Abstract
Background and Aims:
Insufficient liver regeneration causes post-hepatectomy liver failure and small-for-size syndrome. Identifying therapeutic targets to enhance hepatic regenerative capacity remains urgent. Recently, increased IL-33 was observed in patients undergoing liver resection and in mice after partial hepatectomy (PHx). The present study aims to investigate the role of IL-33 in liver regeneration after PHx and to elucidate its underlying mechanisms.
Approach and Results:
We performed PHx in IL-33−/−, suppression of tumorigenicity 2 (ST2)−/−, and wild type control mice, and found deficiency of IL-33 or its receptor ST2 delayed liver regeneration. The insufficient liver regeneration could be normalized in IL-33−/− but not ST2−/− mice by recombinant murine IL-33 administration. Furthermore, we observed an increased level of serotonin in portal blood from wild type mice, but not IL-33−/− or ST2−/− mice, after PHx. ST2 deficiency specifically in enterochromaffin cells recapitulated the phenotype of delayed liver regeneration observed in ST2−/− mice. Moreover, the impeded liver regeneration in IL-33−/− and ST2−/− mice was restored to normal levels by the treatment with (±)-2,5-dimethoxy-4-iodoamphetamine, which is an agonist of the 5 hydroxytrytamine receptor (HTR)2A. Notably, in vitro experiments demonstrated that serotonin/HTR2A-induced hepatocyte proliferation is dependent on p70S6K activation.
Conclusions:
Our study identified that IL-33 is pro regenerative in a non-injurious model of liver resection. The underlying mechanism involved IL-33/ST2-induced increase of serotonin release from enterochromaffin cells to portal blood and subsequent HTR2A/p70S6K activation in hepatocytes by serotonin. The findings implicate the potential of targeting the IL-33/ST2/serotonin pathway to reduce the risk of post-hepatectomy liver failure and small-for-size syndrome.
INTRODUCTION
After tissue loss, the liver is the only organ that can regenerate and regain the original tissue-to–body weight ratio within a short period of time.[1] Due to the regenerative capacity of the liver, partial hepatectomy (PHx) has been an important therapeutic treatment for liver diseases,[2] especially liver cancers.[3] However, post-hepatectomy liver failure (PHLF), one of the most dangerous and life-threatening complications of PHx, causes approximately 10% mortality rate in patients with liver cancer.[4–6] The occurrence and resolution of PHLF are closely related to the regenerative capacity of the remaining liver tissues.[6] Insufficient liver regeneration in patients with PHx often leads to PHLF.[7,8] Partial liver grafts inadequate in sustaining metabolic demands could cause small-for-size syndrome (SFSS) within the first week of liver transplantation.[9] It is reported that the survival rate in patients with SFSS is about 30% less than that in patients without SFSS.[10] Therefore, to prevent PHLF and to improve the clinical outcomes of small-for-size liver transplantation, it is imperative to develop treatment strategies aimed to accelerate liver regeneration.
The underlying mechanisms of liver regeneration after PHx have been intensively studied in rodent models in the past decades.[11,12] Liver regeneration is an orchestrated process involving a plethora of signaling pathways, including IL-6/signal transducer and activator of transcription (STAT)3,[13] HGF/MET,[14] EGF/EGF receptor (EGFR),[15] and pathways mediated by gut-derived factors (e.g., serotonin, bile acids, endotoxin).[1] Furthermore, a number of cytokines play essential roles in liver regeneration.[16] For example, interferon (IFN)γ directly inhibits hepatocyte proliferation,[17] but the type 1 IFN signaling accelerates liver regeneration after PHx.[18] TNFα,[19,20] IL-4,[21] IL-6, [13] IL-17A,[22] and IL-22[23] promote liver regeneration, while IL-10[24] impedes hepatocyte proliferation after PHx in vivo. Recently, it was reported that PHx could increase the release of IL-33 in patients with cholangiocarcinoma undergoing liver resection.[25] Increased levels of IL-33 in the blood were also observed in mice after PHx.[25] Notably, IL-33 has been implicated as an important factor driving wound healing and tissue repair.[26] It is reported that exogenous IL-33 induces cholangiocyte proliferation in hilar bile duct in the liver of naïve mice.[27] However, the role of IL-33 in hepatocyte proliferation and liver regeneration after PHx has not been investigated.
In the current study, we demonstrate the important role of IL-33 and its receptor (suppression of tumor-igenicity 2 [ST2]) in liver regeneration after PHx. Our data indicate that the underlying mechanism involves IL-33/ST2-mediated serotonin release from the small intestine. Our findings suggest that supplementation of IL-33 might be a promising strategy to treat patients with insufficient liver regeneration.
METHODS
Animal experiments and procedures
IL-33−/− and ST2−/− mice were obtained from Merck & Co. and Dr. Shizuo Akira (Osaka Univesity), respectively, via material transfer agreements. Wildtype (WT) C57BL/6N mice were purchased from Taconic Biosciences. The ST2f/f mice were from KOMP and ChgaCreER mice were from EMMA. ST2f/fChgaCre−/− and ST2f/fChgaCre+/− mice were obtained from Dr. Chuan Wu (National Cancer Institute) by crossing ST2f/f and ChgaCreER mice. ST2f/fChgaCre−/− and ST2f/fChgaCre+/− mice were subjected to 1 mg tamoxifen (Sigma-Aldrich) i.p. daily for consecutive 5 days before experiments. Male mice were used in this study, and all animals were housed under a 12-h light/12-h dark cycle. Weight matched mice (8–14 weeks old) were used. PHx in mice was performed as previously described.[28] Recombinant murine IL-33 (rmIL-33) and (±)-2,5-dimethoxy-4-iodoamphetamine (DOI) were purchased from R&D Systems and Sigma-Aldrich, respectively. Bromodeoxyurdine (BrdU) (50 mg/kg; Sigma-Aldrich) was i.p. injected into hepatectomized mice 2 h before sacrifice. All animal studies described have been approved by the Institutional Animal Care and Use Committee at the UTHealth.
Primary hepatocyte culture and treatment
Primary murine hepatocytes were isolated as previously described.[28] Live hepatocytes (1 × 105) were seeded in each well of a 6-well collagen-coated plate. After 4 h, the culture medium was deprived of fetal bovine serum (FBS) for at least 48 h. Subsequently, hepatocytes were treated with 5 μM DOI or 10 μM 70 kDa ribosomal protein S6 kinase (p70S6K) inhibitor, LY-2584702 (MedChemExpress), in 5% FBS.
Immunohistochemistry
Liver samples were harvested and fixed in 10% formalin for at least 24 h. Paraffin-embedded tissues were cut into 5-μm-thick sections. Liver sections were rehydrated and processed for antigen retrieval. Endogenous peroxidase was inactivated, and nonspecific signals were blocked using 2.5% horse serum (Vector Laboratories). Then the liver sections were incubated with primary antibodies at 4°C overnight, followed by horseradish peroxidase–conjugated or alkaline phosphatase–conjugated secondary antibodies (Vector Laboratories). All sections were counterstained with hematoxylin. The primary antibodies used were rabbit anti-Ki67, rabbit anti-ST2 (Abcam), and mouse anti-BrdU (Cell Signaling Technology).
Western blotting
Liver tissues and cell samples were homogenized in radio immunoprecipitation assay buffer (Thermo Fisher Scientific) containing protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific). Protein concentrations were quantified with a commercial BCA protein assay kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. At least 30 μg of total protein from each sample were loaded and separated by gel electrophoresis and then transferred to nitrocellulose membranes. After blocking, membranes were incubated with primary antibodies at 4°C overnight under shaking conditions. The membranes were then incubated with horseradish peroxidase–conjugated secondary antibodies (Jackson ImmunoResearch Laboratories). Detection and quantification of protein bands were performed using a ChemiDoc Imaging System with ImageLab Software (Bio-Rad Laboratories). The primary antibodies used were mouse anti-β-actin (Sigma-Aldrich), mouse anti–proliferating cell nuclear antigen (PCNA), rabbit anti-phospho STAT3 (Tyr705), rabbit anti-STAT3, rabbit anti-phospho EGFR (Tyr1086), rabbit anti-EGFR, rabbit anti-phospho-MET (Thy1234/1235), rabbit anti-MET, rabbit anti-phospho-AKT (Ser473), rabbit anti-AKT, rabbit anti-phospho ERK1/2 (Thr202/Tyr204), rabbit anti-EKR1/2, rabbit anti-phospho-p70S6K (Thr421/Ser424), and rabbit anti-p70S6K (Cell Signaling Technology).
Quantitative real time PCR
Total RNA was purified from liver tissues using Pure-Link RNA Mini Kit (Invitrogen) according to the manufacturer’s protocol, and 1 μg RNA was reverse-transcribed into cDNA using an iScript cDNA Synthesis Kit (Bio-Rad Laboratories). Relative quantitative gene expression was measured with SYBR Green PCR Supermix from Bio-Rad Laboratories. 18S rRNA was used as an internal standard. The primer sequences are as follows (5′-3′): 18S rRNA sense: ACGGAAGGGCACCACCAGGA, anti-sense: CACCACCACCCACGGAATCG; HTR2A sense: TAATGCAATTAGGTGACGACTCG, anti-sense: GCAGGAGAGGTTGGTTCTGTTT; HTR2B sense: GAACAAAGCACAACTTCTGAGC, anti-sense: CCGCGAGTATCAGGAGAGC; and HTR2C sense: CTAATTGGCCTATTGGTTTGGCA, anti-sense: CGGGAATTGAAACAAGCGTCC.
Serotonin measurement
Serotonin levels in portal vein sera and liver tissues were measured using the serotonin ELISA kit (Fitzgerald Industries International) according to the manufacturer’s manual.
Assays of blood samples
Endotoxin and total bile acid levels in portal vein sera were measured using the endotoxin assay kit (Gen-Script) and the total bile acid assay kit (Diazyme Laboratories), respectively.
Statistical analysis
Results are presented as mean ± SEM. The results were assessed using two-tailed unpaired Student’s t test, one-way ANOVA, or two-way ANOVA. p < 0.05 was considered statistically significant.
RESULTS
Deficiency of IL-33 or ST2 delays liver regeneration after PHx
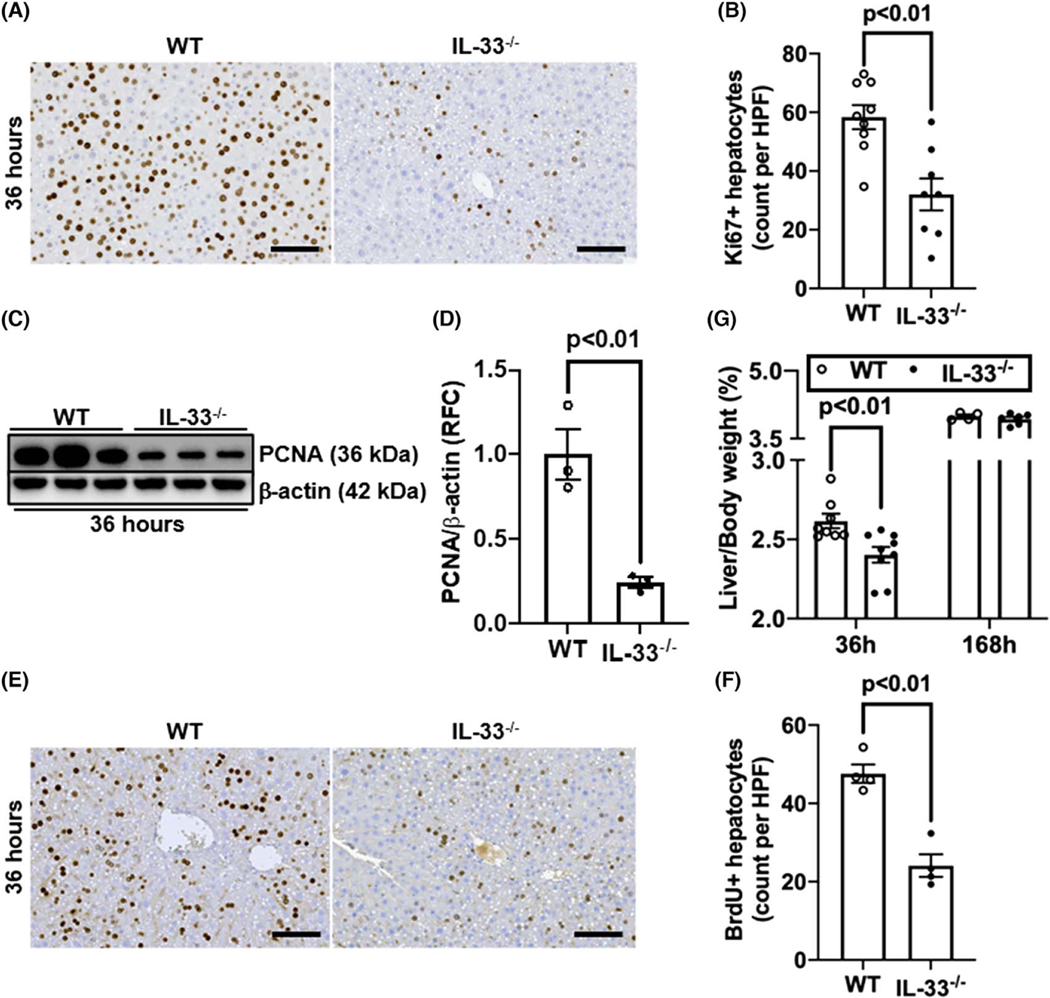
To investigate the role of IL-33 in liver regeneration, we subjected WT and IL-33−/− mice to PHx and measured Ki67-positive proliferating hepatocytes after 36 h, which is the peak time point of hepatocyte proliferation.[12,29] The data showed much fewer Ki67+ hepatocytes in IL-33−/− than WT mice (Figure 1A,B). Western blotting for PCNA and immunostaining for BrdU incorporation further confirmed the attenuation of hepatocyte proliferation in the livers of IL-33−/− mice compared with WT mice (Figure 1C–F). Consistently, the liver-to-body weight ratio was significantly lower in IL-33−/− mice than WT mice at 36 h after PHx (Figure 1G). However, on day 7 after PHx when liver mass was mostly restored,[1] the liver-to-body weight ratio was comparable between WT and IL-33−/− mice (Figure 1G), indicating that IL-33 deficiency delayed, but did not abrogate, liver regeneration after PHx.
FIGURE 1.
IL-33 deletion delays liver regeneration after partial hepatectomy (PHx). Wild-type (WT) and IL-33−/− mice were subjected to PHx. (A,B) Ki67 immunostaining was performed in liver sections collected at 36 h after PHx (scale bar: 100 μm). Ki67-positive hepatocytes were quantified (n = 8-9/group). (C,D) Proliferating cell nuclear antigen (PCNA) levels in liver tissues at 36 h after PHx were measured by immuno-blotting and the ratios of PCNA/β-actin were quantified (n = 3/group). (E,F) Bromodeoxyurdine (BrdU) incorporation was performed in liver sections at 36 h after PHx (scale bar: 100 μm). BrdU positive hepatocytes were quantified (n = 4/group). (G) The ratios of liver/body weight were analyzed at 36 h and 168 h after PHx (n = 4–9/group). Two-tailed unpaired Student’s t test was performed in (B), (D), and (F). Two-way ANOVA was performed in (G). HPF, high-power field; RFC, relative fold change
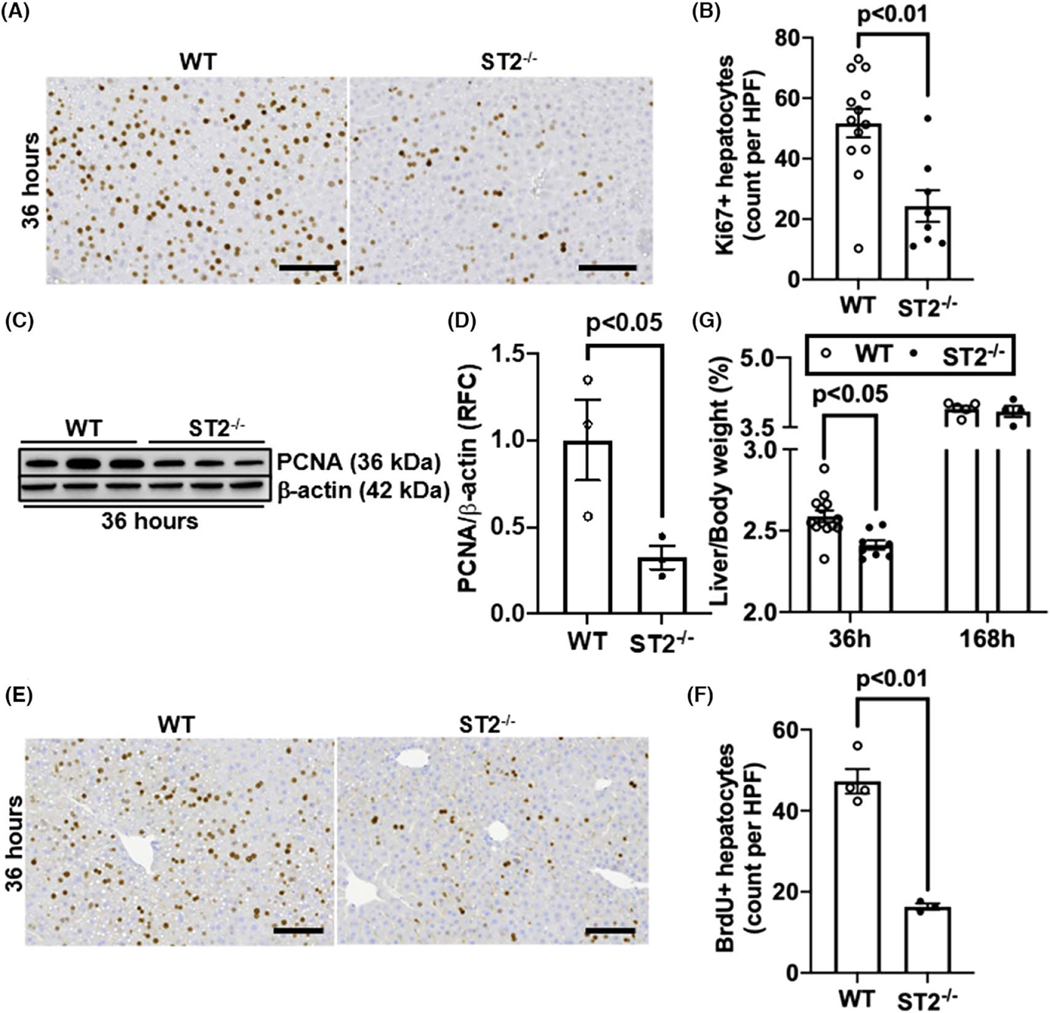
ST2 has been identified as the orphan receptor for IL-33.[30] If the pro-regenerative function of IL-33 is mediated by ST2, we would expect to observe a delayed liver regeneration in ST2−/− mice after PHx. Indeed, our data showed much fewer numbers of Ki67-positive hepatocytes and lower levels of hepatic PCNA expression in ST2−/− mice than WT mice (Figure 2A–D). Consistently, hepatocyte DNA replication measured by BrdU staining was attenuated in ST2−/− mice (Figure 2E,F). Similar to the IL-33−/− mice, ST2−/− mice exhibited lower liver-to-body weight ratio than WT mice at 36 h, but not on day 7, after PHx (Figure 2G).
FIGURE 2.
ST2-deletion delays liver regeneration after PHx. WT and suppression of tumorigenicity 2 (ST2)−/− mice were subjected to PHx. (A, B) Ki67 immunostaining was performed in liver sections collected at 36 h after PHx (scale bar: 100 μm). Ki67-positive hepatocytes were quantified (n = 8–13/group). (C,D) PCNA levels in liver tissues at 36 h after PHx were measured by immunoblotting and the ratios of PCNA/β-actin were quantified (n = 3/group). (E,F) BrdU incorporation was performed in liver sections at 36 h after PHx (scale bar: 100 μm). BrdU positive hepatocytes were quantified (n = 3-4/group). (G) The ratios of liver/body weight were analyzed at 36 h and 168 h after PHx (n = 4–13/group). Two-tailed unpaired Student’s t test was performed in (B), (D), and (F). Two-way ANOVA was performed in (G)
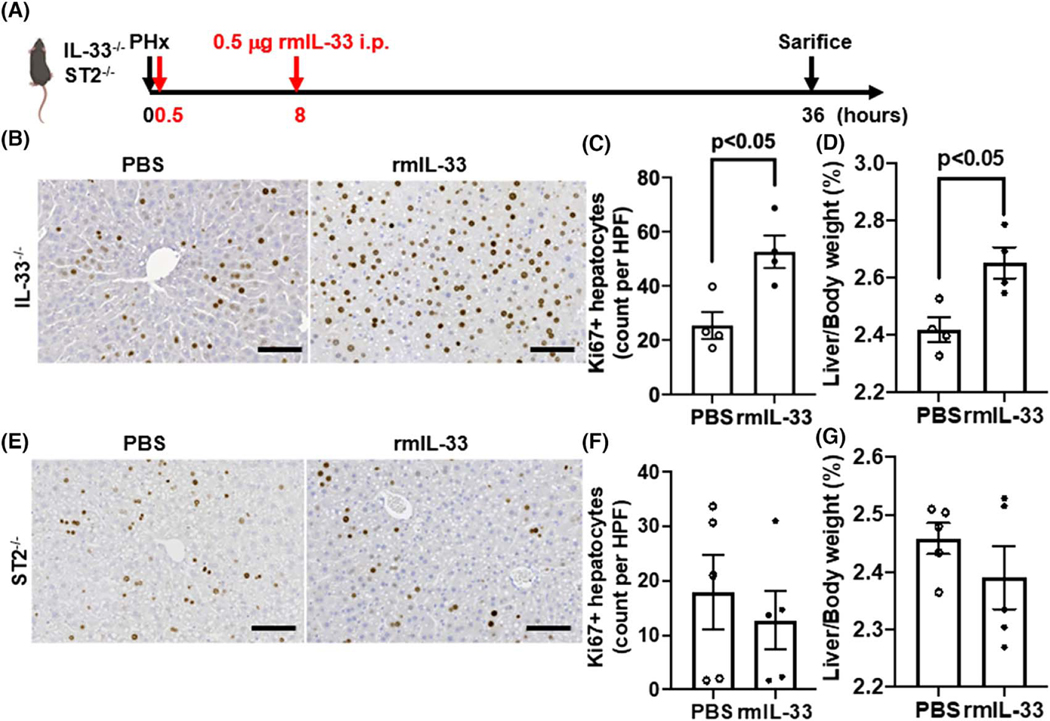
To further corroborate the finding that IL-33, through ST2, plays a critical role in liver regeneration, we treated IL-33−/− and ST2−/− mice with exogenous rmIL-33 after PHx (Figure 3A). Notably, the rmIL-33 treatment of IL-33−/− mice after PHx dramatically increased the number of Ki67-positive hepatocytes and the liver-to-body weight ratio to the levels observed in WT mice (Figure 3B–D). However, rmIL-33 failed to promote hepatocyte proliferation in ST2−/− mice (Figure 3E–G). Together, these data demonstrate that the deficiency of IL-33 or ST2 causes insufficient hepatocyte proliferation after PHx, leading to delayed liver regeneration.
FIGURE 3.
Exogenous IL-33 promotes liver regeneration in IL-33−/− mice after PHx. (A) Strategy of exogenous IL-33 treatments in IL-33−/− and ST2−/− mice after PHx. (B,C) Ki67 immunostaining was performed in liver sections of IL-33−/− mice with PBS or recombinant murine IL-33 (rmIL-33) treatment at 36 h after PHx (scale bar: 100 μm). Ki67-positive hepatocytes were quantified (n = 4/group). (D) The ratios of liver/body weight were analyzed at 36 h after PHx (n = 4/group). (E,F) Ki67 immunostaining was performed in liver sections of ST2−/− mice with PBS or rmIL-33 treatment at 36 h after PHx (scale bar: 100 μm). Ki67-positive hepatocytes were quantified (n = 5/group). (G) The ratios of liver/body weight were analyzed at 36 h after PHx (n = 5/group). Two-tailed unpaired Student’s t test was performed in (C), (D), (F), and (G)
Gut-derived serotonin mediates the effect of IL-33/ST2 on liver regeneration
To elucidate how IL-33/ST2 signaling promotes liver regeneration, we examined signaling pathways reported to contribute to liver regeneration. After PHx, STAT3 is activated at early time points and initiates hepatocytes to enter cell cycle.[16,31] The HGF/MET and EGF/EGFR pathways are directly mitogenic to hepatocytes, and depletion of both signaling pathways results in liver failure after PHx.[1,32] We performed western blotting analysis after PHx to measure the levels of phospho-STAT3/STAT3, phospho-EGFR/EGFR, and phospho-MET/MET, as well as their downstream targets, including phospho-AKT/AKT and phospho-ERK1/2/ERK1/2. The data showed that all of these signaling pathways were activated to similar degrees between WT and ST2−/− livers (Figure S1A–E), suggesting that the STAT3, MET, and EGFR signaling pathways were not involved in the mechanism of IL-33/ST2-regulated liver regeneration.
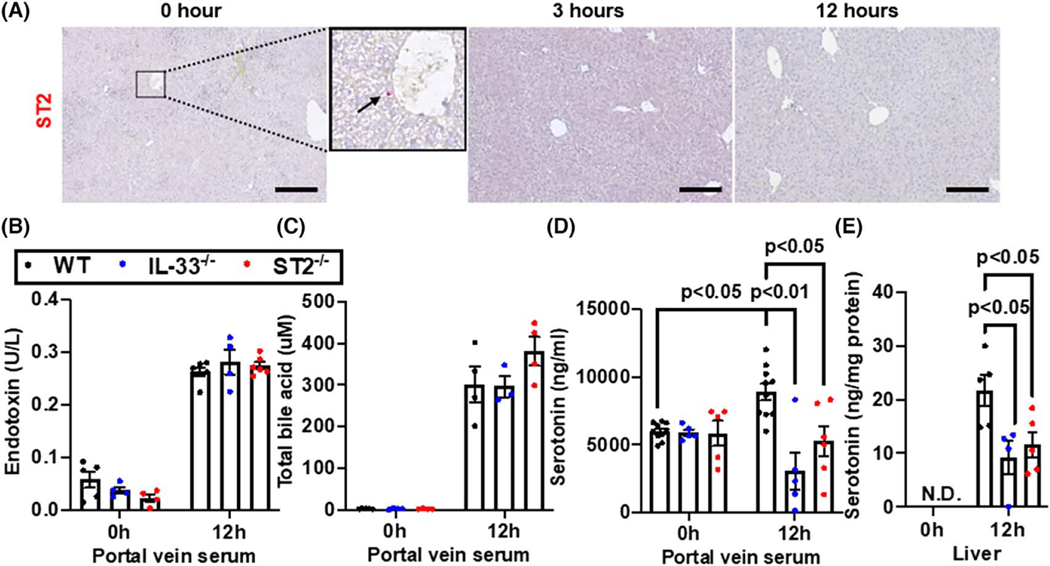
In searching for clues of the underlying mechanism, we first measured serum levels of IL-33. We observed that serum IL-33 levels increased after PHx with a peak at 12 h and then returned to baseline levels by 24 h after surgery (Figure S2). This observation suggests that 12 h after PHx is a good time point to study the mechanistic involvement of IL-33. We next set out to measure the expression of IL-33 receptor (ST2) in the liver; however, we could not detect any ST2-positive cells in the liver after PHx (Figure 4A). This result suggested to us that an extrahepatic signaling might be involved in IL-33-regulated liver regeneration. Gut-liver axis has been widely reported to contribute to liver regeneration.[1,33] Among the gut-derived mediators, endotoxin,[34,35] bile acids,[36,37] and serotonin[38,39] have been associated with liver regeneration. We measured these factors in the portal vein blood at 0 h and 12 h after PHx. Comparable levels of endotoxin and total bile acids were detected among WT, IL-33−/−, and ST2−/− mice (Figure 4B,C). Interestingly, the serotonin levels in portal blood were elevated at 12 h after PHx in WT mice, but not in IL-33−/− and ST2−/− mice (Figure 4D). Administration of exogenous IL-33 could restore serotonin elevation in IL-33−/− mice but not in ST2−/− mice (Figure S3A,B). The levels of serotonin in the liver at 12 h after PHx were also significantly higher in WT mice than IL-33−/− and ST2−/− mice, whereas serotonin was undetectable at 0 h in the livers of all three strains of mice (Figure 4E). These data suggested that a defect in serotonin release from the gut might account for the delayed liver regeneration in IL-33−/− and ST2−/− mice.
FIGURE 4.
Deletion of IL-33 or ST2 attenuates PHx-induced increase of serotonin in the portal blood and liver. (A) Immunohistochemistry (IHC) staining for ST2 in liver sections at 0, 3, and 12 h after PHx. The arrow points to a ST2-positive cell in the inset representative images from n = 3–8 mice/group were shown. Scale bar: 200 μm. (B-E) WT, IL-33−/− and ST2−/− mice were subjected to PHx. Portal vein sera and livers were collected at 0 and 12 h after PHx. Portal blood levels of endotoxin (B), total bile acid (C), and serotonin (D) were measured (n = 3-10/group). (E) Serotonin levels in liver tissues were also measured (n = 4-5/group). Two-way ANOVA was performed in (B), (C), (D), and (E)
Enterochromaffin cell–specific ST2 contributes to the pro-regenerative function of serotonin in PHx
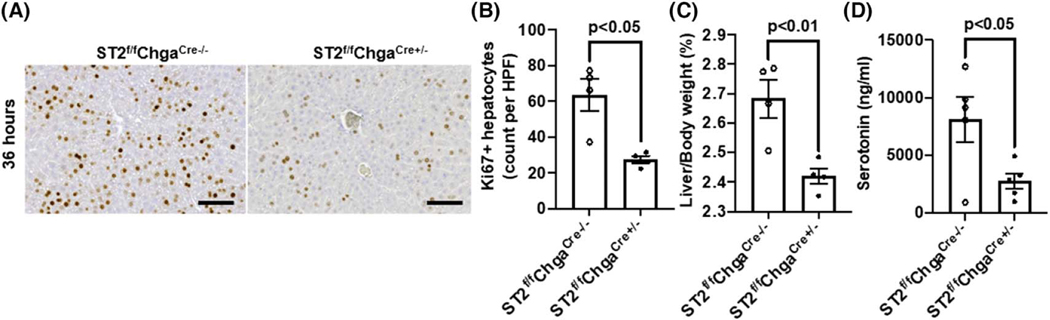
Most of the body’s serotonin (~95%) is synthesized and located in the enterochromaffin cells in the small intestine.[39,40] A recent study demonstrated that the IL-33/ST2 signaling in enterochromaffin cells plays a critical role in serotonin release from these cells.[41] Thus, we hypothesized that mice with enterochromaffin cell–specific deletion of ST2 (ST2f/fChgaCre+/−) would exhibit a delay in liver regeneration after PHx. Indeed, compared with WT littermates (ST2f/fChgaCre−/−), the ST2f/fChgaCre+/− mice showed significantly attenuated hepatocyte proliferation at 36 h after PHx (Figure 5A,B). Consequently, the liver-to-body weight ratio was lower in ST2f/fChgaCre+/− mice than WT littermates at 36 h (Figure 5C). Consistently, the levels of serotonin in portal vein sera were much lower in ST2f/fChgaCre+/− mice than WT littermates at 12 h after PHx (Figure 5D).
FIGURE 5.
Enterochromaffin cell–specific ST2 knockout mice show attenuated hepatocyte proliferation after PHx. ST2f/fChgaCre+/− mice and cohoused ST2f/fChgaCre−/− littermates were subjected to PHx. (A,B) Ki67 immunostaining was performed in liver sections collected at 36 h after PHx (scale bar: 100 μm). Ki67-positive hepatocytes were quantified (n = 4/group). (C) The ratios of liver/body weight were analyzed at 36 h after PHx (n = 4/group). (D) Serotonin levels in portal vein sera were determined at 12 h after PHx (n = 5/group). Two-tailed unpaired Student’s t test was performed in (B), (C), and (D)
Serotonin agonist could normalize liver regeneration in IL-33−/− and ST2−/− mice
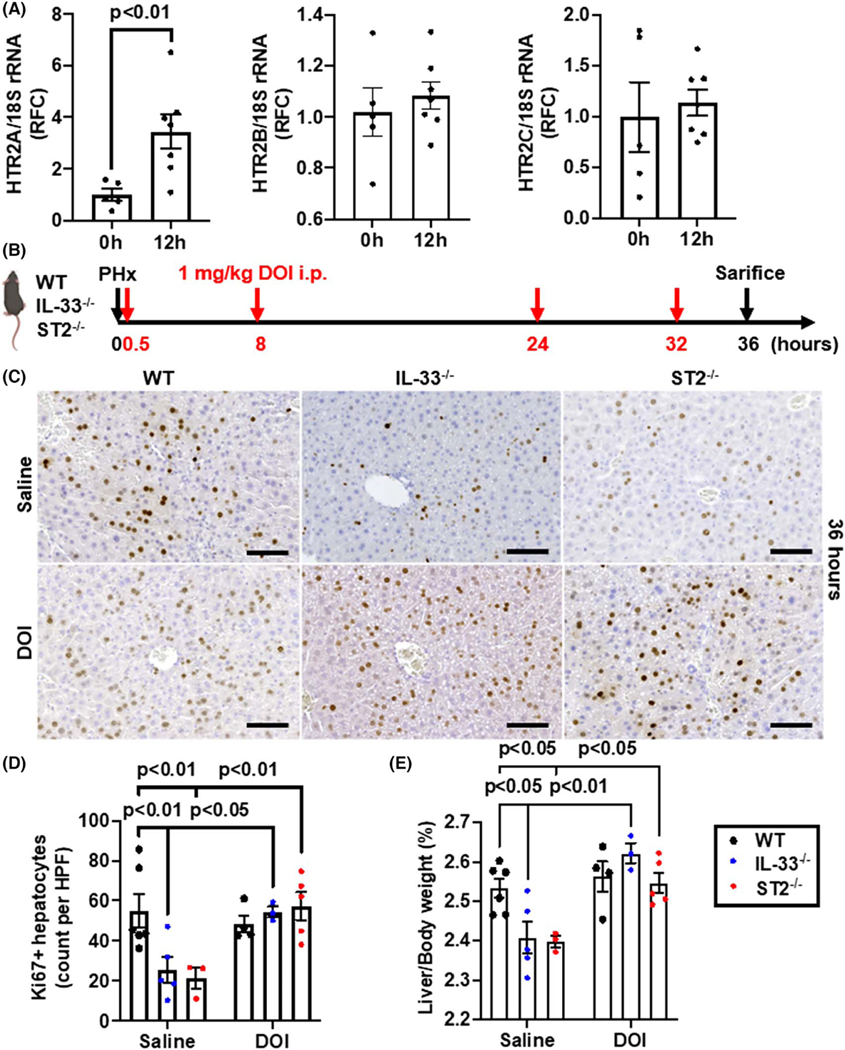
5-hydroxytrytamine receptors (HTR)2 have been implicated in serotonin-mediated liver regeneration.[38,42] Therefore, we measured the mRNA expression of the HTR2 receptors and found that only HTR2A mRNA expression was elevated at 12 h after PHx in WT mice (Figure 6A). Furthermore, our immunofluorescent staining experiments showed that the protein expression levels of HTR2A on hepatocytes were increased at 12 h after PHx (Figure S4). Next, we treated WT, IL-33−/−, and ST2−/− mice with a HTR2A agonist, DOI, after PHx (Figure 6B). Although DOI treatment did not further increase the number of Ki67-positive hepatocytes in WT mice, it significantly enhanced hepatocyte proliferation in IL-33−/− and ST2−/− mice to a degree comparable with that in WT mice (Figure 6C,D). Consistently, the liver-to-body weight ratios were also significantly increased in IL-33−/− and ST2−/− mice after DOI treatment (Figure 6E). These data suggest that IL-33/ST2-regulated liver regeneration is dependent on serotonin/HTR2A signaling.
FIGURE 6.
Serotonin agonist restores liver regeneration in IL-33−/− and ST2−/− mice after PHx. (A) The mRNA levels of 5-hydroxytrytamine receptor 2 (HTR2) were measured in WT livers at 0 and 12 h after PHx (n = 5–7/group). (B) Strategy of (±)-2,5-dimethoxy-4-iodoamphetamine (DOI) treatments in WT, IL-33−/−, and ST2−/− mice after PHx. (C,D) Ki67 immunostaining were performed in liver sections of WT, IL-33−/−, and ST2−/− mice with saline or DOI treatment at 36 h after PHx (scale bar: 100 μm). Ki67-positive hepatocytes were quantified (n = 3–6/group). (E) The ratios of liver/body weight were analyzed at 36 h after PHx (n = 3–6/group). Two-tailed unpaired Student’s t test was performed in (A). Two-way ANOVA was performed in (D) and (E)
P70S6K is involved in IL-33/serotonin-mediated hepatocyte proliferation
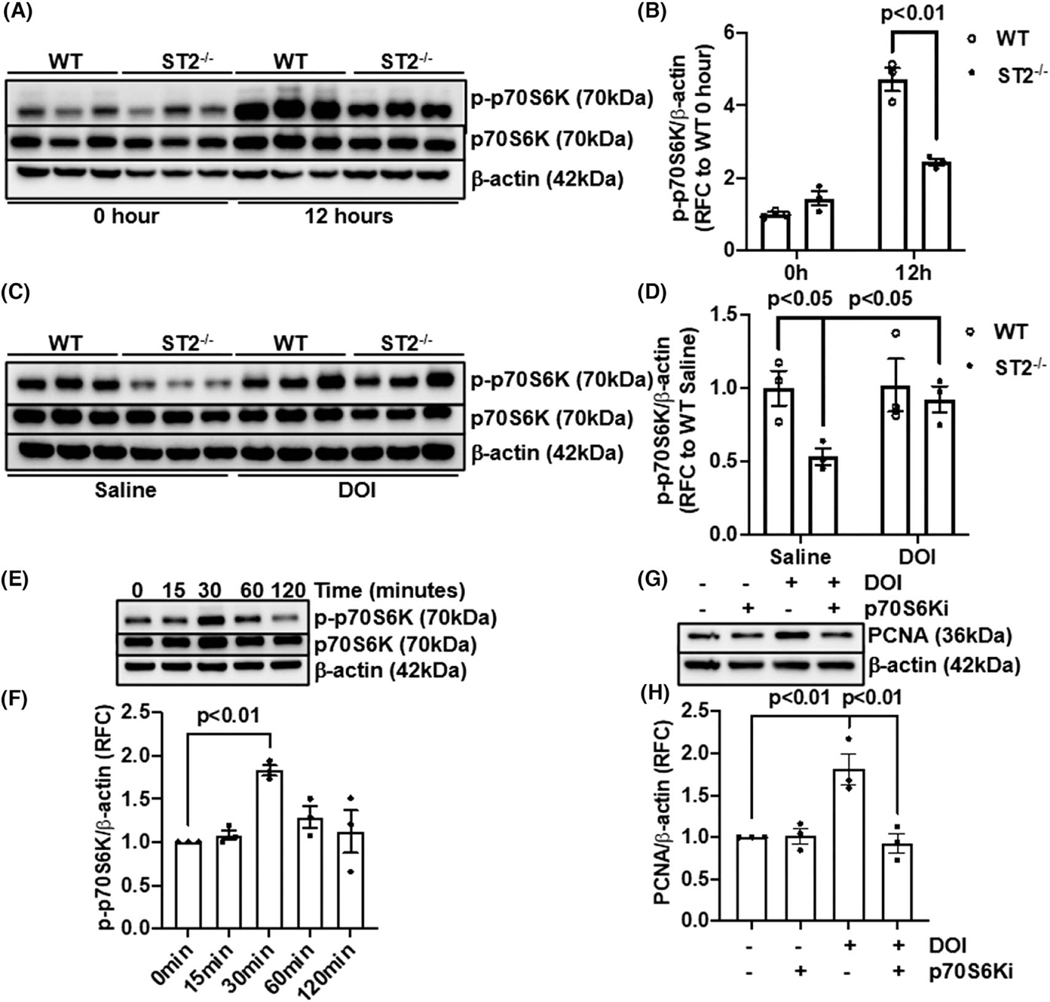
It has been demonstrated that serotonin promotes hepatic cancer cell proliferation through p70S6K activation.[43] To examine whether p70S6K activation might be the downstream of IL-33/serotonin-mediated hepatocyte proliferation, we measured phospho-p70S6K (Thr421/Ser424) in the livers at 12 h after PHx. The levels of phospho-p70S6K were significantly higher in WT than ST2−/− mice (Figure 7A,B). Remarkably, DOI treatment increased p70S6K phosphorylation in ST2−/− mice to a similar level observed in WT mice (Figure 7C,D). Moreover, in vitro treatment of primary murine hepatocytes with DOI for 30 min induced p70S6K phosphorylation (Figure 7E,F). To further demonstrate the involvement of p70S6K in serotonin/HTR2A-regulated hepatocyte proliferation, we treated hepatocytes with DOI in the presence or absence of a p70S6K inhibitor for 24 h. PCNA immunoblotting showed that the DOI treatment could enhance hepatocyte proliferation—an effect that was abrogated by the inhibition of p70S6K (Figure 7G,H). Together, these data suggest that IL-33 promotes hepatocyte proliferation in liver regeneration through enhancing serotonin-induced p70S6K activation.
FIGURE 7.
P70S6K is involved in serotonin mediated hepatocyte proliferation. (A,B) Immunoblotting of phospho-p70S6K/p70S6K was performed in WT and ST2−/− livers at 0 h and 12 h after PHx. The ratios of p-p70S6K/β-actin were quantified (n = 3/group). (C,D) WT and ST2−/− livers treated with saline or DOI were collected at 12 h after PHx. Immunoblotting of phospho-p70S6K/p70S6K was performed and the ratios of p-p70S6K/β-actin quantified (n = 3/group). (E,F) Primary murine hepatocytes were isolated and treated with DOI after serum starvation. Phospho-p70S6K was measured by immunoblotting, and the ratios of p-p70S6K/β-actin from three independent experiments were quantified. (G,H) Primary murine hepatocytes were treated with DOI or an p70S6K inhibitor (p70S6Ki). The levels of PCNA were measured by immunoblotting. The ratios of PCNA/β-actin from three independent experiments were quantified. Two-way ANOVA was performed in (B), (D), (F), and (H)
DISCUSSION
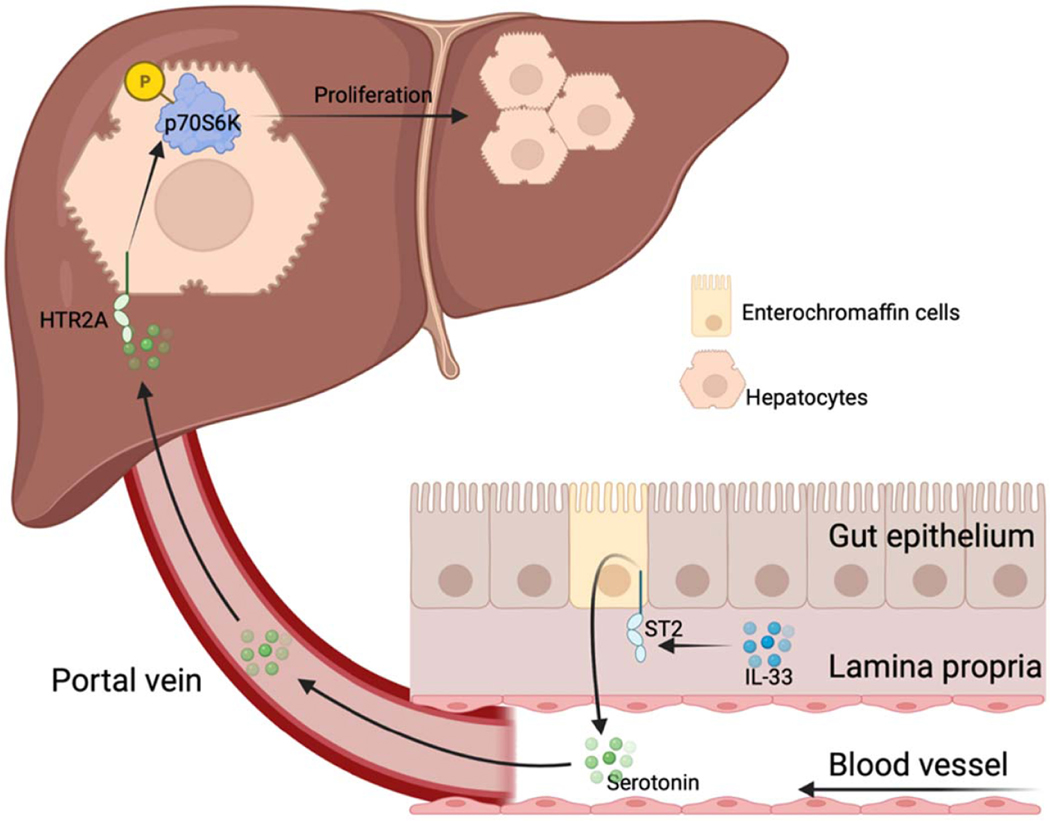
The current study demonstrates that the IL-33/ST2 signaling promotes liver regeneration after PHx through serotonin-involved gut-liver axis (Figure 8). IL-33 enhances the release of serotonin from ST2-positive enterochromaffin cells. Serotonin enters the resected liver through portal blood and binds to HTR2A on hepatocytes, thereby facilitating hepatocyte proliferation. Furthermore, our data show that serotonin-induced hepatocyte proliferation after PHx is dependent on hepatic p70S6K activation.
FIGURE 8.
Proposed mechanism of IL-33/ST2 regulated liver regeneration. After PHx, IL-33/ST2 signaling in enterochromaffin cells enhances serotonin release. Through the portal blood, serotonin travels into the liver where it binds to HTR2A and activates p70S6K, thereby contributing to hepatocyte proliferation
Our finding that IL-33 or ST2 deletion significantly delays liver regeneration indicates that IL-33, signaling through ST2-postive cells, plays an important role in promoting hepatocyte proliferation after PHx. ST2-positive myeloid cells and innate lymphoid cells have been reported to promote epithelial cell proliferation and tissue repair in various injury models, such as naphthalene-induced lung injury, dextran sulfate sodium-induced colitis, and cardiotoxin-induced muscle injury.[44–46] However, we could not detect any ST2-postive cells in the liver after PHx, suggesting an extrahepatic contribution of the IL-33/ST2 signaling in liver regeneration. It is widely reported that ST2 is expressed in both human and murine intestinal epithelium and lamina propria.[47–49] Moreover, emerging studies support a beneficial role of the gut-liver axis in liver regeneration.[50] Notably, simultaneous bowel resection in rats delayed liver regeneration after PHx.[51] Germ-free mice or antibiotics-treated mice exhibited attenuated liver regeneration after PHx.[52–54] PHx in rodents caused an increase of Bacteroidetes and a decrease of Firmicutes during the priming and early phase of hepatocyte proliferation.[55,56] These studies implicate the involvement of gut microbiota in liver regeneration after PHx. However, we cohoused the ST2f/fChgaCre+/− mice with their littermate WT counter-parts and still observed a significant delay in hepatocyte proliferation. The results suggest that the role of IL-33/ST2 in liver regeneration might be independent of gut microbiota.
It is also reported that PHx induces the accumulation of gut-derived endotoxin in the liver. This causes NF-κB activation in Kupffer cells, which initiate hepatocyte proliferation through the release of TNFα and IL-6.[16] Endotoxin neutralization and induction of endotoxin tolerance could significantly impair liver regeneration in rodents after PHx.[35,52] Bile acids, an important factor in the gut-liver axis, have also been implicated in liver regeneration after PHx. An expansion of the bile acid pool has been observed in the liver and circulating blood of rodents after PHx.[57,58] PHx accompanied by ileal resection results in diminished liver regeneration, likely attributable to the loss of bile acid reabsorption in the ileum.[59] Surgical or genetic disruption of normal bile acid enterohepatic circulation, or sequestration of bile acid by cholestyramine, remarkably attenuated liver regeneration.[57,58] However, our data showed that PHx caused similar increases of gut-derived endotoxin and bile acids in both WT and ST2−/− mice, indicating that these factors are not involved in IL-33/ST2-regulated liver regeneration. Interestingly, we uncovered that the serotonin release, up regulated by IL-33/ST2 signaling, served as a gut-derived mediator in regulating liver regeneration.
Serotonin has been reported to promote liver regeneration after PHx. It has been shown that the loss of gut serotonin production in mice with deficiency of tryptophan-hydroxylase 1, critical in serotonin synthesis, attenuates liver regeneration.[38] In the current study, we demonstrated that PHx increased serotonin levels in the portal blood of WT mice, but not in IL-33−/− and ST2−/− mice after PHx, suggesting a critical role of IL-33/ST2 in promoting serotonin release from the gut. A recent elegant study reported that exogenous IL-33 directly triggered calcium influx to promote serotonin release from ST2-positive enterochromaffin cells, and that ST2-deficient enterochromaffin cells failed to respond to IL-33, resulting in a defect of serotonin release.[41] Consistently, we found that the enterochromaffin cell–specific ST2 knockout mice had lower levels of serotonin in the portal blood, correlating with reduced hepatocyte proliferation after PHx compared with WT mice. Moreover, our observation that the DOI treatment could correct the impaired liver regeneration in IL-33−/− and ST2−/− mice further validate that gut-derived serotonin accounts for IL-33/ST2 induced hepatocyte proliferation during liver regeneration.
A previous study detected the induction of both HTR2A and HTR2B in liver after PHx,[38] whereas we only observed an up-regulation of HTR2A, but not HTR2B. However, both the previous study and our current study demonstrate that liver regeneration could be restored by the treatment of DOI (an HTR2A agonist) in mice with defect of serotonin production. Our in vitro experiments also showed that DOI could induce the proliferation of primary murine hepatocytes. Furthermore, it is reported that a selective HTR2A antagonist, ketanserin, impedes liver regeneration after PHx in rats.[42,60] These findings suggest that serotonin-induced hepatocyte proliferation is dependent on HTR2A during liver regeneration.
The serotonin/HTR2A axis has been implicated in activating G1/S transition in rat liver regeneration after PHx.[42] Our data demonstrated that DOI directly promoted hepatocyte proliferation in vitro. We further found that DOI induced p70S6K activation, and that inhibition of p70S6K could suppress the pro-proliferative effect of DOI. It is known that p70S6K activation leads to the phosphorylation of 40S ribosomal S6 and triggers the translation of mRNA with a 5′ oligopyrimidine tract typically encoding components of the protein synthesis machinery, thereby initiating G1 to S transition in cell cycle progression.[61,62] These findings suggest that p70S6K may be important in mediating the pro-proliferative role of serotonin in hepatocytes.
The present study unveiled a role of the IL-33/ST2 pathway in non-injurious liver regeneration. The data also provide insights into the mechanism underlying the involvement of the gut-liver axis in liver regeneration. Our findings suggest that IL-33 supplementation or serotonin agonist might be a promising option for hepatectomized patients with insufficient liver regeneration.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Merck & Co. for providing IL-33−/− mice.
FUNDING INFORMATION
Supported by the National Institutes of Health (DK122708, DK121330, and DK122796) and the German Research Foundation (DFG426793915).
Deutsche Forschungsgemeinschaft, Grant/Award Number: 426793915; National Institute of Diabetes and Digestive and Kidney, Diseases, Grant/Award Number: DK121330, DK122708 and DK122796
Footnotes
CONFLICT OF INTEREST
Nothing to report.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1.Michalopoulos GK, Bhushan B. Liver regeneration: biological and pathological mechanisms and implications. Nat Rev Gastroenterol Hepatol. 2021;18:40–55. [DOI] [PubMed] [Google Scholar]
- 2.Kwon YJ, Lee KG, Choi D. Clinical implications of advances in liver regeneration. Clin Mol Hepatol. 2015;21:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrowsky H, Fritsch R, Guckenberger M, De Oliveira ML, Dutkowski P, Clavien PA. Modern therapeutic approaches for the treatment of malignant liver tumours. Nat Rev Gastroenterol Hepatol. 2020;17:755–72. [DOI] [PubMed] [Google Scholar]
- 4.Jaeck D, Bachellier P, Oussoultzoglou E, Weber JC, Wolf P. Surgical resection of hepatocellular carcinoma. Post-operative outcome and long-term results in Europe: an overview. Liver Transpl. 2004;10:S58–63. [DOI] [PubMed] [Google Scholar]
- 5.Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Post-hepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713–24. [DOI] [PubMed] [Google Scholar]
- 6.Soreide JA, Deshpande R. Post-hepatectomy liver failure (PHLF)—recent advances in prevention and clinical management. Eur J Surg Oncol. 2021;47:216–24. [DOI] [PubMed] [Google Scholar]
- 7.Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540–8. [DOI] [PubMed] [Google Scholar]
- 8.Gong WF, Zhong JH, Lu Z, Zhang QM, Zhang ZY, Chen CZ, et al. Evaluation of liver regeneration and post-hepatectomy liver failure after hemihepatectomy in patients with hepatocellular carcinoma. Biosci Rep. 2019;39:BSR20190088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez-Alejandro R, Sharma H. Small-for-size syndrome in liver transplantation: new horizons to cover with a good launchpad. Liver Transpl. 2016;22:33–6. [DOI] [PubMed] [Google Scholar]
- 10.Shoreem H, Gad EH, Soliman H, Hegazy O, Saleh S, Zakaria H, et al. Small-for-size syndrome difficult dilemma: Lessons from 10 years single centre experience in living donor liver transplantation. World J Hepatol. 2017;9:930–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins GM. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 12.Mitchell C, Willenbring H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc. 2008;3: 1167–70. [DOI] [PubMed] [Google Scholar]
- 13.Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–83. [DOI] [PubMed] [Google Scholar]
- 14.Borowiak M, Garratt AN, Wustefeld T, Strehle M, Trautwein C, Birchmeier C. Met provides essential signals for liver regeneration. Proc Natl Acad Sci U S A. 2004;101:10608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Natarajan A, Wagner B, Sibilia M. The EGF receptor is required for efficient liver regeneration. Proc Natl Acad Sci U S A. 2007; 104:17081–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–53. [DOI] [PubMed] [Google Scholar]
- 17.Sun R, Park O, Horiguchi N, Kulkarni S, Jeong WI, Sun HY, et al. STAT1 contributes to dsRNA inhibition of liver regeneration after partial hepatectomy in mice. Hepatology. 2006;44: 955–66. [DOI] [PubMed] [Google Scholar]
- 18.Wu MS, Kuo YP, Lo YC, Tsai DJ, Lai CY, Chuang TH, et al. Type I interferon signaling accelerates liver regeneration by metabolic modulation in non-infectious conditions. Am J Pathol. 2021;191: 1036–48. [DOI] [PubMed] [Google Scholar]
- 19.Yamada Y, Webber EM, Kirillova I, Peschon JJ, Fausto N. Analysis of liver regeneration in mice lacking type 1 or type 2 tumor necrosis factor receptor: requirement for type 1 but not type 2 receptor. Hepatology. 1998;28:959–70. [DOI] [PubMed] [Google Scholar]
- 20.Yamada Y, Kirillova I, Peschon JJ, Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci U S A. 1997;94:1441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeAngelis RA, Markiewski MM, Kourtzelis I, Rafail S, Syriga M, Sandor A, et al. A complement-IL-4 regulatory circuit controls liver regeneration. J Immunol. 2012;188:641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furuya S, Kono H, Hara M, Hirayama K, Tsuchiya M, Fujii H. Interleukin-17A plays a pivotal role after partial hepatectomy in mice. J Surg Res. 2013;184:838–46. [DOI] [PubMed] [Google Scholar]
- 23.Ren X, Hu B, Colletti LM. IL-22 is involved in liver regeneration after hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2010;298:G74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin S, Wang H, Park O, Wei W, Shen J, Gao B. Enhanced liver regeneration in IL-10-deficient mice after partial hepatectomy via stimulating inflammatory response and activating hepatocyte STAT3. Am J Pathol. 2011;178:1614–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagaoka S, Yamada D, Eguchi H, Yokota Y, Iwagami Y, Asaoka T, et al. The blockade of interleukin-33 released by hepatectomy would be a promising treatment option for cholangiocarcinoma. Cancer Sci. 2021;112:347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lott JM, Sumpter TL, Turnquist HR. New dog and new tricks: evolving roles for IL-33 in type 2 immunity. J Leukoc Biol. 2015; 97:1037–48. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Razumilava N, Gores GJ, Walters S, Mizuochi T, Mourya R, et al. Biliary repair and carcinogenesis are mediated by IL-33-dependent cholangiocyte proliferation. J Clin Invest. 2014;124:3241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen Y, Feng D, Wu H, Liu W, Li H, Wang F, et al. Defective initiation of liver regeneration in osteopontin-deficient mice after partial hepatectomy due to insufficient activation of IL-6/Stat3 pathway. Int J Biol Sci. 2015;11:1236–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia J, Zhou Y, Ji H, Wang Y, Wu Q, Bao J, et al. Loss of histone deacetylases 1 and 2 in hepatocytes impairs murine liver regeneration through Ki67 depletion. Hepatology. 2013;58:2089–98. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Wen Y, Wang L, Wen L, You W, Wei S, et al. Therapeutic opportunities of interleukin-33 in the central nervous system. Front Immunol. 2021;12:654626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cressman DE, Diamond RH, Taub R. Rapid activation of the Stat3 transcription complex in liver regeneration. Hepatology. 1995;21:1443–9. [PubMed] [Google Scholar]
- 32.Paranjpe S, Bowen WC, Mars WM, Orr A, Haynes MM, DeFrances MC, et al. Combined systemic elimination of MET and epidermal growth factor receptor signaling completely abolishes liver regeneration and leads to liver decompensation. Hepatology. 2016;64:1711–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiest R, Albillos A, Trauner M, Bajaj JS, Jalan R. Targeting the gut-liver axis in liver disease. J Hepatol. 2017;67:1084–3. [DOI] [PubMed] [Google Scholar]
- 34.Cornell RP. Gut-derived endotoxin elicits hepatotrophic factor secretion for liver regeneration. Am J Physiol. 1985;249:R551–62. [DOI] [PubMed] [Google Scholar]
- 35.Cornell RP. Restriction of gut-derived endotoxin impairs DNA synthesis for liver regeneration. Am J Physiol. 1985;249:R563–9. [DOI] [PubMed] [Google Scholar]
- 36.Liu HX, Keane R, Sheng L, Wan YJ. Implications of microbiota and bile acid in liver injury and regeneration. J Hepatol. 2015;63: 1502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Haan L, van der Lely SJ, Warps AK, Hofsink Q, Olthof PB, de Keijzer MJ, et al. Post-hepatectomy liver regeneration in the context of bile acid homeostasis and the gut-liver signaling axis. J Clin Transl Res. 2018;4:1–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, et al. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104–7. [DOI] [PubMed] [Google Scholar]
- 39.Papadimas GK, Tzirogiannis KN, Mykoniatis MG, Grypioti AD, Manta GA, Panoutsopoulos GI. The emerging role of serotonin in liver regeneration. Swiss Med Wkly. 2012;142:w13548. [DOI] [PubMed] [Google Scholar]
- 40.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. [DOI] [PubMed] [Google Scholar]
- 41.Chen Z, Luo J, Li J, Kim G, Stewart A, Urban JF Jr, et al. Interleukin-33 promotes serotonin release from enterochromaffin cells for intestinal homeostasis. Immunity. 2021;54:151–63.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papadimas GK, Tzirogiannis KN, Panoutsopoulos GI, Demonakou MD, Skaltsas SD, Hereti RI, et al. Effect of serotonin receptor 2 blockage on liver regeneration after partial hepatectomy in the rat liver. Liver Int. 2006;26:352–61. [DOI] [PubMed] [Google Scholar]
- 43.Soll C, Jang JH, Riener MO, Moritz W, Wild PJ, Graf R, et al. Serotonin promotes tumor growth in human hepatocellular cancer. Hepatology. 2010;51:1244–54. [DOI] [PubMed] [Google Scholar]
- 44.Dagher R, Copenhaver AM, Besnard V, Berlin A, Hamidi F, Maret M, et al. IL-33-ST2 axis regulates myeloid cell differentiation and activation enabling effective club cell regeneration. Nat Commun. 2020;11:4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Millar NL, O’Donnell C, McInnes IB, Brint E. Wounds that heal and wounds that don’t—the role of the IL-33/ST2 pathway in tissue repair and tumorigenesis. Semin Cell Dev Biol. 2017;61:41–50. [DOI] [PubMed] [Google Scholar]
- 46.Faas M, Ipseiz N, Ackermann J, Culemann S, Gruneboom A, Schroder F, et al. IL-33-induced metabolic reprogramming controls the differentiation of alternatively activated macrophages and the resolution of inflammation. Immunity. 2021;54: 2531–46.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahapatro M, Foersch S, Hefele M, He GW, Giner-Ventura E, McHedlidze T, et al. Programming of intestinal epithelial differentiation by IL-33 derived from pericryptal fibroblasts in response to systemic infection. Cell Rep. 2016;15:1743–56. [DOI] [PubMed] [Google Scholar]
- 48.Sedhom MA, Pichery M, Murdoch JR, Foligne B, Ortega N, Normand S, et al. Neutralisation of the interleukin-33/ST2 pathway ameliorates experimental colitis through enhancement of mucosal healing in mice. Gut. 2013;62:1714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia Miguel M, Gonzalez MJ, Quera R, Hermoso MA. Innate immunity modulation by the IL-33/ST2 system in intestinal mucosa. Biomed Res Int. 2013;2013:142492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng Z, Wang B. The gut-liver axis in health and disease: the role of gut microbiota-derived signals in liver injury and regeneration. Front Immunol. 2021;12:775526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyazaki M, Kohda S, Itoh H, Kaiho T, Kimura F, Ambiru S, et al. Inhibition of hepatic regeneration after 70% partial hepatectomy by simultaneous resection of the bowel in rats. Eur Surg Res. 1995;27:396–405. [DOI] [PubMed] [Google Scholar]
- 52.Cornell RP, Liljequist BL, Bartizal KF. Depressed liver regeneration after partial hepatectomy of germ-free, athymic and lipopolysaccharide resistant mice. Hepatology. 1990;11: 916–22. [DOI] [PubMed] [Google Scholar]
- 53.Leng L, Ma J, Lv L, Gao D, Li M, Wang Y, et al. Serum proteome profiling provides a deep understanding of the ‘gut-liver axis’ in relation to liver injury and regeneration. Acta Biochim Biophys Sin (Shanghai). 2021;53:372–80. [DOI] [PubMed] [Google Scholar]
- 54.Wu X, Sun R, Chen Y, Zheng X, Bai L, Lian Z, et al. Oral ampicillin inhibits liver regeneration by breaking hepatic innate immune tolerance normally maintained by gut commensal bacteria. Hepatology. 2015;62:253–64. [DOI] [PubMed] [Google Scholar]
- 55.Liu HX, Rocha CS, Dandekar S, Wan YJ. Functional analysis of the relationship between intestinal microbiota and the expression of hepatic genes and pathways during the course of liver regeneration. J Hepatol. 2016;64:641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bao Q, Yu L, Chen D, Li L. Variation in the gut microbial community is associated with the progression of liver regeneration. Hepatol Res. 2020;50:121–36. [DOI] [PubMed] [Google Scholar]
- 57.Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, et al. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312:233–6. [DOI] [PubMed] [Google Scholar]
- 58.Naugler WE. Bile acid flux is necessary for normal liver regeneration. PLoS One. 2014;9:e97426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Medeiros AC, Azevedo AC, Oseas JM, Gomes MD, Oliveira FG, Rocha KB, et al. The ileum positively regulates hepatic regeneration in rats. Acta Cir Bras. 2014;29:93–8. [DOI] [PubMed] [Google Scholar]
- 60.Balasubramanian S, Paulose CS. Induction of DNA synthesis in primary cultures of rat hepatocytes by serotonin: possible involvement of serotonin S2 receptor. Hepatology. 1998;27:62–6. [DOI] [PubMed] [Google Scholar]
- 61.Lane HA, Fernandez A, Lamb NJ, Thomas G. p70s6k function is essential for G1 progression. Nature. 1993;363:170–2. [DOI] [PubMed] [Google Scholar]
- 62.Pearson RB, Thomas G. Regulation of p70s6k/p85s6k and its role in the cell cycle. Prog Cell Cycle Res. 1995;1:21–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.