Abstract
Objective:
Our study aimed to elucidate the presence, antigen specificities, and potential clinical association of anti-neutrophil extracellular trap (anti-NET) antibodies in a multinational cohort of antiphospholipid antibody (aPL)-positive patients who did not have lupus.
Methods:
Anti-NET IgG/IgM were measured in sera of 389 aPL-positive patients; 308 met the classification criteria for APS. Multivariate logistic regression with best variable model selection was used to determine clinical associations. For a subset of the patients (n=214), we profiled autoantibodies with an autoantigen microarray platform.
Results:
We found elevated levels of anti-NET IgG and/or IgM in 45% of aPL-positive patients. High anti-NET antibody levels are associated with more circulating myeloperoxidase (MPO)-DNA complexes, a biomarker of NETs. When considering clinical manifestations, positive anti-NET IgG was associated with brain white matter lesions even after adjusting for demographic variables and aPL profiles. Anti-NET IgM tracked with complement consumption after controlling for aPL profiles; furthermore, patient serum containing high levels of anti-NET IgM efficiently deposited complement C3d on NETs. As determined by autoantigen microarray, positive testing for anti-NET IgG was significantly associated with several autoantibodies, including those recognizing citrullinated histones, heparan sulfate proteoglycan, laminin, MPO-DNA complexes, and nucleosomes. Anti-NET IgM positivity associated with autoantibodies targeting single-stranded DNA, double-stranded DNA, and proliferating cell nuclear antigen.
Conclusion:
These data reveal high levels of anti-NET antibodies in 45% of aPL-positive patients, where they potentially activate the complement cascade. While anti-NET IgM may especially recognize DNA in NETs, anti-NET IgG species appear more likely to target NET-associated protein antigens.
INTRODUCTION
Antiphospholipid syndrome (APS) is a systemic autoimmune disease that is a leading acquired cause of both thrombosis and late-term pregnancy loss (1). Roughly one-third of APS cases are diagnosed in patients with other autoimmune diseases, such as lupus, with the remainder presenting as a standalone syndrome called primary APS. Current classification criteria for APS seek persistently positive testing for anticardiolipin (aCL) antibodies, anti-β2GPI (aβ2GPI) antibodies, or lupus anticoagulant; these laboratory criteria should also be accompanied by either a thrombotic event (arterial, venous, and/or microvascular) or specific types of pregnancy morbidity (1). While thrombosis and pregnancy morbidity are the traditional hallmarks of APS, a variety of “extra-criteria” clinical phenotypes are also commonly seen in daily practice. Such potentially morbid manifestations include thrombocytopenia, hemolytic anemia, cardiac valve disease, livedo reticularis/racemosa, aPL-associated nephropathy, seizures, brain white matter lesions as determined by MRI, and cognitive dysfunction. There are few, if any, biomarkers to predict which aPL-positive patients are at risk for these extra-criteria features that typically do not respond to anticoagulant medications (1).
While the pathophysiology propels APS remains incompletely understood, potential aPL-mediated mechanisms that have been described include the activation of endothelial cells, monocytes, platelets, coagulation factors, and complement proteins (1). Neutrophil extracellular traps (NETs)—prothrombotic and proinflammatory webs of nuclear DNA, histones, and microbicidal proteins extruded by activated neutrophils—have recently received increasing attention as contributors to thromboinflammation in APS (2). It appears that aPL, especially those recognizing β2GPI, can engage the neutrophil surface, where they circumvent normal homeostatic mechanisms to trigger NET release (2). Interestingly, beyond traditional aPL, we recently found in a small single-center study that some APS patients also develop anti-NET antibodies (anti-NET Abs) that impair NET clearance with the potential to amplify inflammatory responses (3). Unaddressed to date are the antigen specificities of anti-NET Abs and the extent to which these antibodies could serve as biomarkers that would add value to traditional criteria aPL.
The Antiphospholipid Syndrome Alliance for Clinical Trials and InternatiOnal Networking (APS ACTION) was founded in 2010. It is an international research consortium that supports large-scale multicenter clinical studies in individuals who are persistently aPL-positive (4). Here, we sought to elucidate the presence, clinical associations, and antigen specificities of anti-NET Abs in this large multinational cohort. We focused on those individuals who did not have other underlying systemic autoimmune diseases such as lupus.
METHODS
More detailed protocols can be found in the Supplementary Methods. This study complied with all relevant ethical regulations and was approved by the University of Michigan IRB (HUM00200466).
Human samples.
Serum samples from 389 individuals were collected upon enrollment into APS ACTION (Supplementary Table 1). Of these, 175 samples were from the European core lab, 129 were from the North American core lab, and 85 were from the South American core lab.
Generation of neutrophil extracellular traps (NETs).
Human neutrophils were isolated, as we have done previously (5). Purified neutrophils were re-suspended in RPMI culture media (Gibco) supplemented with L-glutamine and 3% fetal bovine serum (Gibco). 1×107 neutrophils were then seeded into each well of a 6-well plate. To induce NETosis, neutrophils were cultured for 4 hours at 37°C and 5% CO2 in the presence of 500 nM phorbol 12-myristate 13-acetate (PMA, Sigma). Following incubation, NETs (both in supernatant and adherent to the plate) were collected and centrifuged at 450xg for 10 minutes at 4°C to pellet large cellular debris. The cell-free NETs-rich supernatants were collected, and NETs were pelleted by centrifuging at 16000xg for 10 minutes at 4°C. The NET pellet was washed once with ice-cold PBS at 16000xg for 10 minutes at 4°C before resuspending in ice-cold PBS at a concentration corresponding to 2×107 neutrophils per milliliter of PBS.
Anti-NET IgG and IgM enzyme-linked immunosorbent assays (ELISAs).
Briefly, a high-binding 96-well EIA/RIA plate was coated overnight at 4°C with micrococcal nuclease-digested NETs diluted to a 5 µg/ml concentration in 0.05 M bicarbonate buffer. The plates were blocked with 4% BSA for 2 hours at room temperature. Serum samples (diluted 1:100 in blocking buffer) were then added to individual wells. After a 1.5-hour incubation at 37°C, NET-bound autoantibodies were detected with an HRP-conjugated secondary antibody (anti-IgG or -IgM). Each experimental sample was compared to a corresponding well containing the same sample but in which no NETs were plated; this created an individual background value for each sample, which was subtracted to obtain the final result. The schematic illustration of the protocol in Figure 1A was created with BioRender software.
Figure 1:
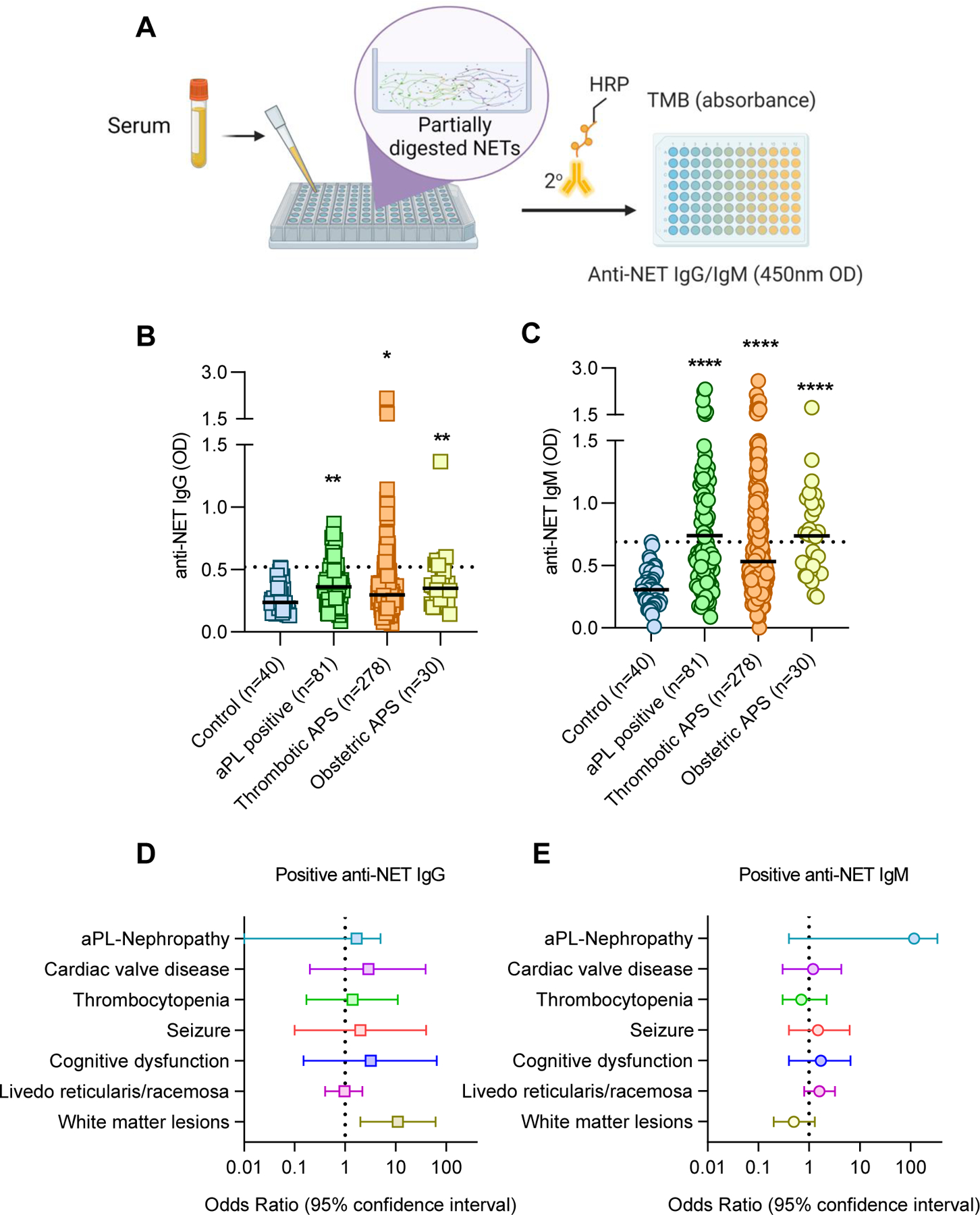
Measurement of anti-NET antibodies in 389 aPL-positive patients. A, Schematic illustration of the anti-NET Ab ELISA. B-C, Anti-NET IgG and IgM were measured in the indicated groups; no patients in this cohort had lupus. Patients were grouped based on no criteria manifestations (“aPL positive”), a history of thrombotic manifestations (47 of these 278 patients also had a history of obstetric manifestations), or a history of obstetric manifestations without history of vascular thrombosis. Levels of anti-NET IgG and IgM at 450-nm optical density (OD) were compared to controls by Kruskal–Wallis test corrected for multiple comparisons by Dunn’s method; *p<0.05, **p<0.01, and ****p<0.0001. Horizontal black lines indicate medians and dashed lines indicate 99th percentile cut-offs. D-E, Association between positive anti-NET IgG (E) or IgM (F) and various extra-criteria APS clinical manifestations were assessed by multivariate logistic regression adjusted for demographic variables (age, sex, ethnicity) and aPL profiles.
Quantification of anti-double strand DNA IgG/IgM and anti-single strand DNA IgG.
Circulating anti-double strand and single-strand DNA were measured with ELISA kits (Cat. No. 3100, Cat. No. 3105, and Cat. No. 3115, Alpha Diagnostics) according to the manufacturer’s instructions.
Quantification of myeloperoxidase (MPO)-DNA complexes.
MPO-DNA complexes were measured as previously described (6).
Quantification of complements C3 and C4.
Circulating complements C3 and C4 were measured with ELISA kits (NBP2-60619 and NBP2-60618, Novus Biologicals) according to the manufacturer’s instructions.
Immunofluorescence microscopy.
The protocol was as we described previously (6).
Autoantigen microarray.
Autoantibody profiling was done with an autoantigen microarray platform developed by Dr. Quan-Zhen Li at the University of Texas Southwestern Medical Center, and data were analyzed using Genepix Pro 7.0 software. See detailed description in Supplementary Materials.
RESULTS
Anti-NET Abs in a multinational population.
We obtained serum samples from 389 persistently aPL-positive patients recruited from Europe, North America, and South American centers. The mean age at the sampling time was 51 years; 70% of patients were females (Supplementary Table 1). 308 patients had primary APS, and 81 patients had persistently positive aPL without “criteria” APS manifestations or another systemic autoimmune disease diagnosis. Forty-seven patients met 3 out of 11 ACR lupus criteria but did not have classifiable lupus (7). Utilizing a custom ELISA platform that we developed (Figure 1A), we measured anti-NET IgG and IgM antibodies in 389 aPL-positive patients alongside 40 healthy controls. As compared with the healthy controls, elevated levels of anti-NET IgG and IgM were detected in patients with both thrombotic and obstetric APS; levels were also high in aPL-positive patients without any criteria manifestations (Figure 1B–C). Based on a positive threshold set at the 99th percentile for healthy-control samples, 61 patients (16%) had high anti-NET IgG activity, while 155 (40%) had high anti-NET IgM activity; 41 patients (11%) had positive testing for both anti-NET IgG and IgM. Overall, at least one of these tests was positive in 175 patients (45%). There was a strong positive relationship between anti-NET IgG and anti-NET IgM (r=0.52, p<0.0001). While no significant difference was seen between lupus anticoagulant positive and negative patients (for anti-NET IgG: p=0.32; for anti-NET IgM: p=0.72), anti-NET activities did demonstrate positive correlations with levels of other traditional aPL (anti-NET IgG vs. aβ2GPI IgG: r=0.21, p<0.0001; anti-NET IgG vs. aCL IgG: r=0.19, p=0.0001; anti-NET IgM vs. aβ2GPI IgM: r=0.18, p=0.0007; and anti-NET IgM vs. aCL IgM: r=0.53, p<0.0001). While none of the participants had classifiable lupus (7), 217 (56%) had positive ANA reported by the participating centers; ANA titers were not available. We tested anti-double strand DNA (dsDNA) IgG and IgM in all APS ACTION samples. We observed a modest positive correlation between anti-NET IgG and anti-dsDNA IgG (r=0.22, p<0.0001) and a stronger correlation between anti-NET IgM and anti-dsDNA IgM (r=0.45, p<0.0001). Targeted testing of anti-single strand DNA (ssDNA) IgG was performed for 80 APS ACTION participants (r=0.18, p=0.1). Among 389 APS ACTION patients, 127 were on hydroxychloroquine at the time of enrollment. The use of hydroxychloroquine was not associated with differences in anti-NET Ab levels (Supplementary Figure 1). A circulating marker of NET release, MPO-DNA complexes, was also quantified in all 389 patients. Anti-NET IgG and IgM both demonstrated positive correlations with MPO-DNA complexes (IgG: r=0.12, p=0.02; IgM: r=0.16, p=0.0012).
Association of anti-NET Abs with extra-criteria APS clinical manifestations.
We next asked whether the presence of anti-NET IgG or IgM might add to traditional aPL in the risk stratification of aPL-positive patients, especially as it relates to extra-criteria manifestations. We found that positive testing for anti-NET IgG was significantly associated with brain white matter lesions assessed by MRI after adjusting for age, sex, ethnicity, and aPL profiles (OR=11, 95% CI 1.9 to 62) (Figure 1D–E). No significant association was found between anti-NET IgM and any extra-criteria manifestations.
Association of anti-NET Abs with complement activation.
Work by our group and others has revealed that one function of anti-NET Abs in patients with lupus (8), APS (3), and COVID-19 (6) is to impair NET degradation, which may present a fertile platform for complement activation (9). We next asked whether anti-NET Abs were associated with complement activation. We measured circulating C3 and C4 among aPL-positive patients. In an unadjusted model, both anti-NET IgG and IgM were inversely correlated with complements C3 (IgG: r=−0.12, p=0.02; IgM: r=−0.18, p=0.0005) and C4 (IgG: r=−0.18, p=0.0003; IgM: r=−0.25, p<0.0001); however, after adjusting for aPL profiles, only the associations between anti-NET IgM and complement levels persisted (C3: r=−0.12, p=0.03; C4: r=−0.13, p=0.03). To look at the potential effect of anti-NET IgM on NET-mediated complement activation, we incubated serum (having either high or low anti-NET IgM activity) with NETs and evaluated complement C3d deposition. Compared with control serum, high anti-NET IgM serum more efficiently deposited complement C3d on NETs (Figure 2A).
Figure 2:
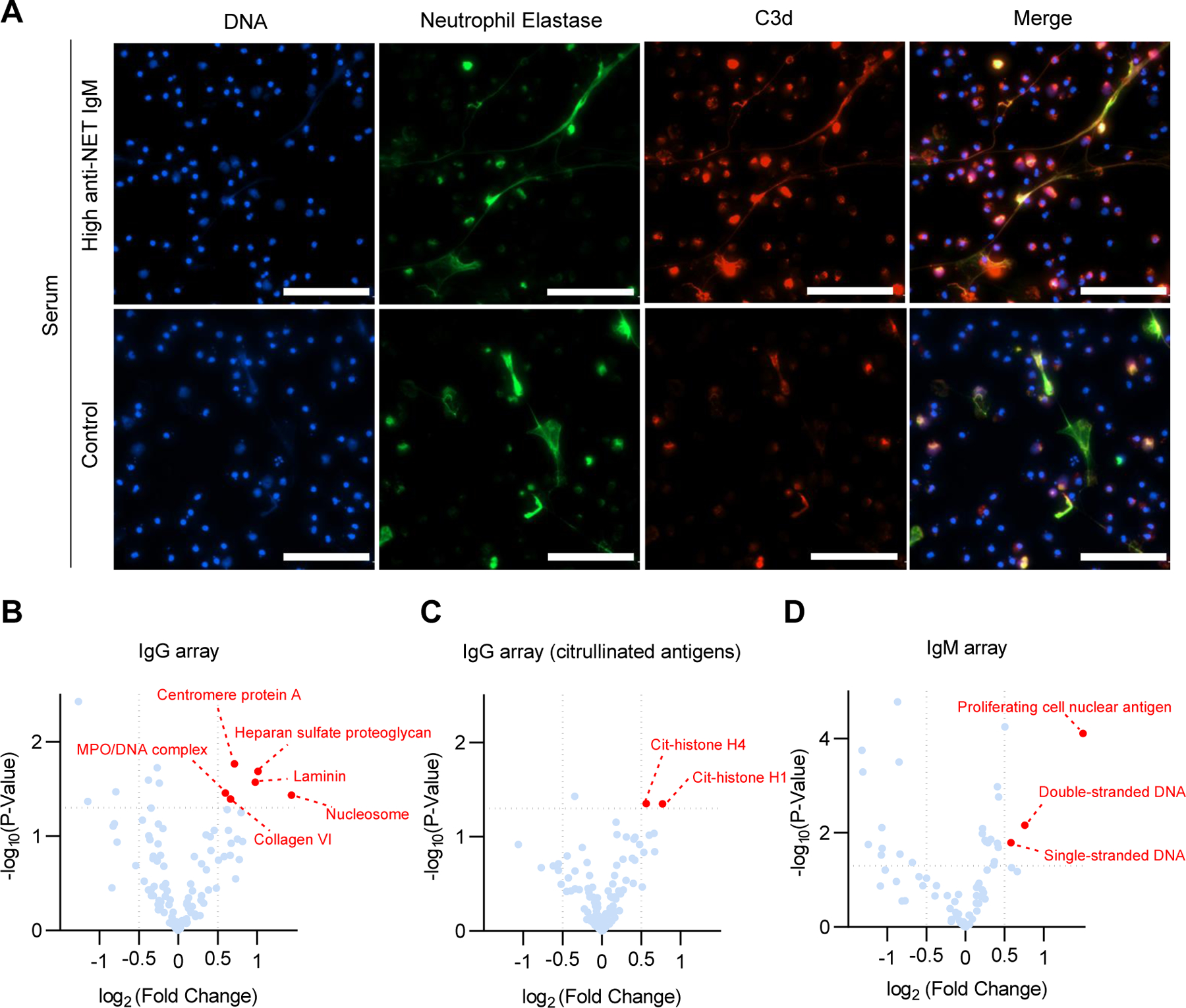
Function and potential antigen specificities of anti-NET antibodies. A, Complement C3d decorating NETs. Control neutrophils were stimulated with PMA to generate NETs, which were then incubated with serum from patients with high (top panels) anti-NET IgM or from healthy controls; scale bars=100 microns. B-D, Autoantibody microarray comparing anti-NET IgG-positive and -negative samples (C-D), and anti-NET IgM-positive and -negative samples (E). Volcano plots demonstrate (in red) antigen specificities that were significantly enriched in the anti-NET-positive groups.
Antigen specificities of anti-NET Abs.
In pursuit of the antigen specificity of anti-NET Abs, we designed a 120-antigen microarray panel with a selection of 65 NET-associated antigens and 55 other autoantigens implicated in human systemic autoimmune diseases (Supplementary Table 2). An on-chip citrullination process was performed to citrullinate 120 autoantigens using a peptidyl-arginine deiminase (PAD) cocktail that contains a mixture of four PAD isoforms (PAD1,2,3,4). Using both the non-citrullinated and citrullinated arrays, we characterized the serum of 214 aPL-positive patients. Positive testing for anti-NET IgG was significantly associated with the following autoantibodies: centromere protein A (a histone H3 variant), citrullinated-histone H1 and H4, collagen VI, heparan sulfate proteoglycan, laminin, MPO-DNA complexes, and nucleosomes. Meanwhile, anti-NET IgM positivity associated with autoantibodies recognizing single-stranded DNA, double-stranded DNA, and proliferating cell nuclear antigen (a DNA clamp) (Figure 2B–D). To validate the microarray findings, we selected two identified antigens (heparan sulfate proteoglycan and nucleosome) and developed ELISAs. We then assessed levels of anti-heparan sulfate proteoglycan IgG and anti-nucleosome IgG among 40 aPL-positive patients. High levels of anti-heparan sulfate proteoglycan IgG and anti-nucleosome IgG were detected in patients who were also positive for anti-NET IgG (Supplementary Figure 2).
DISCUSSION
In this study, we found high levels of anti-NET IgG and/or IgM in almost half of the aPL-positive patients, none of whom had been diagnosed with another systemic autoimmune disease such as lupus. Both anti-NET IgG and IgM tracked with high levels of circulating NETs. Clinically, positive testing for anti-NET IgG was able to identify patients with brain white matter lesions. Meanwhile, anti-NET IgM tracked with complement consumption as measured by low complement C3 and C4; furthermore, APS patient serum with high levels of anti-NET IgM enhanced C3d deposition on NETs. The presented data also suggested that anti-NET IgG activity in aPL-positive patients was more likely to be driven by reactivity with protein antigens such as histones as opposed to the DNA component of NETs. At the same time, anti-NET IgM activity appeared more likely to target DNA or the associated DNA clamp known as proliferating cell nuclear antigen.
Several studies have provided evidence that NET-targeting antibodies are present in various autoimmune diseases, where they may help propel disease pathogenesis. For example, one interesting study demonstrated that serum from lupus patients with IgG activity against NETs degraded NETs poorly; such patients were more likely to have lupus nephritis (8). In a small single-center study of primary APS patients, our group found high anti-NET activity in some APS patients, at levels similar to or higher than those seen in lupus (3). There, we also showed that high levels of anti-NET Abs protected NETs from nuclease digestion in serum (3). In the current study, we not only validated the presence of anti-NET Abs among traditional APS patients in a large, multinational cohort, but also demonstrated for the first time that anti-NET Abs were detectable in many aPL-positive patients who do not have classifiable APS features. Such anti-NET Abs may help identify patients at risk for certain extra-criteria manifestations such as brain white matter lesions.
While complement activation has been suggested to play an important role in the pathophysiology of the thrombotic and obstetric complications of APS, the mechanisms by which the complement system is activated in APS are incompletely understood (10). Existing studies, typically focused on aβ2GPI IgG, have suggested that aPL can bind to C1q, activating the classical complement pathway. However, the predominant subclass of aβ2GPI and aCL among APS patients is IgG2, which has a relatively weak ability to activate complement as compared with IgG1 and IgG3 (10). NETs have long been suggested to serve as a fertile platform for complement activation. For example, complement C3, C1q, factor B, and properdin (complement-activating glycoprotein) have all been shown to decorate the surface of NETs (9, 11). Moreover, NET-associated proteins such as MPO and cathepsin G can directly bind to and activate properdin, thereby promoting complement activation (12). Potentially fueling this vicious cycle, C1q also appears to shield NETs from clearance by DNase (9). Along similar lines, high anti-NET activity may stabilize NETs, which can then serve as a scaffold for complement activation. We were intrigued to find that the IgM isotype of anti-NET Abs was most strongly associated with complement cascade activation. One recent study demonstrated that IgM can directly interact with C1q and C4b to form a complex on liposomal membranes (13), where the assembled structure can then promote complement-mediated inflammatory responses (13). It is certainly possible that anti-NET IgM can interact with NET-bound C1q and other complement factors and further promote complement activation.
Prior to this work, nothing was known about the antigen-specificities of anti-NET IgG and IgM among APS patients. To address this critical question, we designed a custom autoantigen microarray including 120 carefully selected antigens from a bank of over 500 human autoantigens. Priority was given to antigens associated with NETs in the literature. For example, heparan sulfate proteoglycans are well-known cell-surface and extracellular matrix components (14, 15) that have been detected in NETs, where they potentially modulate anti-microbial activities (15). Utilizing this microarray platform, we identified specific antigen targets that were significantly enriched in individuals positive for either anti-NET IgG or IgM. Among those, several interesting targets were identified, including heparan sulfate proteoglycan, laminin, and nucleosomes. Studies in lupus suggest that nucleosomes can bind to glomerular capillaries, mesangial matrix membranes, and glomerular basement membranes via their high affinity for glomerular constituents such as heparan sulfate and laminin (16, 17). Antibodies targeting nucleosomes thereby contribute to lupus nephritis pathogenesis in murine models (16–18). One can speculate that reactivity of anti-NET Abs with some of these antigens might contribute to endothelial activation in APS. While the role of aPL in activating endothelial cells has been demonstrated both in vitro and in vivo, most of these studies have focused on the activation of APS endothelium by traditional aPL, such as aβ2GPI (19). Our data suggest that, beyond aPL, anti-NET Abs with reactivities against constituents of the endothelium might also contribute to APS endothelial activation integral to inflammation and thrombosis. We can speculate that anti-NET Ab-mediated endothelial dysfunction might disrupt the integrity of the blood brain barrier, thereby promoting an influx of inflammatory proteins into the cerebral parenchyma and provoking brain white matter lesions. Future mechanistic studies looking at the role of anti-NET Abs in this and other thromboinflammatory phenotypes of APS are warranted.
It is well known that a subgroup of primary APS patients can eventually develop classifiable lupus (20). One cohort study of 128 primary APS patients followed for eight years found that 13% developed lupus or lupus-like disease (21). A clinically actionable biomarker is lacking to identify this subgroup of primary APS patients. Interestingly, recent advancements in understanding lupus pathogenesis have suggested that exaggerated NET release and impaired NET clearance promote lupus autoantigen expansion, lupus-specific autoantibody production, and organ-damaging type I interferon response (22). Here we found that many primary APS patients or aPL carriers have NET-stabilizing anti-NET antibodies, which are associated with several serological markers of lupus, such as anti-dsDNA and anti-nucleosome antibodies. While we cannot draw definite conclusions on the value of anti-NET antibodies in identifying lupus-prone primary APS patients, we can speculate that these functional anti-NET IgG/IgM could play a role in the evolution of some primary APS patients toward full-blown lupus.
Our study does have some limitations. Due to the cross-sectional design, we are not able to evaluate the durability of anti-NET Abs in aPL-positive patients. Moreover, the interesting association between anti-NET IgG and brain white matter lesions is hypothesis-generating but will need to be confirmed in other cohorts. While we did not see a positive association between the presence of anti-NET Abs and other microvascular complications of APS, such as aPL-associated nephropathy, only 10 patients with this complication were present in the cohort, which hinders the detection power in our model. Furthermore, while we have assembled a large multinational cohort of aPL-positive patients, only 2% of the studied patients were Black. As disparities in healthcare are rightfully receiving increasing attention, the impact of race and ethnicity on the frequency of anti-NET Abs and associated risk with extra-criteria manifestations remains to be elucidated. While we have identified various specific antibodies that preliminarily track with anti-NET activity, the function and clinical significance of those specific autoantibodies need further evaluation. In particular, it will be important to compare the functional role of DNA-targeting anti-NET antibodies versus those that recognize non-DNA antigens.
In summary, we have further defined a new class of autoantibodies among aPL-positive patients, which may promote complement activation and identify certain extra-criteria APS manifestations. Anticoagulation with vitamin K antagonists remains the current mainstay of therapy for APS. While anticoagulation is reasonably efficacious in preventing aPL-associated thrombosis, it often has no bearing on extra-criteria manifestations of APS, such as cardiac, renal, and neurologic complications. Future studies on the role of anti-NET Abs as clinically-relevant biomarkers—which might lead to preemptive immunomodulatory therapy in some patients—are warranted.
Supplementary Material
Acknowledgments
The APS ACTION registry was created using REDCAP provided by the Clinical and Translational Science Center at Weill Cornell Medical College (CTSC grant UL1 TR000457).
We thank JoAnn Vega, CCRC, for her administrative support as the APS ACTION Global Lead Coordinator. We also want to thank all our APS ACION Members: Guillermo Pons-Estel (Santa Fe, Argentina); Bill Giannakopoulos, Steve Krilis (Sydney, Australia); Guilherme de Jesus, Roger Levy, Flavio Signorelli (Rio de Janeiro, Brazil), Danieli Andrade, Gustavo Balbi (Sao Paulo, Brazil); Ann E. Clarke, Leslie Skeith (Calgary, Canada), Paul R. Fortin (Quebec City, Canada); Lanlan Ji, Zhouli Zhang (Beijing, China), Chengde Yang, Hui Shi (Shanghai, China); Stephane Zuily, Denis Wahl (Nancy, France); Maria G. Tektonidou (Athens, Greece); Cecilia Nalli, Laura Andreoli, Angela Tincani (Brescia, Italy), Cecilia B. Chighizola, Maria Gerosa, Pierluigi Meroni (Milan, Italy), Vittorio Pengo, Chunyan Cheng (Padova, Italy), Giulia Pazzola (Reggio Emilia, Italy), Savino Sciascia, Silvia Foddai, Massimo Radin (Turin, Italy); Stacy Davis (Kingston, Jamaica);Olga Amengual, Tatsuya Atsumi (Sapporo, Japan); Imad Uthman (Beirut, Lebanon); Maarten Limper, Philip de Groot (Utrecht, The Netherlands); Guillermo Ruiz - Irastorza, Amaia Ugarte (Barakaldo, Spain), Ignasi Rodriguez-Pinto, Ricard Cervera, Roberto Ríos-Garcés, Giuseppe Barilaro, Jose Pardos-Gea (Barcelona, Spain), Esther Rodriguez Almaraz, Maria Jose Cuadrado (Madrid, Spain), Maria Angeles Aguirre Zamorano, Chary Lopez-Pedrera (Cordoba, Spain); Bahar Artim-Esen, Murat Inanc (Istanbul, Turkey); Maria Laura Bertolaccini, Hannah Cohen, Maria Efthymiou, Munther Khamashta, Ian Mackie, Giovanni Sanna (London, UK); Jason Knight, Yu Zuo (Ann Arbor, Michigan, US), Michelle Petri (Baltimore, Maryland, US), Rebecca K. Leaf (Boston, Massachusetts, US), Robert Roubey (Chapel Hill, North Carolina, US), Thomas Ortel (Durham, North Carolina, US), Emilio Gonzalez, Rohan Willis (Galveston, Texas, US), Nina Kello (New Hyde Park, New York, US), Michael Belmont, Steven Levine, Jacob Rand, Medha Barbhaiya, Doruk Erkan, Jane Salmon, Michael Lockshin (New York City, New York, US), Ali A. Duarte Garcia (Rochester, Minnesota, US), and D. Ware Branch (Salt Lake City, Utah, US).
REFERENCES
- 1.Garcia D, Erkan D. Diagnosis and Management of the Antiphospholipid Syndrome. N Engl J Med 2018;378(21):2010–21. [DOI] [PubMed] [Google Scholar]
- 2.Tambralli A, Gockman K, Knight JS. NETs in APS: Current Knowledge and Future Perspectives. Curr Rheumatol Rep 2020;22(10):67. [DOI] [PubMed] [Google Scholar]
- 3.Zuo Y, Yalavarthi S, Gockman K, Madison JA, Gudjonsson JE, Kahlenberg JM, et al. Anti-Neutrophil Extracellular Trap Antibodies and Impaired Neutrophil Extracellular Trap Degradation in Antiphospholipid Syndrome. Arthritis Rheumatol 2020;72(12):2130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erkan D, Sciascia S, Bertolaccini ML, Cohen H, Committee AAE. Antiphospholipid Syndrome Alliance for Clinical Trials and International Networking (APS ACTION): 10-Year Update. Curr Rheumatol Rep 2021;23(6):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali RA, Gandhi AA, Meng H, Yalavarthi S, Vreede AP, Estes SK, et al. Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat Commun 2019;10(1):1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuo Y, Yalavarthi S, Navaz SA, Hoy CK, Harbaugh A, Gockman K, et al. Autoantibodies stabilize neutrophil extracellular traps in COVID-19. JCI Insight 2021;6(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40(9):1725. [DOI] [PubMed] [Google Scholar]
- 8.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A 2010;107(21):9813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leffler J, Martin M, Gullstrand B, Tyden H, Lood C, Truedsson L, et al. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol 2012;188(7):3522–31. [DOI] [PubMed] [Google Scholar]
- 10.Chaturvedi S, Brodsky RA, McCrae KR. Complement in the Pathophysiology of the Antiphospholipid Syndrome. Front Immunol 2019;10:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuen J, Pluthero FG, Douda DN, Riedl M, Cherry A, Ulanova M, et al. NETosing Neutrophils Activate Complement Both on Their Own NETs and Bacteria via Alternative and Non-alternative Pathways. Front Immunol 2016;7:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Flynn J, Dixon KO, Faber Krol MC, Daha MR, van Kooten C. Myeloperoxidase directs properdin-mediated complement activation. J Innate Immun 2014;6(4):417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharp TH, Boyle AL, Diebolder CA, Kros A, Koster AJ, Gros P. Insights into IgM-mediated complement activation based on in situ structures of IgM-C1-C4b. Proc Natl Acad Sci U S A 2019;116(24):11900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marques C, Reis CA, Vives RR, Magalhaes A. Heparan Sulfate Biosynthesis and Sulfation Profiles as Modulators of Cancer Signalling and Progression. Front Oncol 2021;11:778752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu D, Olson J, Cole JN, van Wijk XM, Brinkmann V, Zychlinsky A, et al. Heparan Sulfate Modulates Neutrophil and Endothelial Function in Antibacterial Innate Immunity. Infect Immun 2015;83(9):3648–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kramers C, Hylkema MN, van Bruggen MC, van de Lagemaat R, Dijkman HB, Assmann KJ, et al. Anti-nucleosome antibodies complexed to nucleosomal antigens show anti-DNA reactivity and bind to rat glomerular basement membrane in vivo. J Clin Invest 1994;94(2):568–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mjelle JE, Rekvig OP, Fenton KA. Nucleosomes possess a high affinity for glomerular laminin and collagen IV and bind nephritogenic antibodies in murine lupus-like nephritis. Ann Rheum Dis 2007;66(12):1661–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller S, Dieker J, Tincani A, Meroni PL. Pathogenic anti-nucleosome antibodies. Lupus 2008;17(5):431–6. [DOI] [PubMed] [Google Scholar]
- 19.Pierangeli SS, Colden-Stanfield M, Liu X, Barker JH, Anderson GL, Harris EN. Antiphospholipid antibodies from antiphospholipid syndrome patients activate endothelial cells in vitro and in vivo. Circulation 1999;99(15):1997–2002. [DOI] [PubMed] [Google Scholar]
- 20.Chen HH, Lin CH, Chao WC. Risk of Systemic Lupus Erythematosus in Patients With Anti-phospholipid Syndrome: A Population-Based Study. Front Med (Lausanne) 2021;8:654791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez-Puerta JA, Martin H, Amigo MC, Aguirre MA, Camps MT, Cuadrado MJ, et al. Long-term follow-up in 128 patients with primary antiphospholipid syndrome: do they develop lupus? Medicine (Baltimore) 2005;84(4):225–30. [DOI] [PubMed] [Google Scholar]
- 22.Yu Y, Su K. Neutrophil Extracellular Traps and Systemic Lupus Erythematosus. J Clin Cell Immunol 2013;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


