Abstract
Purpose:
Adolescent girls and young women (AGYW) are disproportionately affected by STIs. Observation of life course events can describe behavioral and biological factors associated with STI risk.
Methods:
Sexually inexperienced AGYW aged 16–20 in Kenya were followed for five years. Quarterly visits assessed for C. trachomatis (CT), N. gonorrhea (GC), and T. vaginalis (TV), bacterial vaginosis (BV), HSV-2 and HIV. Sexual activity was self-reported but amended if incongruent with results from STI, pregnancy or other testing. Cox regression and Generalized Estimating Equation models were used to determine hazard ratios and relative risks of STI.
Results:
During follow up, 293 of 400 participants reported sex, 163 AGYW experienced an STI, and 72 participants had multiple STIs. Among 163 participants that experienced an STI, there were a total of 259 visits where STIs were detected, 78% (n=201) of which included CT. Cox regression found participants with BV had an over two-fold higher risk of first STI acquisition (adjusted hazard ratio (aHR): 2.35; 95% CI 1.43–3.88; P=0.001). Increased risk for first STI episode was associated with a new partner (aHR: 3.16; 95% CI 1.59–6.28; P=0.001). AGYW who did not disclose sexual activity had the highest risk (aHR: 3.60; 95% CI 1.93–6.70; P<0.001). Condom use was low, with 21% reporting condom use with sex. GEE analysis of all STIs including incident, prevalent, and recurrent, confirmed these risk factors.
Conclusions:
During the critical years after first sex, AGYW with BV, new sexual partners, and those who did not disclose sexual activity were at highest risk for STI events, especially CT.
IMPLICATIONS & CONTRIBUTION
This cohort study followed 400 sexually inexperienced Kenyan young women, who were recruited after reporting only one or no prior sexual partners. During the five year study, we observed high STI incidence, with >50% of participants experiencing STIs, especially Chlamydia.. Our results demonstrated that bacterial vaginosis, new partners, and inability to disclose sexual activity correlated with two-fold or more increased STI risk, identifying specific targets for STI prevention.
The World Health Organization estimates 376 million cases of curable STIs occur among people aged 15–49 years annually [1]. Chlamydia trachomatis (CT) has a global estimated prevalence of 3.8% in women, with 131 million annual incident infections. There is high global incidence of other STIs, including Neisseria gonorrhoeae (GC) (78 million) and Trichomonas vaginalis (TV) (142 million); prevalence of viral STI is similarly high with 417 million people living with herpes simplex type 2 (HSV-2) [1, 2]. Most STIs are asymptomatic for women; despite this, women face disproportionate risk for STI complications compared to men, especially CT which can cause pelvic inflammatory disease and infertility [2]. STI events have long been linked to increased HIV incidence among women and may be key to understanding acquisition of HIV by women.
Sub-Saharan Africa (SSA) accounts for 40% of global STI infections [2]. Adolescent girls and young women (AGYW) bear a disproportionate burden of STI incidence and prevalence [3]. One meta-analysis of STI studies in SSA demonstrated higher chlamydia, gonorrhea, and trichomoniasis prevalence among women aged 15–24 years compared to those aged 25–49 years [3]. The genital mucosa serves as a physical and biological barrier, and vaginal dysbiosis, including bacterial vaginosis (BV) and STI events, can contribute to genital tract inflammation which may compromise the mucosal protections increasing the risk for HIV acquisition [4–6]. In addition to biological factors, BV, STIs, and HIV are influenced by gender-related vulnerabilities, that can be faced by AGYW in sub-Saharan Africa, such as early marriage, school discontinuation, sexual violence, and limited access to sexual health education and services [7, 8].
Adolescent STIs have been characterized primarily in cross-sectional prevalence surveys. Longitudinal cohorts can help identify STI risk factors, but typically enroll sexually active young women. Data representative of all adolescents is needed, including those who appear to be at lower risk, to understand how the interplay of social factors and changing behaviors influence risk of acquiring STIs [2]. We conducted a longitudinal cohort study to enroll girls prior to sexual activity and determine incidence and correlates of STIs. By recruiting AGYW pre-sexual activity, the cohort was designed to observe shifting biological and behavioral risks for AGYW and provide high resolution data on time-varying risk factors for STI acquisition. Understanding the biological and behavioral life course events leading to STI acquisition in AGYW may enable targeted interventions to address adolescent STI risk.
METHODS
Study Design and Setting
The Kenya Girls Study (KGS) is a prospective cohort study among AGYW aged 16–20 years in Thika, Kenya, a municipality northeast of Nairobi with lower HIV incidence and prevalence relative to other regions [9]. Initial enrollment occurred August 2014–April 2016 through community-based recruitment in school and neighborhood settings, reported in [10].
Eligible participants were HIV and HSV–2 seronegative and reported only one or no prior sexual partners at screening. The specific sexual experience required for eligibility was not advertised. Participants ≥ 18 years provided written informed consent to participate; those aged 16–17 obtained written parental informed consent and written assent to participate. Participants received free, confidential, on-demand reproductive health services and transportation reimbursement.
Study Procedures
During quarterly visits, demographic, sexual behavior, and clinical data were collectedParticipants received adolescent-friendly private sexual health care, which included access to free contraception, STI testing and treatment, and regular HIV testing. In 2018, after Kenyan policy changed, free access to oral pre-exposure prophylaxis for HIV was provided. Participants were treated for identified STIs along with their partners. Specimens were collected and assessed quarterly for bacterial vaginosis (BV) from vaginal Gram stains, HSV-2 from genital swabs by polymerase chain reaction (PCR) testing, and HIV and HSV-2 from blood by ELISA, as described previously by Yuh et. al. (2020) [10]. The presence of BV was determined by trained microscopists and scored using the method of Nugent and Hillier [11]; samples with Nugent scores of greater than or equal to seven were classified as BV. Syphilis testing was not done due to low antenatal regional prevalence. Nucleic acid amplification tests (NAAT) of vaginal swabs were performed annually to diagnose CT, GC, and TV, with further tests performed for those with missed visits, STI symptoms, or after STI diagnosis results. NAAT tests were retrospectively performed on samples collected from 2014–2016, and performed prospectively after. Additional samples were tested to ensure no testing gaps greater than 12 months, if possible. Self-reported sexual activity was recorded by questionnaire. During data analysis, a second sexual activity variable was created to account for incongruencies with objective measures includingY-chromosome PCR tests, spermatozoa on Gram stain, pregnancy, or STI test results. This second, more objective measure of sexual activity was used over self-reported sexual activity in downstream analyses; participants were not re-interviewed if their self report of sexual activity differed from objective information.
Study Outcomes
The primary outcome measure was aggregate STI incidence, defined as evidence of infection with CT, GC, TV, or HSV–2. Individual CT, GC, TV, and HSV–2 infections were also examined. Participants were defined as at risk for incident STI if they reported initiating sexual activity, defined as penile-vaginal intercourse, and had a confirmed negative STI result. Date of first sex was self-reported; this date was adjusted if earlier sexual activity was detected using objective testing as above. Participants began contributing time at risk in the analysis at either (1) first study visit, among those who reported sex prior to enrollment, or (2) date of first sex, among those who initiated sex after enrollment. Acquisition of incident STI was defined as first positive STI test. Participants with greater than 18 months between visits were censored at the beginning of the gap, as timing of STI event was unknown.
Statistical Analyses
Kaplan-Meier curves were generated to estimate the cumulative incidence of initial STI events over time. Participants whose first study visit indicated prevalent STIs were excluded. Hazard ratios of potential correlates were estimated using multivariate Cox regression to model STI risk; model covariates included age at enrollment, age of menarche, contraception use, BV status at study visit, new sexual partner in past three months, and non-disclosure of sexual activity. Multivariate regression model was informed by univariate analysis and supported by covariates such as age, contraception, and BV established in literature [2–4, 12]. Statistical significance was determined using a two-sided type one error rate of 0.05.
A Poisson model fit using Generalized Estimating Equations (GEE) with an independent working correlation matrix and robust standard errors was used to estimate relative risks of correlates of all STIs including prevalent, incident, and subsequent STIs. All participants, including those with STI at first study visit, were included in this model. Second STIs were included if a negative STI test was documented after first STI. Statistical analysis was performed using Stata version 16.0.
Ethical Approvals
Ethical approval was obtained from the Kenya Medical Research Institute Scientific Ethics Review Unit and University of Washington Institutional Review Board.
RESULTS
Study cohort characteristics
Among 400 participants, 94 had no evidence of initiating sexual activity before or during the study period and were excluded from analyses (Figure 1). Also excluded from STI incidence analyses were two participants who acquired HIV during the study and 11 participants with missing bacterial STI tests. In total, 293 participants were eligible for analysis. The median age of enrollment was 18.7 years (IQR: 18–19) and median age of first sex was 18.9 years (IQR: 18.3–19.9). Self-reported age at menarche was median 14 years (13–15) and median reported time between menarche and first sex was 4.9 years (IQR: 3.7–6.1)
Figure 1. Flow diagram of longitudinal study cohort of participants.
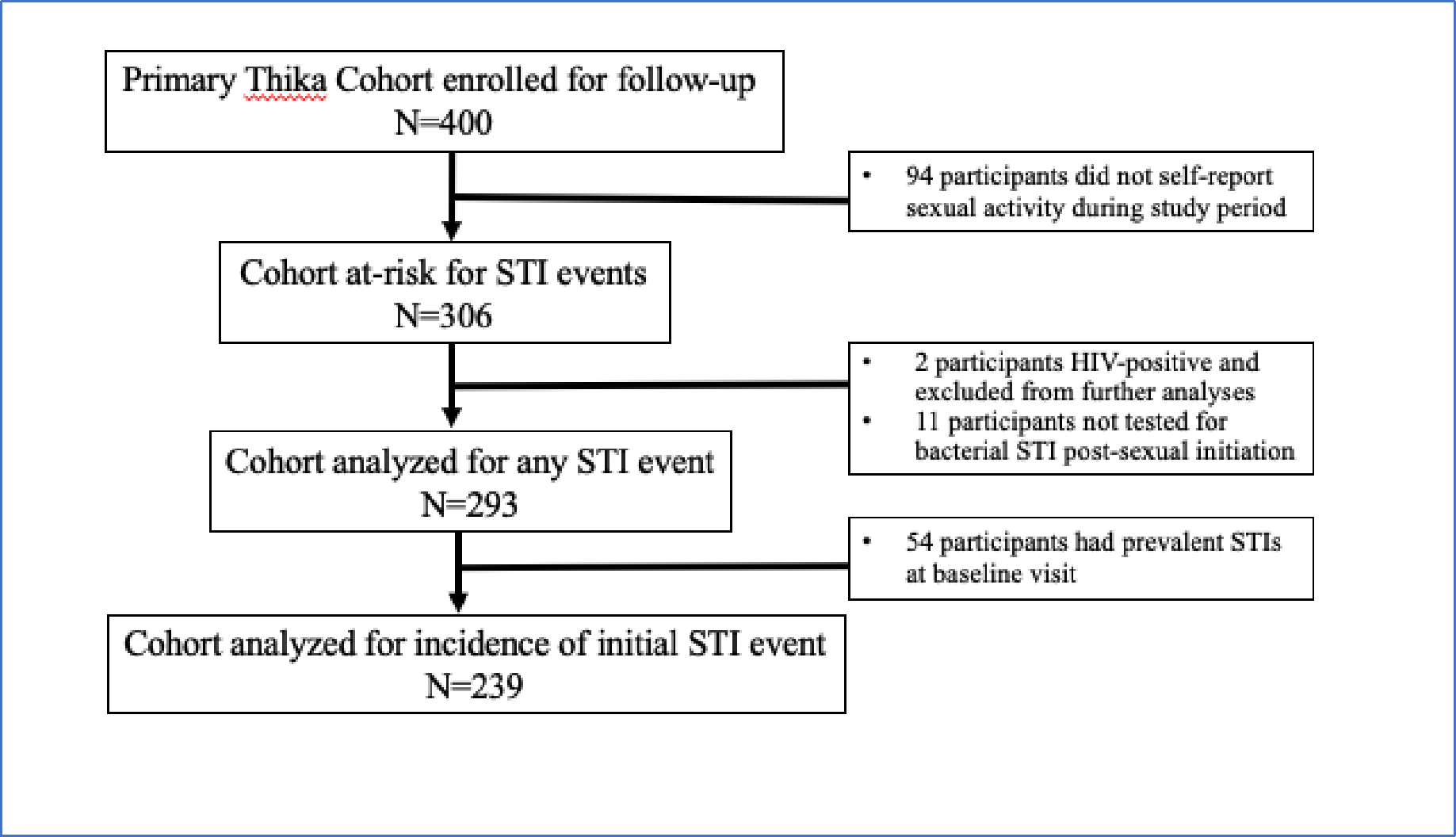
Cohort retention was high, with 85% (341 of 400) of participants retained at one year. At 36 months, 61% (242 of 400) participants were re-consented to participate for an additional 24 months; 91% (220 of 242) of this extended cohort were in the study at 48 months; and 38% (93 of 242) AGYW completed 60 months of follow up. The median follow-up time for participants was 51 months (IQR: 27–57) with 5,278 total study visits. Reasons for attrition were not routinely collected, but a common reason for attrition was relocation out of area. The study ended in April 2020 and participants were exited even if follow up time was less than 60 months.
Detection of STI events
Of 293 sexually active participants, 163 (56%) participants experienced a total of 259 STIs. The median number of STIs was 1 (IQR: 1–2) per participant, with 25% of cases detected at the first assessment, and a median time to first STI of 3.2 months (IQR: 0–17.8) after sexual activity or cohort entry. We observed 54 participants with STI detected at the first study test visit, with prevalent cases reported in Yuh et al. [10], and a further 28 participants with STI at the first visit post-first sex.
STI incidence over time
Among 239 AGYW who were sexually active and eligible for analysis of incident STI, 109 experienced an incident STI over 484.6 person-years of follow-up (incidence rate of 22.5 per 100 person-years). After excluding 54 AGYW with a prevalent STI at first test visit, time to event analysis (Figure 2) demonstrates that within 12 months after first sex, one quarter (24.6%) of participants experienced an incident STI event. Over one third (36.5%) of participants experienced their first STI event by 24 months, which rose to 48.3% by 36 months, and to 58.4% by 48 months. By the end of the study period, 64% of sexually active AGYW had an incident STI event.
Figure 2. Kaplan-Meier estimates of cumulative incidence of first STI infection (GC, CT, TV, and/or HSV-2) after first sex among participants without STI at baseline, over 5 years in Thika, Kenya.
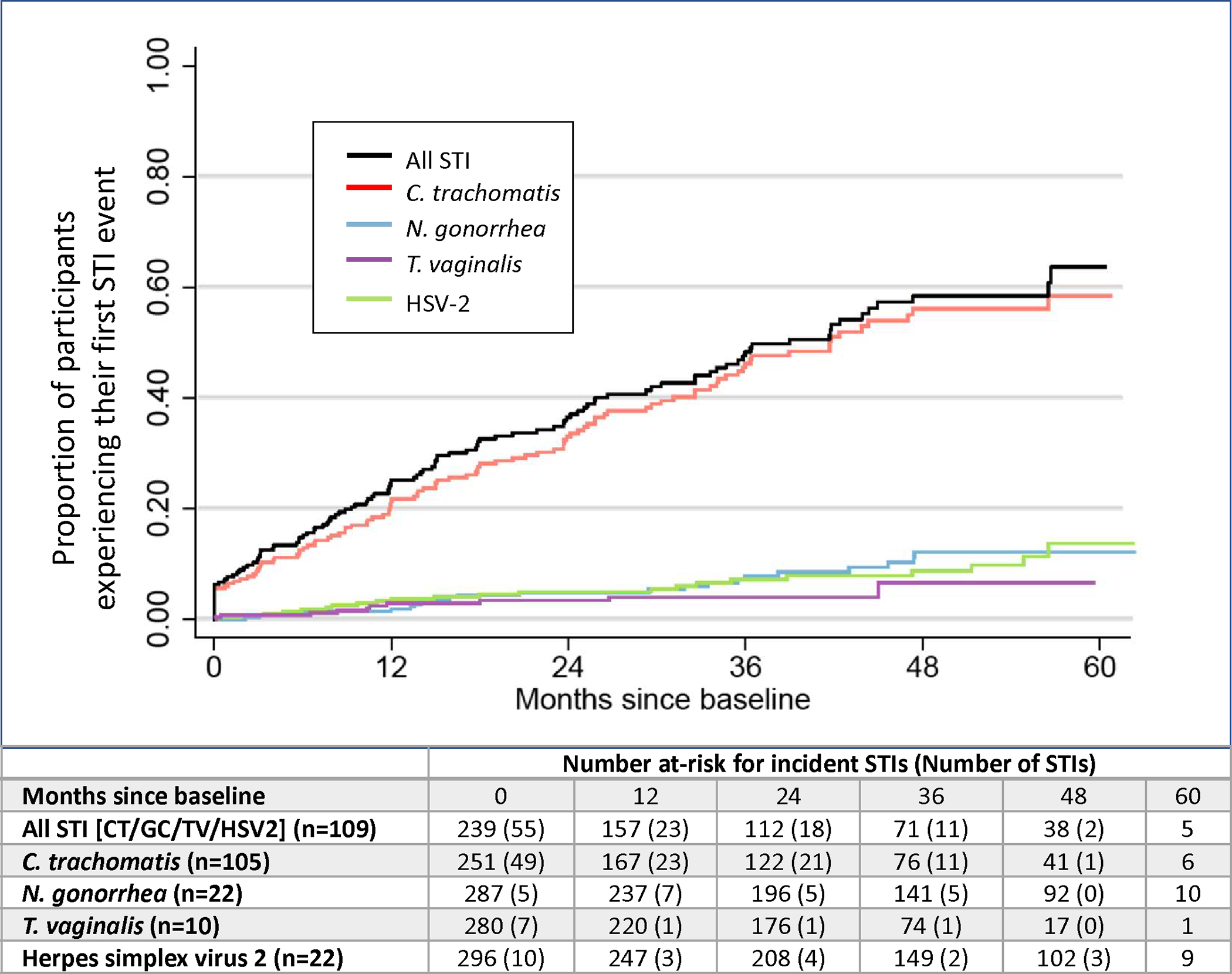
Probability of acquiring each individual STI within the composite STI outcome is displayed with risk table displaying number of participants at risk each year, accompanied with number of STI events at each time point. Since each participant could have incident cases of each STI, denominators are different for each STI.
CT infection comprised the majority (81%) of incident STI events observed over time (Figure 3). Individual time to event analyses were performed for each STI; each analysis has different denominators after excluding baseline infections. Among 251 participants negative for CT at baseline, 105 incident CT infections were observed (42%). Within 3 years of sexual activity, 46.2% of participants had acquired a first CT event, 56.1% within four years, and 58.4% of participants acquired incident CT within 5 years. In contrast to CT, incidence of HSV-2 and GC was relatively low. Among 296 participants negative for HSV-2 at baseline, there were 22 (7.4%) incident cases of HSV-2 infection and among 287 participants negative for GC at baseline, there were 22 (7.7%) incident cases of GC infection. TV was rare, with 10 incident cases detected in 280 participants over 5 years (3.6%).
Figure 3. Visualization of STI events over 60 months of follow-up for those diagnosed with STI (N=163).
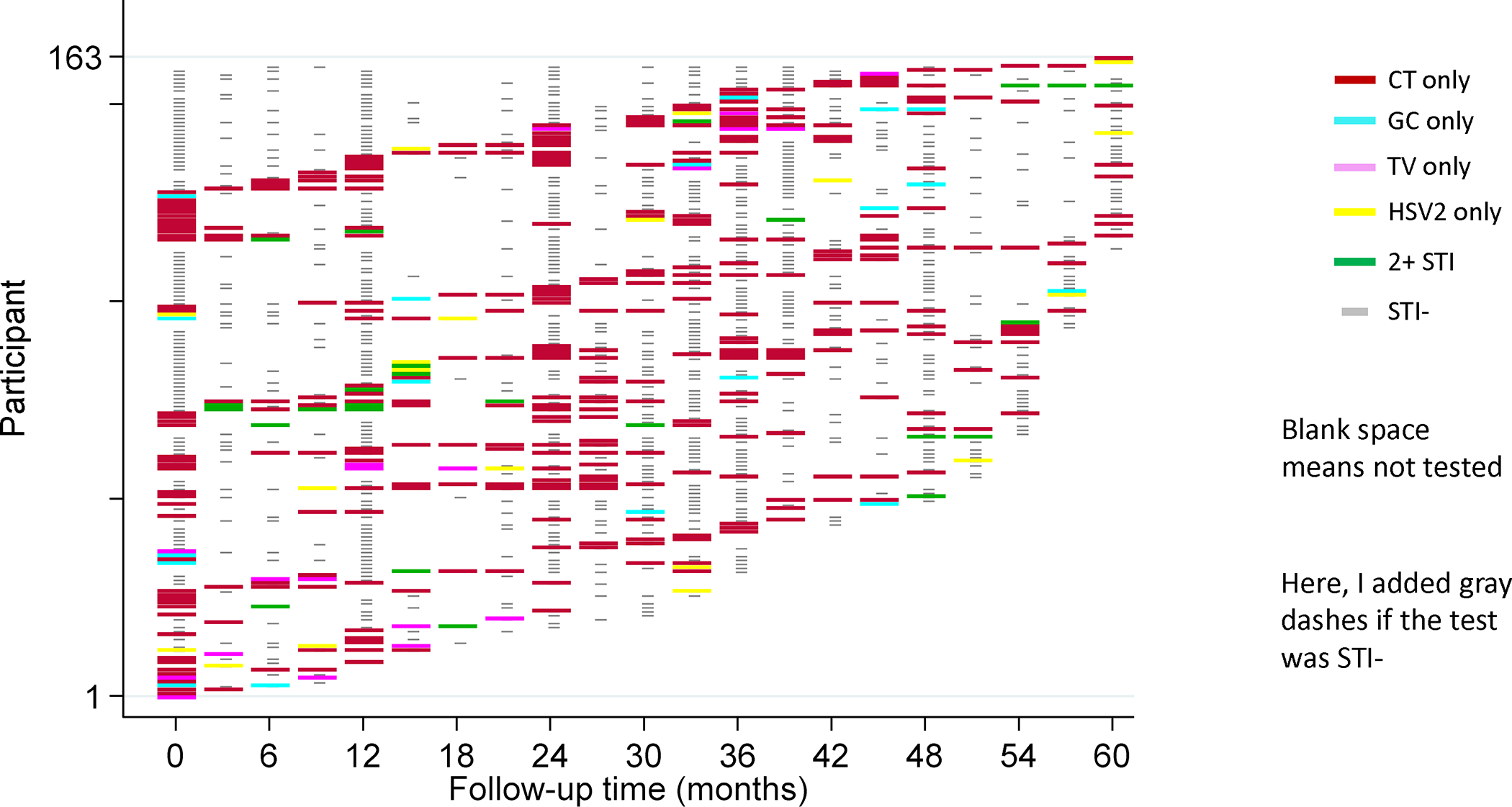
Each participant is represented by one row. Each participant’s STI history can be tracked longitudinally across the heatmap, with color key corresponding to STI test results at each timepoint on the X axis. Study visits with negative test results are denoted by light gray color. Timepoints with no testing are denoted by a blank space.
Correlates of first incident STI event
Cox regression analysis (Table 1) showed that participants with BV at time of STI diagnosis had greater than two-fold higher risk of initial STI acquisition (adjusted hazard ratio (aHR): 2.35; 95% CI 1.43–3.88; P=0.001). AGYW who did not disclose that they were sexually active had more than three-fold increased risk for initial STI (aHR: 3.60; 95% CI 1.93–6.70; P<0.001) compared to those who disclosed sexual activity. Higher STI risk was observed for AGYW reporting a new sexual partner (aHR: 3.16; 95% CI 1.59–6.28; P=0.001) compared to those who did not. Shorter time between menarche and time of first sexual event were not associated with higher risk of STI (aHR: 1.03; 95% CI 0.89–1.18; P=0.72). Older age at study visit was also not associated with initial STI risk (aHR: 0.89; 95% CI 0.72–1.10; P=0.27). Condomless sex in the last 90 days was not associated with risk of first STI (aHR: 0.83; 95% CI 0.51–1.35; P=0.45).
Table 1.
Multivariate Cox regression model of predictors for first ever STI event (CT, GC, TV, HSV-2) among AGYW (N=239) post-first sex.
| Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|
| Covariates | N | HR (95% CI) | P–value | aHR (95% CI)2 | P–value |
|
| |||||
| Older age at visit | 1,094 | 0·80 (0·70 – 0·91) | 0·001** | 0·89 (0·72 – 1·10) | 0·27 |
| Shorter time (years) between menarche and first sex | 985 | 1·12 (1·01 – 1·25) | 0·030* | 1·03 (0·89 – 1·18) | 0·72 |
| Bacterial vaginosis (BV) before or at visit | |||||
| No BV (Nugent 0–6) | 961 | (Ref.) | - | (Ref.) | - |
| BV (Nugent 7–10) | 133 | 1·95 (1·22 – 3·12) | 0·005** | 2·35 (1·43 – 3·88) | 0·001** |
| First sex not disclosed but evident from objective measures | |||||
| Disclosed | 962 | (Ref.) | - | (Ref.) | - |
| Not yet disclosed | 131 | 3·06 (1·90 – 4·93) | <0·001** | 3·60 (1·93 – 6·70) | <0·001** |
| Any new sex partner(s) reported in past 3 months | |||||
| No | 820 | (Ref.) | - | (Ref.) | - |
| Yes | 110 | 2.95 (1·62 – 5·38)1 | <0·001** | 3·16 (1·59 – 6·28) | 0·001** |
| Reported sex without condom in past 3 months | |||||
| No | 501 | (Ref.) | - | (Ref.) | - |
| Yes | 418 | 0·95 (0·61 – 1·49)1 | 0·83 | 0·83 (0·51 – 1·35) | 0·45 |
HR hazard ratio, aHR adjusted hazard ratio, CI confidence interval.
Following disclosure of first sex, participants were asked about sexual behaviors including new partners and condom use. The unadjusted models for new partners and condom use are thus reported among these post-disclosure visits only.
Adjusted for time-fixed covariates (years between age of menarche and first sex) and time-varying covariates (age at study visit, bacterial vaginosis at visit, sex not self-reported but evident from other measures, new sexual partner, and reporting sex without a condom).
p< ·05;
p< ·01
Adjusting for age at study visit, educational achievement was similar among cohort participants and increased years of education was not associated with risk of initial STI event (aHR: 0.86; 95% CI 0.77–0.96; P=0.29). Other socio-economic characteristics such as higher monthly income (HR: 0.95; 95% CI 0.85–1.04; P=0.29) and poorer housing conditions (HR: 1.54; 95% CI 0.98–2.42; P=0.06) were also not associated with risk of initial STI event and were omitted from the final adjusted model.
Multiple and recurrent STI events
Among 163 participants who experienced an STI, 72 participants had multiple STIs and there were a total of 259 visits where 271 STIs were detected. Of these, 78% (n=201) were CT, 11% (n=29) of which included GC, 7% (n=17) of which included TV, and 9% (n=24) of which included HSV–2. At 12 visits, two STIs were detected on the same day (10 CT with GC, and two CT with HSV2). Of the 292 girls at risk for CT infection, 100 experienced only one CT infection, while 46 experienced two or more additional CT infection events after negative CT results; therefore over one third of girls with CT had a recurrent CT event before the end of follow-up. Only 7% (n=2) of participants tested positive for gonorrhea a second time. TV infection prevalence was low (n=18 participants) and was not assessed routinely.
Correlates of multiple STI events over time
GEE model analysis results (Table 2) showed several key biological and behavioral factors were correlated with relative risk of any STI over time, including second and third infections. Similar to the Cox regression model for first STI acquisition, AGYW with BV at the same visit had a significantly increased risk of any STI (CT/GC/TV/HSV-2) (aRR: 1.60; 95% CI 1.19–2.15; P=0.002), as did AGYW reporting a new sexual partner in the last 3 months (aRR: 1.63; 95% CI 1.05–2.52; P=0.028), and AGYW who did not disclose that they were sexually active (aRR: 2.16; 95% CI 1.55–3.00; P<0.001). AGYW with two or more STI events had similar risk factors as those with only one STI, suggesting behaviors were not different between these two groups. Condomless sex was reported at 75% of visits with sex reported in the last 90 days, and did not correlate with STI risk (aRR: 0.91; 95% CI 0.69–1.21; P=0.53). Since CT was overwhelmingly common, we conducted sensitivity analyses to look for risk factors specific to CT; we found that correlates of CT infection were similar to correlates of pooled STI risk.
Table 2.
Relative risk for STI events (CT/GC/TV/HSV-2) among AGYW (N=293) after first sex, over 5 years.
| Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|
| Covariates | N | RR (95% CI) | P–value | aRR (95% CI)2 | P–value |
|
| |||||
| Older age at visit | 1,818 | 0·83 (0·77 – 0·88) | <0·001** | 0·95 (0·86 – 1·04) | 0·23 |
| Shorter time (years) between menarche and first sex | 1,629 | 1·10 (1·03 – 1·18) | 0·006** | 1·06 (0·98 – 1·14) | 0·12 |
| Bacterial vaginosis (BV) before or at visit | |||||
| No BV (Nugent 0–6) | 1,503 | (Ref.) | - | (Ref.) | - |
| BV (Nugent 7–10) | 314 | 1·42 (1·09 – 1·85) | 0·009** | 1·60 (1·19 – 2·15) | 0·002** |
| First sex not disclosed but evident from objective measures | |||||
| Disclosed | 1,579 | (Ref.) | - | (Ref.) | - |
| Not yet disclosed | 237 | 2·48 (1·95 – 3·16) | <0·001** | 2·16 (1·55 – 3·00) | <0·001** |
| Any new sex partner(s) reported in past 3 months | |||||
| No | 1,388 | (Ref.) | - | (Ref.) | - |
| Yes | 148 | 1·88 (1·32 – 2·66)1 | <0·001** | 1·63 (1·05 – 2·52) | 0·028* |
| Reported sex without condom in past 3 months | |||||
| No | 795 | (Ref.) | - | (Ref.) | - |
| Yes | 725 | 0·94 (0·72 – 1·24)1 | 0·69 | 0·91 (0·69 – 1·21) | 0·53 |
RR relative risk, aRR adjusted relative risk, CI confidence interval.
Following disclosure of first sex, participants were asked about sexual behaviors including new partners and condom use. The unadjusted models for new partners and condom use are thus reported among these post-disclosure visits only.
Adjusted for time-fixed covariates (years between age of menarche and first sex) and time-varying covariates (age at study visit, bacterial vaginosis at visit, sex not self-reported but evident from other measures, new sexual partner, and reporting sex without a condom).
p< ·05;
p< ·01
DISCUSSION
Our study examined the incidence and correlates of STI infection among a unique population of sexually inexperienced AGYW, making this one of few prospective studies to measure incidence of STI events in a cohort with a defined time of first sex. Our findings demonstrate with precision that many AGYW experience STI events during the critical years proximal to first sex and multiple and recurrent STI events are common. Further we demonstrated that the window of time is often brief between coitarche and STI acquisition. Both of our statistical models showed that the critical risk factors for STI acquisition were both biological (BV) and behavioral (new sexual partner and non-disclosure of sexual activity), thus identifying new areas to be targeted for STI prevention strategies and research.
The WHO Global Health Sector Strategy on STIs states that STI incidence data is needed across representative populations [2]. This longitudinal study among sexually inexperienced AGYW addresses this evidence gap and demonstrates there is a critical need to adapt existing reproductive health services to meet the unique needs of this population [2]. Our findings are remarkable because AGYW in this cohort were followed for three to five years including time prior to sexual activity, followed with quarterly visits, and had access to preventive, diagnostic, and treatment services. The rates of BV and STI, particularly CT, observed are as high as in many prior studies of AGYW with much higher perceived risk [3, 4, 13–15]. The preponderance of literature describing STIs in “high risk” AGYW has obscured the risks borne by AGYW whose behavioral patterns are not yet established. Several reasons have been proposed to explain increased STI risk of adolescents, including challenges accessing and staying linked to sexual and reproductive health services, higher risk behaviors, social networks with high STI prevalence, and lack of acquired immunity [2, 3, 16, 17]. Our results significantly expand concern for adolescents around the time of first sex, and add impetus for more detailed studies of mucosal events at this critical moment that may lead to enhanced susceptibility.
Extremely high incidence of CT was an especially concerning finding. This infection has spread silently in Kenya and is not currently diagnosed nor treated with standard protocols. Implications of widespread CT infection are sobering: prospective country-wide data from Denmark showed a 30% increased risk of reproductive health complications with a single diagnosis of CT; those with multiple infections had even higher risk of complications including tubal infertility, pelvic inflammatory disease, and ectopic pregnancy, although overall lifetime risk remained low for all the complications (<1%) [25]. It is difficult to tell whether the high incidence measured here is new; other recent studies of Kenyan AGYW have shown high CT burden [15, 16, 26], although those studies included participants recruited for their sexually active behaviors. Our study shows that lower-risk behaviors that made AGYW eligible for our cohort were not protective once sexual activity started and highlights an urgent need to remedy the lack of CT testing for youth in Kenya. The alarmingly high CT incidence demonstrated in our study should be used to advocate for standardized diagnosis and treatment among AGYW, as well as providing empowering sexual health services that address both biological and social determinants of reproductive health disparities.
Another important finding of this work is that non-disclosure of sexual activity among AGYW was a major correlate of STI infection. Our work demonstrates that first sex for AGYW is a time of vulnerability where innovative strategies are needed to increase AGYW agency to seek and accept prevention strategies. This will require rethinking of our approaches for at-risk AGYW, which currently rely on self-reported behavioral risk factors for prioritizing access to services and prevention. There is a lack of strategy for discussing STI with AGYW who do not self-identify as at risk. Further, syndromic management and algorithmic strategies for STI treatment, which are already recognized to be inadequate [2], will miss this non-disclosing population entirely.
Potential strategies to educate and reach AGYW in resource-limited settings have included peer educators, parental support and education on STI screenings, as well as expanding access to comprehensive family planning services and social asset building through female mentor-led group meetings [8, 18, 19]. However, universal STI screening and vaccinations could be a more practical STI reducing strategy that could reach AGYW who are not disclosing sexual activity. Promotion of universal STI screening would be a logical way to de-stigmatize STI diagnosis for AGYW who cannot disclose their activities, yet screening for STIs is still not routinely available even in the highest-risk populations in sub-Saharan Africa. Screening systems that allow for self-collected samples could also address barriers to STI diagnosis and treatment presented by requiring reproductive health exams conducted by healthcare workers before accessing care.
A new relationship is often a time of happiness and pride in the life of an AGYW. But we found that new partners represented moments of elevated STI risk, in alignment with classical STI teaching. STI prevention efforts that describe relationships and normal sexual behavior as “risky” can be alienating. Adolescent-friendly services which provide body- and sex-positive messaging are more likely to be successful. One possible and empowering approach for AGYW could be access to point-of-care diagnostics to identify asymptomatic STIs, especially CT, and to allow AGYW to easily screen partners for infections [20]. This approach would draw attention to the role male partners play in the current STI epidemic and the need to adequately engage them in sexual health to further reduce infection among AGYW.
Similar to other studies, our work also appears to verify that vaginal dysbiosis increases susceptibility to STIs, especially CT [3, 21–23]. It is novel, however, how quickly BV develops after first sex, as most AGYW in our study had no BV at baseline. Further work to characterize changes in the vaginal microbiome is underway from specimens collected in our cohort. Research has shown that periodic presumptive treatment of BV appears to reduce STI risk [24]; however, that study was done among women with many years of sexual experience. Further study is needed to determine whether the vaginal microbiome can be similarly improved during the dynamic period after first sex, and to identify factors which promote resiliency of optimal microbiome despite sexual activity.
This study also has implications for HIV in AGYW. It has been known since the earliest days of the HIV pandemic that concurrent STI greatly increases risk of HIV acquisition; the disproportionate incidence of HIV among sub-Saharan African AGYW has been attributed in large part to these co-infections [16]. Our cohort study took place in an area with lower HIV incidence than most parts of Kenya and recruited AGYW with lower-risk behaviors than their peers. There were two HIV infections over the life of the study, both of which occurred soon after enrollment and before access to HIV pre-exposure prophylaxis (PrEP) in Kenya. Conducting this study in an area with low HIV incidence, and extending access to PrEP as soon as it was available, allowed for ethical longitudinal follow up of AGYW during this highly vulnerable period. As we see similarly high STI rates as in other parts of Kenya with higher HIV incidence, we argue that these data should inform prevention measures for HIV as well as STIs.
Limitations
A potential limitation of this study is that exclusion criteria used for participant recruitment may have resulted in study findings not applicable to adolescents with sexually experience at younger ages.. Conclusions may also not be generalizable to adolescent populations outside of Kenya and among AGYW with different sociodemographic characteristics. There is also possibility of social desirability bias contributing to inaccurate self-reporting of sexual activity and condom use, though study staff were careful to be neutral with wording and prefacing of questions [27, 28].
A potential source of misclassification was that first sex was underreported by participants, and when reported, there were lags in this key variable when compared with objective data such as STI or pregnancy. We successfully were able to re-classify numerous AGYW as sexually active with objective data such as pregnancy or STI. However, misclassification resulting in the exclusion of AGYW from follow up time could have still occurred if they did not disclose sexual activity and no objective data revealed they were sexually active. This could result in an overestimation of the true incidence of STIs and an underestimation of protective behaviors for STIs.
Another concern was that AGYW could have minimized their sexual experience to enter the study; to mitigate against this, we did not advertise the sexual experience needed for study eligibility. Information about male partner factors that could influence STI acquisition was not collected, although we did provide STI treatment for partners. We did not collect data on same-sex activity which may have been occurring. Another potential limitation was that over time, AGYW who remained in the cohort were more consistent with study visits, which might indicate more stable life circumstances and less STI risk. However, we observed steady STI incidence for every year of the cohort, so overrepresentation of persons likely to engage in long term follow up does not appear to have biased the study toward lower risk over time.
CONCLUSION
Sexually inexperienced AGYW demonstrated high STI incidence, especially CT, during the initial years after first sexual activity, with 25% of initial STI events occurring in the first three months after sex. Adolescence presents a crucial transitory life stage and reproductive health services must adapt to meet the unique needs of this vulnerable population. These study findings affirm that BV is correlated with increased susceptibility to CT as well as highlights key behavioral factors, such as non-disclosure of sexual activity and new sexual partners, that have important implications for designing strategies for prevention and control of asymptomatic STI infections among AGYW. Enhanced interventions for CT control are needed for AGYW with limited sexual experience and for those unable or unwilling to disclose sexual activity in a clinic setting.
DATA SHARING
Deidentified participant data, including statistical analysis plan and code, will be made freely available after publication upon request to the corresponding author (aroxby@uw.edu) and with concurrence of the KEMRI Scientific and Ethics Review Unit.
References
- 1.Rowley J, et al. , Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ, 2019. 97(8): p. 548–562P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO, Global Health Sector Strategy on Sexually Transmitted Infections (2016–2021). 2016. [Google Scholar]
- 3.Torrone EA, et al. , Prevalence of sexually transmitted infections and bacterial vaginosis among women in sub-Saharan Africa: An individual participant data meta-analysis of 18 HIV prevention studies. PLoS Med, 2018. 15(2): p. e1002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnabas SL, et al. , Converging epidemics of sexually transmitted infections and bacterial vaginosis in southern African female adolescents at risk of HIV. Int J STD AIDS, 2018. 29(6): p. 531–539. [DOI] [PubMed] [Google Scholar]
- 5.Mwatelah R, et al. , Mechanisms of sexually transmitted infection-induced inflammation in women: implications for HIV risk. J Int AIDS Soc, 2019. 22 Suppl 6: p. e25346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Passmore JA, Jaspan HB, and Masson L, Genital inflammation, immune activation and risk of sexual HIV acquisition. Curr Opin HIV AIDS, 2016. 11(2): p. 156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sumner SA, et al. , revalence of sexual violence against children and use of social services—seven countries, 2007–2013. Morbidity and Mortality Weekly Report 2015. 64,(no. 21: 565.). [PMC free article] [PubMed] [Google Scholar]
- 8.Saul J, et al. , The DREAMS core package of interventions: A comprehensive approach to preventing HIV among adolescent girls and young women. PLoS One, 2018. 13(12): p. e0208167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(NASCOP), M.K.N.A.a.S.C.P., Kenya Population-based HIV Impact Assessment. 2018.
- 10.Yuh T, et al. , Sexually Transmitted Infections Among Kenyan Adolescent Girls and Young Women With Limited Sexual Experience. Front Public Health, 2020. 8: p. 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nugent RP, Krohn MA, and Hillier SL, Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. Journal of Clinical Microbiology, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casmir E, et al. , Protection at First Sexual Intercourse Among Adolescent Girls and Young Women in Kenya. Arch Sex Behav, 2021. 50(1): p. 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jongen VW, et al. , Incidence and risk factors of C. trachomatis and N. gonorrhoeae among young women from the Western Cape, South Africa: The EVRI study. PLoS One, 2021. 16(5): p. e0250871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francis SC, et al. , Prevalence of sexually transmitted infections among young people in South Africa: A nested survey in a health and demographic surveillance site. PLoS Med, 2018. 15(2): p. e1002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deese J, et al. , Sexually transmitted infections among women randomised to depot medroxyprogesterone acetate, a copper intrauterine device or a levonorgestrel implant. Sex Transm Infect, 2021. 97(4): p. 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masese LN, et al. , Screening for Sexually Transmitted Infections in Adolescent Girls and Young Women in Mombasa, Kenya: Feasibility, Prevalence, and Correlates. Sex Transm Dis, 2017. 44(12): p. 725–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chemaitelly H, et al. , Epidemiology of Treponema pallidum, Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, and herpes simplex virus type 2 among female sex workers in the Middle East and North Africa: systematic review and meta-analytics. J Glob Health, 2019. 9(2): p. 020408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahat G and Scoloveno MA, Effectiveness of Adolescent Peer Education Programs on Reducing HIV/STI Risk: An Integrated Review. Research and Theory for Nursing Practice, 2018. 32(2): p. 168–198. [DOI] [PubMed] [Google Scholar]
- 19.Wanje G, et al. , Parents’ and teachers’ views on sexual health education and screening for sexually transmitted infections among in-school adolescent girls in Kenya: a qualitative study. Reprod Health, 2017. 14(1): p. 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niccolai LM, et al. , New sex partner acquisition and sexually transmitted disease risk among adolescent females. Journal of Adolescent Health, 2004. 34(3): p. 216–223. [DOI] [PubMed] [Google Scholar]
- 21.Landon Myer LD, Telerant Robin, de Souza Michelle, Wright Thomas C. Jr, Kuhn Louise, Bacterial Vaginosis and Susceptibility to HIV Infection in South African Women: A Nested Case-Control Study. Journal of Infectious Diseases, 2005. [DOI] [PubMed] [Google Scholar]
- 22.McClelland RS, et al. , Evaluation of the association between the concentrations of key vaginal bacteria and the increased risk of HIV acquisition in African women from five cohorts: a nested case-control study. The Lancet Infectious Diseases, 2018. 18(5): p. 554–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nzomo J, Waiyaki P, and Waihenya R, Bacterial Vaginosis and Correlates in Women of Reproductive Age in Thika, Kenya. Advances in Microbiology, 2013. 03(03): p. 249–254. [Google Scholar]
- 24.Balkus JE, et al. , Impact of Periodic Presumptive Treatment for Bacterial Vaginosis on the Vaginal Microbiome among Women Participating in the Preventing Vaginal Infections Trial. J Infect Dis, 2017. 215(5): p. 723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies B, et al. , Risk of reproductive complications following chlamydia testing: a population-based retrospective cohort study in Denmark. The Lancet Infectious Diseases, 2016. 16(9): p. 1057–1064. [DOI] [PubMed] [Google Scholar]
- 26.Winston SE, et al. , Prevalence of sexually transmitted infections including HIV in street-connected adolescents in western Kenya. Sex Transm Infect, 2015. 91(5): p. 353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rehnstrom Loi U, et al. , Abortion and contraceptive use stigma: a cross-sectional study of attitudes and beliefs in secondary school students in western Kenya. Sex Reprod Health Matters, 2019. 27(3): p. 1652028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Latkin CA, et al. , The relationship between social desirability bias and self-reports of health, substance use, and social network factors among urban substance users in Baltimore, Maryland. Addictive Behaviors, 2017. 73: p. 133–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified participant data, including statistical analysis plan and code, will be made freely available after publication upon request to the corresponding author (aroxby@uw.edu) and with concurrence of the KEMRI Scientific and Ethics Review Unit.


