Abstract
Intrathymic injection is used in several T cell-associated immunological studies to deliver cells or other substances directly into the thymus. Here, we describe the intrathymic injection procedure involving surgical incision of the mouse with or without a thoracotomy. Though this procedure can result in poor recovery, postsurgical complications, and distress to the animal, it is actually a simple procedure that can be carried out relatively easily and quickly with experience.
Keywords: Thymus, Mouse, Intrathymic injection, Thoracotomy, Surgical incision
1. Introduction
Thymus is the essential organ for differentiation and selection of immunocompetent T lymphocytes. It comprises two distinct, symmetrical pyramidal lobes, which are in close contact and connected by an isthmus of connective tissue. This specialized primary lymphoid organ is present in all jawed vertebrates, and is located anterior to the heart and posterior to the sternum in the midline of the thoracic cavity (in the anterior superior mediastinum). Thymus is the first lymphoid organ to develop and in mice, its size is maximal just before puberty followed by a gradual decrease of size and function along with age (age-associated involution) [1].
Intrathymic injection is used to study questions of tolerance induction [2–4], tumor induction [5], cell transplantation [6], T cell-specific gene therapy [7, 8], and T cell development [9–12] in mice. This process can be accomplished by several means including surgical incision of the sternum for direct visualization, accessibility, and injection into the thymus [6–16]; a blind approach following a small skin incision [17, 18] or directly into the thoracic cavity [19]; or an ultrasound-guided approach [20, 21]. As intrathymic injection involving thoracic surgery may cause pain and distress and result in postoperative complications, the latter less invasive techniques, not requiring a thoracotomy, have been developed. While success rates can be low by the blind injection methods, ultrasound-mediated guidance can alleviate these issues, allowing visualization of both the needle and the injected material as it enters the thymus. Nevertheless, many researchers may not have access to ultrasound equipment for the implementation of this procedure. Here, we describe the techniques involving thoracotomy as well as the blind-injection approach involving a small skin incision.
2. Materials
Sterile drape.
Sterile latex surgical gloves.
Sterile gauge sponge or swab.
1 cc insulin syringe, with 29 1/2 G ultrafine needle.
Betadine.
Surgical board.
Rubber bands.
Eye moisture salve.
Forceps, scissors appropriate for incision.
Wound clip.
10 μL syringe (Hamilton Co; Reno, NV), attached with a 27 G needle (no dead volume).
Anesthetics: Ketamine and xylazine (see Notes 1 and 2). Ketamine and xylazine should be diluted in 1× PBS. Ketamine/xylazine anesthetic should be administered at a dose of 0.1 mg/g mouse weight and 0.02 mg/g mouse weight for ketamine and xylazine, respectively.
3. Procedures
3.1. Intrathymic Injection with Thoracotomy
Anesthetize the mice with ketamine and xylazine administration at a dose/volume of 0.01 mL/g mouse weight (~200 μL for a 20 g mouse) via intraperitoneal injection (use 1 cc insulin syringe, with 29 1/2 G ultrafi ne needle) (see Notes 3 and 4).
Rinse the work area with betadine and lay down a sterile drape.
Place the anesthetized mouse on its back on a surgical board and immobilize it by strapping its feet with rubber bands (Fig. 1a) (see Note 5).
Loosely stretch an additional rubber band across the mouth to hold back the head and gently pull out the tongue with forceps so that the mouse does not asphyxiate.
Place the mouse or board so that the head of the animal is toward you.
Apply eye moisture salve to each eye to prevent the cornea from drying out.
Swab the chest and neck area with betadine.
Pinch skin at upper thoracic region with forceps and make a small longitudinal midline cut with a fi ne delicate scissors. Continue incision through the skin with scissors; make one continuous incision up to the xiphoid process visible as white “V” under the skin (from the maxillary to the middle of the rib cage).
Separate skin along fascial plane on either side of incision by gently inserting forceps jaw underneath the skin, and spread the cut skin outward on each side with forceps, creating two “flaps” to expose the sternum.
Carefully lift the salivary gland, lying between the larynx and sternal notch, with forceps, make a single cut in the connective tissue attaching it to the rib cage (at the end pointing away from you), and pull the gland superiorly in your direction to visualize the top of the rib cage and trachea, taking care not to tear them (see Note 6).
Using a clean high-quality fi ne forceps, very carefully pinch thin muscle lying on top of the trachea. Once pinched, do not let it go, and pull muscle inferiorly as far as possible (it should eventually tear) to reveal a small invagination at the top of the rib cage.
By introducing a scissors into the invagination make a vertically oriented 3-mm incision (with an upward movement) down through the sternum to bisect the upper sternum at the centerline (slightly to the right, at the level of first two ribs). Gently spread the opened ribcage sideways using the tip of blunt, curved forceps to expose/reveal the thymus, the milky white-translucent-colored organ pulsing through the opening (Fig. 1b) (see Notes 7 and 8).
Fill 10 μL Hamilton syringe (with a 27 G needle) with 10 μL cell suspension and remove air bubbles.
Maintain the split open by pushing it to the side with forceps and with the other hand insert the needle, bevel up, into the parenchyma of the thymus, 2–3 mm under the thymic capsule. Inject 10 μL of cell suspension or solution in the lobe and withdraw the needle carefully to minimize the backflow. If needed repeat the injection for the other lobe (Fig. 1c) (see Notes 9–11).
Put back down the salivary gland in place, to block the rib cage opening. Release the mouse of rubber bands. Hold the two flaps of skin together with forceps and close the wound by stapling the skin gently with two to three wound clips.
Return mouse to the cage; check to ensure that tongue is still out. Place subsequent mice next to previous ones to conserve heat until they recover from anesthesia (may take up to 2 h for complete recovery) (see Notes 12 and 13).
Fig. 1.
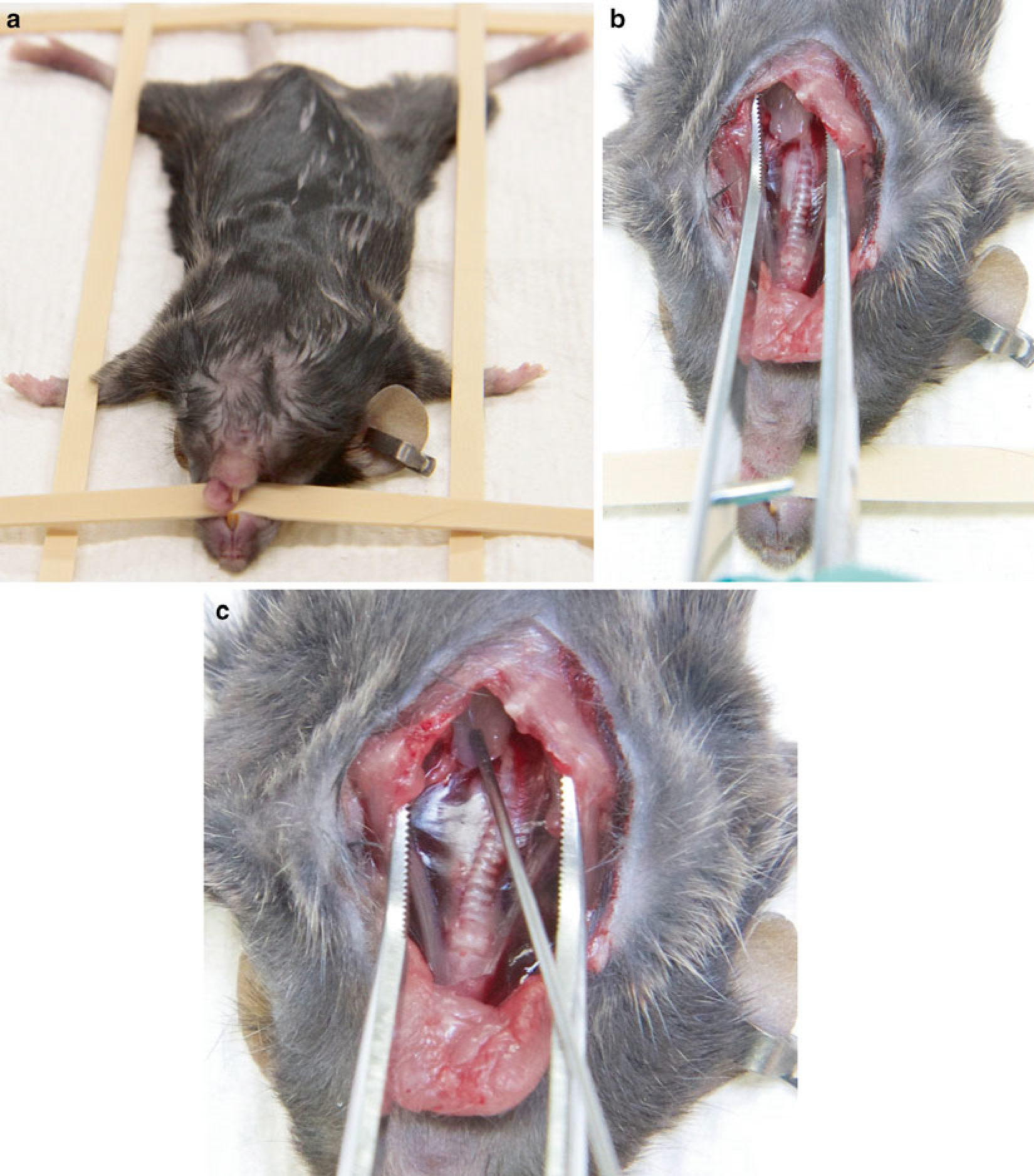
(a) Anesthetized mouse placed and immobilized with rubber bands on surgical board. (b) Midline longitudinal incision, with mouse’s rib cage cut for visualization of thymus. (c) Intrathymic injection, mouse chest held open with forceps and needle with bevel up, is positioned and inserted into the thymus
3.2. Intrathymic Injection Without Thoracotomy
Intrathymic injection can be performed following intercostal injection, a minimally invasive technique, without thoracic cavity exposure.
Anesthetize and immobilize the mice as described above (steps 1–7).
Make a small (4–5 mm) midline incision in the skin above the sternum. Alternatively, you can make a transverse incision in the skin near the second intercostal space, perpendicular to the sternum (Fig. 2a).
Spread the cut skin outward with a forceps.
Position the needle between the third and fourth ribs, at ~30°–40° angle relative to the sternum. With needle vertex pointing toward the manubrium, inject the thymus lobe through the thoracic wall with 10 μL of cells or solution (if the needle does not pass through the thymus, the injection should be successful) (Fig. 2b).
The estimated injection depth is approximately 2–3 mm and both thymic lobes can be accessed through the intercostal space on either side of sternum.
Release the mouse of rubber bands. Hold the two flaps of skin together with forceps and close the wound by stapling the skin gently with two to three wound clips.
Return mouse to the cage for recovery (see Note 14).
Fig. 2.

(a) Small incision made perpendicular to the sternum and across the midline of the upper thoracic region. (b) Intercostal intrathymic injection, the operator inserts the needle between third and fourth ribs
Acknowledgments
We thank N. Taylor and V. Zimmerman for reading and critical comments on this chapter. A special thanks to P. Sarkar for photographs.
4. Notes
Use sterile vials and aseptic techniques to prepare ketamine/xylazine cocktail for surgical anesthesia.
Ketamine and xylazine are controlled substances and therefore should be stored in a locked drawer, at room temperature.
It is easier to inject the thymus of young mice (6-week-old recipients). It can be difficult to inject aged mice due to age-related thymic involution.
Dosing of the anesthetics is crucial and should be evaluated relative to weight, sex, and strain of the mice. Ketamine/xylazine anesthetic should be administered at 100/20 mg/kg mouse weight, ~200 μL for a 20 gm mouse.
Anesthesia takes effect in few minutes and lasts for 20–30 min depending on the mouse strain used. Before starting the procedure, pinch the toe of the mice to assess the anesthetic depth. You can inject a small amount of diluted ketamine solution again if the toe reflex is still active 5 min after ketamine/xylazine anesthetic injection. Do not administer additional ketamine/xylazine anesthetic because the mice will be asleep for a long time and the risk of anesthetic death goes up.
If you are working with more than one mouse, inject next mouse with ketamine/xylazine anesthetic at this point.
At the time of thoracotomy, keep the tip of the scissors away from the heart and lungs. Bleeding may obscure the opening. If necessary soak up the blood with a clean absorbent pad.
Cut only one-third of the sternum. If the tip of the thymus is not visible, you may need to extend the sternum incision.
After administration of cell suspension or solution into the thymus, allow the needle to remain in the lobe for few seconds so that the internal pressure decreases. This gives the injected solution time to redistribute, minimizing leakage after withdrawal.
The injected volume should not exceed 10 μL per lobe (try to inject close to the middle of the lobe ~2–3 mm deep). Due to enormous size variation of the mouse thymus with age, it is difficult to specify the injection depth.
Immediately after injection, flush the syringe three to four times with sterile PBS. Take care not to bend the wire plunger.
To prevent hypothermia after surgery, place animals under a heating lamp while still under anesthesia.
The efficiency of the technique can be improved by rapid surgical incision and injection.
The blind injection technique can be practiced by injecting a dye (e.g., India ink) followed by removal and sectioning of the thymus to observe the site of injection.
References
- 1.Rezzani R, Nardo L, Favero G et al. (2014) Thymus and aging: morphological, radiological, and functional overview. Age 36:313–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen W, Issazadeh S, Sayegh MH et al. (1997) In vivo mechanisms of acquired thymic tolerance. Cell Immunol 179:165–173 [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Sayegh MH, Khoury SJ (1998) Mechanisms of acquired thymic tolerance in vivo: intrathymic injection of antigen induces apoptosis of thymocytes and peripheral T cell anergy. J Immunol 160:1504–1508 [PubMed] [Google Scholar]
- 4.DeMatteo RP, Chu G, Ahn M et al. (1997) Long-lasting adenovirus transgene expression in mice through neonatal intrathymic tolerance induction without the use of immunosuppression. J Virol 71:5330–5335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muto M, Kubo E, Sado T (1987) Development of prelymphoma cells committed to thymic lymphomas during radiation-induced thymic lymphomagenesis in B10 mice. Cancer Res 47:3469–3472 [PubMed] [Google Scholar]
- 6.Goldschneider I, Komschlies KL, Greiner DL (1986) Studies of thymocytopoiesis in rats and mice. I. Kinetics of appearance of thymocytes using a direct intrathymic adoptive transfer assay for thymocyte precursors. J Exp Med 163:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adjali O, Marodon G, Steinberg M et al. (2005) In vivo correction of ZAP-70 immunodeficiency by intrathymic gene transfer. J Clin Invest 115:2287–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seggewiss R, Dunbar CE (2005) A new direction for gene therapy: intrathymic T cell-specific lentiviral gene transfer. J Clin Invest 115:2064–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Obaldia ME, Bell JJ, Bhandoola A (2013) Early T-cell progenitors are the major granulocyte precursors in the adult mouse thymus. Blood 121:64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Obaldia ME, Bell JJ, Wang X et al. (2013) T cell development requires constraint of the myeloid regulator C/EBP-α by the Notch target and transcriptional repressor Hes1. Nat Immunol 14:1277–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scollay RG, Butcher EC, Weissman IL (1980) Thymus cell migration: quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol 10:210–218 [DOI] [PubMed] [Google Scholar]
- 12.Weber BN, Chi AW, Chavez A et al. (2011) A critical role for TCF-1 in T-lineage specification and differentiation. Nature 476:63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guidos CJ, Weissman IL, Adkins B (1989) Intrathymic maturation of murine T lymphocytes from CD8+ precursors. Proc Natl Acad Sci U S A 86:7542–7546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jerabek L, Weissman IL (2002) Intrathymic injection for analysis of T-cell progenitor activity. In: Klug CA, Jordan CT (eds) Hematopoietic stem cell protocols, vol 63, Methods Mol Med. Humana Press, Totowa, NJ, pp 161–165 [DOI] [PubMed] [Google Scholar]
- 15.Hardy RR, Shinton SA (2004) Characterization of B lymphopoiesis in mouse bone marrow and spleen. In: Gu H, Rajewsky K (eds) B cell protocols, Methods in molecular biology. Humana Press, Totowa, NJ, pp 1–24 [DOI] [PubMed] [Google Scholar]
- 16.Rooke R, Benoist C, Mathis D (2000) Intrathymic delivery of MHC genes using recombinant Adenoviruses. In: Kearse KP (ed) T cell protocols: development and activation, Methods in molecular biology. Humana Press, Totowa, NJ, pp 69–79 [DOI] [PubMed] [Google Scholar]
- 17.Liu LL, Du XM, Wang Z et al. (2012) A simplified intrathymic injection technique for mice. Biotech Histochem 87:140–147 [DOI] [PubMed] [Google Scholar]
- 18.de la Cueva T, Naranjo A, de la Cueva E et al. (2007) Refinement of intrathymic injection in mice. Lab Anim (NY) 36:27–32 [DOI] [PubMed] [Google Scholar]
- 19.Adjali O, Montel-Hagen A, Swainson L et al. (2009) In vivo and ex vivo gene transfer in thymocytes and thymocyte precursors. In: Baum C (ed) Genetic modification of hematopoietic stem cells, vol 506, Methods Mol Biol., pp 171–190 [DOI] [PubMed] [Google Scholar]
- 20.Handon RB, Mueller K, Miller SH (2010) An alternative method for intrathymic injections in mice. Lab Anim (NY) 39:248–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuckett AZ, Thornton RH, Shono Y et al. (2014) Image-guided intrathymic injection of multipotent stem cells supports lifelong T-cell immunity and facilitates targeted immunotherapy. Blood 123:2797–2805 [DOI] [PMC free article] [PubMed] [Google Scholar]


