Abstract
Cardiovascular diseases (CVDs) are a leading factor driving mortality worldwide. Iron, an essential trace mineral, is important in numerous biological processes, and its role in CVDs has raised broad discussion for decades. Iron-mediated cell death, namely ferroptosis, has attracted much attention due to its critical role in cardiomyocyte damage and CVDs. Furthermore, ferritinophagy is the upstream mechanism that induces ferroptosis, and is closely related to CVDs. This review aims to delineate the processes and mechanisms of ferroptosis and ferritinophagy, and the regulatory pathways and molecular targets involved in ferritinophagy, and to determine their roles in CVDs. Furthermore, we discuss the possibility of targeting ferritinophagy-induced ferroptosis modulators for treating CVDs. Collectively, this review offers some new insights into the pathology of CVDs and identifies possible therapeutic targets.
Keywords: Cardiovascular disease, Iron, Ferroptosis, Ferritinophagy, Therapeutic target
Abstract
心血管疾病(CVDs)在全球范围内是死亡的主要驱动因素。铁是一种必需的微量元素,在多种生物过程中很重要。几十年来,铁对心血管疾病的作用引起了广泛的讨论。由铁介导的细胞死亡方式,即铁死亡,在心肌细胞损伤和心血管疾病中发挥着重要的作用,因此受到广泛关注。此外,铁自噬是诱导铁死亡的上游机制,与心血管疾病密切相关。本文就铁死亡和铁自噬的过程、机制、铁自噬的调控途径和分子靶点进行综述,并总结其对心血管疾病的作用。此外,我们讨论了针对铁自噬诱导的铁死亡调节剂治疗心血管疾病的可能性。总之,本综述将为心血管疾病的病理学机制提供新的见解,并提供一系列潜在治疗靶点。
Keywords: 心血管疾病, 铁, 铁死亡, 铁自噬, 治疗靶点
1. Introduction
Cardiovascular diseases (CVDs) are a leading cause of mortality worldwide. The epidemic is predicted to spread rapidly in developed and developing countries, in association with a high tendency to develop hypertension, diabetes, and obesity (Luan et al., 2021c). A myriad of risk factors, both heritable and behavioral, are believed to result in the high incidence of CVDs. Multiple pathways might contribute to the pathogenesis of CVD, such as the generation of reactive oxygen species (ROS), mitochondrial dysfunction, calcium imbalance, phosphorylation signaling pathway defects, cellular senescence, and genomic instability (Luan et al., 2021a). Metal ions play essential roles in cardiovascular development. Homeostasis of many metals, such as iron and copper, is critical for maintaining the function of the cardiovascular system, and its imbalance is a fundamental cause of CVDs (Corradi and Mutti, 2011).
Iron is an essential mineral element required in numerous biological processes. Deficiency or excess iron accumulation can cause many pathological disorders (Puig et al., 2017). The role of iron in CVDs has elicited broad debate for decades. Imbalances in iron homeostasis, such as deficiency and overload, can lead to CVDs (Koleini et al., 2021). Increasing evidence shows that deficiency of iron can contribute to adverse outcomes for heart failure (HF) patients (von Haehling et al., 2015). Iron shortage leads to impaired cardiomyocyte mitochondrial function, energy deficits, and cardiac dysfunction (Savarese et al., 2023). However, iron overload can also cause harmful outcomes by generating hydroxyl radicals, leading to the oxidative damage to DNA, lipids, and proteins (Lakhal-Littleton, 2019).
Ferroptosis, iron-mediated cell death, has attracted much attention because of its critical role in cardiomyocyte damage and CVDs (Wang et al., 2022). Additionally, ferroptosis is known to be an iron-dependent autophagic cell death process. Ferritinophagy can initiate ferroptosis by modulating iron homeostasis and the production of ROS in cells, thereby contrib‑uting to the development of CVDs (Qin YH et al., 2021).
2. Ferroptosis
Ferroptosis is an autophagic cell death process that depends on iron. It is induced by over production of ROS or lipid peroxides and abnormal iron metabolism (Han et al., 2023). Some morphological alterations have been observed in ferroptosis, including mild chromatin condensation, disruption of plasma membrane integrity, cytoplasm swelling, and mitochondrial alterations (such as an increase in membrane density, interrupted cristae, and rupture of the outer membrane) (Lv et al., 2022). Ferroptosis is usually associated with cell proliferation, differentiation, and senescence, and is relevant to numerous pathological disorders (Liu X et al., 2022; Lv et al., 2022; Prasad et al., 2023).
Core events of ferroptosis are ROS production and lipid peroxidation, which damage the plasma membrane. A hallmark of ferroptosis is the presence of phospholipid hydroperoxides, comprising phospholipids containing polyunsaturated fatty acids (PUFAs), in the membranes of cells (Li SY et al., 2022). Lipidomics results revealed that among the phospholipid types, phosphatidylethanolamine is the most closely associated with ferroptosis (Lv et al., 2022). Long-chain acyl-coenzyme A (CoA) synthetase 4 (ACSL4) is responsible for phosphatidylethanolamine production by esterifying adrenal acid and arachidonic acid (Reeves et al., 2021). Binding of ACSL4 and PUFA-CoAs inhibits ferroptosis (Gan, 2022). Currently, two main pathways are involved in ferroptosis regulation: the glutathione peroxidase 4 (GPX4)-glutathione (GSH) and ferroptosis suppressor protein 1 (FSP1)-coenzyme Q (CoQ) pathways.
Among ferroptosis regulators, GPX4, an enzyme dependent on GSH, contains selenocysteine and is responsible for catalyzing lipid hydroperoxide reduction (Ma et al., 2022). Constitutive depletion or inactive mutation of GPX4 leads to early embryonic mortality (Liu JP et al., 2022). Conditional depletion of GPX4 results in mitochondrial damage and ferroptosis events (Li C et al., 2022). In contrast, GPX4 overexpression prevents oxidative damage in various cell types (Li FJ et al., 2022; Zhang et al., 2023). GPX4-overexpressing ApoE-depleted mice exhibit fewer oxidized lipids and atherosclerotic lesions than ApoE -/- mice (Guo et al., 2008). GPX4 overexpression is also beneficial for reducing cardiac ischemia/reperfusion (I/R) damage (Li N et al., 2021).
GSH is derived from glutamate, glycine, and cysteine through GSH synthetase and glutamate-cysteine ligase, and mediates ferroptosis (Li FJ et al., 2022). Cysteine is the limiting substrate for generating GSH (Banjac et al., 2008). Therefore, inhibiting cysteine by limiting a cystine/glutamate antiporter across the plasma membrane, system Xc-, is sufficient to induce ferroptosis. Suppression of system Xc- results in a shortage of GSH and attenuates the effect of GPX4 in protecting against lipid peroxidation and ferroptosis (Liu et al., 2021). The FSP1-coenzyme Q10 (CoQ10) pathway acts synergistically with GPX4-GSH to alleviate ferroptosis, and exists as an independent parallel system (Doll et al., 2019).
FSP1, namely apoptosis-inducing factor mitochondrion-associated 2 (AIFM2), is presumably involved in apoptosis independent of caspase 1 because of its structural similarity to AIFM1 (Sevrioukova, 2011). FSP1 is translocated to the cell membrane and serves as an oxidoreductase that promotes CoQ10 regeneration instead of stimulating apoptosis (Bersuker et al., 2019). A further study revealed that CoQ10 is responsible for inhibition of ferroptosis by FSP1. Pharmacological inhibition of FSP1 is strongly synergistic with GPX4 inhibitors in inducing ferroptosis in several types of cancer (Zheng et al., 2021). Furthermore, FSP1 can protect cells from detrimental lipid peroxidation and ferroptosis (Mishima et al., 2022).
Dihydroorotate dehydrogenase (DHODH), an inner mitochondrial membrane-located flavin-dependent enzyme, is responsible for the 4th step of pyrimidine nucleotide synthesis to orotate, and reduction of ubiquinone to dihydroubiquione (CoQH2) (Liu M et al., 2022). DHODH can block mitochondrial ferroptosis by modulating the CoQH2 level. DHODH ablation promotes lipid peroxidation in cancer cells with low GPX4 levels (Mao et al., 2021).
2.1. Function of mitochondria in ferroptosis
The occurrence of ferroptosis is correlated with an imbalance of ROS production and antioxidant defense, as well as the disturbance of various regulatory signals in some organelles (Tang DL et al., 2021). Changes in mitochondrial morphology occur during ferroptosis, including contraction, cristae enlargement, and outer membrane rupture. Abnormal mitochondrial dynamics and dysfunction increase cellular susceptibility to ferroptosis, but mitochondria also have unique defense mechanisms against oxidative damage caused by ferroptosis (Javadov, 2022).
Mitochondria are the main source of ROS, and significant ROS accumulation makes cells prone to ferroptosis (Luan et al., 2021a, 2021b). In addition, the breakdown of mitochondrial glutamine promotes iron death caused by amino acid starvation (Jiang et al., 2021). Mitochondria play a critical role in cellular iron metabolism, and the high concentration of iron makes mitochondria prime sites for inducing ferroptosis (Gao et al., 2021). Earlier studies have shown that tumor cell lines with mitochondrial DNA deletion are just as sensitive to iron death as wild-type cells, while cells that undergo mitophagy and thus consume mitochondria can still undergo ferroptosis (Kopinski et al., 2021).
On the other hand, mitochondria resist cell ferroptosis (Wang and Jiang, 2023). Fatty acid β-oxidation occurs mainly in mitochondria and suppresses lipid peroxidation by reducing PUFAs (Xiao et al., 2022). In addition, mitochondrial DHODH could protect cells from ferroptosis by inhibiting lipid peroxidation under conditions of GPX4 inactivation (Gan, 2021). Some proteins, such as cysteine desulfurase (NFS1) and frataxin (FXN), play roles in Fe–S cluster synthesis and are also resistant to ferroptosis (Fox et al., 2015). Mitochondrial DNA loss and subsequent mitochondrial dysfunction increase the susceptibility of liver cells with mitochondrial DNA loss to iron overload. Mitochondrial DNA stress also triggers ferroptosis via activating the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon gene (STING) pathway (Lin et al., 2022). Therefore, fully functioning mitochondria help cells resist ferroptosis.
Previous research has demonstrated that dysregulated ferroptosis affects the progression of various CVDs (Wang et al., 2022). A thorough understanding of ferroptosis regulatory mechanisms and related networks might offer additional strategies for combating CVDs associated with ferroptosis.
2.2. Ferroptosis in cardiovascular disorders
Increasing evidence shows that ferroptosis is relevant to the progression of CVDs. Aberrant regulation of ferroptosis-related genes is implicated in the aggravation of CVDs (Wang et al., 2022). Association between ferroptosis and heart diseases has been widely reported, but the theory of iron death has provided an opportunity to elucidate the molecular mechanism. Recently, ferroptosis has been associated with cardiac diseases such as myocardial ischemia/reperfusion (MIR) injury (MIRI) and adriamycin cardiomyopathy (Li and Zhang, 2021). Consequently, intervention of ferroptosis might provide a strategy for treating various CVDs.
2.2.1. Ferroptosis and cardiomyocyte proliferation
Ferroptosis is crucial for the proliferation of cardiomyocytes (Sugezawa et al., 2022). Erastin is a cystine transporter inhibitor that induces ferroptotic cell death and greatly suppresses cardiomyoblast growth (Shibata et al., 2019). Ectonucleotide pyrophosphatase/phosphodiesterase 2 (ENPP2) overexpression mildly induces H9C2 cell proliferation and migration by modulating critical ferroptosis-related genes, including ACSL4, GPX4,and nuclear factor erythroid-2-related factor 2 (Nrf2), and suppresses ferroptosis induced by erastin (Bai et al., 2018).
2.2.2. Ferroptosis and myocardial infarction
Numerous studies revealed that several antioxidants can alleviate myocardial infarction (MI) symptoms, implicating the role of ROS accumulation in MI progression (Aladağ et al., 2021). Tandem mass tag (TMT) quantitative proteomic analysis led by Park et al. (2019) showed that ferroptosis might be the underlying pathogenic factor in the hearts of MI patients (Zhao et al., 2021). A further bioinformatic study revealed down-regulation of the GSH metabolic pathway and ROS accumulation, accompanied by reduced GPX4 levels in the MI process (Yang and Lian, 2020). Depletion of GPX4 led to lipid peroxidation, contributing to ferroptosis in H9C2 cardiomyoblasts (Imai et al., 2017). MiR-30d was reduced in myocardial tissues by interacting with autophagy-related gene 5 (ATG5). Activation of ATG5 further triggered ferroptosis in H9C2 cells (Yang et al., 2013). Furthermore, mammalian target of rapamycin (mTOR) is believed to be critical in preventing iron overload and ferroptosis, suggesting that it has potential value in MI protection (Yi et al., 2020). Deciphering the potential mechanism of ferroptosis and the related regulatory network could provide novel therapeutic targets for the prevention of MI.
2.2.3. Ferroptosis in heart failure
Studies indicate that ferroptosis is potentially associated with the absence of cardiomyocytes and leads to HF (Leng et al., 2022). Activation of ferroptosis promotes elevated iron in HF rats and triggers disease progression (Yan et al., 2021). Enhanced interactions between Toll-like receptor 4 (TLR4) and NADPH oxidase 4 (NOX4) were observed in HF by integrated bioinformatics analysis (Chen et al., 2019). Down-regulation of TLR4 or NOX4 significantly abrogated activated ferroptosis, indicating a potential therapeutic approach for targeting HF (Chen et al., 2019). Similarly, cardiac hypertrophy in cellular and animal models exhibits an excess accumulation of lipid peroxidation, increased iron production, and reduced cell viability (Han JW et al., 2020). The function of circular RNAs (circRNAs) in iron metabolism was documented by Zheng et al.(2021).CircSnx12 was found to regulate ferroptosis associated with iron metabolism by interacting with miR-224-5p, which could target the ferroptosis-associated ferritin heavy chain (FTH) gene (Qin YH et al., 2021). Therefore, targeting circRNAs might act as a treatment for HF.
In conclusion, the recently discovered ferroptosis-related regulatory network might provide a new target for treating HF. Acetaldehyde dehydrogenase 2 (ALDH2) transgenes have been shown to promote cardiac abnormality in β-amyloid precursor protein (APP)/presenilin 1 (PS1)mutant mice by mediating lipid peroxidation as well as ferroptosis (Wang et al., 2020). Elevation of the essential lipid peroxidative enzymes ACSL4 and nuclear receptor coactivator 4 (NCOA4) and reduced solute carrier family 7 member 11 (SLC7A11)/GPX4 were observed simultaneously in the myocardium, but could be eliminated by the ALDH2 transgenes. ALDH2 relieved the abnormal left ventricular end-systolic size (Lee HL et al., 2021).
2.2.4. Ferroptosis in ischemia/reperfusion injury
Revascularization remains the most prominent approach to ischemic cardiomyopathy. MIR damage benefits little from this therapy. Numerous types of cell death have been observed during I/R injury, including ferroptosis. Ferroptosis is involved in the etiology of I/R damage (Wang et al., 2023). Activated ferroptosis is featured by overproduction of iron and lipid peroxidation and reduced GPX4 activity in H9C2 cells induced by hypoxia/reoxygenation. Treatment with an iron chelator and a glutaminolysis inhibitor significantly alleviated myocardial infarct size and attenuated cardiac injury in an I/R injury mice model (Lee et al., 2016).
Reperfusion is responsible for marked ferroptosis found in murine hearts (Tang LJ et al., 2021a). Production of oxidized and extremely toxic phosphatidylcholines in the reperfusion process is believed to be involved in ferroptosis (Stamenkovic et al., 2021). Ubiquitin-specific protease 7 (USP7) participates in the regulation of ferroptosis by activating the p53/transferrin receptor 1 (TfR1) pathway during cardiac I/R stimulation (Tang LJ et al., 2021b). Additionally, ferroptosis is highly associated with endoplasmic reticulum stress (ERS) following I/R stimulation (Lee et al., 2018). The inhibitor of ferroptosis, ferrostatin-1 (Fer-1), reduced ERS markers (Shi et al., 2022). A combination treatment targeting necrosis and ferroptosis using ponatinib and deferoxamine (DFO) evoked a beneficial synergistic cardioprotective effect and attenu‑ated I/R injury (Lu et al., 2021).
2.2.5. Ferroptosis and cardiac injury
Previous research showed that administering aferroptosis inhibitor to block iron death can dramatically reduce heart damage induced by I/R (Lan et al., 2022). A chemotherapeutic anthracycline drug, doxorubicin (DOX), induces chronic and lethal cardiotoxins, leading to DOX-induced cardiomyopathy (DIC), and since DIC is lethal, the clinical use of DOX is highly restricted (Henninger and Fritz, 2018). Ferroptosis was found to be the underlying mechanism of DIC. DOX therapy induced remarkable ferroptosis-associated symptoms in mice lacking apoptosis and necroptosis, and Fer-1 rescued the deleterious effects (Kitakata et al., 2022). Specifically, DOX administration led to Nrf2-mediated upregulation of heme oxygenase-1 (HO-1), acting as the primary cause of iron overload (Qin YH et al., 2021). DOX treatment also reduced GPX4 levels and promoted the excess lipid peroxidation accumulation in the mitochondria of cardiomyocytes (Tsubouchi et al., 2019). GPX4 transgenic mice had an ameliorated cardiac ventricular ejection fraction (Yoo et al., 2012). In conclusion, accumulation of lipid peroxidation in the mitochondria induces ferroptosis, and GPX4 alleviates DOX-induced ferroptosis. Additionally, overexpression of acyl-CoA thioesterase 1 (Acot1), a gene which mediates catalytic hydrolysis of acyl-CoA, ameliorates DIC by weakening ferroptosis (Liu YC et al., 2020). Currently, dexrazoxane (DXZ) is the only US Food and Drug Administration (FDA)-approved iron chelator in clinical use to alleviate DIC (Yu et al., 2020).
Liu XM et al. (2022) showed that ferroptosis is associated with cardiac myocyte death in DOX-induced culture. DOX-induced ferroptosis is triggered in mitochondria. Chelation with ferrous ions (Fe2+) in mitochondria, but not with iron ions (Fe3+), protects against DOX-induced lipopolysaccharide (LPS) and ferroptosis. DOX-Fe2+ is critical in DOX-associated Fenton reactions, and the chelating agents targeting Fe2+ on mitochondria (but not Fe3+) can effectively prevent DOX-induced LPS and mitochondria-dependent ferroptosis (Tadokoro et al., 2020). Therefore, ferroptosis is the primary cell death modulator in DOX cardiotoxicity. However, Fang et al. (2019) and Tadokoro et al. (2020) showed that DOX-induced cell death in cultured cardiomyoblast cell lines could not be rescued by ferroptosis inhibitors and showed positivity for cleaved caspase (Fang et al., 2019; Tadokoro et al., 2020). The reasons behind the inconsistency of DOX-induced toxicity between in vitro and in vivo conditions must be explored.
2.2.6. Ferroptosis and atherosclerosis
Ferroptosis is involved in the progression of atherosclerosis (Wang et al., 2021) and is believed to be relevant to its pathogenesis. Ferroptosis inhibitors lessen atherosclerosis, and ameliorate endothelial function (Meng et al., 2021). In ApoE -/-mice fed a high-fat diet, Fer-1 treatment partially relieved atherosclerotic plaque formation by rescuing decreased SLC7A11 and GPX4, and inhibiting iron-induced lipid peroxidation (Bai et al., 2020). Fer-1 and DFO reversed the decrease in aortic endothelial cell viability in response to oxidized low-density lipoprotein (Zhang et al., 2012). A human study indicated that lipid metabolism-related pathways were highly enriched in advanced atherosclerotic samples (Beaumont et al., 1958). In summary, ferroptosis is believed to be involved in the pathology of atherosclerosis. In addition, inhibition of iron-induced ROS has been shown to be a potential strategy for treating ferroptosis-mediated CVDs.
Several selective forms of autophagy, such as ferritinophagy, have been shown to be involved in the process of ferroptotic cell death by degrading anti-ferroptosis proteins or organelles (Ajoolabady et al., 2021).
3. Ferritinophagy
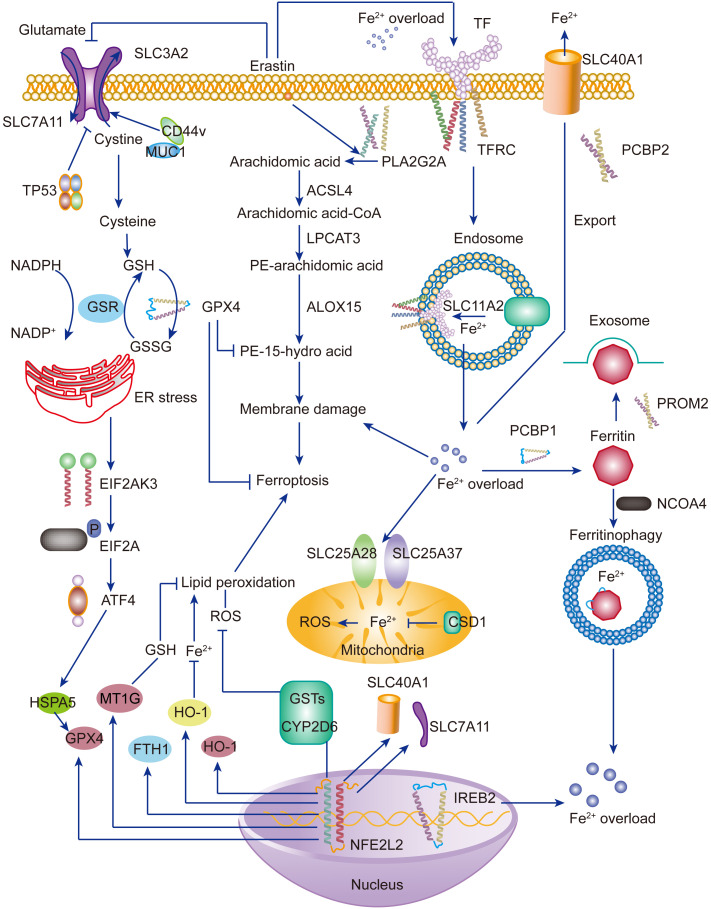
Ferritinophagy, a particular form of autophagic degradation, leads to ferroptosis by ferritin degradation, which also contributes to unstable iron overload, lipid peroxidation, membrane rupture, and cell death (Ajoolabady et al., 2021). Iron is critical for many proteins in fundamental cellular processes (Chua et al., 2007). Both iron insufficiency and overload cause adverse effects (Xiong et al., 2014). When the iron content is inadequate, the body modulates iron release and replenishes ferritin by selective autophagic degradation (Fig. 1) (Maine, 1979). Once autophagy is activated, NCOA4 recruits ferritin to the lysosome, inducing ferritin degradation to produce free iron. This process is known as ferritinophagy (Fleming, 2008). However, cells without NCOA4 are unable to degrade ferritin, leading to insufficient intracellular iron. Therefore, NCOA4 acts as a critical modulator of iron homeostasis.
Fig. 1. Ferroptosis: lipid peroxidation and iron overload. Upon iron overload, Fe3+-bound transferrin binds to transferrin receptor and forms endosome, where Fe3+ turns to Fe2+ and subsequently induces Fe2+ overload in the cytosol ( Yu et al., 2021 ). Iron overload induces ROS production, mediating lipid peroxidation via the Fenton reaction ( Tang et al., 2020 ). Consequently, lipid peroxidation induces membrane damage and ferroptosis. Mammalian cells are apt to prevent lipid peroxidation. Cystine/glutamate antiporter systems activate GPX4 and resist lipid peroxidation and ferroptosis ( Lee JY et al., 2021 ; Li FJ et al., 2022 ). Under some conditions, these anti-ferroptosis systems are unable to exert normal function, which makes cells susceptible to ferroptosis. Moreover, proper induction of ferritinophagy complements Fe2+ by mediating ferritin degradation, thus as a protective way. However, when excessive ferritinophagy, Fe2+ is over accumulated and induces lipid peroxidation and ferroptosis. SLC: solute carrier family; TF: transferrin; TP53: tumor protein p53; CD44v: cluster of differentiation 44 variant; MUC1: mucin 1; GSH: glutathione; GSR: glutathione reductase; GSSG: oxidized glutathione; NADPH/NADP+ : nicotinamide adenine dinucleotide phosphate; ER: endoplasmic reticulum; EIF2A: eukaryotic initiation factor 2α; EIF2AK3: EIF2A kinase 3; ATF4: activating transcription factor 4; HSPA5: heat shock 70 kDa protein 5; GPX4: glutathione peroxidase 4; MT1G: metallothionein 1G; FTH1: ferritin heavy chain 1; HO-1: heme oxygenase 1; ROS: reactive oxygen species; PLA2G2A: phospholipase A2 group IIA; ACSL4: long-chain acyl-coenzyme A (CoA) synthetase 4; LPCAT3: lysophosphatidylcholine acyltransferase 3; ALOX15: arachidonate 15-lipoxygenase; CSD1: the first cold-shock domain; GST: glutathione S-transferase; CYP2D6: cytochrome P450 family 2 subfamily D member 6; NFE2L2: nuclear factor erythroid 2-related factor 2; IREB2: iron-responsive element-binding protein 2; TFRC: transferrin receptor; PCBP: poly(rC)-binding protein; PROM2: prominin 2; NCOA4: nuclear receptor coactivator 4.
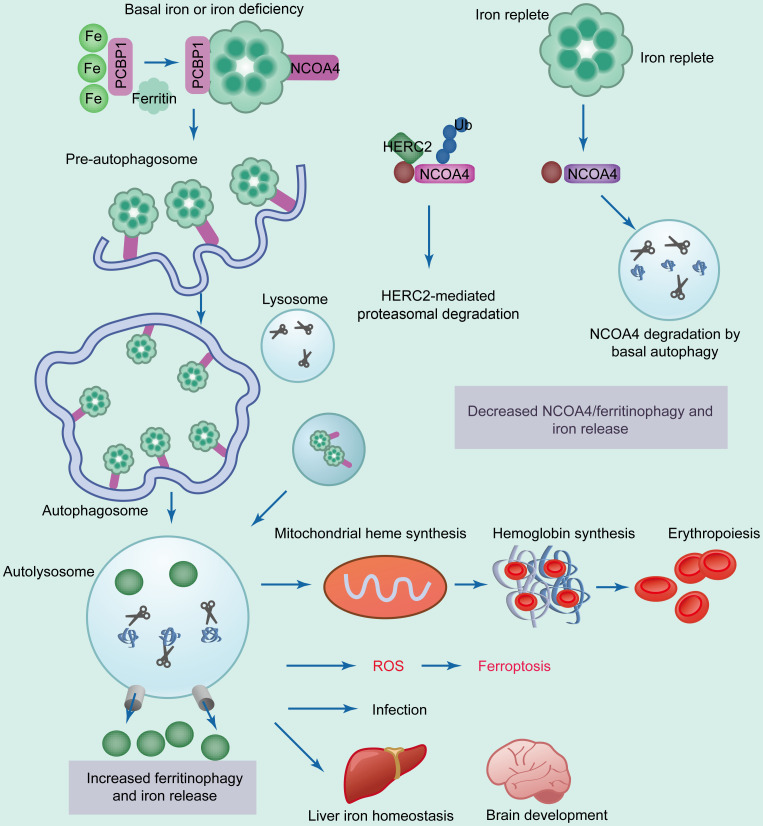
Two transcript variants of NCOA4 have been identified in humans: NCOA4α and the splice isoform NCOA4β (Kollara and Brown, 2012). Isoforms have the same N-terminal coiled-coil domain and a small part of the C-terminus. The C-terminal domain, present only in NCOA4α, is responsible for binding with a conserved surface on ferritin heavy chain 1 (FTH1) (Santana-Codina and Mancias, 2018). The ferritinophagy pathway is precisely mediated via intracellular iron levels (Liu MZ et al., 2022). Under basal iron or iron-deficient conditions, poly(rC)-binding protein 1 (PCBP1) binds to iron and transfers it to ferritin (Fig. 2) (Protchenko et al., 2021). NCOA4 binds to ferritin and transfers it to the autophagosome (Fujimaki et al., 2019).
Fig. 2. Modulation of NCOA4-mediated ferritinophagy by the intercellular level of iron. Under iron deficiency, PCBP1 recruits iron to ferritin, containing heavy and light chains. NCOA4 binds to ferritin and transfers it to the nascent autophagosome through unknown mechanisms ( Santana-Codina and Mancias, 2018 ). Subsequent fusion of the autophagosome and lysosome leads to ferritinophagy, degradation of ferritin, and induction of iron release. Increased ferritinophagy is positively correlated with increased ROS and ferroptosis. Iron is also critical in iron homeostasis of the liver and in brain development. During iron-abundant conditions, HERC2 binds to NCOA4 and mediates its proteasomal degradation, leading to decreased NCOA4 level, ferritinophagy, and intracellular iron level ( Mancias et al., 2015 ). PCBP1: poly(rC)-binding protein 1; NCOA4: nuclear receptor coactivator 4; HERC2: HECT and RLD domain containing E3 ubiquitin protein ligase 2; Ub: ubiquitin; ROS: reactive oxygen species.
Autophagosome and lysosome fusion results in the degradation of ferritin and the release of free iron mediated by “ferritinophagy” (Liu R et al., 2015). Increased ferritinophagy might induce excessive ROS accumulation and ferroptosis (Fig. 1) (Tang et al., 2018). Under iron-replete conditions, the binding of NCOA4 and HECT and RLD domain containing E3 ubiquitin protein ligase 2 (HERC2), an E3 ubiquitin ligase, is enhanced, resulting in proteasomal degradation of NCOA4, followed by inhibition of ferritinophagy and increased ferritin iron storage (Fig. 2) (del Rey and Mancias, 2019). The binding of NCOA4 and FTH1 is weakened following iron overload, suppressing ferritinophagy and promoting ferritin iron storage (Santana-Codina et al., 2021). The binding site in NCOA4 overlaps with the site for binding FTH1, suggesting the possibility of competitive binding between HERC2 and FTH1 (Wu et al., 2010). Further studies are needed to determine whether they can bind simultaneously to NCOA4.
The binding of HERC2 to NCOA4 is alleviated upon iron depletion, inducing increased stabilization of NCOA4, ferritin degradation, and iron release (Li XQ et al., 2020). The mechanisms underlying the recruitment of NCOA4-FTH1 to the lysosome remain largely unclear. Previous research suggested that an endosomal sorting complex required for transport (ESCRT)-dependent endosomal sorting pathway is ATG8-independent lysosomal trafficking for NCOA4 and ferritin (Tu et al., 2010).
NCOA4 can be activated by ATG, which mediates the binding of FTH and ferritin light chain (FTL) to the autophagosome (del Rey and Mancias, 2019). Depletion of NCOA4 and ATGs can significantly suppress ferritinophagy and ferroptosis (Hou et al., 2016). Furthermore, intracellular iron levels can be modulated by post-transcriptional levels of iron-regulatory protein 1 (IRP1) and IRP2 (Park and Chung, 2019). When cells have an adequate iron content, IRP1 is converted to aconitase containing an Fe–S cluster, and IRP2 is subjected to proteasomal degradation, thereby inhibiting the IRP system (Clarke et al., 2006). The iron preservation route is activated during iron deficiency and supplies intracellular iron (Erber et al., 2021). Moreover, the NCOA4 level is elevated during erythropoiesis, and its expression is relevant to heme biosynthesis genes (Fig. 2). Generally, ferritinophagy occurs in the cytoplasm, while ferritin degradation and ferroptosis occur in the mitochondria (Tang et al., 2018).
Free iron in the cytoplasm enters mitochondria via mitochondrial ferritin (MFRN), and excessive iron can be preserved in a specific form of mitochondrial ferritin (Gao and Chang, 2014). Increased ferritin in hypoxic macrophages correlates with reduced NCOA4 levels (Drysdale et al., 2002). During hypoxia, NCOA4 levels are inhibited by miR-6862-5p, which can be modulated by c-Jun N-terminal kinase (Fuhrmann et al., 2020). Iron depletion induces MFRN via the hypoxia-inducible factor-1α (HIF-1α)-specific protein 1 axis and promotes mitophagy in damaged mitochondria (Peyssonnaux et al., 2007). Ferritinophagy maintains iron homeostasis by modulating iron release (Santana-Codina and Mancias, 2018). Once iron deficiency occurs, transferrin activity is activated, ferritinophagy is enhanced, and free intracellular iron is increased by different means (Park and Chung, 2019).
When iron is in excess, the C-terminus of NCOA4 is recruited to HERC2, leading to ubiquitin-dependent NCOA4 degradation in a ubiquitin-dependent manner, accompanied by inhibited ferritinophagy and blocked iron release from ferritin (Philpott, 2020). Increased Fe2+ levels can negatively modulate NCOA4 abundance via two independent mechanisms: promoting its ubiquitination by interacting with HERC2 or participating in its degradation by unknown mechanisms (Santana-Codina et al., 2021). When ferritinophagy is excessively provoked, excess Fe2+ accumulates, contributing to lipid peroxidation (particularly PUFAs), plasma membrane damage, and downstream ferroptotic cell death (Fig. 1) (Santana-Codina et al., 2021). Thus, suppression of ferritinophagy can significantly block Fe2+ overload and ferroptosis (Ajoolabady et al., 2021).
3.1. Signaling pathways and molecular targets involved in ferritinophagy
Ferritinophagy inhibition may block ferroptosis in metabolic diseases (Ajoolabady et al., 2021). NCOA4 depletion inhibits ferritinophagy and protects against erastin-induced ferroptosis (Gryzik et al., 2021). NCOA4 overexpression delays Ras-selective lethal 3 (RSL3)-induced ferroptotic cell death (Gryzik et al., 2021). Therefore, NCOA4 depletion blocks ferritinophagy, making cells more resistant to erastin but more sensitive to RSL3 (Gryzik et al., 2021). Additionally, NCOA4 ablation reduces sideroflexin 1 (SFXN1) and suppresses SFXN1-mediated mitochondrial Fe2+ accumulation. This protects against apelin-13-induced cardiomyocyte hypertrophy, indicating that depletion of ferritinophagy-specific genes inhibits ferroptosis (Tang et al., 2019).
Hypoxia was found to activate mitogen-activated protein kinase 8 (MAPK8) and sequentially activate miR-6862-5p in primary human macrophages (Fuhrmann et al., 2020). Subsequently, miR-6862-5p promotes NCOA4 degradation, suggesting an inhibitory role of the MAPK8–miR-6862-5p–NCOA4 axis in ferritinophagy and iron overload (Fuhrmann et al., 2020). This cascade also increases ferritin to prevent ferroptosis. Moreover, ubiquitination of NCOA4 can inhibit ferritinophagy as well as ferroptosis (Li C et al., 2021). Therapeutic approaches that aim to improve NCOA4 degradation or ubiquitination by HERC2 remain challenging (Li C et al., 2021). In HepG2 cells, high expression of NCOA4 and low expression of FTH1 resulted in more obvious formosanin C-induced ferritinophagy and ferroptosis (Lin et al., 2020). FTH1 overexpression led to attenuated ferritinophagy, decreased NCOA4 and microtubule-associated protein 1 light chain 3α (MAP1LC3A/LC3A), and inhibited ferroptosis in a PC-12 cell line and a Parkinson’s disease (PD) rat model (Gryzik et al., 2021).
Consequently, modulation of FTH1 can affect the levels of other genes involved in ferritinophagy, indicating that genetic interference is a promising therapeutic approach for suppression of ferritinophagy and ferroptosis (Xiu et al., 2022). Defects in cargo degradation also inhibit ferritinophagy (Hou et al., 2016). Similarly, targeting ferritin overload in the outer retina by disrupting hydrolase trafficking to synuclein α (SNCA) inhibits ferritinophagy in retinal degeneration in PD both in vitro and in vivo(Zhang et al., 2020).
Ras-related protein Rab-1A (RAB1A) overexpression can abolish SNCA-mediated ferritinophagy inhibition (Yang et al., 2016). Therefore, therapeutic intervention with RAB1A and SNCA alleviates ferritinophagy, ferritin degradation, and subsequent ferroptosis (Ajoolabady et al., 2021). Moreover, SNCA inhibits ferritinophagy and affects common autophagy, triggering autophagosome accumulation in the pathogenesis of certain diseases (Minakaki et al., 2018). Overexpression of ELAV-like protein 1 (ELAVL1) induces autophagosome formation, ferritinophagy, and ferroptosis by increasing the stability of Beclin-1 (BECN1) messenger RNA (mRNA) in liver stellate cells (Ren et al., 2022). Moreover, cold-inducible RNA-binding protein (CIRBP) promotes ferroptosis by binding with ELAVL1 and activating ferritinophagy (Sui et al., 2021). Although many studies have revealed the involvement of ferritinophagy in the progression of several human diseases, the mechanisms involved remain unclear.
Since ferritinophagy is a specialized form of autophagy, modulation of general autophagy pathways could affect ferritinophagy and, subsequently, ferroptosis (Ajoolabady et al., 2021). Mice with ATG5 depletion exhibit reduced iron storage, increased GSH levels and GPX4 activity during early brain injury, suggesting that inhibition of autophagy can block ferroptosis by alleviating ferritinophagy and ferritin degradation (Lv et al., 2014). Conversely, modulation of ferritinophagy may manipulate general autophagy pathways. Di-2-pyridyl ketone dithiocarbamate (an iron chelator) facilitates ferritinophagy and ferritin degradation, contributing to ROS accumulation, further leading to upregulation of tumor protein p53 (TP53) and, finally, downregulation of the protein kinase B (AKT)–mammalian target of rapamycin C1 (mTORC1) axis, indicating a modulatory effect of the ROS–TP53–AKT–mTORC1 axis in ferritinophagy (Huang et al., 2018). In this respect, manipulation of general autophagy might offer a promising opportunity to attenuate ferritinophagy and ferroptosis.
The pharmacological agents that can be used for inhibition of autophagy can be categorized into four groups: (1) phosphatidylinositol 3-kinase catalytic subunit type 3 (PIK3C3/VPS34) complex blockers; (2) AAA adenosine triphosphatase (ATPase) blockers; (3) V-type ATPase blockers; and (4) lysosomal alkalizers (Pridham et al., 2017). For instance, spautin-1 activates deubiquitinating enzymes, such as USP10 and USP13, promoting the degradation of the PIK3C3 complex and inhibiting autophagy (Pesce et al., 2018). Xanthohumol suppresses autophagy by binding to valosin-containing protein, which participates in autophagosome maturation and belongs to a subunit of the AAA ATPase family (Sasazawa et al., 2012). Concanamycin A and bafilomycin A1 block autophagic flux by inactivating lysosomal hydrolase (Kane et al., 1999). Furthermore, monensin, matrine, and chloroquine are effective lysosomal alkalizers that can block lysosomal acidification and autophagic degradation (Stenseth and Thyberg, 1989). However, inhibition of autophagy can affect basal autophagic flux, which is essential for normal physiological processes in mammalian cells. Therefore, future investigations targeting the precise manipulation of ferritinophagy with minimal effects on the general autophagy process are crucial.
To fulfill this goal, we could search for ferritinophagy-specific molecules in addition to NCOA4, which can selectively recruit ATG proteins and autophagic compartments around ferritin during ferritinophagy, thereby discriminating ferritinophagy from other types of autophagy. High mobility group box 1 (HMGB1) has been proven to trigger ferritinophagy in renal tubules (Mohanty et al., 2021). Its inhibition relieves acute lung injury (ALI) induced by LPS by reducing ferritinophagy and ferroptosis (Liu PF et al., 2020). Overall, ferritinophagy-specific autophagy can distinguish ferritinophagy from general autophagy pathways (Ajoolabady et al., 2021). Since ferritinophagy-specific molecules, except NCOA4 and perhaps HMGB1, remain largely undefined, we suggest two possible research directions, namely the ferritin degradation process during ferritinophagy and the recruitment of ATGs during autophagosome formation, to search for potential ferritinophagy-specific molecules. Identifying such molecules would be the preferred approach for targeting ferritinophagy while keeping basal autophagy intact. Only by manipulating ferritinophagy in this way can we make progress in ferritinophagy-mediated human diseases.
Ferritinophagy alters intercellular iron content and induces ROS accumulation, leading to ferroptosis. This cell death pattern is related to the progression of many pathological diseases.
3.2. Ferritinophagy in various diseases
Ferroptosis is correlated with several pathological disorders, including neurodegenerative diseases (NDs), infectious diseases, cancers, and CVDs (Han C et al., 2020). A thorough understanding of the underlying mechanisms may provide pharmacological means to block ND or induce cancer ferroptosis for therapeutic purposes.
3.2.1. Neurodegenerative diseases
NDs have previously been linked to iron overload and oxidative stress (Han C et al., 2020). Ferroptosis has been verified to be involved in the pathophysiology of neurodegeneration. In PD, ferroptosis and ROS can induce dopamine oxidation and dopaminergic neuronal cell death (Guo et al., 2018). Elevation of divalent metal transporter 1 (DMT1) expression and activation of mutations in transferrin both induce aggravated ferroptosis and correlate with poor prognosis in PD patients (Shindo et al., 2006). In contrast, TfR mutation reduces the import of cellular iron and links with good outcomes. Similar phenomena have been witnessed in Alzheimer’s disease (AD). Iron has been shown to colocalize with deleterious amyloid deposits (van Bergen et al., 2016).
Ferroptosis was shown to directly induce neuronal cell death in immediately derived rat organotypic hippocampal slices cultured with glutamate (Lai et al., 2022). Iron chelators and ferrostatin can block glutamate-induced ferroptosis (Li et al., 2017). Although ferritinophagy makes cells sensitive to ferroptosis, the connection between autophagy and ND indicates that defective global autophagy tightly correlates with the development of ND (Santana-Codina and Mancias, 2018). For example, WD repeat domain 45 (WDR45) mutation impairs autophagy, which has been observed in several ND patients with brain iron accumulation (Xiong et al., 2021).
Generally, autophagy weakens with age, along with an increase in iron levels (Park and Chung, 2019). Long-term depletion of NCOA4 induces a higher serum iron content and increased susceptibility to iron overload. This finding indicates that long-term defective ferritinophagy leads to iron overload in vivo, although iron utilization in erythropoiesis is deficient, leading to anemia (Santana-Codina et al., 2019). Further studies focused on the regulation of NCOA4 expression, and its function in the central nervous system (CNS) is warranted.
3.2.2. Cancers
A large amount of iron accumulates in various cancers to sustain irregular proliferation. Preliminary data suggest that NCOA4 is probably involved in tumorigenesis and that the cooperation of ferroptosis and ferritinophagy could offer a novel insight into cancer therapy (Santana-Codina et al., 2021). NOCA4 levels positively correlate with transformation in ovarian carcinoma. Overexpression of oncogenes, including v-Myc avian myelocytomatosis viral oncogene homolog (MYC) and H-Ras, induces transformation and upregulation of NCOA4 levels (Shroff et al., 2015).
Furthermore, NCOA4 modulates cell survival, since the knockdown of NCOA4 in transformed cells inhibits cell survival and its overexpression reduces colony formation (Rockfield et al., 2018). In prostate cancer, NCOA4, a tumor suppressor, correlates with proliferation as well as invasion. The different functioning of NCOA4 in tumors may be attributed to the various cancer contexts or the specific role of NCOA4 in ferritinophagy (Gu et al., 2022).
Although the link between ferritinophagy and tumorigenesis is unclear, the importance of autophagy in cancers is well documented. Autophagy is associated with ferroptosis sensitivity and tumor autophagy dependence (Xie et al., 2023). The role of autophagy is complicated and can be both tumor suppression and tumor progression, depending on the cancer context (Zalpoor et al., 2022). In K-Ras-driven cancers, such as pancreatic cancer, autophagy is highly activated, along with iron accumulation (Yang et al., 2011). Furthermore, cell lines from pancreatic cancer show elevated expression of NCOA4 as well as consequent activation of the ferritinophagy pathway (Santana-Codina et al., 2022). These features suggest hypersensitivity of ferroptosis in pancreatic cancer.
The direct role of NCOA4 in modulating ferroptosis in pancreatic cancer cells was raised by Yang et al. (2014), who showed that NCOA4 depletion attenuated artesunate-mediated ferroptosis. Generally, autophagy (and therefore ferritinophagy) has been established to modulate ferroptosis positively. Therefore, triggering ferroptosis in cancers with high autophagy levels can potentially indicate greater vulnerability of tumor cells (Lei et al., 2022). Accordingly, increasing iron flux via the ferritinophagy pathway may be a possible therapeutic strategy by increasing labile iron and ROS, thereby making cancer cells vulnerable to ferroptosis-inducing agents.
3.2.3. Infectious diseases
Ferritinophagy mediates susceptibility to the occurrence of infectious diseases. Uropathogenic Escherichia coli propagates by establishing reservoirs within urothelial cell autophagosomes (Bauckman and Mysorekar, 2016). These bacteria proliferate according to the traffic of ferritin-bound iron to the autophagosome. Ferritin trafficking is based on NCOA4, and the depletion of NCOA4 significantly alleviates the bacterial load (Ryu et al., 2018). Autophagy inhibitors and iron chelators could weaken bacterial infection and relieve host cell death, implicating a potential therapeutic approach in certain bacterial infections by modulating NCOA4-dependent ferritinophagy (Pullarkat et al., 2014). Human cytomegalovirus (HCMV) protein pUL38 inhibits the function of USP24 to protect against iron-dependent, stress-induced premature death of the endoplasmic reticulum (ER) (Sun et al., 2018). USP24 deubiquitinase activity promotes NCOA4 stabilization and subsequently induces ferritinophagy, leading to increased cellular iron levels and iron-dependent premature cell death (Santana-Codina and Mancias, 2018).
In summary, pUL38 negatively modulates ferritinophagy to prevent premature death of HCMV-infected cells by regulating USP24 activity (Savaryn et al., 2013). These studies revealed that NCOA4-mediated ferritinophagy may act as an effective strategy in combating certain infectious diseases.
Ferritinophagy plays a critical role in the induction of ferroptosis and promotes numerous pathological disorders in CVDs, including HF, cardiac hypertrophy, and cardiac I/R injury (Fang et al., 2023).
3.2.4. Ferritinophagy in nanoparticle-induced endothelial cytotoxicity
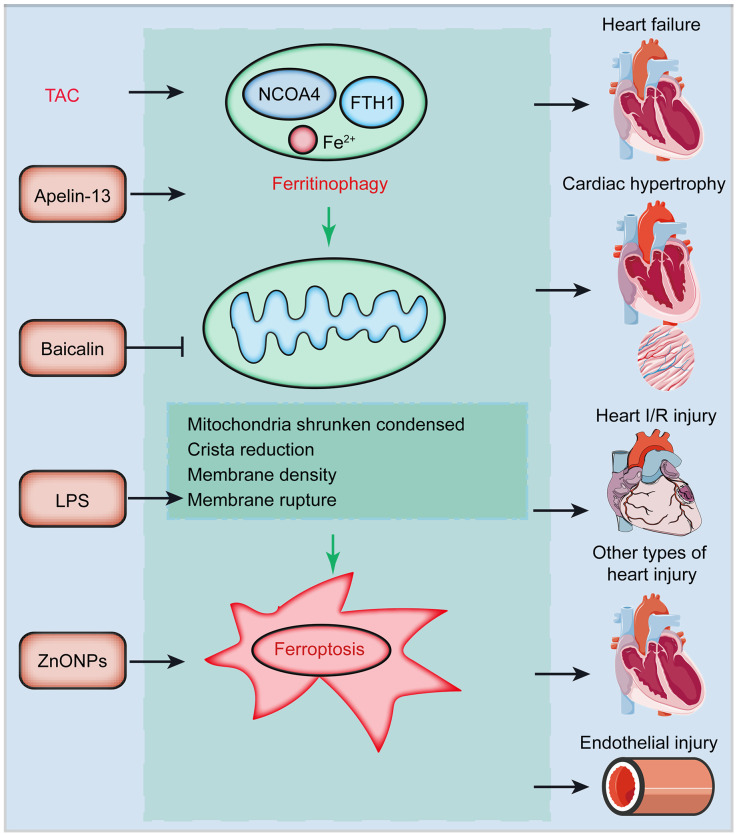
ZnO nanoparticles (ZnONPs) show great potential in mediating drug delivery and bioimaging because of their high biocompatibility and ease of production (Xiong, 2013). ZnONPs were also found to induce endothelial cytotoxicity via undetermined underlying mechanisms. Qin et al. (2020) discovered that ZnONPs lead to endothelial cell toxicity by promoting ferroptosis in vitro and in vivo(Qin et al., 2020). NCOA4 depletion attenuates ZnONP-induced lipid peroxidation and ferroptosis, indicating that ferritinophagy affects the progression of ZnONP-induced ferroptosis (Fig. 3) (Gryzik et al., 2021).
Fig. 3. Potential induction of ferritinophagy in CVDs. Ferritinophagy and its induced ferroptosis are involved in the pathogenesis of several CVDs. For instance, NCOA4-mediated ferritinophagy is activated in TAC-induced pressure overload failed heart tissues (Omiya et al., 2021). Apelin-13 induces ferritinophagy and cardiac hypertrophy (Yang et al., 2022). Administration of baicalin relieves ferritinophagy and injured cardiac function in heart I/R rats. LPS affects the mitochondrial shrinkage and condensation, induces crista reduction, mediates membrane density and rupture, and further induces ferroptosis. ZnONPs induce endothelial cell injury by promoting ferroptosis in vitro and in vivo (Qin X et al., 2021). CVDs: cardiovascular diseases; NCOA4: nuclear receptor coactivator 4; TAC: transverse aortic constriction; I/R: ischemia/reperfusion; ZnONPs: ZnO nanoparticles; FTH1: ferritin heavy chain 1; LPS: lipopolysaccharide.
3.2.5. Ferritinophagy and heart failure
NCOA4-mediated ferritinophagy is activated in failing hearts induced by transverse aortic constriction (TAC) (Santana-Codina and Mancias, 2018). NCOA4 depletion significantly relieves left ventricle chamber size, fibrosis, and cardiac function induced by pressure overload (Ito et al., 2021). Cardiac remodeling markers were dramatically reduced in TAC-induced NCOA4-depleted mice (Silva et al., 2021). Reduced FTH1 levels and enhanced ferritinophagy in NCOA4 +/+ hearts were rescued in NCOA4 -/- mice after TAC induction (Qin YH et al., 2021). Furthermore, isoproterenol-induced cardiomyocyte ferritinophagy depends on NCOA4 and an increased unstable iron pool (Zhuo et al., 2013). Therefore, NCOA4 mediates pressure overload-induced cardiac remodeling by regulating ferritinophagy.
3.2.6. Ferritinophagy and cardiac hypertrophy
Activation of the apelin/angiotensin receptor-like 1 (APJ) system is believed to initiate cardiac hypertrophy (Lu et al., 2017). Apelin-13/APJ induces the generation of ferritinophagy-associated free iron and ROS production in H9C2 cells (Tang et al., 2019). Moreover, apelin-13 promotes elevation of SFXN1, a mitochondrial protein that delivers iron to mitochondria, leading to mitochondrial iron overload and ferritinophagy, which finally aggravates cardiac hypertrophy (Fig. 3) (Tang et al., 2019).
3.2.7. Ferritinophagy and myocardial ischemia-reperfusion injury
Cardiac I/R injury and subsequent percutaneous coronary intervention are serious complications of acute MI, leading to severe cardiomyocyte death and impairment of cardiac function (Silvain et al., 2021). In myocardial I/R animal and cell models, ferroptosis was enhanced and NCOA4-mediated ferritinophagy was activated, but could be blocked by baicalin treatment (Fig. 3) (Gao et al., 2020).
3.2.8. Ferritinophagy in sepsis-induced cardiac injury
Septic cardiomyopathy is associated with high morbidity and mortality and is a common complication in sepsis patients (Hollenberg and Singer, 2021). Previous research indicated that ferritinophagy-induced ferroptosis is required to drive sepsis-induced cardiac injury. The ferroptosis marker cyclooxygenase-2 was increased and typical mitochondrial morphological alterations were observed in the hearts of LPS-induced septic mice (Li N et al., 2020). Mechanistically, LPS promotes NCOA4 expression and its binding with ferritin, and induces NCOA4-mediated ferritinophagy, resulting in the accumulation of free iron, mitochondrial ROS overload, and ferroptosis (Fig. 3) (Santana-Codina et al., 2021). Therefore, ferritinophagy and related ferroptosis might be promising targets for preventing sepsis-mediated cardiac injury.
4. Targeting ferritinophagy-induced ferroptosis modulators in cardiovascular diseases
Ferroptosis dysfunction has been implicated in multiple CVDs. Elucidation of the underlying mechanisms of ferroptosis may help combat ferroptosis-induced cardiovascular disorders. Here, we present a summary of promising ferroptosis- and ferritinophagy-associated modulators in CVDs.
4.1. Ferroptosis-associated compounds
Three main iron chelators, deferiprone, DFO, and deferasirox, treat iron overload-induced cardiomyopathy (Table 1) (Taher et al., 2016). Deferiprone is an iron chelator, which is cardioprotective in cardiac I/R injury. Oral deferiprone therapy alleviated myocardial iron accumulation and resulted in cardioprotective effect in patients treated with deferasirox or desferrioxamine in iron overload-correlated thalassemia (Fisher et al., 2013b). Deferoxamine, a ferroptosis inhibitor, was proved to alleviate HF and cardiac infarction (Jin et al., 2022). Dexazoxane, an ethylene diamine tetraacetic acid (EDTA) cyclic derivative, is the only approved iron-chelating drug for DIC that inhibits ferroptosis (Zhang et al., 2021). In the I/R model, Fer-1 treatment significantly abrogated cell death during I/R injury (Luo et al., 2022). Liproxstatin-1 (Lip-1) mitigates heart I/R injury by increasing GPX4 levels (Feng et al., 2019). Cyanidin-3-glucoside (C3G), an agent derived from purple or red vegetables and fruits, exhibits anti-inflammatory, antioxidant, and heart-protecting effects (Ding et al., 2006). C3G has also been proven to suppress ferroptosis in cardiomyocytes by reducing iron content and TfR1 expression and elevating FTH1 and GPX4 expression, ultimately protecting cardiac function. Resveratrol has also been found to function similarly (Ding et al., 2006).
Table 1.
Compounds modulating ferroptosis in cardiovascular diseases
| Compound | Mechanism | Model | Effect | Reference |
|---|---|---|---|---|
| DFO | Reduce iron overload | Mice | Alleviates cardiac infarction and mitigates HF | Jin et al., 2022 |
| Nanochelators | Mice | The same as DFO, but have fewer side-effects and rapid renal excretion | Kang et al., 2019 | |
| Desferrioxamine mesylate | Human | Improves LV ejection fraction | Fisher et al., 2013a | |
| Deferiprone | Human | Improves LV ejection fraction, and alleviates myocardial iron accumulation | Fisher et al., 2013b | |
| Dexazoxane | Human | Alleviates DIC | Zhang et al., 2021 | |
| Deferasirox | Human | Improves LV ejection fraction | Fisher et al., 2013b | |
| DXZ | Mice | Alleviates DIC by suppressing ferroptosis | Zhang et al., 2021 | |
| C3G | Rats | Protects from MIRI via ferroptosis | Ding et al., 2006 | |
| Rapamycin | Mice | Protects ferroptotic cardiomyocytes by targeting mTOR | Baba et al., 2018 | |
| Fer-1 | Reduce lipid ROS | Mice | Protects cardiomyocytes from ferroptotic damage | Fang et al., 2020 |
| Fer-1 | Mice | Promotes SLC7A11 and GPX4 expression | Bai et al., 2020 | |
| Fer-1 | Mice | Alleviates DIC | Shizukuda et al., 2005 | |
| Lip-1 | Mice | Protects from heart I/R injury by reducing VDAC1 levels and increasing GPX4 levels | Feng et al., 2019 | |
| Vitamin E | Mice | Protects from ferroptotic cell damage | Hinman et al., 2018 | |
| Zileuton | Mice | Protects from neurodegenerative disease by inhibiting 5-lipoxygenase | Liu Y et al., 2015 | |
| Mito-TEMPO | Mice | Inhibits DOX-related ferroptosis | Jiang et al., 2022 | |
| Puerarin | Rats/cell | Exerts cardioprotective effects in H9C2 myocytes and an HF rat model | Liu et al., 2018 |
DFO: deferoxamine; HF: heart failure; LV: left ventricular; DIC: DOX-induced cardiomyopathy; DXZ: dexrazoxane; C3G: cyanidin-3-glucoside; MIRI: myocardial ischemia-reperfusion injury; mTOR: mammalian target of rapamycin; Fer-1: ferrostatin-1; ROS: reactive oxygen species; SLC7A11: solute carrier family 7 member 11; GPX4: glutathione peroxidase 4; Lip-1: liproxstatin-1; I/R: ischemia/reperfusion; VDAC1: voltage-dependent anion channel 1; TEMPO: 2,2,6,6-tetramethylpiperidine-1-oxyl.
N-Acetylcysteine (NAC), a GSH donor, can accelerate antioxidant therapy (Dodd et al., 2008). HO-1 can degrade heme into ferrous iron. HO-1 overexpression can relieve hypertrophy, fibrosis, and oxidative stress in HF (Allwood et al., 2014). Fer-1 is protective in DOX-induced animals. Mito-2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO), a mitochondrial ROS scavenger, inhibits DOX-associated ferroptosis, and protects cardiomyocytes (Jiang et al., 2022). DXZ, an iron chelator, is protective in DIC by inhibiting ferroptosis (Zhang et al., 2021). mTOR can act as a target to protect cardiomyocytes from ferroptosis (Baba et al., 2018). Statins suppress the production of GPX4 and CoQ10, thereby promoting mesenchymal cell ferroptosis (Littarru and Langsjoen, 2007).
4.2. Exosomes
Recently, exosomes extracted from mesenchymal stem cells (MSCs) were found to exert cardioprotective effects by inhibiting ferroptosis (Song et al., 2021). Administration of human umbilical cord blood (HUCB)-MSC-derived exosomes significantly attenuated the increased level of DMT1 and myocardial injury (Song et al., 2021). Protective effects of HUCB-MSC-derived exosomes were dependent onmiR-23a-3p.
4.3. Puerarin
Targeting lipid peroxidation exerts cardioprotective effects in CVDs (Gianazza et al., 2021). Administration of puerarin suppresses iron-dependent lipid peroxidation and prevents cardiomyocyte loss, indicating that puerarin may offer a promising approach to combat HF (Liu et al., 2018).
4.4. Nanomaterials
Nanoparticles (NPs) are considered highly potent drug-delivery materials (Qi et al., 2017). Resveratrol-NPs protect against erastin-induced ferroptosis and prevent intracerebral hemorrhage (Mo et al., 2021). Given the function of NCOA4 in ferritinophagy-associated ferroptosis, it is likely that NP-mediated ferritinophagy and ferroptosis might act as potential therapies against CVDs. Due to the risk of ferroptosis-induced cardiac damage, additional studies are needed.
5. Conclusions
Ferroptosis differs from other cell death types in terms of morphology and mechanism. Increasingly, studies report that ferroptosis is a key factor in CVDs. Currently, there are many shortcomings to be overcome. The first is exploration of the mechanism of ferroptosis. Ferroptosis is involved in multiple signaling pathways. However, identifying the precise mechanism or coordination between various mechanisms, including ferroptosis and autophagy, remains challenging. The second is the development of drugs acting on the process of ferroptosis in cells. Ferritinophagy is related to a newly discovered form of cell death, ferroptosis, and plays an essential regulatory role in CVDs. While there have been some studies on the treatment of cancer, there has been less research in other areas. Therefore, exploring the mechanism of ferroptosis will provide a sufficient basis for clinical diagnosis and prevention.
In the future, cell morphology will be observed directly by transmission electron microscopy (TEM) to monitor the occurrence of ferroptosis (Graham and Orenstein, 2007). During ferroptosis, iron overload occurs in cells, and detecting significantly increased iron levels can be a key indicator for monitoring ferroptosis. The occurrence of ferroptosis can be used as a biomarker of CVDs and provides critical information for their prevention and diagnosis. In the future, these biomarkers could be used as early monitoring indicators for various CVDs.
Ferritinophagy and ferroptosis are related to the development of most CNS diseases (Qin YH et al., 2021). Inhibition of ferritinophagy and ferroptosis can reduce the damage to myocardial cells and increase the recovery of the function of myocardial cells and endothelial cells, suggesting that ferritinophagy and ferroptosis mediate the damage to cells. However, the regulatory mechanism by which ferritinophagy and ferroptosis mediate the damage to myocardial cells needs further study. The initiation, signal transduction, and specificity of ferritinophagy and ferroptosis to cell damage are not yet fully understood. Further research on the molecular mechanisms and pathways of ferritinophagy and ferroptosis in CVDs is expected to provide a new theoretical basis for future targeted treatment of ferritinophagy- and ferroptosis-related CVDs. Targeting ferritinophagy and ferroptosis and its critical regulatory molecules for the treatment of CVDs requires more animal and preclinical studies to evaluate its therapeutic efficacy.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 82204389 and 82000454), the Medical Science and Technology Research Project of Henan Province (No. SBGJ202103079), and the Henan Medical Science and Technology Joint Building Program (Nos. LHGJ20230283, LHGJ20190236, LHGJ20190227, LHGJ20190092, LHGJ20200310, and LHGJ20200284), China. We thank the Home for Researchers (https://www.home-for-researchers.com) for their language modification service.
Author contributions
Yi LUAN contributed to the conception of the study. Yi LUAN and Yang YANG contributed significantly to data analysis and manuscript preparation. Yang YANG, Kaidi REN, Bo QIN, and Hengdao LIU wrote the manuscript. Kaidi REN, Bo QIN, Hengdao LIU, Yi LUAN, and Yang YANG provided ideas and financial support for the review. Ying LUAN, Hui LIU, Han XING, and Jinyan PEI helped perform the analysis with constructive discussion and provided substantive guidance on the paper’s ideas and the entire writing process. All authors have read and approved the final manuscript.
Compliance with ethics guidelines
Yi LUAN, Yang YANG, Ying LUAN, Hui LIU, Han XING, Jinyan PEI, Hengdao LIU, Bo QIN, and Kaidi REN declare that they have no conflict of interest.
This review does not contain any studies with human or animal subjects performed by any of the authors.
References
- Ajoolabady A, Aslkhodapasandhokmabad H, Libby P, et al. , 2021. Ferritinophagy and ferroptosis in the management of metabolic diseases. Trends Endocrinol Metab, 32(7): 444-462. 10.1016/j.tem.2021.04.010 [DOI] [PubMed] [Google Scholar]
- Aladağ N, Asoğlu R, Ozdemir M, et al. , 2021. Oxidants and antioxidants in myocardial infarction (MI): investigation of ischemia modified albumin, malondialdehyde, superoxide dismutase and catalase in individuals diagnosed with ST elevated myocardial infarction (STEMI) and non-STEMI (NSTEMI). J Med Biochem, 40(3): 286-294. 10.5937/jomb0-28879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allwood MA, Kinobe RT, Ballantyne L, et al. , 2014. Heme oxygenase-1 overexpression exacerbates heart failure with aging and pressure overload but is protective against isoproterenol-induced cardiomyopathy in mice. Cardiovasc Pathol, 23(4): 231-237. 10.1016/j.carpath.2014.03.007 [DOI] [PubMed] [Google Scholar]
- Baba Y, Higa JK, Shimada BK, et al. , 2018. Protective effects of the mechanistic target of rapamycin against excess iron and ferroptosis in cardiomyocytes. Am J Physiol Heart Circ Physiol, 314(3): H659-H668. 10.1152/ajpheart.00452.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai T, Li MX, Liu YF, et al. , 2020. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radic Biol Med, 160: 92-102. 10.1016/j.freeradbiomed.2020.07.026 [DOI] [PubMed] [Google Scholar]
- Bai YT, Chang R, Wang H, et al. , 2018. ENPP2 protects cardiomyocytes from erastin-induced ferroptosis. Biochem Biophys Res Commun, 499(1): 44-51. 10.1016/j.bbrc.2018.03.113 [DOI] [PubMed] [Google Scholar]
- Banjac A, Perisic T, Sato H, et al. , 2008. The cystine/cysteine cycle: a redox cycle regulating susceptibility versus resistance to cell death. Oncogene, 27(11): 1618-1628. 10.1038/sj.onc.1210796 [DOI] [PubMed] [Google Scholar]
- Bauckman KA, Mysorekar IU, 2016. Ferritinophagy drives uropathogenic Escherichia coli persistence in bladder epithelial cells. Autophagy, 12(5): 850-863. 10.1080/15548627.2016.1160176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont JL, Beaumont V, Lenegre J, 1958. Research on lipid metabolism in human atherosclerosis. II. Multiple aspects of blood lipids in angina pectoris. Rev Fr Etud Clin Biol, 3(8): 852-868. [PubMed] [Google Scholar]
- Bersuker K, Hendricks JM, Li ZP, et al. , 2019. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature, 575(7784): 688-692. 10.1038/s41586-019-1705-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XQ, Xu SD, Zhao CX, et al. , 2019. Role of TLR4/NADPH oxidase 4 pathway in promoting cell death through autophagy and ferroptosis during heart failure. Biochem Biophys Res Commun, 516(1): 37-43. 10.1016/j.bbrc.2019.06.015 [DOI] [PubMed] [Google Scholar]
- Chua ACG, Graham RM, Trinder D, et al. , 2007. The regulation of cellular iron metabolism. Crit Rev Clin Lab Sci, 44(5-6): 413-459. 10.1080/10408360701428257 [DOI] [PubMed] [Google Scholar]
- Clarke SL, Vasanthakumar A, Anderson SA, et al. , 2006. Iron-responsive degradation of iron-regulatory protein 1 does not require the Fe–S cluster. EMBO J, 25(3): 544-553. 10.1038/sj.emboj.7600954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradi M, Mutti A, 2011. Metal ions affecting the pulmonary and cardiovascular systems. Met Ions Life Sci, 8: 81-105. [PubMed] [Google Scholar]
- del Rey MQ, Mancias JD, 2019. NCOA4-mediated ferritinophagy: a potential link to neurodegeneration. Front Neurosci, 13: 238. 10.3389/fnins.2019.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding M, Feng RT, Wang SY, et al. , 2006. Cyanidin-3-glucoside, a natural product derived from blackberry, exhibits chemopreventive and chemotherapeutic activity. J Biol Chem, 281(25): 17359-17368. 10.1074/jbc.M600861200 [DOI] [PubMed] [Google Scholar]
- Dodd S, Dean O, Copolov DL, et al. , 2008. N-Acetylcysteine for antioxidant therapy: pharmacology and clinical utility. Expert Opin Biol Ther, 8(12): 1955-1962. 10.1517/14728220802517901 [DOI] [PubMed] [Google Scholar]
- Doll S, Freitas FP, Shah R, et al. , 2019. FSP1 is a glutathione-independent ferroptosis suppressor. Nature, 575(7784): 693-698. 10.1038/s41586-019-1707-0 [DOI] [PubMed] [Google Scholar]
- Drysdale J, Arosio P, Invernizzi R, et al. , 2002. Mitochondrial ferritin: a new player in iron metabolism. Blood Cells Mol Dis, 29(3): 376-383. 10.1006/bcmd.2002.0577 [DOI] [PubMed] [Google Scholar]
- Erber LN, Luo A, Gong Y, et al. , 2021. Iron deficiency reprograms phosphorylation signaling and reduces O-GlcNAc pathways in neuronal cells. Nutrients, 13(1): 179. 10.3390/nu13010179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang XX, Wang H, Han D, et al. , 2019. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci USA, 116(7): 2672-2680. 10.1073/pnas.1821022116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang XX, Cai ZX, Wang H, et al. , 2020. Loss of cardiac ferritin H facilitates cardiomyopathy via Slc7a11-mediated ferroptosis. Circ Res, 127(4): 486-501. 10.1161/CIRCRESAHA.120.316509 [DOI] [PubMed] [Google Scholar]
- Fang XX, Ardehali H, Min JX, et al. , 2023. The molecular and metabolic landscape of ironand ferroptosis in cardiovascular disease. Nat Rev Cardiol, 20: 7-23. 10.1038/s41569-022-00735-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng YS, Madungwe NB, Aliagan ADI, et al. , 2019. Liproxstatin-1 protects the mouse myocardium against ischemia/reperfusion injury by decreasing VDAC1 levels and restoring GPX4 levels. Biochem Biophys Res Commun, 520(3): 606-611. 10.1016/j.bbrc.2019.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SA, Brunskill SJ, Doree C, et al. , 2013a. Desferrioxamine mesylate for managing transfusional iron overload in people with transfusion-dependent thalassaemia. Cochrane Database Syst Rev, (8): CD004450. 10.1002/14651858.CD004450.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SA, Brunskill SJ, Doree C, et al. , 2013b. Oral deferiprone for iron chelation in people with thalassaemia. Cochrane Database Syst Rev, (8): CD004839. 10.1002/14651858.CD004839.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MD, 2008. The regulation of hepcidin and its effects on systemic and cellular iron metabolism. Hematology Am Soc Hematol Educ Program, 2008(1): 151-158. 10.1182/asheducation-2008.1.151 [DOI] [PubMed] [Google Scholar]
- Fox NG, Das D, Chakrabarti M, et al. , 2015. Frataxin accelerates [2Fe-2S] cluster formation on the human Fe–S assembly complex. Biochemistry, 54(25): 3880-3889. 10.1021/bi5014497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann DC, Mondorf A, Beifuß J, et al. , 2020. Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis. Redox Biol, 36: 101670. 10.1016/j.redox.2020.101670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimaki M, Furuya N, Saiki S, et al. , 2019. Iron supply via NCOA4-mediated ferritin degradation maintains mitochondrial functions. Mol Cell Biol, 39(14): e00010-19. 10.1128/MCB.00010-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan BY, 2021. Mitochondrial regulation of ferroptosis. J Cell Biol, 220(9): e202105043. 10.1083/jcb.202105043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan BY, 2022. ACSL4, PUFA, and ferroptosis: new arsenal in anti-tumor immunity. Signal Transduct Target Ther, 7: 128. 10.1038/s41392-022-01004-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao GF, Chang YZ, 2014. Mitochondrial ferritin in the regulation of brain iron homeostasis and neurodegenerative diseases. Front Pharmacol, 5: 19. 10.3389/fphar.2014.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao JY, Zhou QL, Wu D, et al. , 2021. Mitochondrial iron metabolism and its role in diseases. Clin Chim Acta, 513: 6-12. 10.1016/j.cca.2020.12.005 [DOI] [PubMed] [Google Scholar]
- Gao Z, Gao Q, Lv XD, 2020. MicroRNA-668-3p protects against oxygen-glucose deprivation in a rat H9c2 cardiomyocyte model of ischemia-reperfusion injury by targeting the stromal cell-derived factor-1 (SDF-1)/CXCR4 signaling pathway. Med Sci Monit, 26: e919601. 10.12659/MSM.919601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianazza E, Brioschi M, Fernandez AM, et al. , 2021. Lipid peroxidation in atherosclerotic cardiovascular diseases. Antioxid Redox Signal, 34(1): 49-98. 10.1089/ars.2019.7955 [DOI] [PubMed] [Google Scholar]
- Graham L, Orenstein JM, 2007. Processing tissue and cells for transmission electron microscopy in diagnostic pathology and research. Nat Protoc, 2(10): 2439-2450. 10.1038/nprot.2007.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryzik M, Asperti M, Denardo A, et al. , 2021. NCOA4-mediated ferritinophagy promotes ferroptosis induced by erastin, but not by RSL3 in HeLa cells. Biochim Biophys Acta Mol Cell Res, 1868(2): 118913. 10.1016/j.bbamcr.2020.118913 [DOI] [PubMed] [Google Scholar]
- Gu CZ, Chang WJ, Wu JL, et al. , 2022. NCOA4: an immunomodulation-related prognostic biomarker in colon adenocarcinoma and pan-cancer. J Oncol, 2022: 5242437. 10.1155/2022/5242437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JD, Zhao X, Li Y, et al. , 2018. Damage to dopaminergic neurons by oxidative stress in Parkinson’s disease (Review). Int J Mol Med, 41(4): 1817-1825. 10.3892/ijmm.2018.3406 [DOI] [PubMed] [Google Scholar]
- Guo ZM, Ran QT, Roberts LJ II, et al. , 2008. Suppression of atherogenesis by overexpression of glutathione peroxidase-4 in apolipoprotein E-deficient mice. Free Radic Biol Med, 44(3): 343-352. 10.1016/j.freeradbiomed.2007.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Liu YY, Dai RJ, et al. , 2020. Ferroptosis and its potential role in human diseases. Front Pharmacol, 11: 239. 10.3389/fphar.2020.00239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JW, Kang C, Kim Y, et al. , 2020. Isoproterenol-induced hypertrophy of neonatal cardiac myocytes and H9c2 cell is dependent on TRPC3-regulated Cav1.2 expression. Cell Calcium, 92: 102305. 10.1016/j.ceca.2020.102305 [DOI] [PubMed] [Google Scholar]
- Han XJ, Zhang J, Liu J, et al. , 2023. Targeting ferroptosis: a novel insight against myocardial infarction and ischemia-reperfusion injuries. Apoptosis, 28: 108-123. 10.1007/s10495-022-01785-2 [DOI] [PubMed] [Google Scholar]
- Henninger C, Fritz G, 2018. Statins in anthracycline-induced cardiotoxicity: Rac and Rho, and the heartbreakers. Cell Death Dis, 8: e2564. 10.1038/cddis.2016.418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman A, Holst CR, Latham JC, et al. , 2018. Vitamin E hydroquinone is an endogenous regulator of ferroptosis via redox control of 15-lipoxygenase. PLoS ONE, 13(8): e0201369. 10.1371/journal.pone.0201369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg SM, Singer M, 2021. Pathophysiology of sepsis-induced cardiomyopathy. Nat Rev Cardiol, 18(6): 424-434. 10.1038/s41569-020-00492-2 [DOI] [PubMed] [Google Scholar]
- Hou W, Xie YC, Song XX, et al. , 2016. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy, 12(8): 1425-1428. 10.1080/15548627.2016.1187366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TF, Sun YJ, Li YL, et al. , 2018. Growth inhibition of a novel iron chelator, DpdtC, against hepatoma carcinoma cell lines partly attributed to ferritinophagy-mediated lysosomal ROS generation. Oxid Med Cell Longev, 2018: 4928703. 10.1155/2018/4928703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai H, Matsuoka M, Kumagai T, et al. , 2017. Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. Curr Top Microbiol Immunol, 403: 143-170. 10.1007/82_2016_508 [DOI] [PubMed] [Google Scholar]
- Ito J, Omiya S, Rusu MC, et al. , 2021. Iron derived from autophagy-mediated ferritin degradation induces cardiomyocyte death and heart failure in mice. eLife, 10: e62174. 10.7554/eLife.62174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadov S, 2022. Mitochondria and ferroptosis. Curr Opin Physiol, 25: 100483. 10.1016/j.cophys.2022.100483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JJ, Zhang GF, Zheng JY, et al. , 2022. Targeting mitochondrial ROS-mediated ferroptosis by quercetin alleviates high-fat diet-induced hepatic lipotoxicity. Front Pharmacol, 13: 876550. 10.3389/fphar.2022.876550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang XJ, Stockwell BR, Conrad M, 2021. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol, 22(4): 266-282. 10.1038/s41580-020-00324-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, He Q, Cheng C, et al. , 2022. UAMC-3203 or/and deferoxamine improve post-resuscitation myocardial dysfunction through suppressing ferroptosis in a rat model of cardiac arrest. Shock, 57(3): 344-350. 10.1097/SHK.0000000000001869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MD, Schwarz RD, St. Pierre L, et al. , 1999. Inhibitors of V-type ATPases, bafilomycin A1 and concanamycin A, protect against β-amyloid-mediated effects on 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem, 72(5): 1939-1947. 10.1046/j.1471-4159.1999.0721939.x [DOI] [PubMed] [Google Scholar]
- Kang H, Han MR, Xue J, et al. , 2019. Renal clearable nanochelators for iron overload therapy. Nat Commun, 10: 5134. 10.1038/s41467-019-13143-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitakata H, Endo J, Ikura H, et al. , 2022. Therapeutic targets for DOX-induced cardiomyopathy: role of apoptosis vs. ferroptosis. Int J Mol Sci, 23(3): 1414. 10.3390/ijms23031414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleini N, Shapiro JS, Geier J, et al. , 2021. Ironing out mechanisms of iron homeostasis and disorders of iron deficiency. J Clin Invest, 131(11): e148671. 10.1172/JCI148671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollara A, Brown TJ, 2012. Expression and function of nuclear receptor co-activator 4: evidence of a potential role independent of co-activator activity. Cell Mol Life Sci, 69(23): 3895-3909. 10.1007/s00018-012-1000-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopinski PK, Singh LN, Zhang SP, et al. , 2021. Mitochondrial DNA variation and cancer. Nat Rev Cancer, 21(7): 431-445. 10.1038/s41568-021-00358-w [DOI] [PubMed] [Google Scholar]
- Lai YF, Dong J, Wu Y, et al. , 2022. Lipid peroxides mediated ferroptosis in electromagnetic pulse-induced hippocampal neuronal damage via inhibition of GSH/GPX4 axis. Int J Mol Sci, 23(16): 9277. 10.3390/ijms23169277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhal-Littleton S, 2019. Iron deficiency as a therapeutic target in cardiovascular disease. Pharmaceuticals, 12(3): 125. 10.3390/ph12030125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan HY, Gao Y, Zhao ZY, et al. , 2022. Ferroptosis: redox imbalance and hematological tumorigenesis. Front Oncol, 12: 834681. 10.3389/fonc.2022.834681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HL, Hee SW, Hsuan CF, et al. , 2021. A novel ALDH2 activator AD-9308 improves diastolic and systolic myocardial functions in streptozotocin-induced diabetic mice. Antioxidants, 10(3): 450. 10.3390/antiox10030450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Chiang KC, Feng TH, et al. , 2016. The iron chelator, Dp44mT, effectively inhibits human oral squamous cell carcinoma cell growth in vitro and in vivo. Int J Mol Sci, 17(9): 1435. 10.3390/ijms17091435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Kim WK, Bae KH, et al. , 2021. Lipid metabolism and ferroptosis. Biology, 10(3): 184. 10.3390/biology10030184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Lee DH, Choudry HA, et al. , 2018. Ferroptosis-induced endoplasmic reticulum stress: cross-talk between ferroptosis and apoptosis. Mol Cancer Res, 16(7): 1073-1076. 10.1158/1541-7786.MCR-18-0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei G, Zhuang L, Gan BY, 2022. Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer, 22(7): 381-396. 10.1038/s41568-022-00459-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng YL, Luo X, Yu JY, et al. , 2022. Ferroptosis: a potential target in cardiovascular disease. Front Cell Dev Biol, 9: 813668. 10.3389/fcell.2021.813668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Sun GC, Chen BL, et al. , 2021. Nuclear receptor coactivator 4-mediated ferritinophagy contributes to cerebral ischemia-induced ferroptosis in ischemic stroke. Pharmacol Res, 174: 105933. 10.1016/j.phrs.2021.105933 [DOI] [PubMed] [Google Scholar]
- Li C, Wu ZY, Xue H, et al. , 2022. Ferroptosis contributes to hypoxic-ischemic brain injury in neonatal rats: role of the SIRT1/Nrf2/GPx4 signaling pathway. CNS Neurosci Ther, 28(12): 2268-2280. 10.1111/cns.13973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FJ, Long HZ, Zhou ZW, et al. , 2022. System Xc-/GSH/GPX4 axis: an important antioxidant system for the ferroptosis in drug-resistant solid tumor therapy. Front Pharmacol, 13: 910292. 10.3389/fphar.2022.910292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Wang W, Zhou H, et al. , 2020. Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radic Biol Med, 160: 303-318. 10.1016/j.freeradbiomed.2020.08.009 [DOI] [PubMed] [Google Scholar]
- Li N, Jiang WY, Wang W, et al. , 2021. Ferroptosis and its emerging roles in cardiovascular diseases. Pharmacol Res, 166: 105466. 10.1016/j.phrs.2021.105466 [DOI] [PubMed] [Google Scholar]
- Li Q, Han XN, Lan X, et al. , 2017. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight, 2(7): e90777. 10.1172/jci.insight.90777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SY, Wang R, Wang YX, et al. , 2022. Ferroptosis: a new insight for treatment of acute kidney injury. Front Pharmacol, 13: 1065867. 10.3389/fphar.2022.1065867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SZ, Zhang XY, 2021. Iron in cardiovascular disease: challenges and potentials. Front Cardiovasc Med, 8: 707138. 10.3389/fcvm.2021.707138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XQ, Lozovatsky L, Sukumaran A, et al. , 2020. NCOA4 is regulated by HIF and mediates mobilization of murine hepatic iron stores after blood loss. Blood, 136(23): 2691-2702. 10.1182/blood.2020006321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MM, Liu N, Qin ZH, et al. , 2022. Mitochondrial-derived damage-associated molecular patterns amplify neuroinflammation in neurodegenerative diseases. Acta Pharmacol Sin, 43(10): 2439-2447. 10.1038/s41401-022-00879-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PL, Tang HH, Wu SY, et al. , 2020. Saponin formosanin C-induced ferritinophagy and ferroptosis in human hepatocellular carcinoma cells. Antioxidants, 9(8): 682. 10.3390/antiox9080682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littarru GP, Langsjoen P, 2007. Coenzyme Q10 and statins: biochemical and clinical implications. Mitochondrion, 7: S168-S174. 10.1016/j.mito.2007.03.002 [DOI] [PubMed] [Google Scholar]
- Liu B, Zhao CX, Li HK, et al. , 2018. Puerarin protects against heart failure induced by pressure overload through mitigation of ferroptosis. Biochem Biophys Res Commun, 497(1): 233-240. 10.1016/j.bbrc.2018.02.061 [DOI] [PubMed] [Google Scholar]
- Liu JP, Cen SY, Xue ZA, et al. , 2022. A class of disulfide compounds suppresses ferroptosis by stabilizing GPX4. ACS Chem Biol, 17(12): 3389-3406. 10.1021/acschembio.2c00445 [DOI] [PubMed] [Google Scholar]
- Liu M, Kong XY, Yao Y, et al. , 2022. The critical role and molecular mechanisms of ferroptosis in antioxidant systems: a narrative review. Ann Transl Med, 10(6): 368. 10.21037/atm-21-6942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MR, Zhu WT, Pei DS, 2021. System Xc-: a key regulatory target of ferroptosis in cancer. Invest New Drugs, 39(4): 1123-1131. 10.1007/s10637-021-01070-0 [DOI] [PubMed] [Google Scholar]
- Liu MZ, Kong N, Zhang GY, et al. , 2022. The critical role of ferritinophagy in human disease. Front Pharmacol, 13: 933732. 10.3389/fphar.2022.933732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PF, Feng YT, Li HW, et al. , 2020. Ferrostatin-1 alleviates lipopolysaccharide-induced acute lung injury via inhibiting ferroptosis. Cell Mol Biol Lett, 25: 10. 10.1186/s11658-020-00205-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Zhi XY, Zhong Q, 2015. ATG14 controls SNARE-mediated autophagosome fusion with a lysosome. Autophagy, 11(5): 847-849. 10.1080/15548627.2015.1037549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Du SW, Wang SD, et al. , 2022. Ferroptosis in osteosarcoma: a promising future. Front Oncol, 12: 1031779. 10.3389/fonc.2022.1031779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XM, Li DL, Pi WH, et al. , 2022. LCZ696 protects against doxorubicin-induced cardiotoxicity by inhibiting ferroptosis via AKT/SIRT3/SOD2 signaling pathway activation. Int Immunopharmacol, 113: 109379. 10.1016/j.intimp.2022.109379 [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang W, Li YY, et al. , 2015. The 5-lipoxygenase inhibitor zileuton confers neuroprotection against glutamate oxidative damage by inhibiting ferroptosis. Biol Pharm Bull, 38(8): 1234-1239. 10.1248/bpb.b15-00048 [DOI] [PubMed] [Google Scholar]
- Liu YC, Zeng LP, Yang Y, et al. , 2020. Acyl-CoA thioesterase 1 prevents cardiomyocytes from Doxorubicin-induced ferroptosis via shaping the lipid composition. Cell Death Dis, 11(9): 756. 10.1038/s41419-020-02948-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LQ, Wu D, Li LF, et al. , 2017. Apelin/APJ system: a bifunctional target for cardiac hypertrophy. Int J Cardiol, 230: 164-170. 10.1016/j.ijcard.2016.11.215 [DOI] [PubMed] [Google Scholar]
- Lu LQ, Tian J, Luo XJ, et al. , 2021. Targeting the pathways of regulated necrosis: a potential strategy for alleviation of cardio-cerebrovascular injury. Cell Mol Life Sci, 78: 63-78. 10.1007/s00018-020-03587-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan Y, Luan Y, Feng Q, et al. , 2021a. Emerging role of mitophagy in the heart: therapeutic potentials to modulate mitophagy in cardiac diseases. Oxid Med Cell Longev, 2021: 3259963. 10.1155/2021/3259963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan Y, Ren KD, Luan Y, et al. , 2021b. Mitochondrial dynamics: pathogenesis and therapeutic targets of vascular diseases. Front Cardiovasc Med, 8: 770574. 10.3389/fcvm.2021.770574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan Y, Luan Y, Yuan RX, et al. , 2021c. Structure and function of mitochondria-associated endoplasmic reticulum membranes (MAMs) and their role in cardiovascular diseases. Oxid Med Cell Longev, 2021: 4578809. 10.1155/2021/4578809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Apaijai N, Liao SC, et al. , 2022. Therapeutic potentials of cell death inhibitors in rats with cardiac ischaemia/reperfusion injury. J Cell Mol Med, 26(8): 2462-2476. 10.1111/jcmm.17275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv XH, Jiang HH, Li BG, et al. , 2014. The crucial role of Atg5 in cortical neurogenesis during early brain development. Sci Rep, 4: 6010. 10.1038/srep06010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv YH, Wu MY, Wang Z, et al. , 2022. Ferroptosis: from regulation of lipid peroxidation to the treatment of diseases. Cell Biol Toxicol, 39: 827-851. 10.1007/s10565-022-09778-2 [DOI] [PubMed] [Google Scholar]
- Ma TY, Du JT, Zhang YF, et al. , 2022. GPX4-independent ferroptosis—a new strategy in disease’s therapy. Cell Death Discov, 8: 434. 10.1038/s41420-022-01212-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maine MD, 1979. Role of trace metals in regulation of cellular heme and hemoprotein metabolism: sensitizing effects of chronic iron treatment on acute gold toxicity. Drug Metab Rev, 9(2): 237-255. 10.3109/03602537908993893 [DOI] [PubMed] [Google Scholar]
- Mancias JD, Vaites LP, Nissim S, et al. , 2015. Ferritinophagy via NCOA4 is required for erythropoiesis and is regulated by iron dependent HERC2-mediated proteolysis. Elife, 4: e10308. 10.7554/eLife.10308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C, Liu XG, Zhang YL, et al. , 2021. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature, 593(7860): 586-590. 10.1038/s41586-021-03539-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng ZJ, Liang HP, Zhao JL, et al. , 2021. HMOX1 upregulation promotes ferroptosis in diabetic atherosclerosis. Life Sci, 284: 119935. 10.1016/j.lfs.2021.119935 [DOI] [PubMed] [Google Scholar]
- Minakaki G, Menges S, Kittel A, et al. , 2018. Autophagy inhibition promotes SNCA/alpha-synuclein release and transfer via extracellular vesicles with a hybrid autophagosome-exosome-like phenotype. Autophagy, 14(1): 98-119. 10.1080/15548627.2017.1395992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima E, Ito J, Wu ZJ, et al. , 2022. A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature, 608(7924): 778-783. 10.1038/s41586-022-05022-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo YS, Duan LN, Yang YN, et al. , 2021. Nanoparticles improved resveratrol brain delivery and its therapeutic efficacy against intracerebral hemorrhage. Nanoscale, 13(6): 3827-3840. 10.1039/d0nr06249a [DOI] [PubMed] [Google Scholar]
- Mohanty SK, Donnelly B, Temple H, et al. , 2021. High mobility group box 1 release by cholangiocytes governs biliary atresia pathogenesis and correlates with increases in afflicted infants. Hepatology, 74(2): 864-878. 10.1002/hep.31745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omiya S, Ito J, Otsu L, 2021. Labile iron derived from autophagy-mediated ferritin degradation in cardiomyocytes under pressure overload increases myocardial oxidative stress and develops heart failure in mice. Eur Heart J, 42(Suppl_1): ehab724.0747. 10.1093/eurheartj/ehab724.0747 [DOI] [Google Scholar]
- Park E, Chung SW, 2019. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis, 10(11): 822. 10.1038/s41419-019-2064-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TJ, Park JH, Lee GS, et al. , 2019. Quantitative proteomic analyses reveal that GPX4 downregulation during myocardial infarction contributes to ferroptosis in cardiomyocytes. Cell Death Dis, 10: 835. 10.1038/s41419-019-2061-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce E, Sondo E, Ferrera L, et al. , 2018. The autophagy inhibitor spautin-1 antagonizes rescue of mutant CFTR through an autophagy-independent and USP13-mediated mechanism. Front Pharmacol, 9: 1464. 10.3389/fphar.2018.01464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyssonnaux C, Zinkernagel AS, Schuepbach RA, et al. , 2007. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs). J Clin Invest, 117(7): 1926-1932. 10.1172/JCI31370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott CC, 2020. Iron on the move: mobilizing liver iron via NCOA4. Blood, 136(23): 2604-2605. 10.1182/blood.2020007971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad MK, Mohandas S, Mohanram RK, 2023. Role of ferroptosis inhibitors in the management of diabetes. BioFactors, 49(2): 270-296. 10.1002/biof.1920 [DOI] [PubMed] [Google Scholar]
- Pridham KJ, Varghese RT, Sheng Z, 2017. The role of class IA phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunits in glioblastoma. Front Oncol, 7: 312. 10.3389/fonc.2017.00312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protchenko O, Baratz E, Jadhav S, et al. , 2021. Iron chaperone poly rC binding protein 1 protects mouse liver from lipid peroxidation and steatosis. Hepatology, 73(3): 1176-1193. 10.1002/hep.31328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig S, Ramos-Alonso L, Romero AM, et al. , 2017. The elemental role of iron in DNA synthesis and repair. Metallomics, 9(11): 1483-1500. 10.1039/c7mt00116a [DOI] [PubMed] [Google Scholar]
- Pullarkat V, Meng Z, Donohue C, et al. , 2014. Iron chelators induce autophagic cell death in multiple myeloma cells. Leuk Res, 38(8): 988-996. 10.1016/j.leukres.2014.06.005 [DOI] [PubMed] [Google Scholar]
- Qi RG, Wang YH, Bruno PM, et al. , 2017. Nanoparticle conjugates of a highly potent toxin enhance safety and circumvent platinum resistance in ovarian cancer. Nat Commun, 8: 2166. 10.1038/s41467-017-02390-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Tang QH, Jiang XJ, et al. , 2020. Zinc oxide nanoparticles induce ferroptotic neuronal cell death in vitro and in vivo. Int J Nanomedicine, 15: 5299-5315. 10.2147/IJN.S250367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Zhang J, Wang B, et al. , 2021. Ferritinophagy is involved in the zinc oxide nanoparticles-induced ferroptosis of vascular endothelial cells. Autophagy, 17(12): 4266-4285. 10.1080/15548627.2021.1911016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin YH, Qiao Y, Wang D, et al. , 2021. Ferritinophagy and ferroptosis in cardiovascular disease: mechanisms and potential applications. Biomed Pharmacother, 141: 111872. 10.1016/j.biopha.2021.111872 [DOI] [PubMed] [Google Scholar]
- Reeves AR, Sansbury BE, Pan MX, et al. , 2021. Myeloid-specific deficiency of long-chain acyl CoA synthetase 4 reduces inflammation by remodeling phospholipids and reducing production of arachidonic acid-derived proinflammatory lipid mediators. J Immunol, 207(11): 2744-2753. 10.4049/jimmunol.2100393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren YF, Yang MW, Wang XD, et al. , 2022. ELAV-like RNA binding protein 1 regulates osteogenesis in diabetic osteoporosis: involvement of divalent metal transporter 1. Mol Cell Endocrinol, 546: 111559. 10.1016/j.mce.2022.111559 [DOI] [PubMed] [Google Scholar]
- Rockfield S, Flores I, Nanjundan M, 2018. Expression and function of nuclear receptor coactivator 4 isoforms in transformed endometriotic and malignant ovarian cells. Oncotarget, 9(4): 5344-5367. 10.18632/oncotarget.23747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu MS, Duck KA, Philpott CC, 2018. Ferritin iron regulators, PCBP1 and NCOA4, respond to cellular iron status in developing red cells. Blood Cells Mol Dis, 69: 75-81. 10.1016/j.bcmd.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana-Codina N, Mancias JD, 2018. The role of NCOA4-mediated ferritinophagy in health and disease. Pharmaceuticals, 11(4): 114. 10.3390/ph11040114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana-Codina N, Gableske S, del Rey MQ, et al. , 2019. NCOA4 maintains murine erythropoiesis via cell autonomous and non-autonomous mechanisms. Haematologica, 104(7): 1342-1354. 10.3324/haematol.2018.204123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana-Codina N, Gikandi A, Mancias JD, 2021. The role of NCOA4-mediated ferritinophagy in ferroptosis. Adv Exp Med Biol, 1301: 41-57. 10.1007/978-3-030-62026-4_4 [DOI] [PubMed] [Google Scholar]
- Santana-Codina N, del Rey MQ, Kapner KS, et al. , 2022. NCOA4-mediated ferritinophagy is a pancreatic cancer dependency via maintenance of iron bioavailability for iron–sulfur cluster proteins. Cancer Discov, 12(9): 2180-2197. 10.1158/2159-8290.CD-22-0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasazawa Y, Kanagaki S, Tashiro E, et al. , 2012. Xanthohumol impairs autophagosome maturation through direct inhibition of valosin-containing protein. ACS Chem Biol, 7(5): 892-900. 10.1021/cb200492h [DOI] [PubMed] [Google Scholar]
- Savarese G, von Haehling S, Butler J, et al. , 2023. Iron deficiency and cardiovascular disease. Eur Heart J, 44(1): 14-27. 10.1093/eurheartj/ehac569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaryn JP, Reitsma JM, Bigley TM, et al. , 2013. Human cytomegalovirus pUL29/28 and pUL38 repression of p53-regulated p21CIP1 and caspase 1 promoters during infection. J Virol, 87(5): 2463-2474. 10.1128/JVI.01926-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevrioukova IF, 2011. Apoptosis-inducing factor: structure, function, and redox regulation. Antioxid Redox Signal, 14(12): 2545-2579. 10.1089/ars.2010.3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Liu R, Chen L, 2022. Ferroptosis inhibitor ferrostatin‑1 alleviates homocysteine‑induced ovarian granulosa cell injury by regulating TET activity and DNA methylation. Mol Med Rep, 25(4): 130. 10.3892/mmr.2022.12645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y, Yasui H, Higashikawa K, et al. , 2019. Erastin, a ferroptosis-inducing agent, sensitized cancer cells to X-ray irradiation via glutathione starvation in vitro and in vivo . PLoS ONE, 14(12): e0225931. 10.1371/journal.pone.0225931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo M, Torimoto Y, Saito H, et al. , 2006. Functional role of DMT1 in transferrin-independent iron uptake by human hepatocyte and hepatocellular carcinoma cell, HLF. Hepatol Res, 35(3): 152-162. 10.1016/j.hepres.2006.03.011 [DOI] [PubMed] [Google Scholar]
- Shizukuda Y, Matoba S, Mian OY, et al. , 2005. Targeted disruption of p53 attenuates doxorubicin-induced cardiac toxicity in mice. Mol Cell Biochem, 273(1-2): 25-32. 10.1007/s11010-005-5905-8 [DOI] [PubMed] [Google Scholar]
- Shroff EH, Eberlin LS, Dang VM, et al. , 2015. MYC oncogene overexpression drives renal cell carcinoma in a mouse model through glutamine metabolism. Proc Natl Acad Sci USA, 112(21): 6539-6544. 10.1073/pnas.1507228112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MM, de Souza-Neto FP, de Jesus ICG, et al. , 2021. Alamandine improves cardiac remodeling induced by transverse aortic constriction in mice. Am J Physiol Heart Circ Physiol, 320(1): H352-H363. 10.1152/ajpheart.00328.2020 [DOI] [PubMed] [Google Scholar]
- Silvain J, Zeitouni M, Paradies V, et al. , 2021. Procedural myocardial injury, infarction and mortality in patients undergoing elective PCI: a pooled analysis of patient-level data. Eur Heart J, 42(4): 323-334. 10.1093/eurheartj/ehaa885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YF, Wang BC, Zhu XL, et al. , 2021. Human umbilical cord blood-derived MSCs exosome attenuate myocardial injury by inhibiting ferroptosis in acute myocardial infarction mice. Cell Biol Toxicol, 37: 51-64. 10.1007/s10565-020-09530-8 [DOI] [PubMed] [Google Scholar]
- Stamenkovic A, O'Hara KA, Nelson DC, et al. , 2021. Oxidized phosphatidylcholines trigger ferroptosis in cardiomyocytes during ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol, 320(3): H1170-H1184. 10.1152/ajpheart.00237.2020 [DOI] [PubMed] [Google Scholar]
- Stenseth K, Thyberg J, 1989. Monensin and chloroquine inhibit transfer to lysosomes of endocytosed macromolecules in cultured mouse peritoneal macrophages. Eur J Cell Biol, 49(2): 326-333. [PubMed] [Google Scholar]
- Sugezawa K, Morimoto M, Yamamoto M, et al. , 2022. GPX4 regulates tumor cell proliferation via suppressing ferroptosis and exhibits prognostic significance in gastric cancer. Anticancer Res, 42(12): 5719-5729. 10.21873/anticanres.16079 [DOI] [PubMed] [Google Scholar]
- Sui MX, Xu D, Zhao WY, et al. , 2021. CIRBP promotes ferroptosis by interacting with ELAVL1 and activating ferritinophagy during renal ischaemia-reperfusion injury. J Cell Mol Med, 25(13): 6203-6216. 10.1111/jcmm.16567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YM, Bao QC, Xuan BQ, et al. , 2018. Human cytomegalovirus protein pUL38 prevents premature cell death by binding to ubiquitin-specific protease 24 and regulating iron metabolism. J Virol, 92(13): e00191-18. 10.1128/JVI.00191-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro T, Ikeda M, Ide T, et al. , 2020. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight, 5(9): e132747. 10.1172/jci.insight.132747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taher AT, Porter JB, Kattamis A, et al. , 2016. Efficacy and safety of iron-chelation therapy with deferoxamine, deferiprone, and deferasirox for the treatment of iron-loaded patients with nontransfusion-dependent thalassemia syndromes. Drug Des Devel Ther, 10: 4073-4078. 10.2147/DDDT.S117080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DL, Chen X, Kang R, et al. , 2021. Ferroptosis: molecular mechanisms and health implications. Cell Res, 31(2): 107-125. 10.1038/s41422-020-00441-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang LJ, Luo XJ, Tu H, et al. , 2021a. Ferroptosis occurs in phase of reperfusion but not ischemia in rat heart following ischemia or ischemia/reperfusion. Naunyn Schmiedebergs Arch Pharmacol, 394(2): 401-410. 10.1007/s00210-020-01932-z [DOI] [PubMed] [Google Scholar]
- Tang LJ, Zhou YJ, Xiong XM, et al. , 2021b. Ubiquitin-specific protease 7 promotes ferroptosis via activation of the p53/TfR1 pathway in the rat hearts after ischemia/reperfusion. Free Radic Biol Med, 162: 339-352. 10.1016/j.freeradbiomed.2020.10.307 [DOI] [PubMed] [Google Scholar]
- Tang MZ, Chen Z, Wu D, et al. , 2018. Ferritinophagy/ferroptosis: iron-related newcomers in human diseases. J Cell Physiol, 233(12): 9179-9190. 10.1002/jcp.26954 [DOI] [PubMed] [Google Scholar]
- Tang MZ, Huang Z, Luo XL, et al. , 2019. Ferritinophagy activation and sideroflexin1-dependent mitochondria iron overload is involved in apelin-13-induced cardiomyocytes hypertrophy. Free Radic Biol Med, 134: 445-457. 10.1016/j.freeradbiomed.2019.01.052 [DOI] [PubMed] [Google Scholar]
- Tang SC, Gao F, Chen HM, et al. , 2020. The role of iron, its metabolism and ferroptosis in traumatic brain injury. Front Cell Neurosci, 14: 590789. 10.3389/fncel.2020.590789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubouchi K, Araya J, Yoshida M, et al. , 2019. Involvement of GPx4-regulated lipid peroxidation in idiopathic pulmonary fibrosis pathogenesis. J Immunol, 203(8): 2076-2087. 10.4049/jimmunol.1801232 [DOI] [PubMed] [Google Scholar]
- Tu C, Ortega-Cava CF, Winograd P, et al. , 2010. Endosomal-sorting complexes required for transport (ESCRT) pathway-dependent endosomal traffic regulates the localization of active src at focal adhesions. Proc Natl Acad Sci USA, 107(37): 16107-16112. 10.1073/pnas.1009471107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bergen JMG, Li X, Hua J, et al. , 2016. Colocalization of cerebral iron with amyloid beta in mild cognitive impairment. Sci Rep, 6: 35514. 10.1038/srep35514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Haehling S, Jankowska EA, van Veldhuisen DJ, et al. , 2015. Iron deficiency and cardiovascular disease. Nat Rev Cardiol, 12(11): 659-669. 10.1038/nrcardio.2015.109 [DOI] [PubMed] [Google Scholar]
- Wang H, Jiang XJ, 2023. cGASing mitochondria to fend off ferroptosis. Cell Res, 33(4): 263-264. 10.1038/s41422-023-00791-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Chen XZ, Wang YH, et al. , 2022. Emerging roles of ferroptosis in cardiovascular diseases. Cell Death Discov, 8: 394. 10.1038/s41420-022-01183-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SY, Wang L, Qin X, et al. , 2020. ALDH2 contributes to melatonin-induced protection against APP/PS1 mutation-prompted cardiac anomalies through cGAS-STING-TBK1-mediated regulation of mitophagy. Signal Transduct Target Ther, 5: 119. 10.1038/s41392-020-0171-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YQ, Zhao YJ, Ye T, et al. , 2021. Ferroptosis signaling and regulators in atherosclerosis. Front Cell Dev Biol, 9: 809457. 10.3389/fcell.2021.809457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Li YN, Ye YZ, et al. , 2023. NLRP3 inflammasome deficiency attenuates cerebral ischemia-reperfusion injury by inhibiting ferroptosis. Brain Res Bull, 193: 37-46. 10.1016/j.brainresbull.2022.11.016 [DOI] [PubMed] [Google Scholar]
- Wu WW, Sato K, Koike A, et al. , 2010. HERC2 is an E3 ligase that targets BRCA1 for degradation. Cancer Res, 70(15): 6384-6392. 10.1158/0008-5472.CAN-10-1304 [DOI] [PubMed] [Google Scholar]
- Xiao LL, Ma XZ, Ye LQ, et al. , 2022. IL-9/STAT3/fatty acid oxidation-mediated lipid peroxidation contributes to Tc9 cell longevity and enhanced antitumor activity. J Clin Invest, 132(7): e153247. 10.1172/JCI153247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie YC, Hou T, Liu JY, et al. , 2023. Autophagy-dependent ferroptosis as a potential treatment for glioblastoma. Front Oncol, 13: 1091118. 10.3389/fonc.2023.1091118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong HM, 2013. ZnO nanoparticles applied to bioimaging and drug delivery. Adv Mater, 25(37): 5329-5335. 10.1002/adma.201301732 [DOI] [PubMed] [Google Scholar]
- Xiong QH, Li X, Li WJ, et al. , 2021. WDR45 mutation impairs the autophagic degradation of transferrin receptor and promotes ferroptosis. Front Mol Biosci, 8: 645831. 10.3389/fmolb.2021.645831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Wang L, Yu FL, 2014. Regulation of cellular iron metabolism and its implications in lung cancer progression. Med Oncol, 31(7): 28. 10.1007/s12032-014-0028-2 [DOI] [PubMed] [Google Scholar]
- Xiu ZR, Zhu YL, Han JC, et al. , 2022. Caryophyllene oxide induces ferritinophagy by regulating the NCOA4/FTH1/LC3 pathway in hepatocellular carcinoma. Front Pharmacol, 13: 930958. 10.3389/fphar.2022.930958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HF, Zou T, Tuo QZ, et al. , 2021. Ferroptosis: mechanisms and links with diseases. Signal Transduct Target Ther, 6: 49. 10.1038/s41392-020-00428-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ND, Tan SH, Ng S, et al. , 2014. Artesunate induces cell death in human cancer cells via enhancing lysosomal function and lysosomal degradation of ferritin. J Biol Chem, 289(48): 33425-33441. 10.1074/jbc.M114.564567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Wang XX, Contino G, et al. , 2011. Pancreatic cancers require autophagy for tumor growth. Genes Dev, 25(7): 717-729. 10.1101/gad.2016111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SS, Lian GJ, 2020. ROS and diseases: role in metabolism and energy supply. Mol Cell Biochem, 467(1-2): 1-12. 10.1007/s11010-019-03667-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XJ, Zhong XM, Tanyi JL, et al. , 2013. mir-30d Regulates multiple genes in the autophagy pathway and impairs autophagy process in human cancer cells. Biochem Biophy Res Commun, 431(3): 617-622. 10.1016/j.bbrc.2012.12.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XZ, Li XX, Zhang YJ, et al. , 2016. Rab1 in cell signaling, cancer and other diseases. Oncogene, 35(44): 5699-5704. 10.1038/onc.2016.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YY, Zhang K, Huang SF, et al. , 2022. Apelin-13/APJ induces cardiomyocyte hypertrophy by activating the Pannexin-1/P2X7 axis and FAM134B-dependent reticulophagy. J Cell Phys, 237(4): 2230-2248. 10.1002/jcp.30685 [DOI] [PubMed] [Google Scholar]
- Yi JM, Zhu JJ, Wu J, et al. , 2020. Oncogenic activation of PI3K-AKT-mToR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc Natl Acad Sci USA, 117(49): 31189-31197. 10.1073/pnas.2017152117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SE, Chen LJ, Na R, et al. , 2012. Gpx4 ablation in adult mice results in a lethal phenotype accompanied by neuronal loss in brain. Free Radic Biol Med, 52(9): 1820-1827. 10.1016/j.freeradbiomed.2012.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XX, Ruan Y, Shen T, et al. , 2020. Dexrazoxane protects cardiomyocyte from doxorubicin-induced apoptosis by modulating miR-17-5p. BioMed Res Int, 2020: 5107193. 10.1155/2020/5107193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Yan Y, Niu FL, et al. , 2021. Ferroptosis: a cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov, 7: 193. 10.1038/s41420-021-00579-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalpoor H, Akbari A, Jazi NN, et al. , 2022. Possible role of autophagy induced by COVID-19 in cancer progression, chemo-resistance, and tumor recurrence. Infect Agents Cancer, 17: 38. 10.1186/s13027-022-00450-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Ma GS, Yao YY, et al. , 2012. Olmesartan attenuates the impairment of endothelial cells induced by oxidized low density lipoprotein through downregulating expression of LOX-1. Int J Mol Sci, 13(2): 1512-1523. 10.3390/ijms13021512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Wang Z, Liu ZX, et al. , 2021. Protective effects of dexazoxane on rat ferroptosis in doxorubicin-induced cardiomyopathy through regulating HMGB1. Front Cardiovasc Med, 8: 685434. 10.3389/fcvm.2021.685434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang XY, Guan BY, et al. , 2023. Qing-Xin-Jie-Yu Granule inhibits ferroptosis and stabilizes atherosclerotic plaques by regulating the GPX4/xCT signaling pathway. J Ethnopharmacol, 301: 115852. 10.1016/j.jep.2022.115852 [DOI] [PubMed] [Google Scholar]
- Zhang PY, Park HJ, Zhang J, et al. , 2020. Translation of the intrinsically disordered protein α-synuclein is inhibited by a small molecule targeting its structured mRNA. Proc Natl Acad Sci USA, 117(3): 1457-1467. 10.1073/pnas.1905057117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao WK, Zhou Y, Xu TT, et al. , 2021. Ferroptosis: opportunities and challenges in myocardial ischemia-reperfusion injury. Oxid Med Cell Longev, 2021: 9929687. 10.1155/2021/9929687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JS, Sato M, Mishima E, et al. , 2021. Sorafenib fails to trigger ferroptosis across a wide range of cancer cell lines. Cell Death Dis, 12(7): 698. 10.1038/s41419-021-03998-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo XZ, Wu Y, Ni YJ, et al. , 2013. Isoproterenol instigates cardiomyocyte apoptosis and heart failure via AMPK inactivation-mediated endoplasmic reticulum stress. Apoptosis, 18(7): 800-810. 10.1007/s10495-013-0843-5 [DOI] [PubMed] [Google Scholar]