Abstract
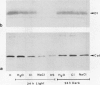
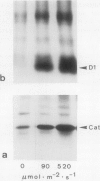
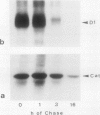
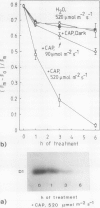
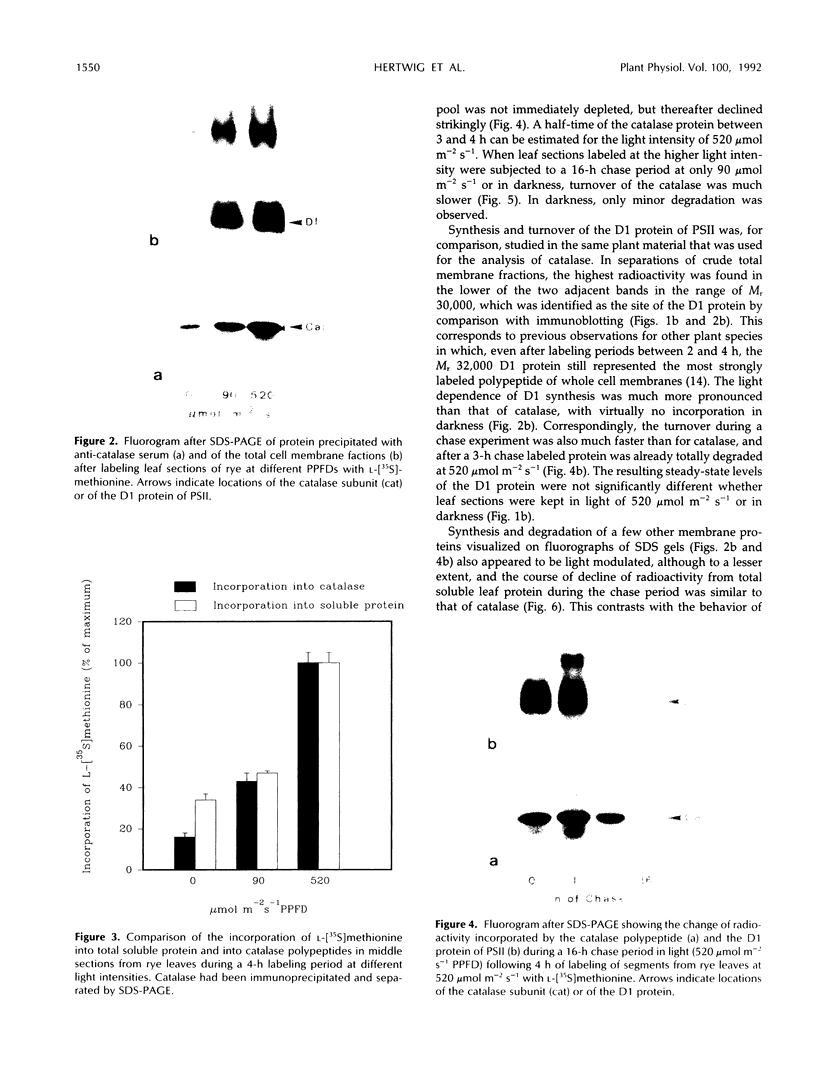
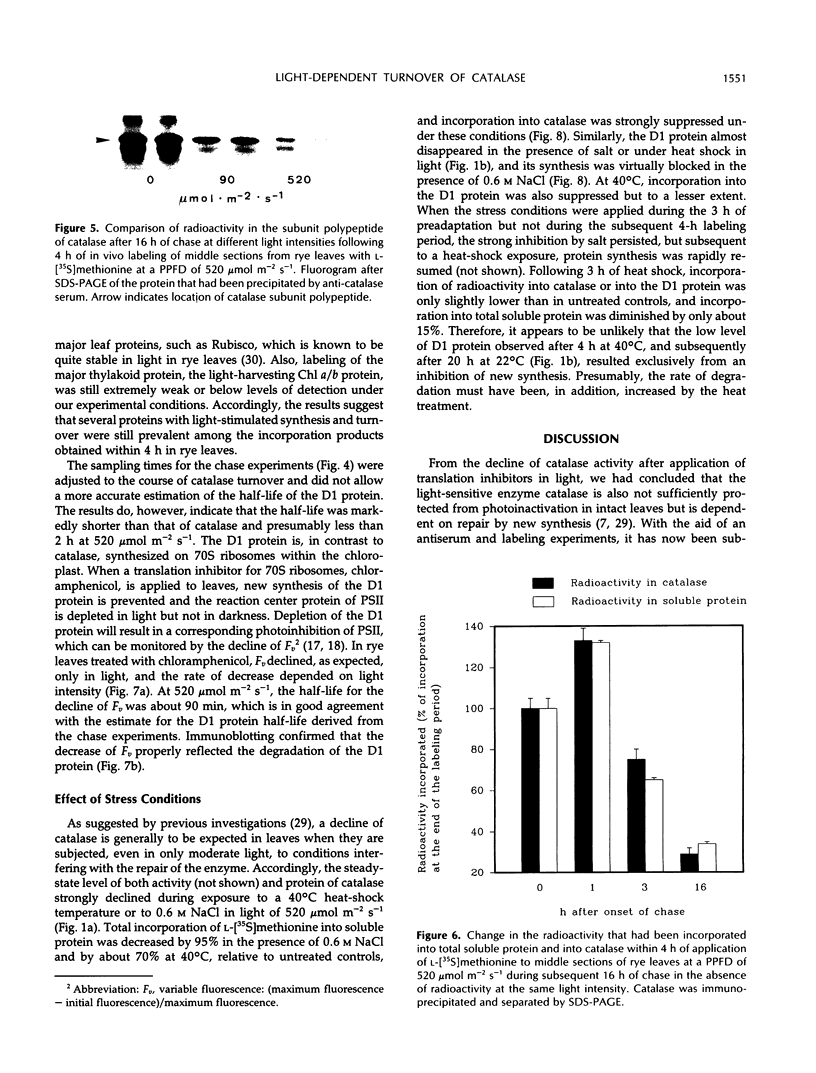
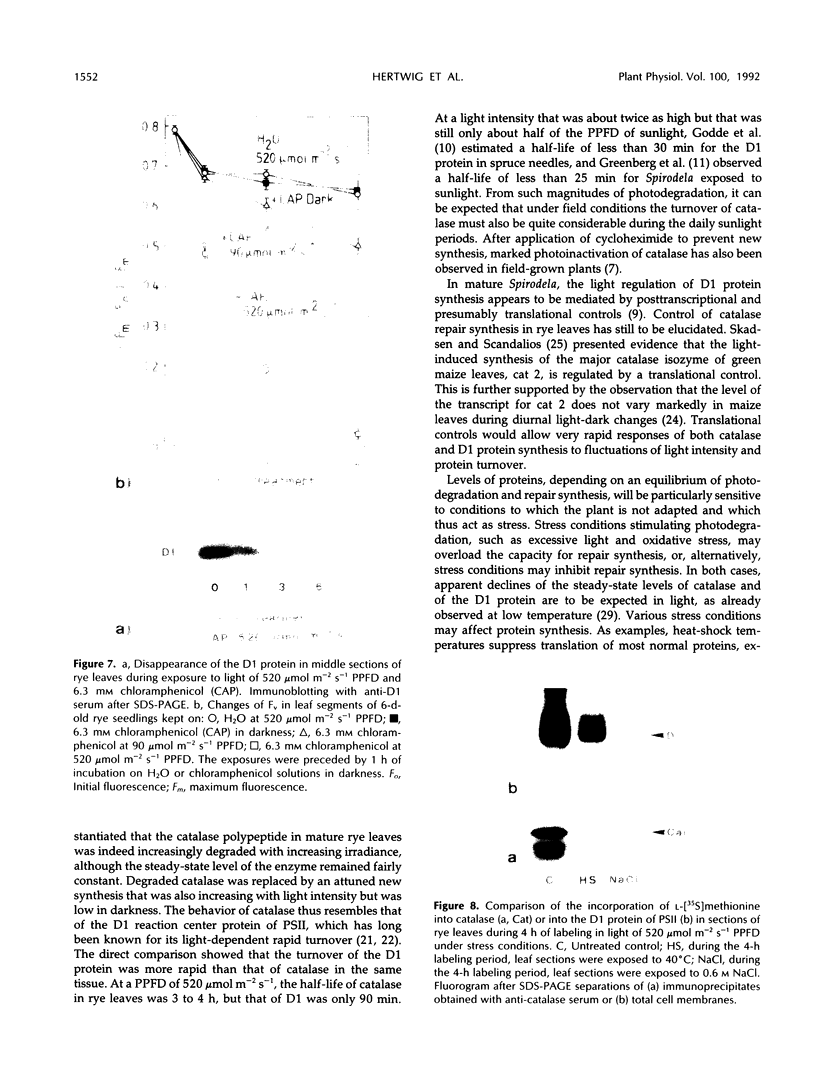
The enzyme catalase (EC 1.11.1.6) is light sensitive and subject to a rapid turnover in light, similar to the D1 reaction center protein of photosystem II. After 3 h of preadaptation to darkness or to different light intensities (90 and 520 μmol m−2 s−1 photosynthetic photon flux density), sections of rye leaves (Secale cereale L.) were labeled for 4 h with l-[35S]methionine. From leaf extracts, catalase was immunoprecipitated with an antiserum prepared against the purified enzyme from rye leaves. Both incorporation into catalase and degradation of the enzyme polypeptide during a subsequent 16-h chase period increased with light intensity. At a photon flux density of 520 μmol m−2 s−1, the apparent half-time of catalase in rye leaves was 3 to 4 h, whereas that of the D1 protein was much shorter, about 1.5 h. Exposure to stress conditions, such as 0.6 m NaCl or a heat-shock temperature of 40°C, greatly suppressed both total protein synthesis and incorporation of the label into catalase and into the D1 protein. Immunoblotting assays indicated that in light, but not in darkness, steady-state levels of catalase and of the D1 protein strongly declined during treatments with salt, heat shock, or translation inhibitors that block repair synthesis. Because of the common property of rapid photodegradation and the resulting dependence on continuous repair, declines in catalase as well as of the D1 protein represent specific and sensitive indicators for stress conditions that suppress the translational activities of leaves.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biekmann S., Feierabend J. Synthesis and degradation of unassembled polypeptides of the coupling factor of photophosphorylation CF1 in 70S ribosome-deficient rye leaves. Eur J Biochem. 1985 Nov 4;152(3):529–535. doi: 10.1111/j.1432-1033.1985.tb09228.x. [DOI] [PubMed] [Google Scholar]
- Björn L. O. Photoinactivation of catalases from mammal liver, plant leaves and bacteria. Comparison of inactivation cross sections and quantum yields at 406 nm. Photochem Photobiol. 1969 Aug;10(2):125–129. doi: 10.1111/j.1751-1097.1969.tb07229.x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cheng L. Y., Packer L. Photodamage to hepatocytes by visible light. FEBS Lett. 1979 Jan 1;97(1):124–128. doi: 10.1016/0014-5793(79)80066-6. [DOI] [PubMed] [Google Scholar]
- Cheng L., Kellogg E. W., 3rd, Packer L. Photoinactivation of catalase. Photochem Photobiol. 1981 Jul;34(1):125–129. [PubMed] [Google Scholar]
- Eyster H. C. CATALASE ACTIVITY IN CHLOROPLAST PIGMENT DEFICIENT TYPES OF CORN. Plant Physiol. 1950 Oct;25(4):630–638. doi: 10.1104/pp.25.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierabend J., Engel S. Photoinactivation of catalase in vitro and in leaves. Arch Biochem Biophys. 1986 Dec;251(2):567–576. doi: 10.1016/0003-9861(86)90365-6. [DOI] [PubMed] [Google Scholar]
- Fromm H., Devic M., Fluhr R., Edelman M. Control of psbA gene expression: in mature Spirodela chloroplasts light regulation of 32-kd protein synthesis is independent of transcript level. EMBO J. 1985 Feb;4(2):291–295. doi: 10.1002/j.1460-2075.1985.tb03628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg B. M., Gaba V., Canaani O., Malkin S., Mattoo A. K., Edelman M. Separate photosensitizers mediate degradation of the 32-kDa photosystem II reaction center protein in the visible and UV spectral regions. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6617–6620. doi: 10.1073/pnas.86.17.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory L. P. Polonium-210 in leaf tobacco from four countries. Science. 1965 Oct 1;150(3692):74–76. doi: 10.1126/science.150.3692.74-a. [DOI] [PubMed] [Google Scholar]
- Hoffman-Falk H., Mattoo A. K., Marder J. B., Edelman M., Ellis R. J. General occurrence and structural similarity of the rapidly synthesized, 32,000-dalton protein of the chloroplast membrane. J Biol Chem. 1982 Apr 25;257(8):4583–4587. [PubMed] [Google Scholar]
- Kahane I., Poljakoff-Mayber A. Effect of substrate salinity on the ability for protein synthesis in pea roots. Plant Physiol. 1968 Jul;43(7):1115–1119. doi: 10.1104/pp.43.7.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle D. J., Ohad I., Arntzen C. J. Membrane protein damage and repair: Selective loss of a quinone-protein function in chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4070–4074. doi: 10.1073/pnas.81.13.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mattoo A. K., Hoffman-Falk H., Marder J. B., Edelman M. Regulation of protein metabolism: Coupling of photosynthetic electron transport to in vivo degradation of the rapidly metabolized 32-kilodalton protein of the chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1380–1384. doi: 10.1073/pnas.81.5.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo A. K., Marder J. B., Edelman M. Dynamics of the photosystem II reaction center. Cell. 1989 Jan 27;56(2):241–246. doi: 10.1016/0092-8674(89)90897-0. [DOI] [PubMed] [Google Scholar]
- Redinbaugh M. G., Sabre M., Scandalios J. G. Expression of the maize Cat3 catalase gene is under the influence of a circadian rhythm. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6853–6857. doi: 10.1073/pnas.87.17.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skadsen R. W., Scandalios J. G. Translational control of photo-induced expression of the Cat2 catalase gene during leaf development in maize. Proc Natl Acad Sci U S A. 1987 May;84(9):2785–2789. doi: 10.1073/pnas.84.9.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tel-Or E., Huflejt M. E., Packer L. Hydroperoxide metabolism in cyanobacteria. Arch Biochem Biophys. 1986 Apr;246(1):396–402. doi: 10.1016/0003-9861(86)90485-6. [DOI] [PubMed] [Google Scholar]
- Winter U., Feierabend J. Multiple coordinate controls contribute to a balanced expression of ribulose-1,5-bisphosphate carboxylase/oxygenase subunits in rye leaves. Eur J Biochem. 1990 Jan 26;187(2):445–453. doi: 10.1111/j.1432-1033.1990.tb15324.x. [DOI] [PubMed] [Google Scholar]