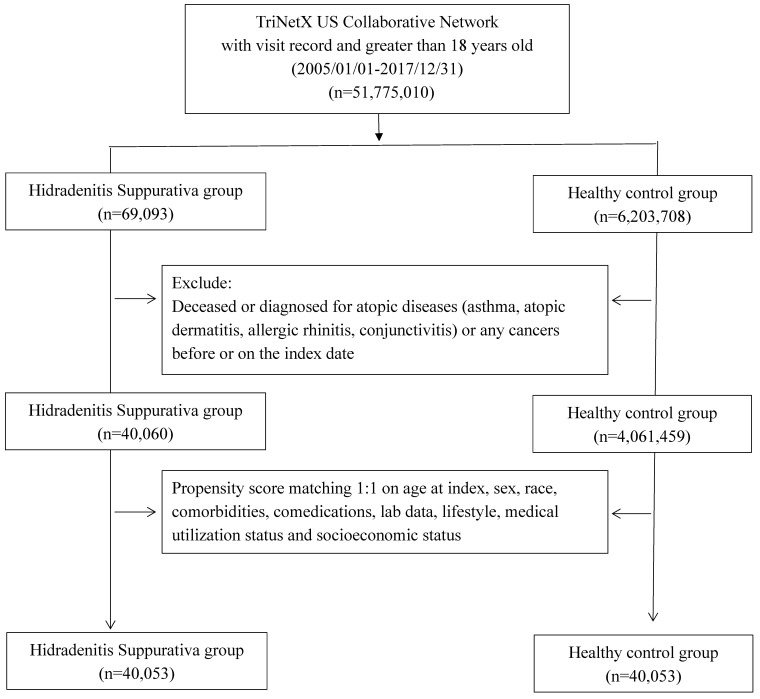
All participants were enrolled during the study period, spanning from January 1, 2005, to December 31, 2017. Exclusions were applied to individuals under 18 years old, those who had passed away before the index date, and those with a history of cancer or atopic diseases (including asthma, atopic dermatitis, allergic rhinitis, and conjunctivitis) prior to the index date. The HS group consisted of individuals diagnosed with HS before the index date, while the control group comprised those who underwent general examinations and had no prior HS diagnosis. Propensity score matching was performed in a 1:1 ratio, aligning critical covariates such as age, sex, race, comorbidities, comedications, socioeconomic status, lab data, and medical utilization status. The study's outcome event was defined as incident atopic diseases, excluding events occurring between the index date and 6 months thereafter. Following 1:1 PSM, the study included 40,053 HS patients and 40,053 controls for assessment. Hazard ratios (HR) for various atopic diseases in the HS group compared to the controls were assessed. Stratification analyses were conducted based on age and sex subgroups to further examine the observed associations. Different matching algorithms and wash-out periods were applied as sensitivity analyses to mitigate potential biases.
All statistical analyses were conducted using the TriNetX research network system. For each analysis, we calculated a 95% confidence interval (95% CI) to indicate the significance of the HR value. To assess the baseline characteristics of participants and the significance between the control group and HS group, we used the standardized difference (SD). When the presented SD value exceeded 0.1 between the groups, the difference was deemed statistically significant.
Results
The study included a population of 40,053 HS patients and an equal number of matched control individuals. The mean age of the HS patients was 34.2 years. Within the HS group, 27.0% were male, and 72.3% were female. The HS group predominantly consisted of white and Black or African American individuals, accounting for 45.4% and 35.0% of the group, respectively. Before matching, there was a significant difference in the baseline distribution of diabetes mellitus between the HS and control cohorts. However, most other comorbidities, including hypertension and hyperlipidemia, did not show significant differences before matching. Following matching, the baseline disparities between the HS and non-HS groups became insignificant (Table 1).
Table 1.
Baseline characteristics of study subjects (before and after propensity score matching)
| Before matching | After matchinga | ||||||
|---|---|---|---|---|---|---|---|
| HS cohort (n=40,060) |
Control cohort (n=4,061,459) |
Std diff |
HS cohort (n=40,053) |
Control cohort (n=40,053) |
Std diff |
||
| Age at index | |||||||
| Mean±SD | 34.2±14.2 | 39.3±20.4 | 0.29 | 34.2±14.2 | 34.6±14.6 | 0.02 | |
| Sex, n (%) | |||||||
| Male | 10798 (27.0) | 1767975 (43.5) | 0.35 | 10798 (27.0) | 10753 (26.8) | 0.00 | |
| Female | 28976 (72.3) | 2213467 (54.5) | 0.38 | 28969 (72.3) | 28980 (72.4) | 0.00 | |
| Unknown sex | 286 (0.7) | 80017 (2.0) | 0.11 | 286 (0.7) | 320 (0.8) | 0.01 | |
| Race, n (%) | |||||||
| White | 18186 (45.4) | 2455819 (60.5) | 0.31 | 18186 (45.4) | 18123 (45.2) | 0.00 | |
| Black or African American | 13639 (34.0) | 563360 (13.9) | 0.49 | 13632 (34.0) | 13889 (34.7) | 0.01 | |
| Asian | 615 (1.5) | 136698 (3.4) | 0.12 | 615 (1.5) | 1073 (2.7) | 0.08 | |
| American Indian or Alaska Native | 182 (0.5) | 12976 (0.3) | 0.02 | 182 (0.5) | 130 (0.3) | 0.02 | |
| Native Hawaiian or Other Pacific Islander | 54 (0.1) | 5335 (0.1) | 0.00 | 54 (0.1) | 39 (0.1) | 0.01 | |
| Other Race | 1473 (3.7) | 147230 (3.6) | 0.00 | 1473 (3.7) | 1291 (3.2) | 0.02 | |
| Unknown Race | 5911 (14.8) | 740041 (18.2) | 0.09 | 5911 (14.8) | 5508 (13.8) | 0.03 | |
| Social economic status | |||||||
| Socioeconomic/psychosocial circumstances problem | 526 (1.3) | 21657 (0.5) | 0.08 | 524 (1.3) | 495 (1.2) | 0.01 | |
| Lifestyle | |||||||
| Alcohol dependence, smoking and substance use | 3723 (9.3) | 114918 (2.8) | 0.27 | 3717 (9.3) | 3751 (9.4) | 0.00 | |
| Comorbidities | |||||||
| Hypertension | 4163 (10.4) | 373134 (9.2) | 0.04 | 4162 (10.4) | 4200 (10.5) | 0.00 | |
| Diabetes mellitus | 2505 (6.3) | 160770 (4.0) | 0.10 | 2503 (6.2) | 2467 (6.2) | 0.00 | |
| Hyperlipidemia | 2097 (5.2) | 241296 (5.9) | 0.03 | 2097 (5.2) | 2012 (5.0) | 0.01 | |
| Psoriasis | 310 (0.8) | 11973 (0.3) | 0.07 | 308 (0.8) | 304 (0.8) | 0.00 | |
| Urticaria | 297 (0.7) | 13663 (0.3) | 0.06 | 297 (0.7) | 254 (0.6) | 0.01 | |
| Medications | |||||||
| Glucocorticoids | 5656 (14.1) | 310671 (7.6) | 0.21 | 5650 (14.1) | 5599 (14.0) | 0.00 | |
| Adalimumab | 149 (0.4) | 2226 (0.1) | 0.07 | 145 (0.4) | 102 (0.3) | 0.02 | |
| Infliximab | 86 (0.2) | 1392 (0.0) | 0.05 | 84 (0.2) | 60 (0.2) | 0.01 | |
| Medical Utilization Status | |||||||
| Emergency visit | 8237 (20.6) | 403967 (9.9) | 0.30 | 8232 (20.6) | 8342 (20.8) | 0.01 | |
| Inpatient visit | 5241 (13.1) | 395464 (9.7) | 0.11 | 5236 (13.1) | 5270 (13.2) | 0.00 | |
| Laboratory data | |||||||
| BMI, n (%) | |||||||
| ≧ 35 (kg/m2) | 2602 (6.5) | 73254 (1.8) | 0.24 | 2596 (6.5) | 2622 (6.5) | 0.00 | |
| C reactive protein, n (%) | |||||||
| ≧ 3 (mg/L) | 1224 (3.1) | 49620 (1.2) | 0.13 | 1220 (3.0) | 1122 (2.8) | 0.01 | |
Bold font represents a standardized difference was more than 0.1
HS: Hidradenitis Suppurativa; BMI, body mass index; SD, standardized difference
aPropensity score matching was performed on age at index, sex, race, status of comorbidities (including diabetes mellitus, chronic kidney disease, heart failure), status of smoking, alcoholism and substance use, medical utilization status, lab data regarding inflammation status (CRP) and social economic status (problems related to housing and economic circumstances, persons with potential health hazards related to socioeconomic and psychosocial circumstances).
Risk of atopic dermatitis, asthma and allergic rhinitis
Compared to non-HS controls, HS patients were associated with a higher risk of developing atopic dermatitis. In the short-term follow-up (3 years), HS patients exhibited a 1.84-fold risk of atopic dermatitis (95% CI, 1.47-2.31), a 1.41-fold risk of asthma (95% CI, 1.33-1.49), and a 1.08-fold risk of allergic rhinitis (95% CI, 1.03-1.13). Over a 10-year follow-up, the HR for HS patients developing atopic dermatitis, asthma, and allergic rhinitis was 1.65 (95% CI, 1.43-1.90), 1.40 (95% CI, 1.32-1.49), and 1.08 (95% CI, 1.02-1.14), respectively. This risk persisted during the 15-year follow-up (Table 1). In the crude model without propensity score matching, the HR for atopic dermatitis was 1.87 (95% CI, 1.72-2.04), and the HR for asthma was 1.85 (95% CI, 1.78-1.92). This association also held in sensitivity models using various matching variables (Table S1). Additionally, the 15-year risk of atopic diseases in HS patients remained significant even when applying longer wash-out periods. When excluding incident atopic dermatitis cases diagnosed within 3 years after the index date, the 15-year risk of atopic dermatitis in HS patients was 1.57 times higher than in controls (95% CI, 1.34-1.85), and the risk of asthma was 1.39 times higher than in controls (95% CI, 1.30-1.48) (Table S2).
Stratification analysis
The observed associations between HS and atopic dermatitis, as well as HS and asthma, were significant in both male and female HS subgroups. In male HS patients, the risk of developing atopic dermatitis and asthma in the subsequent 15 years was 1.56 (95% CI, 1.17-2.07) and 1.40 (95% CI, 1.22-1.60), respectively. For female HS patients, the HR for atopic dermatitis was 1.56 (95% CI, 1.34-1.82), and the HR for asthma was 1.42 (95% CI, 1.33-1.51). Similarly, in both the younger (18-64 years old) and older (above 65 years old) HS subgroups, the risk of developing asthma and atopic dermatitis was significantly higher compared to their respective control groups (Table 3).
Table 3.
Stratification analysis of age and sex
| New-onset atopic dermatitis | New-onset asthma | ||||||
|---|---|---|---|---|---|---|---|
| Subgroups | HS cohort (No. of event/ HS patient amount in each subgroup) |
Control cohort (No. of event/ non-HS patient amount in each subgroup) |
HR (95% CI)a | HS cohort (No. of event/ HS patient amount in each subgroup) |
Control cohort (No. of event/ non-HS patient amount in each subgroup) |
HR (95% CI)a | |
| Sex | |||||||
| Male | 121/10794 | 77/10794 | 1.56 (1.17,2.07) | 495/10794 | 358/10794 | 1.40 (1.22,1.60) | |
| Female | 410/28971 | 262/28971 | 1.56 (1.34,1.82) | 2340/28971 | 1667/28971 | 1.42 (1.33,1.51) | |
| Age at index date | |||||||
| 18-64 years old | 485/36068 | 314/36068 | 1.54 (1.33,1.77) | 2595/36068 | 1827/36068 | 1.43 (1.35,1.52) | |
| ≥ 65 years old | 55/3985 | 27/3985 | 2.06 (1.30,3.27) | 278/3985 | 188/3985 | 1.53 (1.27,1.84) | |
a Propensity score matching was performed on age at index, sex, race, body mass index, status of comorbidities (including diabetes mellitus, hypertension, hyperlipidemia, urticaria, psoriasis), status of comedication use (glucocorticoid, TNF alpha inhibitors), status of smoking, alcoholism and substance use, medical utilization status, lab data regarding inflammation status (CRP) and social economic status (problems related to housing and economic circumstances, persons with potential health hazards related to socioeconomic and psychosocial circumstances).
Discussion
Several studies have previously reported a close association between HS and atopic diseases. However, our study takes a step further by revealing a long-term association between HS, atopic dermatitis, asthma, and allergic rhinitis. Unlike previous investigations, which often relied on cross-sectional methods, our study offers robust long-term data, addressing the evidence gap in this field.
HS is known to be associated with various inflammatory comorbidities 15-17. Apart from inflammatory conditions, HS has also been linked to dysfunctions in different organ systems, including renal diseases, hypertension, dyslipidemia, and diabetes 16, 18. The pathogenesis of HS involves the IL-23, Th17 pathway, and Th1 pathway 19, 20. Excessive keratinization and epithelial hyperplasia of hair follicles lead to follicular obstruction 21. The presence of bacteria and Damage-Associated Molecular Pattern Molecules (DAMPs) in obstructed follicles triggers the activation of macrophages, resulting in the release of IL-1β and TNF 22. IL-1β facilitates the migration of neutrophils into the skin 23, while TNF increases the Th17/Treg ratio, leading to immune dysregulation and immune cell infiltration in HS patients 24. Dendritic cells produce IL-12 and IL-23, activating the Th1 and Th17 pathways, respectively 25, 26. This process eventually establishes the chronic inflammation cycle in HS.
Atopic dermatitis is influenced by both lifestyle and environmental factors, with adult prevalence reported to range from 7% to 14% worldwide 27-30. It primarily occurs between the ages of 20 and 40, with a higher incidence in females until the age of 65. The role of Th2 immunity in the immunological reactions of atopic dermatitis is well-established 31. Moreover, the etiology of atopic dermatitis varies among different ethnic groups 32, with Asian atopic dermatitis patients exhibiting activation of both Th2 and Th17 pathways, while those of Europeans and Africans primarily involve the Th2 pathway 33.
Asthma, a common clinical disease, has a prevalence ranging from 1% to 18%. In asthma patients older than 13 years, the prevalence is higher in females than in males 34. Apart from the typical Th2-mediated asthma, there are other forms of asthma induced by Th17 or Th1 pathways 35. It is evident that HS, atopic dermatitis, and asthma are all highly associated with immune dysregulation. They share a common mechanism involving the dysregulation of the Th17 pathway. Furthermore, recent research has suggested a potential genetic association between HS and atopic dermatitis 8, although further studies are needed to provide conclusive evidence.
Some data in our study warrants further consideration. Among HS patients, the risk of developing atopic dermatitis appeared to be numerically higher in those aged over 65 when compared to individuals aged 18 to 64. This could be attributed to the higher prevalence of atopic dermatitis in individuals over the age of 65 36. When we conducted stratification analysis by age, we excluded younger individuals, who tend to have a higher incidence of atopic dermatitis, potentially amplifying the association between older HS patients and atopic dermatitis.
Another noteworthy point concerns the elevated risk of atopic dermatitis in HS patients. While both atopic dermatitis and asthma are considered allergic diseases resulting from a Th1/Th2 imbalance, the risk of atopic dermatitis was numerically higher than that of asthma in HS patients. This observed trend aligns with findings in previous literature. In a previous meta-analysis, despite the high heterogeneity in the existing evidence, the odds ratio for developing atopic dermatitis in HS patients was calculated to be 4.10 (95% CI, 2.16-8.18), whereas the odds ratio for asthma was 1.50 (95% CI, 1.24-1.81) 6. In studies with a longitudinal design, a recent Korean study reported a 1.54-fold risk (95% CI, 1.47-1.61) for atopic dermatitis and a 1.21-fold risk (95% CI, 1.16-1.26) for asthma in HS patients 37. The higher risk of atopic dermatitis in HS may be partially attributed to a potential genetic link between the two conditions, as suggested by a two-sample Mendelian randomization study 8. However, current evidence remains insufficient to confirm the precise genetic association between HS and atopic dermatitis.
Our study has several notable limitations. Firstly, it is an observational study, and therefore, we cannot establish causation between HS, atopic dermatitis, and asthma. Secondly, despite our matching efforts, our study sample is predominantly composed of individuals of White and African ethnic backgrounds, with smaller proportions of Asian and Native American individuals. Given the variation in clinical disease presentation among different racial groups, the generalizability of our findings may be limited. Thirdly, due to data limitations, we were unable to classify HS patients by the severity of their condition, which hinders our ability to assess the impact of different disease states on our outcomes. Fourthly, despite our confounder matching and sensitivity analyses, misclassification bias and residual confounding may still exist, including the possibility of misdiagnosed HS or unaccounted potential confounders. Therefore, our results should be interpreted with caution. Notwithstanding these limitations, our findings suggest a potential long-term association between HS and atopic diseases, providing valuable evidence of HS as a risk factor for atopic diseases. This discovery may contribute to refining current HS treatment guidelines and increasing awareness of this clinical condition.
Supplementary Material
Supplementary tables.
Figure 1.
Flowchart of participant selection
Table 2.
Risk of atopic diseases under different follow-up timea
| Outcomes | Hazard ratio (95% Confidence interval)b | ||
|---|---|---|---|
| 3 years | 10 years | 15 years | |
| Atopic dermatitis | 1.84 (1.47,2.31) | 1.65 (1.43,1.90) | 1.65 (1.44,1.90) |
| Asthma | 1.43 (1.31,1.56) | 1.40 (1.32,1.49) | 1.41 (1.33,1.49) |
| Allergic rhinitis | 1.10 (1.02,1.19) | 1.08 (1.02,1.14) | 1.08 (1.03,1.13) |
HS: hidradenitis suppurativa
aData present here were the value of follow up from 180 days after index date to the respective following up years.
b Propensity score matching was performed on age at index, sex, race, body mass index, status of comorbidities (including diabetes mellitus, hypertension, hyperlipidemia, urticaria, psoriasis), status of comedication use (glucocorticoid, TNF alpha inhibitors), status of smoking, alcoholism and substance use, medical utilization status, lab data regarding inflammation status (CRP) and social economic status (problems related to housing and economic circumstances, persons with potential health hazards related to socioeconomic and psychosocial circumstances).
Acknowledgments
Funding
This work was supported by a grant from Taichung Veterans General Hospital Research Foundation.
Specific author contributions
All the authors involved in drafting or revising the article and approved of the submitted version.
Study conception and design: Chang HC, Wu MC, Lin CY, Guo YC, Lu HY, Lee CY and Gau SY.
Data acquisition: Chang HC and Gau SY.
Data analysis and demonstration: Chang HC, Wu MC and Gau SY.
Original draft preparation: Chang HC, Wu MC, Lin CY, Guo YC, Lu HY, Lee CY and Gau SY.
Data sharing statement
Data in this study were retrieved from TriNetX Research Network. All data available in the database were administrated by the TriNetX platform. Detailed information can be retrieved at the official website of the research network (https://trinetx.com).
References
- 1.Sabat R, Jemec GBE, Matusiak Ł, Kimball AB, Prens E, Wolk K. Hidradenitis suppurativa. Nat Rev Dis Primers. 2020;6(1):18. doi: 10.1038/s41572-020-0149-1. [DOI] [PubMed] [Google Scholar]
- 2.Posso-De Los Rios CJ, Sarfo A, Ghias M, Alhusayen R, Hamzavi I, Lowes MA, Alavi A. Proceeding report of the third symposium on Hidradenitis Suppurativa advances (SHSA) 2018. Exp Dermatol. 2019;28(7):769–775. doi: 10.1111/exd.13928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strunk A, Midura M, Papagermanos V, Alloo A, Garg A. Validation of a Case-Finding Algorithm for Hidradenitis Suppurativa Using Administrative Coding from a Clinical Database. Dermatology. 2017;233(1):53–57. doi: 10.1159/000468148. [DOI] [PubMed] [Google Scholar]
- 4.Pawankar R, Canonica GW, Holgate ST, Lockey RF, Blaiss M. WAO white book on allergy: update 2013. World Allergy Organization. 2013. 248.
- 5.Le Floc'h A, Allinne J, Nagashima K, Scott G, Birchard D, Asrat S, Bai Y, Lim WK, Martin J, Huang T. et al. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy. 2020;75(5):1188–1204. doi: 10.1111/all.14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gau SY, Chan WL, Tsai JD. Risk of Atopic Diseases in Patients with Hidradenitis Suppurativa: A Systematic Review and Meta-Analysis of Observational Studies. Dermatology. 2023;239(2):314–322. doi: 10.1159/000528920. [DOI] [PubMed] [Google Scholar]
- 7.Sherman S, Kridin K, Bitan DT, Leshem YA, Hodak E, Cohen AD. Hidradenitis suppurativa and atopic dermatitis: A 2-way association. Journal of the American Academy of Dermatology. 2021;85(6):1473–1479. doi: 10.1016/j.jaad.2020.12.051. [DOI] [PubMed] [Google Scholar]
- 8.Tang Z, Shen M, Man X. Association between atopic dermatitis and hidradenitis suppurativa: A two-sample Mendelian randomization study. J Dermatol. 2023;50(9):e287–e288. doi: 10.1111/1346-8138.16796. [DOI] [PubMed] [Google Scholar]
- 9.Akkoc T, de Koning PJA, Rückert B, Barlan I, Akdis M, Akdis CA. Increased activation-induced cell death of high IFN-γ-producing TH1 cells as a mechanism of TH2 predominance in atopic diseases. Journal of Allergy and Clinical Immunology. 2008;121(3):652–658.e651. doi: 10.1016/j.jaci.2007.12.1171. [DOI] [PubMed] [Google Scholar]
- 10.Hadi Y, Dulai PS, Kupec J, Mohy-Ud-Din N, Jairath V, Farraye FA, Kochhar GS. Incidence, outcomes, and impact of COVID-19 on inflammatory bowel disease: propensity matched research network analysis. Aliment Pharmacol Ther. 2022;55(2):191–200. doi: 10.1111/apt.16730. [DOI] [PubMed] [Google Scholar]
- 11.Schmale IL, Poulakis A, Abend A, Luitje ME, Man LX. Chronic Rhinosinusitis With Nasal Polyposis Treated With Dupilumab: Real-World Use and Outcomes. J Allergy Clin Immunol Pract. 2023. [DOI] [PubMed]
- 12.Yong SB, Gau SY, Li CJ, Tseng CW, Wang SI, Wei JCC. Associations between COVID-19 outcomes and asthmatic patients with inhaled corticosteroid. Front Pharmacol. 2023;14:1204297. doi: 10.3389/fphar.2023.1204297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joseph Bailey AM, Oi-Yee Li H, Tan MG, Kirchhof MG. Hidradenitis suppurativa and major adverse cardiac events: A systematic review and meta-analysis. J Am Acad Dermatol. 2021;84(3):844–848. doi: 10.1016/j.jaad.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Taquet M, Sillett R, Zhu L, Mendel J, Camplisson I, Dercon Q, Harrison PJ. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry. 2022;9(10):815–827. doi: 10.1016/S2215-0366(22)00260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dauden E, Lazaro P, Aguilar MD, Blasco AJ, Suarez C, Marin I, Queiro R, Bassas-Vila J, Martorell A, Garcia-Campayo J. Recommendations for the management of comorbidity in hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2018;32(1):129–144. doi: 10.1111/jdv.14517. [DOI] [PubMed] [Google Scholar]
- 16.Shlyankevich J, Chen AJ, Kim GE, Kimball AB. Hidradenitis suppurativa is a systemic disease with substantial comorbidity burden: a chart-verified case-control analysis. J Am Acad Dermatol. 2014;71(6):1144–1150. doi: 10.1016/j.jaad.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Gau SY, Preclaro IAC, Wei JC, Lee CY, Kuan YH, Hsiao YP, Juang SE, Ma KS. Risk of psoriasis in people with hidradenitis suppurativa: A systematic review and meta-analysis. Front Immunol. 2022;13:1033844. doi: 10.3389/fimmu.2022.1033844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gau SY. Increased risk of renal diseases in people with hidradenitis suppurativa: a systematic review and meta-analysis. Int J Dermatol. 2023;62(1):e4–e6. doi: 10.1111/ijd.16423. [DOI] [PubMed] [Google Scholar]
- 19.Sherman S, Kridin K, Bitan DT, Leshem YA, Hodak E, Cohen AD. Hidradenitis suppurativa and atopic dermatitis: A 2-way association. J Am Acad Dermatol. 2021;85(6):1473–1479. doi: 10.1016/j.jaad.2020.12.051. [DOI] [PubMed] [Google Scholar]
- 20.Aarts P, Dudink K, Vossen A, van Straalen KR, Ardon CB, Prens EP, van der Zee HH. Clinical Implementation of Biologics and Small Molecules in the Treatment of Hidradenitis Suppurativa. Drugs. 2021;81(12):1397–1410. doi: 10.1007/s40265-021-01566-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Laffert M, Helmbold P, Wohlrab J, Fiedler E, Stadie V, Marsch WC. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp Dermatol. 2010;19(6):533–537. doi: 10.1111/j.1600-0625.2009.00915.x. [DOI] [PubMed] [Google Scholar]
- 22.van der Zee HH, de Ruiter L, van den Broecke DG, Dik WA, Laman JD, Prens EP. Elevated levels of tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-alpha and IL-1beta. Br J Dermatol. 2011;164(6):1292–1298. doi: 10.1111/j.1365-2133.2011.10254.x. [DOI] [PubMed] [Google Scholar]
- 23.Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, Vom Baur E, Witte K, Warszawska K, Philipp S. et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med (Berl) 2009;87(5):523–536. doi: 10.1007/s00109-009-0457-0. [DOI] [PubMed] [Google Scholar]
- 24.Moran B, Sweeney CM, Hughes R, Malara A, Kirthi S, Tobin AM, Kirby B, Fletcher JM. Hidradenitis Suppurativa Is Characterized by Dysregulation of the Th17:Treg Cell Axis, Which Is Corrected by Anti-TNF Therapy. J Invest Dermatol. 2017;137(11):2389–2395. doi: 10.1016/j.jid.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 25.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3(2):133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 26.Schlapbach C, Hanni T, Yawalkar N, Hunger RE. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65(4):790–798. doi: 10.1016/j.jaad.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Bylund S, Kobyletzki LB, Svalstedt M, Svensson A. Prevalence and Incidence of Atopic Dermatitis: A Systematic Review. Acta Derm Venereol. 2020;100(12):adv00160. doi: 10.2340/00015555-3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stander S. Atopic Dermatitis. N Engl J Med. 2021;384(12):1136–1143. doi: 10.1056/NEJMra2023911. [DOI] [PubMed] [Google Scholar]
- 29.Silvestre Salvador JF, Romero-Perez D, Encabo-Duran B. Atopic Dermatitis in Adults: A Diagnostic Challenge. J Investig Allergol Clin Immunol. 2017;27(2):78–88. doi: 10.18176/jiaci.0138. [DOI] [PubMed] [Google Scholar]
- 30.Yong SB, Gau SY, Guo YC, Wei JC. Allergy from perspective of environmental pollution effects: from an aspect of atopic dermatitis, immune system, and atmospheric hazards-a narrative review of current evidences. Environ Sci Pollut Res Int. 2022;29(38):57091–57101. doi: 10.1007/s11356-022-21582-3. [DOI] [PubMed] [Google Scholar]
- 31.Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):345–360. doi: 10.1016/S0140-6736(20)31286-1. [DOI] [PubMed] [Google Scholar]
- 32.Suaini NHA, Tan CPT, Loo EXL, Tham EH. Global differences in atopic dermatitis. Pediatr Allergy Immunol. 2021;32(1):23–33. doi: 10.1111/pai.13335. [DOI] [PubMed] [Google Scholar]
- 33.Brunner PM, Guttman-Yassky E. Racial differences in atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122(5):449–455. doi: 10.1016/j.anai.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Chowdhury NU, Guntur VP, Newcomb DC, Wechsler ME. Sex and gender in asthma. Eur Respir Rev. 2021. 30(162) [DOI] [PMC free article] [PubMed]
- 35.Gans MD, Gavrilova T. Understanding the immunology of asthma: Pathophysiology, biomarkers, and treatments for asthma endotypes. Paediatr Respir Rev. 2020;36:118–127. doi: 10.1016/j.prrv.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Chan LN, Magyari A, Ye M, Al-Alusi NA, Langan SM, Margolis D, McCulloch CE, Abuabara K. The epidemiology of atopic dermatitis in older adults: A population-based study in the United Kingdom. PLoS One. 2021;16(10):e0258219. doi: 10.1371/journal.pone.0258219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JW, Heo YW, Lee JH, Lee S. Epidemiology and comorbidity of hidradenitis suppurativa in Korea for 17 years: A nationwide population-based cohort study. J Dermatol. 2023;50(6):778–786. doi: 10.1111/1346-8138.16747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables.