Abstract
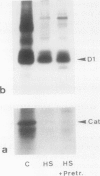
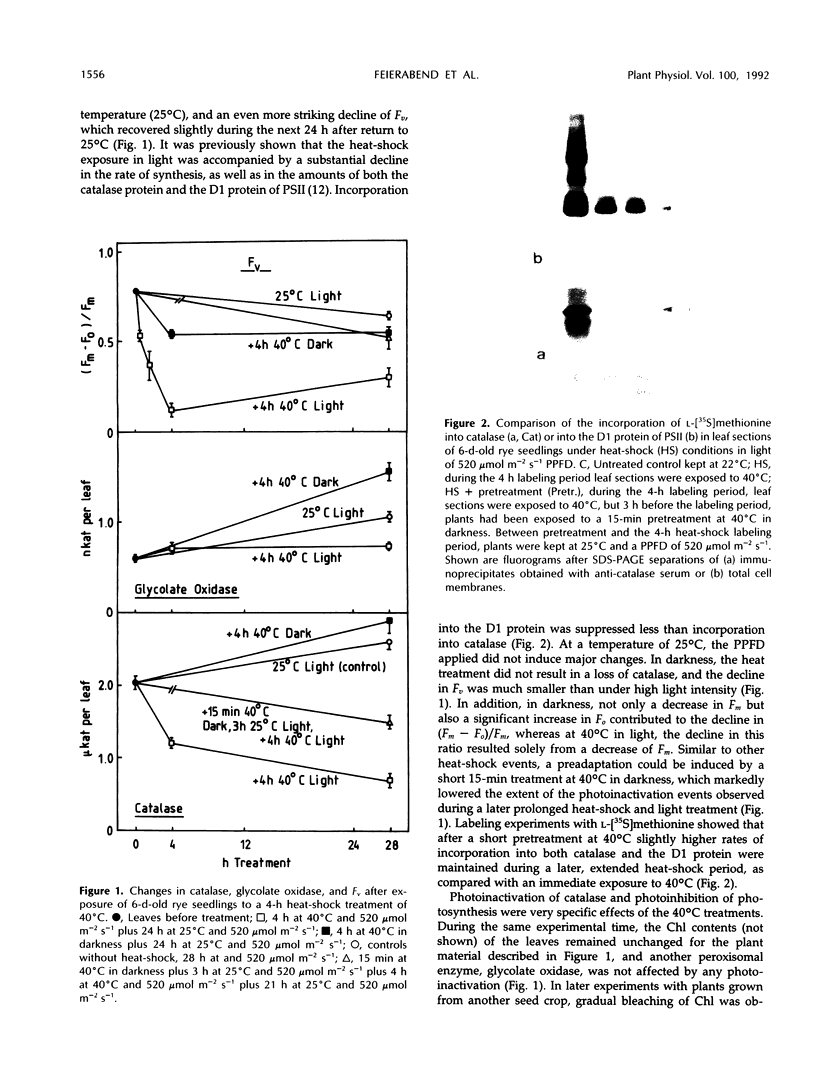
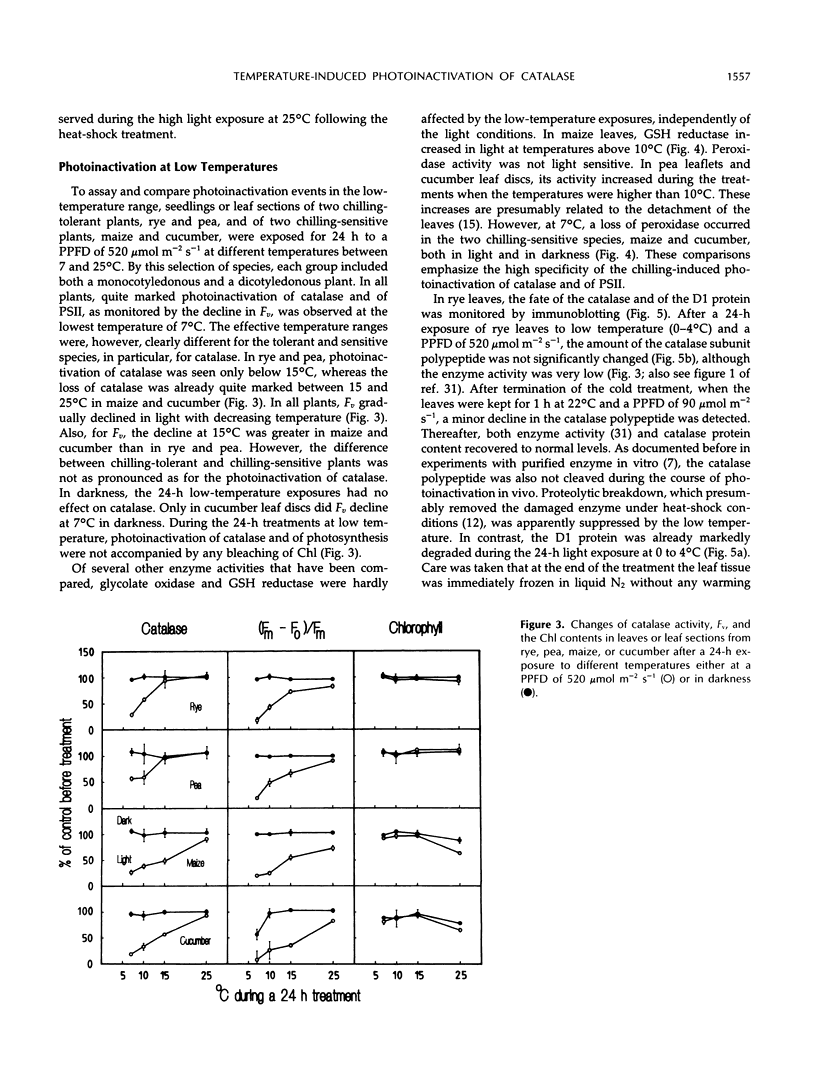
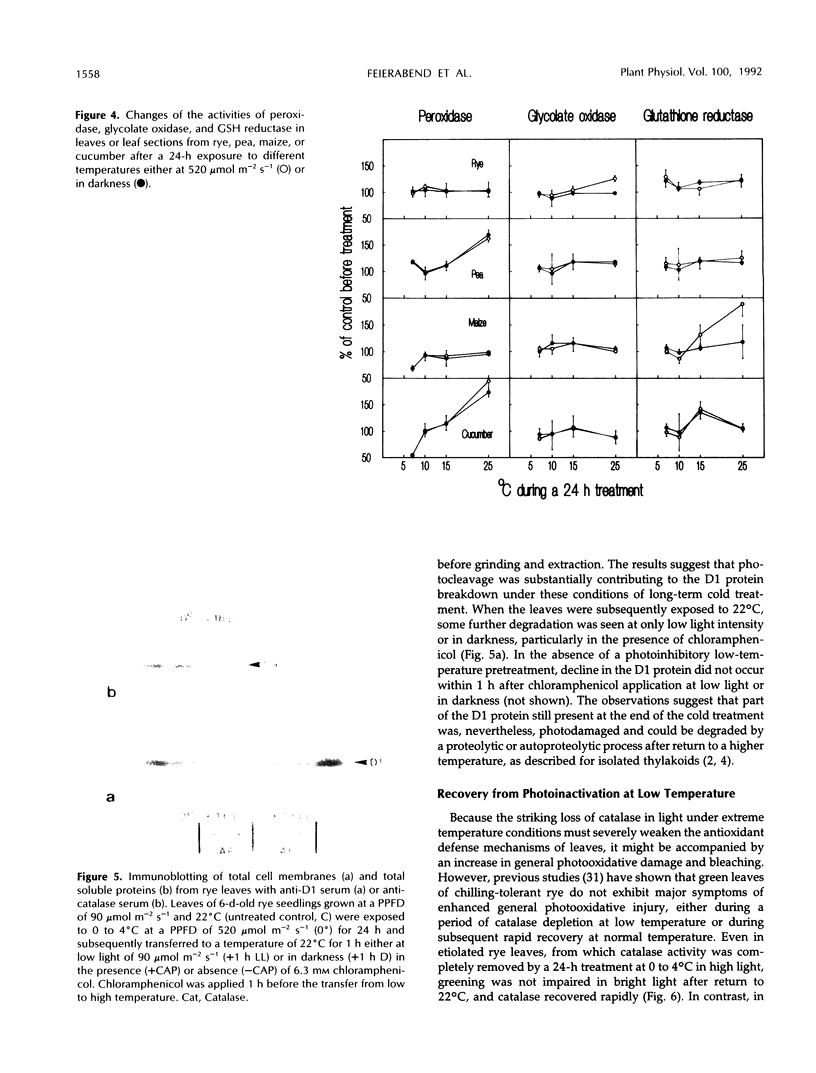
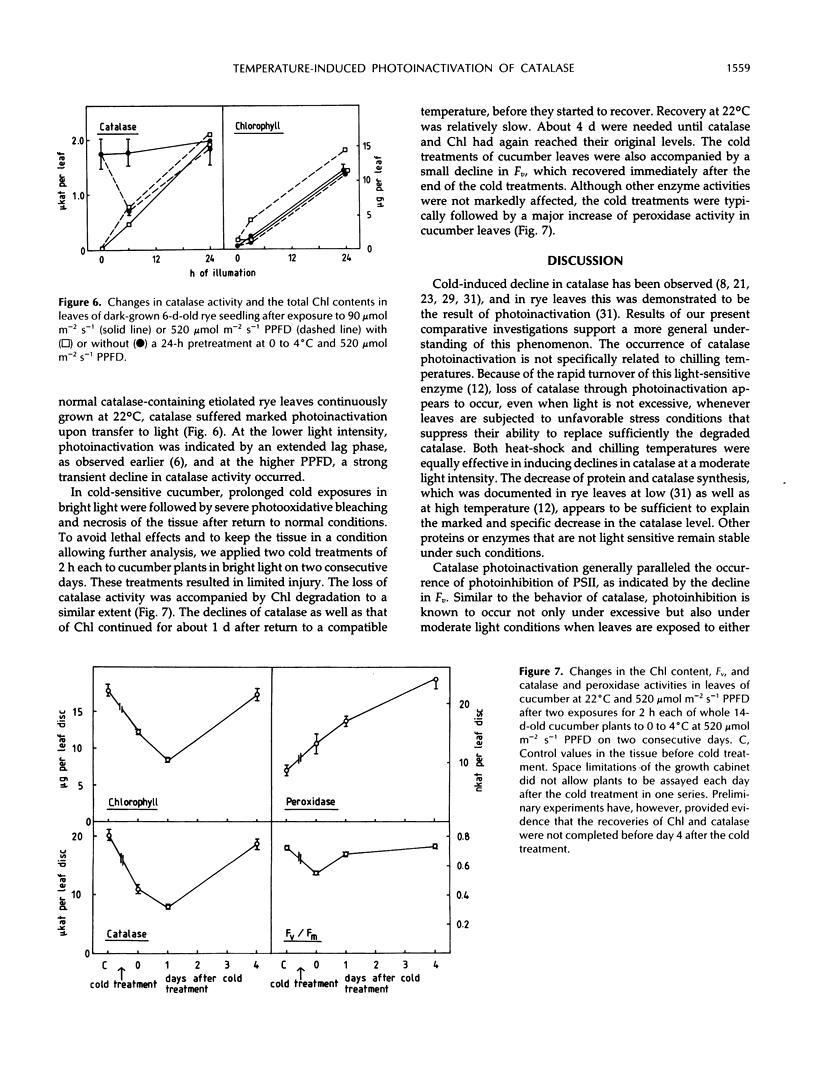
Severe photoinactivation of catalase (EC 1.11.1.6) and a decline of variable fluorescence (Fv), indicating photoinhibition of photosynthesis, were observed as rapid and specific symptoms in leaves exposed to a high heat-shock temperature of 40°C as well as in leaves exposed to low chilling temperatures in white light of only moderately high photosynthetic photon flux density of 520 μE m−2 s−1. Other parameters, such as peroxidase (EC 1.11.1.7), glycolate oxidase (EC 1.1.3.1), glutathione reductase (EC 1.6.4.2), or the chlorophyll content, were hardly affected under these conditions. At a compatible temperature of 22°C, the applied light intensity did not induce severe photoinactivations. In darkness, exposures to high or low temperatures did not affect catalase levels. Also, decline of Fv in light was not related to temperature sensitivity in darkness. The effective low-temperature ranges inducing photoinactivation of catalase differed significantly for chilling-tolerant and chilling-sensitive plants. In leaves of rye (Secale cereale L.) and pea (Pisum sativum L.), photoinactivation occurred only below 15°C, whereas inactivation occurred at 15°C in cucumber (Cucumis sativus L.) and maize (Zea mays L.). The behavior of Fv was similar, but the difference between chilling-sensitive and chilling-tolerant plants was less striking. Whereas the catalase polypeptide, although photoinactivated, was not cleaved at 0 to 4°C, the D1 protein of photosystem II was greatly degraded during the low-temperature treatment of rye leaves in light. Rye leaves did not exhibit symptoms of any major general photodamage, even when they were totally depleted of catalase after photoinactivation at 0 to 4°C, and catalase recovered rapidly at normal temperature. In cucumber leaves, the decline of catalase after exposures to bright light at 0 to 4°C was accompanied by bleaching of chlorophyll, and the recovery observed at 25°C was slow and required several days. Similar to the D1 protein of photosystem II, catalase differs greatly from other proteins by its inactivation and high turnover in light. Inasmuch as catalase and D1 protein levels depend on continuous repair synthesis, preferential and rapid declines are generally to be expected in light whenever translation is suppressed by stress actions, such as heat or chilling, and recovery will reflect the repair capacity of the plants.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber J., Andersson B. Too much of a good thing: light can be bad for photosynthesis. Trends Biochem Sci. 1992 Feb;17(2):61–66. doi: 10.1016/0968-0004(92)90503-2. [DOI] [PubMed] [Google Scholar]
- Feierabend J., Beevers H. Developmental studies on microbodies in wheat leaves : I. Conditions influencing enzyme development. Plant Physiol. 1972 Jan;49(1):28–32. doi: 10.1104/pp.49.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierabend J., Engel S. Photoinactivation of catalase in vitro and in leaves. Arch Biochem Biophys. 1986 Dec;251(2):567–576. doi: 10.1016/0003-9861(86)90365-6. [DOI] [PubMed] [Google Scholar]
- Hertwig B., Streb P., Feierabend J. Light dependence of catalase synthesis and degradation in leaves and the influence of interfering stress conditions. Plant Physiol. 1992 Nov;100(3):1547–1553. doi: 10.1104/pp.100.3.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington S. E., He J., Smillie R. M. Photoinhibition at low temperature in chilling-sensitive and -resistant plants. Plant Physiol. 1989 Aug;90(4):1609–1615. doi: 10.1104/pp.90.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ohad I., Adir N., Koike H., Kyle D. J., Inoue Y. Mechanism of photoinhibition in vivo. A reversible light-induced conformational change of reaction center II is related to an irreversible modification of the D1 protein. J Biol Chem. 1990 Feb 5;265(4):1972–1979. [PubMed] [Google Scholar]
- Omran R. G. Peroxide Levels and the Activities of Catalase, Peroxidase, and Indoleacetic Acid Oxidase during and after Chilling Cucumber Seedlings. Plant Physiol. 1980 Feb;65(2):407–408. doi: 10.1104/pp.65.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. O., Slack C. R., McPherson H. G. Plants under Climatic Stress: VI. Chilling and Light Effects on Photosynthetic Enzymes of Sorghum and Maize. Plant Physiol. 1974 Nov;54(5):696–701. doi: 10.1104/pp.54.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]