Abstract
An NADP-dependent methylene tetrahydromethanopterin (H4MPT) dehydrogenase has recently been proposed to be involved in formaldehyde oxidation to CO2 in Methylobacterium extorquens AM1. We report here on the purification of this novel enzyme to apparent homogeneity. Via the N-terminal amino acid sequence, it was identified to be the mtdA gene product. The purified enzyme catalyzed the dehydrogenation of methylene H4MPT with NADP+ rather than with NAD+, with a specific activity of approximately 400 U/mg of protein. It also catalyzed the dehydrogenation of methylene tetrahydrofolate (methylene H4F) with NADP+. With methylene H4F as the substrate, however, the specific activity (26 U/mg) and the catalytic efficiency (Vmax/Km) were approximately 20-fold lower than with methylene H4MPT. Whereas the dehydrogenation of methylene H4MPT (E0 = −390 mV) with NADP+ (E0 = −320 mV) proceeded essentially irreversibly, the dehydrogenation of methylene H4F (E0 = −300 mV) was fully reversible. Comparison of the primary structure of the NADP-dependent dehydrogenase from M. extorquens AM1 with those of methylene H4F dehydrogenases from other bacteria and eucarya and with those of methylene H4MPT dehydrogenases from methanogenic archaea revealed only marginally significant similarity (<15%).
Methylobacterium extorquens AM1 is an aerobic methylotrophic bacterium which belongs to the α-subgroup of the proteobacteria (22, 30). In this organism methanol has been proposed to be metabolized to CO2 via formaldehyde, N5,N10-methylene tetrahydrofolate (methylene H4F), N5,N10-methenyl tetrahydrofolate (methenyl H4F), N10-formyl tetrahydrofolate (formyl H4F), and formate as intermediates in reactions involving pyrroloquinolinequinone-dependent methanol dehydrogenase, NADP-dependent methylene H4F dehydrogenase, methenyl H4F cyclohydrolase, formyl H4F synthetase, and NAD-dependent formate dehydrogenase (7, 19, 21).
Recently, M. extorquens AM1 was shown to contain genes thought to be unique for methanogenic archaea, namely, genes predicted to encode methenyl tetrahydromethanopterin (H4MPT) cyclohydrolase, formylmethanofuran:H4MPT formyltransferase, and formylmethanofuran dehydrogenase (6). These enzymes catalyze the first three steps of methanogenesis during the growth of methanogens on CO2 and H2 and the last three steps of CO2 formation during the growth of methanogens on methanol (15, 28). The genes in M. extorquens AM1 encoding the three methanogenic enzymes were shown by mutagenesis to be required for growth on C1 compounds, suggesting an involvement of the corresponding enzymes in formaldehyde oxidation to CO2 via N5,N10-methylene H4MPT (methylene H4MPT), N5,N10-methenyl H4MPT (methenyl H4MPT), N5-formyl H4MPT (formyl H4MPT), and formylmethanofuran as intermediates (6).
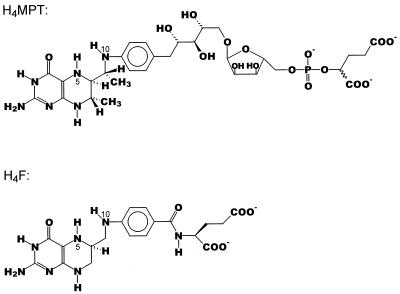
The genetic evidence for this novel metabolic pathway was substantiated by the finding that cell extracts of methanol-grown M. extorquens AM1 exhibit methenyl H4MPT cyclohydrolase activity and formylmethanofuran:H4MPT formyltransferase activity and contain dephospho-H4MPT (6). H4MPT is an H4F analogue previously found only in methanogenic and sulfate-reducing archaea. Its properties are significantly different from those of H4F (Fig. 1). Thus, the redox potential of the N5,N10-methenyl H4MPT–N5,N10-methylene H4MPT couple (−390 mV) is 90 mV more negative than that of the N5,N10-methenyl H4F–N5,N10-methylene H4F couple (−300 mV) (13, 29, 37).
FIG. 1.
Structures of H4MPT (25, 32, 33) and H4F. Functionally, the most important difference between H4MPT and H4F is that H4MPT has an electron-donating methylene group in conjugation to N10 via the aromatic ring, whereas H4F has an electron-withdrawing carbonyl group in this position. One consequence is that the redox potential of the N5,N10-methenyl H4MPT–N5,N10-methylene H4MPT couple (−390 mV) is almost 100 mV more negative than that of the N5,N10-methenyl H4F–N5,N10-methylene H4F couple (−300 mV) (29). The H4MPT derivative found in M. extorquens AM1 lacks the α-hydroxyglutaryl phosphate unit (6). The dephospho-H4MPT is also present in methanogenic archaea (35).
Further support for the novel metabolic pathway came from the finding that cell extracts of M. extorquens AM1 contain an NADP-dependent methylene H4MPT dehydrogenase activity catalyzing the formation of methenyl H4MPT from methylene H4MPT (6). The presence of an NADP-dependent methylene H4MPT dehydrogenase in M. extorquens AM1 is of special interest because the methylene H4MPT dehydrogenases in methanogenic and sulfate-reducing archaea are specific for coenzyme F420, which is a 5′ deazaflavin derivative (8). The redox potential of the F420–F420H2 couple (−360 mV) is 40 mV more negative than that of the NAD(P)+–NAD(P)H couple (−320 mV) (12, 34). Apparently, M. extorquens AM1 contains a novel enzyme combining features of both the F420-dependent methylene H4MPT dehydrogenase from methanogens and the NAD(P)-dependent methylene H4F dehydrogenase found in bacteria and eucarya. We report here on the purification and the characterization of the novel enzyme.
MATERIALS AND METHODS
Coenzymes.
H4MPT, methenyl H4MPT, and coenzyme F420 were purified from Methanobacterium thermoautotrophicum Marburg (DSM 2133) (5). H4F was purchased from Sigma. Anoxic stock solutions of H4MPT and H4F were prepared in 50 mM Tricine-KOH (pH 7.0) containing 2 mM dithiothreitol. In giving concentrations of H4F, it was considered that less than 50% of the commercially available H4F was biologically active as determined enzymatically, due to the presence of the biologically inactive R isomer and dihydrofolate (36). Methenyl H4F was generated from methylene H4F by dehydrogenation via NADP-dependent methylene H4MPT dehydrogenase from M. extorquens AM1 and was purified by high-performance liquid chromatography (HPLC). Methylene H4F was generated from H4F and formaldehyde by spontaneous reaction. Methylene H4F was not purified prior to the enzymatic conversion to methenyl H4F.
Growth of bacteria.
M. extorquens AM1 was grown on methanol (100 mM) at 30°C in minimal medium as described previously (11). The cultures were harvested in the late-exponential phase at a cell concentration of 3 g (wet mass)/liter. Cells were pelleted by centrifugation at 5,000 × g and stored at −20°C.
Preparation of cell extracts.
Frozen cells (9 g) were suspended in 18 ml of 50 mM morpholinepropanesulfonic acid (MOPS)-KOH (pH 7.0) at 4°C and passed three times through a French pressure cell at 1.2 × 108 Pa. Cell debris and unbroken cells were removed by centrifugation at 27,000 × g for 30 min. The resulting supernatant is referred to as cell extract.
Protein concentration was determined by the Bradford assay (4) by using the Bio-Rad reagent with bovine serum albumin as the standard.
Determination of methylene H4MPT dehydrogenase activity.
The assays were performed routinely at 30°C in 1-ml cuvettes (depth, 1 cm) in a total volume of 0.7 ml. The reactions were monitored photometrically by measuring the increase or decrease in absorbance at 340 nm. For the calculations, ɛ340 values of 6.2 mM−1 cm−1 for NADPH, 20.8 mM−1 cm−1 for methenyl H4MPT (10), and 21.7 mM−1 cm−1 for methenyl H4F (18) were used. Units of enzyme activities are defined as 1 μmol/min at 30°C.
Methylene H4MPT dehydrogenation with NADP+ was routinely measured in 120 mM potassium phosphate (pH 6.0) at a methylene H4MPT concentration of 35 μM and an NADP+ concentration of 0.2 mM. The methylene H4MPT was generated in the cuvette by a spontaneous reaction of H4MPT (35 μM) with formaldehyde (3 mM). The excess of formaldehyde did not adversely affect the enzyme activity.
Methylene H4F dehydrogenation with NADP+ was measured in 120 mM potassium phosphate (pH 6.0) at a methylene H4F concentration of 70 μM and an NADP+ concentration of 0.2 mM. The methylene H4F was generated in the cuvette by a spontaneous reaction of H4F (70 μM) with formaldehyde (3 mM). The excess of formaldehyde did not adversely affect the enzyme activity.
Methenyl H4MPT reduction with NADPH was measured in 120 mM potassium phosphate (pH 7.0) at a methenyl H4MPT concentration of 30 μM and an NADPH concentration of 50 μM. The NADPH was regenerated with glucose-6-phosphate (2 mM) and glucose-6-phosphate dehydrogenase (10 U).
Methenyl H4F reduction with NADPH was measured in 120 mM potassium phosphate (pH 7.0) at a methenyl H4F concentration of 30 μM and an NADPH concentration of 50 μM.
Purification of methylene H4MPT dehydrogenase.
All purification steps were performed at 4°C under aerobic conditions. To 23 ml of cell extract stirred on ice, 34.5 ml of saturated ammonium sulfate in 50 mM Tris-HCl (pH 7.0) was added to a final concentration of 60% saturation. After 20 min of stirring, the precipitated protein was removed by 30 min of centrifugation at 20,000 × g. The supernatant was applied to a phenyl Sepharose (High Performance 26/10; Pharmacia Biotech) column equilibrated with 2 M ammonium sulfate in 50 mM Tris-HCl (pH 7.0). With a linear gradient decreasing from 2 to 0 M (NH4)2SO4 (600 ml), the dehydrogenase activity eluted at about 0.25 M (NH4)2SO4. Combined active fractions were concentrated with Centricon 30 microconcentrators (Millipore), washed with 50 mM MOPS-KOH (pH 7.0) (1:5), and subjected to anion-exchange chromatography on a Q Sepharose column (High Performance 16/10; Pharmacia Biotech). The enzyme activity was recovered in the flowthrough of the column (50 mM MOPS-KOH [pH 7.0]). Active fractions were pooled, washed, and concentrated by using Centricon 30 microconcentrators and 50 mM morpholineethanesulfonic acid (MES)-NaOH (pH 5.5). The enzyme was further purified by cation-exchange chromatography on a Mono S column (5/5; Pharmacia Biotech) via a linear gradient from 0 to 0.2 M NaCl in 50 mM MES-NaOH (pH 5.5) (57 ml). Methylene H4MPT dehydrogenase was recovered at 0.16 M NaCl, diluted (1:3) with 50 mM MES-NaOH (pH 5.5), and rechromatographed on a Mono S column by using the same gradient.
Determination of the N-terminal amino acid sequence.
Purified enzyme was electrophoresed in the presence of sodium dodecyl sulfate (SDS), and the 32-kDa band was electroblotted onto a poly(vinyl trifluoride) membrane (Applied Biosystems). Sequence determination was performed on a 477 protein/peptide sequencer from Applied Biosystems by D. Linder, Giessen, Germany.
Determination of dephospho-H4MPT and H4F concentrations.
A cell extract (3 ml) of M. extorquens AM1 was ultrafiltrated with Centricon 3 microconcentrators. The filtrate, containing the low-molecular-mass compounds, was then supplemented with 15 mM formaldehyde, 2 mM NADP+, and 2 U of purified methylene H4MPT dehydrogenase in order to convert dephospho-H4MPT and H4F into dephospho-methenyl H4MPT and methenyl H4F, respectively. Up to this point, all steps were performed under strictly anaerobic conditions. Subsequently, the filtrate was subjected to HPLC with a LiChrospher RP-18 column (14 mm by 125 mm) (Merck). Absorbed compounds were eluted (1 ml/min) with a linear gradient (30 ml) of 0 to 50% methanol in 25 mM sodium formate (pH 3.0). The eluate was continuously monitored for absorbance at 335, 356, and 274 nm, and UV-visible spectra were recorded by using a Hewlett-Packard 1050 diode array detector. The retention times were 20.0 min for dephospho-methenyl H4MPT, 18.8 min for methenyl H4MPT, and 17.9 min for methenyl H4F. The methenyl H4MPT had been added to the filtrate for calibration. The methenyl derivatives were analyzed by matrix-assisted laser desorption ionization time-of-flight (MALDI/TOF) mass spectrometry by J. Kahnt, Marburg, Germany, using Voyager-DE RP (PerSeptive Biosystems).
RESULTS
Cell extracts of M. extorquens AM1 grown on methanol catalyzed the reduction of NADP+ with methylene H4MPT (2.6 U/mg), the reduction of NADP+ with methylene H4F (0.2 U/mg), and the reduction of NAD+ with methylene H4MPT (0.6 U/mg) rather than with methylene H4F (<0.01 U/mg). The activities were associated with the soluble cell fraction. The two NADP-dependent activities were found to copurify and to be separated from the NAD-dependent activity upon ammonium sulfate precipitation. Purification was possible under aerobic conditions.
Cell extracts of M. extorquens AM1 did not catalyze the reduction of coenzyme F420 with methylene H4MPT or methylene H4F. They also appeared not to contain coenzyme F420, as determined spectrofluorometrically.
Purification and molecular properties.
The two NADP-dependent activities were purified by hydrophobic chromatography on a phenyl Sepharose column, by anion-exchange chromatography on a Q Sepharose column, and by cation-exchange chromatography on a Mono S column. In each step a complete copurification of NADP-dependent methylene H4MPT dehydrogenase and NADP-dependent methylene H4F dehydrogenase was observed. Purification of both activities was approximately 130-fold with 24% yields (Table 1).
TABLE 1.
Copurification of NADP-dependent methylene H4F dehydrogenase and NADP-dependent methylene H4MPT dehydrogenase activities from M. extorquens AM1 grown on methanol
| Purification step | Protein (mg) | Activity (U)a with:
|
Fold purifi-cationb | Yield (%)b | |
|---|---|---|---|---|---|
| H4F | H4MPT | ||||
| Cell extract | 642 | 127 | 1,652 | 1 | 100 |
| (NH4)2SO4 supernatant | 129 | 107 | 1,408 | 4 | 85 |
| Phenyl Sepharose | 28 | 88 | 1,278 | 18 | 77 |
| Q Sepharose | 3.4 | 61 | 1,020 | 115 | 62 |
| Mono S Ic | 2.5 | 55 | 812 | 125 | 49 |
| Mono S II | 1.2 | 31 | 495 | 128 | 24 |
Determined at 30°C under standard assay conditions.
Calculated for activities determined with methylene H4MPT.
Mono S I and Mono S II, first and second chromatographies, respectively, on a Mono S column.
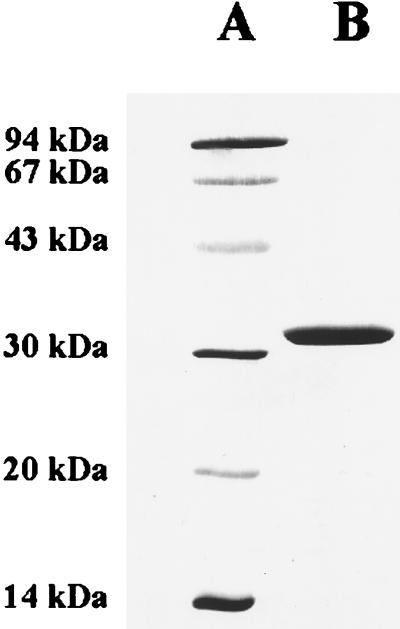
After the second chromatography on Mono S, the preparation contained only one polypeptide, with an apparent molecular mass of 32 kDa, as revealed by SDS-polyacrylamide gel electrophoresis (PAGE) (Fig. 2). The N-terminal amino acid sequence (SKKLLFQFDTDATPSVFDVV) determined by Edman degradation and the apparent molecular mass conformed with those predicted for the mtdA product (7).
FIG. 2.
SDS-PAGE analysis of purified methylene H4MPT dehydrogenase from M. extorquens AM1. Protein was separated on a 16% polyacrylamide gel and subsequently stained with Coomassie brilliant blue (17). Lane A, low-molecular-mass standards (Pharmacia Biotech); lane B, 5 μg of purified MtdA.
The purified enzyme eluted from Superdex 200, a gel filtration column, with an apparent molecular mass of 93 kDa (data not shown), suggesting that the dehydrogenase has a homotrimeric structure.
The UV-visible spectrum of the enzyme was that of a protein lacking a chromophoric prosthetic group (data not shown). The ɛ280 determined and the ɛ280 calculated from the tyrosine, phenylalanine, and tryptophan contents of the enzyme coincided within a 5% range.
The calculated pI for MtdA is 7.2, conforming with the property of the enzyme to bind to cation-exchange resins at pHs below 7.
The enzyme could be stored at −20°C under aerobic conditions for several weeks without loss of activity.
Catalytic properties.
The purified enzyme was found to be similarly active with the H4MPT and the dephospho-H4MPT derivatives, allowing for determination of the catalytic properties by using the more readily available H4MPT. Apparently, the modification in the side chain (Fig. 1) has little effect on the enzyme activity, a phenomenon also observed for the various H4MPT-dependent enzymes in methanogenic archaea (14, 23, 26, 27).
Under the standard assay conditions described in Materials and Methods, the purified enzyme catalyzed the dehydrogenation of methylene H4MPT with NADP+ with a specific activity of 413 U/mg and the dehydrogenation of methylene H4F with NADP+ with a specific activity of 26 U/mg. It also catalyzed the reverse reactions, the reduction of methenyl H4MPT to methylene H4MPT (9 U/mg) and of methenyl H4F to methylene H4F (15 U/mg) using NADPH. For thermodynamic reasons (see below), the reduction of methenyl H4MPT to methylene H4MPT could be monitored only when an NADPH-regenerating system was employed.
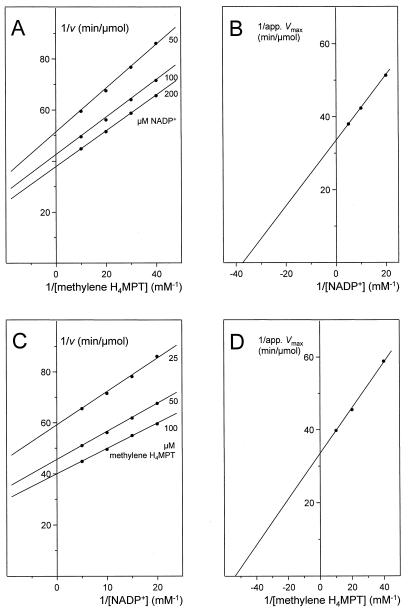
The dependence of the rate of methylene H4MPT dehydrogenation with NADP+ on the concentrations of the substrates was determined. Reciprocal plots of the initial rates versus the concentration of one substrate at different fixed concentrations of the second substrate yielded straight lines (Fig. 3A and C). From reciprocal plots of 1/apparent Vmax versus 1/[S], where [S] is the concentration of the substrate, a Vmax of 600 U/mg, a Km for methylene H4MPT of approximately 20 μM, and a Km for NADP+ of approximately 30 μM were obtained (Fig. 3B and D). With methylene H4F as the substrate, the Vmax was approximately 30 U/mg, the Km for methylene H4F was approximately 30 μM, and the Km for NADP+ was approximately 10 μM (data not shown). Thus, the enzyme catalyzed the dehydrogenation of methylene H4MPT with an approximately 20-fold-higher catalytic efficiency (Vmax/Km) than the dehydrogenation of methylene H4F.
FIG. 3.
Kinetics of methylene H4MPT dehydrogenation with NADP+ as catalyzed by NADP+-dependent methylene H4MPT dehydrogenase from M. extorquens AM1. (A and C) Plots of 1/v versus 1/[S], where velocity (v) is given as micromoles of substrate converted per minute. (B and D) Replots of 1/apparent (app.) Vmax versus 1/[S]. Assay conditions were as follows: temperature, 30°C; pH 6.0; NADP+ and methylene H4MPT concentrations as indicated; 50 ng of purified methylene H4MPT dehydrogenase.
Due to the relatively low Km values for both substrates, it was not possible to accurately measure the rate dependence on the substrate concentration at concentrations much lower than the Km. Such measurements would be required to ascertain whether the lines in the double reciprocal plots shown in Fig. 3A and C really converge or are parallel, which would indicate a ternary complex (sequential) or a ping-pong catalytic mechanism, respectively. The absence of a chromophoric prosthetic group argues for a ternary complex (sequential) catalytic mechanism.
The optimum pH for methylene H4MPT dehydrogenation was near 6.0, and the optimum temperature was near 45°C. The activity was slightly stimulated by salts, and maximal activity was reached at a potassium phosphate concentration of 120 mM (pH 6.0). NaCl concentrations higher than 100 mM were inhibitory.
The purified enzyme did not catalyze the reduction of NAD+, flavin adenine dinucleotide, flavin mononucleotide, coenzyme F420, or dyes such as methylene blue or benzyl viologen using either methylene H4MPT or methylene H4F. It also did not exhibit methenyl H4MPT cyclohydrolase or methenyl H4F cyclohydrolase activity.
Thermodynamics.
Methylene H4MPT dehydrogenation using NADP+ was found to proceed almost to completion. The reverse reaction, the reduction of methenyl H4MPT using NADPH, ceased after less than 1% of the methylene H4MPT had been reduced, as predicted from the E0 of −390 mV of the N5,N10-methenyl H4MPT–N5,N10-methylene H4MPT couple and the E0 of −320 mV of the NADP+-NADPH couple. In the case of methylene H4F dehydrogenation, equilibrium was attained when about 30% of the methylene H4F had been converted to methenyl H4F, in agreement with the E0 of −300 mV of the methylene H4F-methenyl H4F couple. From the concentrations at equilibrium, free energy changes (ΔG° values) of −13 and +3 kJ/mol were calculated for methylene H4MPT dehydrogenation and methylene H4F dehydrogenation, respectively.
Intracellular concentrations of dephospho-H4MPT and H4F.
Dephospho-H4MPT and H4F in cell extracts of M. extorquens AM1 were converted into more stable and readily detectable methenyl derivatives, which were subsequently subjected to reversed-phase HPLC and quantitated spectroscopically. Per milligram of cell extract protein, 1.4 nmol of dephospho-methenyl H4MPT and 0.5 nmol of methenyl H4F were detected, corresponding to intracellular concentrations of 0.4 and 0.15 mM, respectively, assuming that the intracellular volume is 3.3 μl/mg of protein, as in other procaryotes (3).
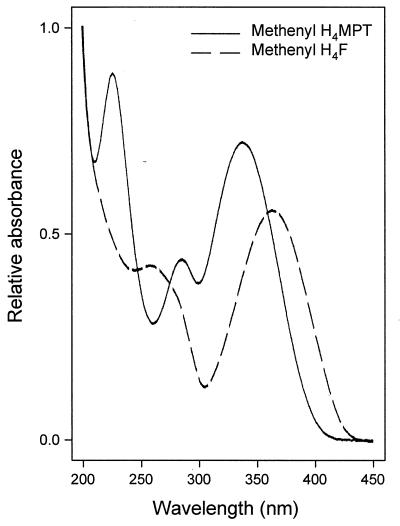
Dephospho-methenyl H4MPT and methenyl H4F were identified by their retention times on the HPLC column (internal standardization [data not shown]) and by the UV-visible spectra (Fig. 4), and their masses were determined by MALDI/TOF mass spectrometry (data not shown). The dephospho-methenyl H4MPT isolated from M. extorquens AM1 was used as the substrate by the NADP-dependent methylene H4MPT dehydrogenase with a specific activity similar to that for methenyl H4MPT.
FIG. 4.
UV-visible spectra of dephospho-methenyl H4MPT and methenyl H4F isolated from M. extorquens AM1 grown on methanol. The compounds were separated by HPLC, and their spectra were recorded online by diode array detection.
The HPLC analysis did not yield evidence for the presence of tetrahydropterin derivatives other than dephospho-H4MPT and H4F.
DISCUSSION
The NADP-dependent methylene H4MPT dehydrogenase from M. extorquens AM1 has several properties in common with the two kinds of methylene H4MPT dehydrogenases found in methanogenic archaea, the F420-dependent methylene H4MPT dehydrogenase (16) and the H2-forming methylene H4MPT dehydrogenase (29). The three types of dehydrogenases are all composed of only one type of subunit with a molecular mass between 30 and 40 kDa, and they all lack a chromophoric prosthetic group and exhibit a ternary complex (sequential) catalytic mechanism. However, the three enzymes show only a very low degree of sequence similarity (<15%). Only minor sequence similarities are also found with NAD(P)-dependent methylene H4F dehydrogenases from bacteria and eucarya, which, with a few exceptions (2, 20, 24, 31, 38), are generally more complex, multifunctional enzymes containing additionally methenyl H4F cyclohydrolase in bacteria and both methenyl H4F cyclohydrolase and N5-formyl H4F synthetase activities (1, 9) in eucarya. Therefore, based on differences in amino acid sequence and coenzyme specificity, four families of methylene tetrahydropterin dehydrogenases can be defined; they catalyze the following reactions: methylene H4MPT + NADP+ ⇌ methenyl H4MPT+ + NADPH (ΔG° = −13 kJ/mol) methylene H4MPT + F420 + H+ ⇌ methenyl H4MPT+ + F420H2 (ΔG° = −5.5 kJ/mol) methylene H4MPT + H+ ⇌ methenyl H4MPT+ + H2 (ΔG° = +5.5 kJ/mol) methylene H4F + NAD(P)+ ⇌ methenyl H4F+ + NAD(P)H (ΔG° = +3.5 kJ/mol)
Within each of the four families, all enzymes show sequence similarity, even when they belong to organisms that are very distantly related phylogenetically.
In addition to the NADP-dependent methylene H4MPT dehydrogenase, M. extorquens AM1 cell extracts contain an NAD-dependent methylene H4MPT dehydrogenase activity. Genetic evidence indicates that this enzyme is encoded by orfX (6). The amino acid sequence of OrfX is 30% identical to that of the NADP-dependent methylene H4MPT dehydrogenase. While the NADP-dependent dehydrogenase (MtdA) exhibits some activity with methylene H4F, the NAD-dependent methylene H4MPT dehydrogenase is specific for H4MPT (unpublished results).
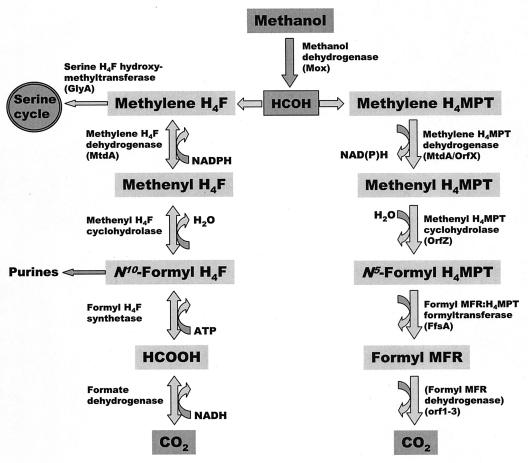
For understanding possible functions of the pyridine nucleotide-dependent methylene H4MPT dehydrogenases found so far only in M. extorquens AM1, it is important that they preferentially catalyze the C1 oxidation reaction, whereas the three other methylene tetrahydropterin dehydrogenases can, in principle, operate in both directions. It is also of importance that the NADP-dependent methylene H4MPT dehydrogenase exhibits some NADP-dependent methylene H4F dehydrogenase activity and that M. extorquens AM1 contains dephospho-H4MPT and H4F at similar concentrations. Under certain conditions the NADP-dependent methylene H4MPT dehydrogenase could therefore also function in the reversible dehydrogenation of methylene H4F. There is evidence that methanol-grown M. extorquens AM1 contains an H4F-specific serine hydroxymethyltransferase and an H4F-specific methenyl H4F cyclohydrolase (unpublished results). There is also evidence for the presence of an H4MPT-specific methenyl H4MPT cyclohydrolase and an H4MPT-specific formylmethanofuran:H4MPT formyltransferase. It therefore appears that in M. extorquens AM1 two independent C1 transfer pathways are simultaneously operative, one that involves dephospho-H4MPT as the C1 carrier and is most likely to operate in the oxidative direction and one that involves H4F and might operate in either direction, depending upon cellular pools of intermediates (Fig. 5). Purification and characterization of the enzymes involved in these pathways are an important step in the elucidation of the roles of the two C1 transfer pathways in the anabolism and catabolism of M. extorquens AM1 growing on methanol.
FIG. 5.
Possible role of methylene H4MPT dehydrogenase (MtdA) and other enzymes in growth of M. extorquens AM1 on methanol. Note that the presence of methanofuran in M. extorquens AM1 has not been proven. It is not known whether N5-formyl H4MPT can support purine biosynthesis.
ACKNOWLEDGMENTS
This work was supported by the Max-Planck-Gesellschaft, the Deutsche Forschungsgemeinschaft, and the Fonds der Chemischen Industrie (R.K.T.) and by an NIH grant to M.E.L. (GM36296).
REFERENCES
- 1.Allaire M, Li Y G, MacKenzie R E, Cygler M. The 3-D structure of a folate-dependent dehydrogenase/cyclohydrolase bifunctional enzyme at 1.4 Å resolution. Structure. 1998;6:173–182. doi: 10.1016/s0969-2126(98)00019-7. [DOI] [PubMed] [Google Scholar]
- 2.Barlowe C K, Appling D R. Isolation and characterization of a novel eucaryotic monofunctional NAD+-dependent 5,10-methylenetetrahydrofolate dehydrogenase. Biochemistry. 1990;29:7089–7094. doi: 10.1021/bi00482a020. [DOI] [PubMed] [Google Scholar]
- 3.Blaut M, Gottschalk G. Coupling of ATP synthesis and methane formation from methanol and molecular hydrogen in Methanosarcina barkeri. Eur J Biochem. 1984;141:217–222. doi: 10.1111/j.1432-1033.1984.tb08178.x. [DOI] [PubMed] [Google Scholar]
- 4.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Breitung J, Börner G, Scholz S, Linder D, Stetter K O, Thauer R K. Salt dependence, kinetic properties and catalytic mechanism of N-formylmethanofuran:tetrahydromethanopterin formyltransferase from the extreme thermophile Methanopyrus kandleri. Eur J Biochem. 1992;210:971–981. doi: 10.1111/j.1432-1033.1992.tb17502.x. [DOI] [PubMed] [Google Scholar]
- 6.Chistoserdova L, Vorholt J A, Thauer R K, Lidstrom M E. C1-transfer enzymes and coenzymes linking methylotrophic bacteria and methanogenic archaea. Science. 1998;281:99–102. doi: 10.1126/science.281.5373.99. [DOI] [PubMed] [Google Scholar]
- 7.Chistoserdova L V, Lidstrom M E. Genetics of the serine cycle in Methylobacterium extorquens AM1: identification of sgaA and mtdA and sequences of sgaA, hprA, and mtdA. J Bacteriol. 1994;176:1957–1968. doi: 10.1128/jb.176.7.1957-1968.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eirich L D, Vogels G D, Wolfe R S. Proposed structure for coenzyme F420 from Methanobacterium. Biochemistry. 1978;17:4583–4593. doi: 10.1021/bi00615a002. [DOI] [PubMed] [Google Scholar]
- 9.Enßle M, Zirngibl C, Linder D, Thauer R K. Coenzyme F420 dependent N5,N10-methylenetetrahydromethanopterin dehydrogenase in methanol-grown Methanosarcina barkeri. Arch Microbiol. 1991;155:483–490. [Google Scholar]
- 10.Escalante-Semerena J C, Rinehart K L, Wolfe R S. Tetrahydromethanopterin, a carbon carrier in methanogenesis. J Biol Chem. 1984;259:9447–9455. [PubMed] [Google Scholar]
- 11.Fulton G L, Nunn D N, Lidstrom M E. Molecular cloning of a malyl coenzyme A lyase gene from Pseudomonas sp. strain AM1, a facultative methylotroph. J Bacteriol. 1984;160:718–723. doi: 10.1128/jb.160.2.718-723.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gloss L M, Hausinger R P. Reduction potential characterization of methanogen factor 390. FEMS Microbiol Lett. 1987;48:143–145. [Google Scholar]
- 13.Keltjens J T, Vogels G D. Methanopterin and methanogenic bacteria. BioFactors. 1988;1:95–103. [PubMed] [Google Scholar]
- 14.Keltjens J T, Brugman A J A M, Kesseleer J M A, te Brömmelstroet B W J, van der Drift C, Vogels G D. 5-Formyl-5,6,7,8-tetrahydromethanopterin is the intermediate in the process of methanogenesis in Methanosarcina barkeri. BioFactors. 1992;3:249–255. [PubMed] [Google Scholar]
- 15.Keltjens J T, Vogels G D. Conversion of methanol and methylamines to methane and carbon dioxide. In: Ferry J G, editor. Methanogenesis. New York, N.Y: Chapman & Hall; 1993. pp. 253–303. [Google Scholar]
- 16.Klein A R, Thauer R K. Overexpression of the coenzyme-F420-dependent N5,N10-methylenetetrahydromethanopterin dehydrogenase gene from the hyperthermophilic Methanopyrus kandleri. Eur J Biochem. 1997;245:386–391. doi: 10.1111/j.1432-1033.1997.t01-1-00386.x. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.MacKenzie R E. Biogenesis and interconversion of substituted tetrahydrofolates. In: Blakley R L, Benkovic S J, editors. Folates and pterins. New York, N.Y: John Wiley & Sons; 1984. pp. 256–306. [Google Scholar]
- 19.Marison I W, Attwood M M. A possible alternative mechanism of the oxidation of formaldehyde to formate. J Gen Microbiol. 1982;128:1441–1446. [Google Scholar]
- 20.Moore M R, O’Brien W E, Ljungdahl L G. Purification and characterization of nicotinamide adenine dinucleotide-dependent methylenetetrahydrofolate dehydrogenase from Clostridium formicoaceticum. J Biol Chem. 1974;249:5250–5253. [PubMed] [Google Scholar]
- 21.Nunn D N, Lidstrom M E. Isolation and complementation analysis of 10 methanol oxidation mutant classes and identification of the methanol dehydrogenase structural gene of Methylobacterium sp. strain AM1. J Bacteriol. 1986;166:581–590. doi: 10.1128/jb.166.2.581-590.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peel D, Quayle J R. Microbial growth on C1 compounds. 1. Isolation and characterization of Pseudomonas AM1. Biochem J. 1961;81:465–469. doi: 10.1042/bj0810465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raemakers-Franken P C, Kortstee A J, van der Drift C, Vogels G D. Methanogenesis involving a novel carrier of C1 compounds in Methanogenium tationis. J Bacteriol. 1990;172:1157–1159. doi: 10.1128/jb.172.2.1157-1159.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ragsdale S W, Ljungdahl L G. Purification and properties of NAD-dependent 5,10-methylenetetrahydrofolate dehydrogenase from Acetobacterium woodii. J Biol Chem. 1984;259:3499–3503. [PubMed] [Google Scholar]
- 25.Schleucher J, Griesinger C, Schwörer B, Thauer R K. H2-forming N5,N10-methylenetetrahydromethanopterin dehydrogenase from Methanobacterium thermoautotrophicum catalyzes a stereoselective hydride transfer as determined by two-dimensional NMR spectroscopy. Biochemistry. 1994;33:3986–3993. doi: 10.1021/bi00179a027. [DOI] [PubMed] [Google Scholar]
- 26.te Brömmelstroet B W, Hensgens C M H, Geerts W J, Keltjens J T, van der Drift C, Vogels G D. Purification and properties of 5,10-methenyltetrahydromethanopterin cyclohydrolase from Methanosarcina barkeri. J Bacteriol. 1990;172:564–571. doi: 10.1128/jb.172.2.564-571.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.te Brömmelstroet B W, Geerts W J, Keltjens J T, van der Drift C, Vogels G D. Purification and properties of 5,10-methylenetetrahydromethanopterin dehydrogenase and 5,10-methylenetetrahydromethanopterin reductase, two coenzyme F420-dependent enzymes, from Methanosarcina barkeri. Biochim Biophys Acta. 1991;1079:293–302. doi: 10.1016/0167-4838(91)90072-8. [DOI] [PubMed] [Google Scholar]
- 28.Thauer R. Biodiversity and unity in biochemistry. Antonie Leeuwenhoek. 1997;71:21–32. doi: 10.1023/a:1000149705588. [DOI] [PubMed] [Google Scholar]
- 29.Thauer R K, Klein A R, Hartmann G C. Reactions with molecular hydrogen in microorganisms: evidence for a purely organic hydrogenation catalyst. Chem Rev. 1996;96:3031–3042. doi: 10.1021/cr9500601. [DOI] [PubMed] [Google Scholar]
- 30.Tsuji K, Tsien H C, Hanson R S, DePalma S R, Scholtz R, LaRoche S. 16S ribosomal RNA sequence analysis for determination of phylogenetic relationship among methylotrophs. J Gen Microbiol. 1990;136:1–10. doi: 10.1099/00221287-136-1-1. [DOI] [PubMed] [Google Scholar]
- 31.Uyeda K, Rabinowitz J C. Enzymes of clostridial purine fermentation. Methylenetetrahydrofolate dehydrogenase. J Biol Chem. 1967;242:4378–4385. [PubMed] [Google Scholar]
- 32.Van Beelen P, Stassen A P M, Bosch J W G, Vogels G D, Guijt W, Haasnoot C A G. Elucidation of the structure of methanopterin, a coenzyme from Methanobacterium thermoautotrophicum, using two-dimensional nuclear-magnetic-resonance techniques. Eur J Biochem. 1984;138:563–571. doi: 10.1111/j.1432-1033.1984.tb07951.x. [DOI] [PubMed] [Google Scholar]
- 33.Van Beelen P, Labro J F A, Keltjens J T, Geerts W J, Vogels G D, Laarhoven W H, Guijt W, Haasnoot C A G. Derivatives of methanopterin, a coenzyme involved in methanogenesis. Eur J Biochem. 1984;139:359–365. doi: 10.1111/j.1432-1033.1984.tb08014.x. [DOI] [PubMed] [Google Scholar]
- 34.Walsh C. Naturally occurring 5-deazaflavin coenzymes: biological redox roles. Acc Chem Res. 1986;19:216–221. [Google Scholar]
- 35.White R H. Methanopterin biosynthesis: methylation of the biosynthetic intermediates. Biochim Biophys Acta. 1998;1380:257–267. doi: 10.1016/s0304-4165(97)00148-7. [DOI] [PubMed] [Google Scholar]
- 36.Wohlfarth G, Geerligs G, Diekert G. Purification and properties of a NADH-dependent 5,10-methylenetetrahydrofolate reductase from Peptostreptococcus productus. Eur J Biochem. 1990;192:411–417. doi: 10.1111/j.1432-1033.1990.tb19242.x. [DOI] [PubMed] [Google Scholar]
- 37.Wohlfarth G, Diekert G. Thermodynamics of methylenetetrahydrofolate reduction to methyltetrahydrofolate and its implications for the energy metabolism of homoacetogenic bacteria. Arch Microbiol. 1991;155:378–381. [Google Scholar]
- 38.Wohlfarth G, Geerligs G, Diekert G. Purification and characterization of NADP+-dependent 5,10-methylenetetrahydrofolate dehydrogenase from Peptostreptococcus productus Marburg. J Bacteriol. 1991;173:1414–1419. doi: 10.1128/jb.173.4.1414-1419.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]