Abstract
Giant cell tumor of bone (GCTB) is a locally aggressive tumor that shows predilection for the metaphysis/epiphysis of long bones, with an incidence of 4–5% of primary bone tumors. GCTB shows two main populations of cells: mononuclear cells and non-neoplastic multi-nucleated giant cells, with or without fibrous background. On the other hand, giant-cell-poor GCTB are rare with only few reports in the literature. These cases offer a diagnostic challenge, given the absence of giant cells and such cases have consistently been shown to harbor the H3F3A gene mutation by sequencing. The H3.3 G34W mutation specific monoclonal antibody has shown high specificity in the diagnosis of GCTB. Two cases of giant-cell-poor GCTB are presented in this study, in which giant cells were absent or sparse and the diagnosis of GCTB was confirmed by the expression of H3.3 G34W monoclonal antibody in the mononuclear cells by immunohistochemistry. Whether this represents a histologic variant of GCTB or partial involution of GCTB is not yet fully understood, however, an immune response, infectious/inflammatory reaction and/or anti-tumor cytokine production have been purported to be factors inciting disease regression in GCTB.
Keywords: Giant cell tumor of bone, involuted giant cell tumor of bone, H3F3A gene mutation, giant-cell-poor giant cell tumor of bone
Introduction
Giant cell tumor of bone (GCTB) is a locally aggressive neoplasm and can metastasize rarely. It accounts for 4–5% of primary bone tumors. They arise most commonly at the epi-metaphysis of long bones. GCTB in flat bones like the pelvis and sacrum, are far less common and more challenging to treat [1, 2]. On imaging, GCTB typically presents as an osteolytic and eccentric intramedullary lesion that extends to the articular surface with sharply demarcated and well-defined borders. Bone scan shows increased metabolic activity at the periphery of the lesion [3, 4].
Morphologically, classic findings of GCTB include two main populations of cells: 1) mononuclear cells and 2) reactive multinucleated osteoclast-like giant cells, with or without a fibrous background [5, 6]. Giant cell poor-GCTBs are extremely rare and have been described as few case reports in the literature [7, 8]. These cases pose a diagnostic challenge, given their paucity of giant cells however, they have consistently been shown to harbor the H3F3A gene mutation by sequencing [7]. H3F3A histone gene mutation commonly H3.3 p.Gly34Trp is found in approximately 95% of GCTB [9, 10]. The use of H3.3 G34W mutation specific monoclonal antibody has shown high specificity in giant cell tumors and has been widely utilized as a pathognomonic marker in such cases [11, 12]. The antibody highlights the true neoplastic cell population which is often not readily visible on H&E sections.
While the standard treatment of GCTB is curettage with adjuvant therapy such as bone cement, denosumab, a fully humanized monoclonal antibody against the receptor activator of NF-kB ligand (RANKL) which prevents bone resorption mediated by osteoclastic giant cells, can be used for the treatment of unresectable tumors. This results in a striking decrease in giant cells, maturation of mononuclear neoplastic cells and bone formation, a process which gets reversed following the cessation of treatment, with a high risk of recurrence [13–17]. The histologic changes seen in giant cell tumor cases treated with denosumab are challenging since they can resemble bone forming tumors including osteosarcoma. There have been few reports of malignant transformation of giant cell tumors following denosumab therapy [18, 19].
The natural course of GCTB under conservative treatment includes stable disease, involution, tumor ossification post therapy, local recurrence, and rarely malignant transformation [20 – 23]. Self-involuted giant cell tumor is very rare and ill-understood.
In this study, we present two cases of diagnostically challenging giant cell tumors of bone, showing unusual morphology and rare to absent giant cells, and the diagnosis of GCTB was confirmed by H3.3 G34W protein expression.
Case Report
Following approval of the study by Institutional Review Board (IRB) # 16–1682, we searched in the archives of our institution (2010–2021) for cases with a diagnosis of regressed and involuted giant cell tumor of bone confirmed by H3.3 G34W immunohistochemistry (IHC) and/or H3F3A gene mutation detection and, fibrohistiocytic bone lesions involving the epi-metaphysis in adults without prior G34W immunohistochemical or molecular studies. In the latter group, corresponding tissue blocks were retrieved, and full tissue sections were obtained at 4 μ thickness. Immunohistochemistry was performed using Histone H3.3 (G34W) RM263 antibody clone on a BOND III automated instrument with appropriate positive and negative controls. There were 5 benign fibrous histiocytomas, and we excluded all non epi-metaphyseal lesions. Only in one case, the lesion involved femoral head. Of 110 (2010–2021) giant cell tumors there were only two with diagnosis of involuted/regressed giant cell tumor and all others were excluded. Demographic data collection including age, gender, clinical presentation, tumor size and tumor location were recorded. Additionally, radiologic images of all cases were reviewed.
In the two cases of giant-cell-poor GCTB identified, the diagnosis of GCTB could only be confirmed after immunohistochemical confirmation of positivity for H3.3 G34W antibody. The fibrohistiocytic tumor was negative for G34W IHC on testing and excluded from the study.
Case 1
A 56-year-old woman presented with a 6-month history of left knee pain which started after an episode of bending. The pain was felt predominantly posteriorly and then migrated to the medial joint line. The radiograph and CT studies showed a lytic lesion in the medial femoral condyle with internal thickened trabeculae and fat attenuation in the CT images. MRI exam demonstrated fat suppressed areas and cystic areas and fluid levels, were suggestive of secondary aneurysmal bone cyst (figure 1 a–f). Differential diagnosis based on imaging findings, included intraosseous ganglion, healing bone cyst, intraosseous lipoma, and hemangioma. The patient underwent intralesional curettage. Histologic examination showed predominantly adipose tissue with sheets of ovoid to spindle-shaped monomorphic mononuclear cells with areas of hemorrhage, hemosiderin deposition, fibrosis, and reactive bone formation. Focal areas of aneurysmal bone cyst (ABC)-like changes were present. No osteoclast-type giant cells were seen. The histological findings of fibrosis, hemosiderin deposition, mononuclear cells raised the possibility of secondary changes in a giant cell tumor of bone. Immunohistochemical stain for H3.3 G34W was performed and showed positivity in the mononuclear cells supporting the diagnosis of giant cell-poor GCTB (figure 1 g–j).
Fig. 1. (Case 1).


a: Frontal radiograph shows a lesion with internal thickened trabeculae (arrows). b: Axial CT images shows the lesion is lytic with thickened trabeculae (arrows) and fat attenuation (arrowheads). c: Axial PD fat-suppressed image shows low signal suggestive of fat suppression (*) and multiple cystic areas (arrowheads). d, e: Sagittal PD and PD fat-suppressed images show confluent area of fat suppression (*) and cystic areas (arrowheads). f: Sagittal PD fat-suppressed image shows multiple fluid levels (arrows). g, h: H&E (2X magnification) shows predominant adipose tissue with focal hemorrhage and cystic changes. i: (20X magnification) shows clusters of mononuclear cells that are oval to spindle-shaped with no atypia, no giant cells seen. j: Neoplastic cells are positive for the G34W monoclonal antibody.
Case 2
A 68-year-old woman presented with a one-year history of left knee pain without improvement despite multiple corticosteroid injections. The initial frontal radiograph showed a lytic lesion without a sclerotic rim in the medial femoral condyle abutting the subchondral bone. Seven months later, a follow up radiograph and CT scan showed that the lesion had increased in size with a new sclerotic rim and periosteal reaction with cortical fenestration. Bone scan showed an intense radiotracer uptake at the periphery of the lesion and relative less uptake at the center. MR coronal images showed mixture of hyperintense and isointense signal in T1-weighted images and heterogenous hypo- and hyperintense signal in STIR images. The rim of the lesion (arrows) was hypointense in both MR sequences (figure 2 a–f). The radiographic differential diagnoses included brown tumor, giant cell tumor of bone, and plasmacytoma. Upon biopsy, histologic examination showed fibrovascular tissue with a mixed inflammatory infiltrate, mostly plasma cells, and scattered mononuclear stromal cells in the background. Immunohistochemical workup to exclude myeloma was performed which showed polyclonality of the plasma cells. Due to the location and imaging differential diagnosis of GCTB, a H3.3 G34W immunostain was performed, which showed multifocal nuclear positivity in the background mononuclear cells, which were completely effaced on the H&E by the rich plasma cell infiltrate. This finding raised the possibility of sampling the edge of giant-cell-poor giant cell tumor of bone, despite the non-specific morphologic findings (figure 2 g–j).
Fig. 2. (Case 2).




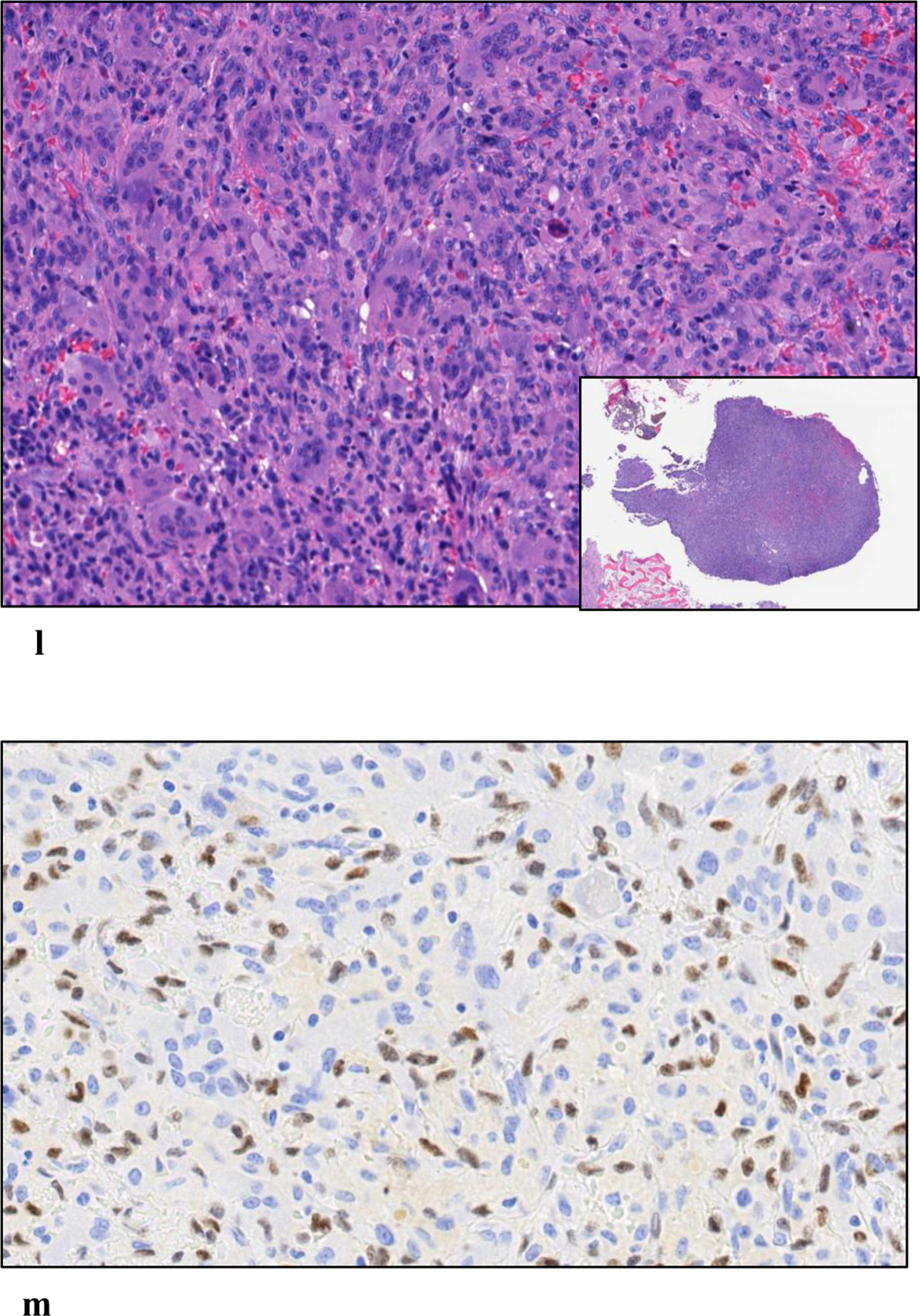
a: Initial frontal radiograph shows a lytic lesion (arrowheads) without a sclerotic rim in the medial femoral condyle abutting the subchondral bone. b, c: Follow up frontal radiograph and coronal CT images performed seven months later, show increased lesion size with a new sclerotic rim (arrowheads) and periosteal reaction with cortical fenestration (arrow). d: Bone scan showed an intense radiotracer uptake at the periphery of the lesion and relative less uptake at the center (arrow). e: Coronal T1-weighted image demonstrates mixture of hyperintense and isointense signal. f: Coronal STIR image demonstrates heterogenous hypo- and hyperintense signal. The rim of the lesion (arrows) is hypointense in both images. g: H&E (4X magnification) shows a predominance of collagenous stroma, plasma cells and scant mononuclear cells. h: CD138 Immunostain shows abundant plasma cells which were polytypic by kappa and lambda stains. i: (40X magnification) showing plasma cells with scattered mononuclear cells. j: Few neoplastic cells are positive for the G34W monoclonal antibody. k: H&E of curettage specimen showing the areas of partial involution and predominant plasma cells. l: H&E of curettage specimen showing the areas of conventional GCTB with a predominance of giant cells. m: In areas with diffuse giant cells, the neoplastic cells are positive for the G34W monoclonal antibody.
Upon excision of the lesion with curettage, microscopic examination of the specimen showed predominantly plasma cell infiltrates with a small area of conventional GCTB confirming the diagnosis of a giant cell tumor with very brisk inflammatory response and predominant giant cell poor areas (figure 2 k–m).
Discussion
Under normal conditions, proliferation, and activation of osteoclast-like giant cells in GCTB is mediated by RANKL, (expressed by the mononuclear neoplastic cells) which binds with RANK (expressed on the cell membrane of osteoclasts and its precursors) and this process is regulated by osteoprotegerin (OPG) negative feedback inhibition [26]. Jiang et al. [27] showed that Runx2, a key factor in the RANKL-RANK pathway, is a target for regulating bone destruction in GCTB. This is driven by the stromal cells that demonstrated significant upregulation of Runt-related transcription factor 2 (Runx2) and Twist-related protein 1 (TWIST) expression. Osteoclast-like giant cells in large numbers and diffuse distribution has been a consistent histologic finding in untreated giant cell tumor of bone. On radiography and CT, GCTB commonly presents as a lytic and eccentric lesion at the end of long or flat bones, and its border is well-defined without sclerosis or mineralized matrix [5]. On bone scans, the lesion frequently shows peripheral radiotracer uptake with central photopenia [5]. On MRI, GCTB exhibits low to intermediate T2 signal due to chronic hemorrhage and fluid levels are occasionally present [5]. The diagnosis is made in conjunction with the imaging finding of an epi-metaphyseal lytic lesion in a skeletally mature individual. Pathologically GCTB consistently express H3.3 G34W monoclonal antibody in 91% of cases and/or H3F3A gene mutation detected by genetic sequencing [9–11]. The specificity of G34W antibody has led to the recognition of rare cases of GCTB which present as diagnostically challenging giant cell poor lesions. Benign osteolytic bone lesions involving the cortex or medulla of metaphysis and/or the epiphysis, were classified as non-ossifying fibroma or fibrous histiocytoma, with morphological features comprising bland, spindle cells arranged in storiform pattern with scattered osteoclast-like giant cells. Majority of these lesions (80%) harbor mutations in gene Kirsten rat sarcoma viral oncogene homolog (KRAS) and fibroblast growth factor receptor 1 (FGFR1) genes. [24]. In the new WHO 2020, lesions with similar morphology arising in epiphyses in skeletally mature individuals are regarded as GCTB [5, 25]. Thus, it appears that GCTB does not always conform to its classic morphology of syncytial growth of giant cells with interspersed mononuclear cells and can show scattered osteoclast-like giant cells to complete disappearance of giant cells as seen in one of our cases. While the causative factors for loss/disappearance of giant cells is not known, a brisk inflammatory response as seen in one of our cases may imbalance the RANK-RANKL pathway interaction [28]. Whether this is involution or regressing phenomena is debatable.
Leinauer et al. [7] described three cases of giant cell tumor of bone without giant cells, which had a common morphologic feature of isomorphic oval mononuclear cells in a background of collagenous stroma and bone formation, these histologic findings were similar to case 1 in our study, however our case in addition showed secondary ABC- like changes, a finding occasionally seen in giant cell tumor of bone [29]. However, the predominant fatty appearance with thickened trabeculae and sclerotic rim on MRI and CT are highly unusual for GCTB and its diagnosis was not considered on the initial imaging interpretation. Our case 2 on the other hand, showed predominantly an inflammatory infiltrate composed of plasma cells with only a focal presence of classic GCTB, suggesting the loss of giant cells is triggered by the inflammatory response raising the possibility of regression or partial involution. Of note, the radiologic imaging appearance of this lesion on presentation was typical for GCTB. An inflammatory response as a feature of regression has not been previously described. Spontaneous regression of GCTB is extremely rare and was reported as an isolated case [8], where a primary GCTB of distal radius, a diagnosis suggested on fine needle aspiration cytology specimen, showed complete absence of both mononuclear cells and giant cells upon wide local excision. Sections showed chronic inflammation comprising lymphocytes, plasma cells and histiocytes with bone necrosis and new bone formation. The complete regression was seen after 8 months without treatment, which was attributed to an immune response, inhibition of tumor cells by cytokines, tumor necrosis or angiogenesis inhibition. Despite its rarity, spontaneous regression of GCTB lung metastasis has been reported in multiple studies [21, 30–31]. One study [32] reported six patients with pulmonary metastasis of GCTB, one of which showed marked decrease of both lung parenchymal and extra-pleural nodules after two years of follow-up without treatment. Regression of lung metastasis in GCTB following RANK Ligand inhibitor (denosumab) has also been reported [33]. There was a reduction in size and number of pulmonary nodules with calcification, in a case of metastatic GCTB after 2 cycles of denosumab, showing histologic features of giant cells loss.
Spontaneous regression of tumors is a known occurrence and has been described in several tumors. With an approximate rate of 1 in 100,000, spontaneous regression has been reported in some tumors like embryonal tumors in children, carcinoma of the breast and kidney, melanoma, and sarcomas [34]. This may be due to recognition of cancer cells as non-self and harmful by the immune system with subsequent control of its local progression and metastasis [35]. An association between acute infection and spontaneous regression of tumors has been documented, where pathogens such as bacteria play a role in activating immune system and fighting cancer cells [36]. Non-infectious inflammatory reaction can also trigger an immune response and initiate an antitumor cascade of events [37, 38]. One can postulate a non-infectious inflammatory response as a trigger for spontaneous partial regression in our case 2.
Conclusion
We have presented 2 cases of giant cell poor giant cell tumor of bone whose diagnosis could be established by confirmation with mutation specific antibody (H3.3 G34W) despite the lack of classic morphological features and imaging correlation. At last follow up, Patient 1 is two years post curettage with bone grafting and experiences no pain, has full range of motion, and has had a return to normal activities. Radiographs show excellent incorporation of the graft. Patient 2 is one year post curettage with minimal discomfort in the region of the metal plate with full range of motion and a normal knee exam. There is no radiologic evidence of recurrence.
Acknowledgment
The study is supported by the Department of Pathology at Memorial Sloan Kettering Cancer Center R&D fund and in part by the NIH/National Cancer Institute Cancer Center Support grant under award P30CA008748.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Raskin KA, Schwab JH, Mankin HJ, Springfield DS, Hornicek FJ. Giant cell tumor of bone. J Am Acad Orthop Surg. 2013. Feb;21(2):118–26. [DOI] [PubMed] [Google Scholar]
- 2.Karpik M. Giant Cell Tumor (tumor gigantocellularis, osteoclastoma)-epidemiology, diagnosis, treatment. Ortopedia, traumatologia, rehabilitacja. 2010. May 1;12(3):207–15. [PubMed] [Google Scholar]
- 3.Montgomery C, Couch C, Emory CL, Nicholas R. Giant cell tumor of bone: review of current literature, evaluation, and treatment options. The journal of knee surgery. 2019. Apr;32(04):331–6. [DOI] [PubMed] [Google Scholar]
- 4.Murphey MD, Nomikos GC, Flemming DJ, Gannon FH, Temple HT, Kransdorf MJ. Imaging of giant cell tumor and giant cell reparative granuloma of bone: radiologic-pathologic correlation. Radiographics. 2001. Sep;21(5):1283–309. [DOI] [PubMed] [Google Scholar]
- 5.Flanagan AM, Larousserie F, O’Donnell PG, Yoshida A. Giant cell tumour of bone. In WHO Classification of Tumours Editorial Board ed. Soft tissue and bone tumours, vol. 3. 5th ed. Lyon: IARC Press, 2020; 440–446. [Google Scholar]
- 6.Noh BJ, Park YK. Giant cell tumor of bone: updated molecular pathogenesis and tumor biology. Human pathology. 2018. Nov 1;81:1–8. [DOI] [PubMed] [Google Scholar]
- 7.Leinauer B, Wolf E, Werner M, et al. H3F3A-mutated giant cell tumour of bone without giant cells-clinical presentation, radiology and histology of three cases. Histopathology. 2021. Nov;79(5):720–730. [DOI] [PubMed] [Google Scholar]
- 8.Mallya V, Gupta L, Khurana N, Maini L. Spontaneous regression of giant cell tumor of the wrist: Myth or fact? A case report. Indian Journal of Pathology & Microbiology: an Official Organ of Indian Association of Pathologists & Microbiologists. 2019. Apr 1;62(2):346–8. [DOI] [PubMed] [Google Scholar]
- 9.Behjati S, Tarpey PS, Presneau N, et al. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nature genetics. 2013. Dec;45(12):1479–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Presneau N, Baumhoer D, Behjati S, et al. Diagnostic value of H3F3A mutations in giant cell tumour of bone compared to osteoclast-rich mimics. The Journal of Pathology: Clinical Research. 2015. Apr;1(2):113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amary F, Berisha F, Ye H, et al. H3F3A (Histone 3.3) G34W immunohistochemistry: a reliable marker defining benign and malignant giant cell tumor of bone. The American journal of surgical pathology. 2017. Aug;41(8):1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto H, Iwasaki T, Yamada Y, et al. Diagnostic utility of histone H3. 3 G34W, G34R, and G34V mutant-specific antibodies for giant cell tumors of bone. Human pathology. 2018. Mar 1;73:41–50. [DOI] [PubMed] [Google Scholar]
- 13.Roux S, Amazit L, Meduri G, Guiochon-Mantel A, Milgrom E, Mariette X. RANK (receptor activator of nuclear factor kappa B) and RANK ligand are expressed in giant cell tumors of bone. American journal of clinical pathology. 2002. Feb 1;117(2):210–6. [DOI] [PubMed] [Google Scholar]
- 14.Branstetter DG, Nelson SD, Manivel JC, et al. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin Cancer Res. 2012. Aug 15;18(16):4415–24. [DOI] [PubMed] [Google Scholar]
- 15.Girolami I, Mancini I, Simoni A, et al. Denosumab treated giant cell tumour of bone: a morphological, immunohistochemical and molecular analysis of a series. J Clin Pathol. 2016. Mar;69(3):240–7. [DOI] [PubMed] [Google Scholar]
- 16.Hayashida K, Kawabata Y, Kato I, et al. Clinical and pathological analysis of giant cell tumor of bone with denosumab treatment and local recurrence. J Orthop Sci. 2022. Jan;27(1):215–221. [DOI] [PubMed] [Google Scholar]
- 17.Tariq MU, Umer M, Khan Z, Saeed J, Siddiqui MA, Din NU. Spectrum of histological features of Denosumab treated Giant Cell Tumor of Bone; potential pitfalls and diagnostic challenges for pathologists. Ann Diagn Pathol. 2020. Apr;45:151479. [DOI] [PubMed] [Google Scholar]
- 18.Aponte-Tinao LA, Piuzzi NS, Roitman P, Farfalli GL. A high-grade sarcoma arising in a patient with recurrent benign giant cell tumor of the proximal tibia while receiving treatment with denosumab. Clinical Orthopaedics and Related Research®. 2015. Sep;473(9):3050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broehm CJ, Garbrecht EL, Wood J, Bocklage T. Two Cases of Sarcoma Arising in Giant Cell Tumor of Bone Treated with Denosumab. Case reports in medicine. 2015 Dec 22;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rock MG, Sim FH, Unni KK, et al. Secondary malignant giant-cell tumor of bone. Clinicopathological assessment of nineteen patients. The Journal of Bone and Joint surgery. American Volume. 1986. Sep 1;68(7):1073–9. [PubMed] [Google Scholar]
- 21.Goldenberg RR, Campbell CJ, Bonfiglio M. Giant-cell tumor of bone: an analysis of two hundred and eighteen cases. JBJS. 1970. Jun 1;52(4):619–64. [PubMed] [Google Scholar]
- 22.Leggon RE, Zlotecki R, Reith J, Scarborough MT. Giant cell tumor of the pelvis and sacrum: 17 cases and analysis of the literature. Clin Orthop Relat Res. 2004. Jun; (423):196–207. [DOI] [PubMed] [Google Scholar]
- 23.Burke C, Link T, O’Donnell RJ, Cho SJ, Motamedi D. Giant cell tumor of bone: documented progression over 4 years from its origin at the metaphysis to the articular surface. Case Reports in Radiology. 2016 Aug 17;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bovée JV, Hogendoorn PC. Non-ossifying fibroma: A RAS-MAPK driven benign bone neoplasm. J Pathol. 2019. Jun;248(2):127–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baumhoer D, Rogozhin DV. Non-ossifying fibroma. In WHO Classification of Tumours Editorial Board. Soft tissue and bone tumours. 5th ed. Lyon, France: IARC; 2020:447–448. [Google Scholar]
- 26.Wu PF, Tang JY, Li KH. RANK pathway in giant cell tumor of bone: pathogenesis and therapeutic aspects. Tumour Biol. 2015. Feb;36(2):495–501. [DOI] [PubMed] [Google Scholar]
- 27.Jiang ZY, Jiang JJ, Ma YS, et al. Downregulation of miR-223 and miR-19a induces differentiation and promotes recruitment of osteoclast cells in giant-cell tumor of the bone via the Runx2/TWISTRANK/RANKL pathway. Biochemical and Biophysical Research Communications. 2018. Nov 10;505(4):1003–9. [DOI] [PubMed] [Google Scholar]
- 28.Papadaki M, Rinotas V, Violitzi F, et al. New Insights for RANKL as a Proinflammatory Modulator in Modeled Inflammatory Arthritis. Front Immunol. 2019. Feb 5;10:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Çomunoğlu N, Kepil N, Dervişoğlu S. Histopathology of giant cell tumors of the bone: With special emphasis on fibrohistiocytic and aneurysmal bone cyst like components. Acta Orthop Traumatol Turc. 2019. Jan;53(1):35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maloney WJ, Vaughan LM, Jones HH, Ross J, Nagel DA. Benign metastasizing giant-cell tumor of bone. Report of three cases and review of the literature. Clin Orthop Relat Res. 1989. Jun; (243):208–15 [PubMed] [Google Scholar]
- 31.Tubbs WS, Brown LR, Beabout JW, Rock MG, Unni KK. Benign giant-cell tumor of bone with pulmonary metastases: clinical findings and radiologic appearance of metastases in 13 cases. AJR Am J Roentgenol. 1992. Feb;158(2):331–4. [DOI] [PubMed] [Google Scholar]
- 32.Kay RM, Eckardt JJ, Seeger LL, Mirra JM, Hak DJ. Pulmonary metastasis of benign giant cell tumor of bone. Six histologically confirmed cases, including one of spontaneous regression. Clin Orthop Relat Res. 1994. May; (302):219–30. [PubMed] [Google Scholar]
- 33.Dietrich MF, Cavuoti D, Landay M, Arriaga YE. Histological Regression of Giant Cell Tumor of Bone Following RANK Ligand Inhibition. J Investig Med High Impact Case Rep. 2014. Nov 23;2(4):2324709614560216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fauvet J, Campagne J, Chavy A, Piet G. [Spontaneous cures, regressions and remissions of cancers]. Rev Prat. 1960. Sep 1;10:2349–84. [PubMed] [Google Scholar]
- 35.Jessy T. Immunity over inability: The spontaneous regression of cancer. Journal of natural science, biology, and medicine. 2011. Jan;2(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coley WB. Late results of the treatment of inoperable sarcoma by the mixed toxins of erysipelas and Bacillus prodigiosus. Am J Med Sci. 1906;131(3):375–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010. Mar 19; 140 (6): 883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pasvolsky O, Berger T, Bernstine H, Hayman L, Raanani P, Vidal L. Spontaneous regression of hodgkin lymphoma: case report and review of the literature. Acta haematologica. 2019; 141 (1): 14–8. [DOI] [PubMed] [Google Scholar]


