Abstract
A 54-year-old man with noncontributory medical history presented to an ophthalmologist in January 2022 after 10 days of irritation in his right eye. The patient recounts having felt something get into his eye and under his contact lens (CL) while he was climbing into his car, but he was unsure what the foreign body may have been. Initial examination by the clinician found uncorrected distance visual acuity of 20/100–2 with a corneal abrasion, 4+ corneal edema, and 3+ conjunctival injection, for which he was placed on topical antibiotics (ocuflox and tobradex) with a bandage CL. 1 week later, visual acuity was 20/80, corneal edema had improved, and he was noted to have corneal scarring and an epithelial defect. Tobradex was continued while prednisolone drops and preservative-free artificial tears were started. 1 week later, the patient had worsening visual acuity to 20/250 and was referred to our tertiary center.
On initial consultation, the patient had an uncorrected distance visual acuity of 20/500 and an uncorrected near visual acuity of >J10 in the right eye. Slitlamp examination of the right eye was significant for vortex keratopathy and mild corneal pannus with 360-degree subtle conjunctivalization of the limbus (Figure 1). The corneal topograph was obtained showing significant surface irregularity on the Placido image (Figure 2). Examination of the left eye was unremarkable. The ocular history is significant for myopia of −4.0 diopters and CL use for 20 years. The patient admits to regularly wearing soft CLs for several days straight and only removing them for a few hours. Antibiotics were discontinued, corticosteroid drops were reduced in frequency, and the patient was continued on preservative-free artificial tears.
What imaging might you consider? What is your differential diagnosis at this point? What would be the most appropriate surgical and/or medical interventions? What would you counsel in prognosis for this patient?
This is a case of a 54-year-old man who presented with acute epithelial keratopathy in the setting of chronic corneal changes including scarring, conjunctivalization, and neovascularization, consistent with underlying limbal stem-cell deficiency (LSCD).
In practice, LSCD is largely a clinical diagnosis, and therefore, it is most important to obtain a thorough history and careful slitlamp examination to detect typical characteristics, including epitheliopathy and conjunctival changes adjacent to the limbus.1 To confirm the diagnosis, impression cytology with in vivo markers can show a combination of corneal cells with conjunctivalized epithelial cells. Goblet cells may also be visualized in the cornea. In vivo confocal microscopy may also be used for diagnosis and disease monitoring to identify an absence of limbal stem cells and replacement of these cells with vascular and fibrotic tissue.
Given the noncontributory medical history and inappropriate CL use described, CL overwear and consequent hypoxia are likely the underlying etiologies of LSCD.2 We can rule out toxic or traumatic causes of LSCD, for example, there is no mention of prior chemotherapy, radiation, bone marrow transplant, benzalkonium chloride-containing eyedrop use, or prior ocular surgery. There is no infiltrate to suggest an infectious cause, and there is no history of prior episodes to suggest viral etiology. That said, herpes cannot definitively be ruled out, and given the unilateral disease, it should be kept in mind. The left eye examination is unremarkable, but CL-induced LSCD can be asymmetric or subclinical in the fellow eye.
In this particular case, LSCD should be approached conservatively in a stepwise fashion with a focus on medical interventions first.3 CL wear should be discontinued immediately. The cornea should be lubricated aggressively with preservative-free artificial tears and lubricating ointments. This may be augmented with punctual occlusion. Any coexisting eyelid disease should be addressed. If there is inadequate improvement, serum tears may be tried. A vaulted lens such as a prosthetic replacement of the ocular surface ecosystem lens can be tried to provide a good tear reservoir, to reduce symptoms, and to improve healing.
If medical therapy is insufficient at this point, surgical options exist. If the eyelids are malpositioned, surgical correction may be indicated. For the ocular surface, amniotic membrane transplantation may be used. If the condition is truly unilateral, autologous limbal stem-cell transplant is a consideration; however, caution should be taken if the patient has any early changes associated with LSCD in the other eye. If allogenic limbal stem-cell transplant seems necessary, it may be prudent to identify a viable living donor.
With discontinuation of CL wear, adherence to the above regimen, and consistent follow-up, moderate improvement is expected because, although there is 360-degree subtle conjunctivalization, more severe LSCD seems to be limited to a few clock hours. The patient should expect to wear spectacles to correct his refractive error for the foreseeable future with limited use of daily disposable soft CL as a cautious consideration later on.
This patient has a history of an uncertain traumatic or spontaneous erosion and subsequent healing problems in the setting of presumed CL-related keratopathy and extended-wear CL use. The process is essentially unilateral based on history and examination. The photographs demonstrate partial opacification of what appears to be mainly the superficial layers of the cornea, involving the visual axis, and with surrounding diffuse epitheliopathy and extension to the limbus. There is no mention or obvious signs of actinic damage, pingueculum, pterygium, or other findings suggesting neoplasia at the involved or adjacent limbus.
Possible causes of the inciting event could include spontaneous erosion or an abrasion caused by removal of the CL or perceived foreign body, again, likely in the setting of an eye compromised by extended-wear soft CL use. Exacerbating factors could include delayed epithelialization, medication, or preservative toxicity from the eyedrops used in the early treatment. No mention is made of epithelial basement membrane dystrophy in either eye, nor does the patient have a known neurotrophic state, floppy eyelid syndrome, a history of herpetic corneal disease, or dystrophic calcification such as band keratopathy.
Anterior segment optical coherence tomography (AS-OCT) with corneal epithelial mapping, sensation testing, and very careful examination of the fellow eye could be perhaps helpful. It would be important to determine with serial examinations if there is indeed stromal scarring or rather just opacification and irregularity of the overlying epithelium and basement membrane.
Initial intervention should include complete avoidance of CLs, withdrawal of any toxic or preserved eyedrops, use of frequent preservative-free lubricants, treatment of possible comorbidities such as blepharitis, and careful observation. Empiric treatment with oral antivirals should be considered in the early stages of care unless contraindicated, given the unusual and apparently unilateral presentation. Depending on findings of above testing and serial examinations, the more probable working differential diagnosis will presume partial/sectoral LSCD vs ocular surface squamous neoplasia (OSSN). If the former is mainly suspected, serum tears, topical cyclosporine, and/or preservative-free corticosteroids (compounded dexamethasone 0.1% or loteprednol ointment) could be tried as secondary measures. With either working diagnosis, scraping of the opaque area with cytologic analysis could be helpful and perhaps even therapeutic by removing metaplastic/dysplastic cells and allowing healthy adjacent epithelium to fill in. If OSSN is highly suspected or diagnosed, topical chemotherapy should be considered. Interferon alpha 2B is the most gentle and therefore preferred in this case but is difficult to get in the United States. Mitomycin or 5-FU could also be considered with caution, once the other limbal stem cells are healthier after the above supportive treatment.
If resolution is attained, soft CL should only be resumed with caution and careful follow-up. Spectacles, scleral CLs, or perhaps even vision correction surgery may be safer in the long run.
The most likely diagnosis is unilateral partial LSCD, given the patient’s long history of CL overwear, delayed corneal epithelialization, corneal pannus with 360-degree subtle conjunctivalization of the limbus, and vortex keratopathy. Another possibility is neurotrophic keratopathy, which often coexists with LSCD and shares the risk factor of CL overwear.1 One possibility that is less likely but important to rule out is OSSN, especially given the opalescent appearance of the cornea in the slitlamp photograph. OSSN may occur from chronic ocular inflammation induced by long-term CL overwear.2 Clinical examination with fluorescein staining to visualize the classic vortex keratopathy pattern and clock hours of limbus involved is helpful for confirming LSCD and determining its extent, which seems partial from approximately 4:00 to 10:00 in this patient. Lissamine green staining will highlight areas of keratinization (leukoplakia), which would raise the suspicion for OSSN. Checking corneal sensation with a Cochet-Bonnet esthesiometer or dental floss will determine whether there is neurotrophic keratopathy.
Additional imaging to consider includes AS-OCT and in vivo confocal microscopy. AS-OCT in LSCD shows loss of both the palisades of Vogt and limbal crypts on limbal scans and epithelial thinning and irregularity with subepithelial fibrosis on central corneal scans.3 This is in contrast to the classic AS-OCT findings of epithelial thickening and hyperreflectivity with an abrupt transition seen in OSSN.4 In vivo confocal microscopy of the cornea in LSCD can demonstrate epithelial cells with conjunctival morphology and the presence of goblet cells.3 If the diagnosis remains inconclusive, impression cytology of the cornea with immunohistochemistry for cytokeratins (cytokeratin 3 for corneal epithelium and cytokeratin 19 for conjunctival epithelium) can be informative.1
If conservative management with discontinuation of soft CL and use of frequent preservative-free lubrication fail, next steps to consider would be autologous serum tears and scleral lenses. Autologous simple limbal epithelial transplantation is the surgical management of choice for unilateral LSCD, with multiple studies citing long-term clinical success and survival rates of approximately 80%.5
The appearance of this case is consistent with a limbal disease process with a whorl pattern of epitheliopathy and opaque sheets of epithelium arising from the limbus toward the central cornea. The main differential diagnoses are toxic keratopathy related to topical medications and CL-induced LSCD. The differential of vortex/hurricane keratopathy also includes corneal epithelial dystrophies such as Lisch epithelial corneal dystrophy, however, the corneal lesions typically have more feathery borders. Another less likely diagnosis on the differential for an opaque epithelial sheet arising from the limbus is corneal intraepithelial neoplasia of the cornea, however, the acute presentation and epithelial appearance in this case is not typical for corneal intraepithelial neoplasia. Overall, LSCD (“deficiency”) is top on the differential, especially for this patient with a history of long-term soft CL wear.1 This diagnosis necessitates careful examination of the other eye for subtle signs of limbal disease. These patients can have asymmetric/unilateral disease, although bilateral is more common. Preservatives in ophthalmic drops are an important cause of limbal/epithelial stress and in these cases and the use of multiple benzalkonium preserved drops including tobramycin, which can be toxic to the epithelium, likely contributed to the worsening of the epithelial disease.
In the setting of LSCD, the opaque epithelium extending toward the central cornea likely represents conjunctival-type epithelium. Fluorescein staining is extremely useful in this setting as the conjunctival epithelium has different barrier properties compared to corneal epithelium and will pick up the stain that becomes more apparent after a minute or so. The vortex pattern of the epithelium, which actually follows the pattern of the corneal innervation, becomes apparent due to the different barrier properties and the phenotype of the epithelial cells growing over the cornea. This pattern can also be seen in the setting of toxicity (from drops, etc.) as well as in the healing epithelium of extensive epithelial defects (that require significant increase in the epithelial proliferation).
As far as imaging, although not absolutely required, in vivo confocal microscopy would be useful for this patient to visualize the phenotype of the epithelium (corneal vs conjunctival). Of course, this is not readily available in most clinical settings. It would also be helpful to perform AS-OCT, including an epithelial thickness map. Typically, in the setting of LSCD, the epithelium is thinner in the conjunctivalized areas. Impression cytology is rarely performed to detect goblet cells on the cornea.
As already done in this case, we would discontinue/minimize medications with preservatives. If infectious etiologies are excluded, we use topical steroids, preferably without preservatives along with nonpreservative artificial tears. The patient is advised to stop wearing his CLin the affected eye. In terms of prognosis, with stopping CL wear and minimizing toxicity from drops, there is a good chance that a healthier corneal epithelium will be restored but this can take several months.1 The number of clock hours of limbal involvement will determine the ultimate prognosis. In persistent cases, we may perform epithelial debridement (with histopathologic examination) with/without amniotic membrane or using the sequential sectoral conjunctival epitheliectomy technique described by Dua. In terms of long-term CL wear, we generally recommend rigid gas-permeable or properly fitted scleral lenses which are less stressful to the limbus compared to soft CL. Finally, in rare cases with recalcitrant disease, limbal transplantation can be considered.
Editors’ Comments
We agree with our colleagues regarding CL-induced LSCD being the most likely etiology for this patient’s condition. We began this patient on preservative-free artificial tears and a topical steroid, followed by corneal scraping with MMC and placement of amniotic membrane. A sample from the scraping was sent to pathology and resulted with no evidence of neoplasia. The patient was also started on daily oral doxycycline. A 4-month regimen of topical interferon 4 times a day was started to address any potential contribution from occult OSSN or viral etiologies. The patient has done very well, and after 4 months he was able to achieve 20/15–2 corrected distance visual acuity in the right eye with near total resolution of the vortex keratopathy on slitlamp examination (Figure 3). The patient is regularly counseled to not wear CLs in either eye.
Figure 3.
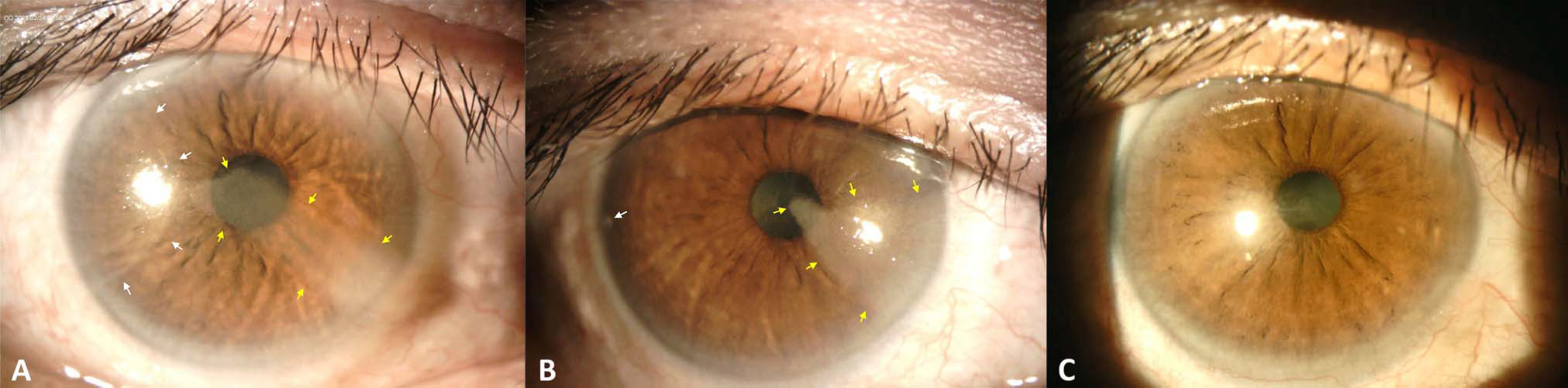
Slitlamp photographs of the right eye over time. (A) Initial consultation with arrows pointing to 2 separate sectoral corneal lesions. (B) 3 weeks after corneal scraping (1 month after initial presentation) with arrows pointing to areas of residual lesion. (C) 4 months after initial presentation showing near complete resolution.
Figure 1.
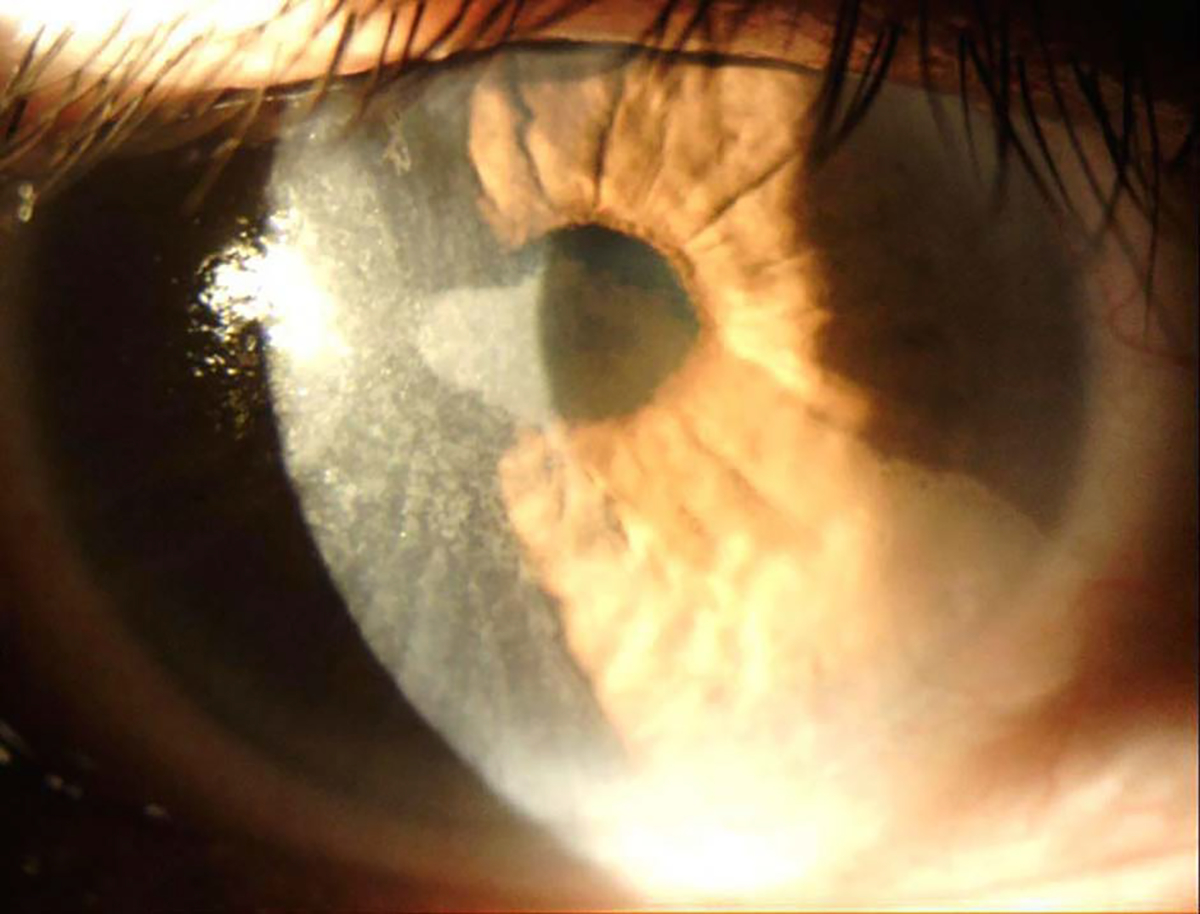
Slitlamp photograph of the right eye as seen on initial consultation.
Figure 2.
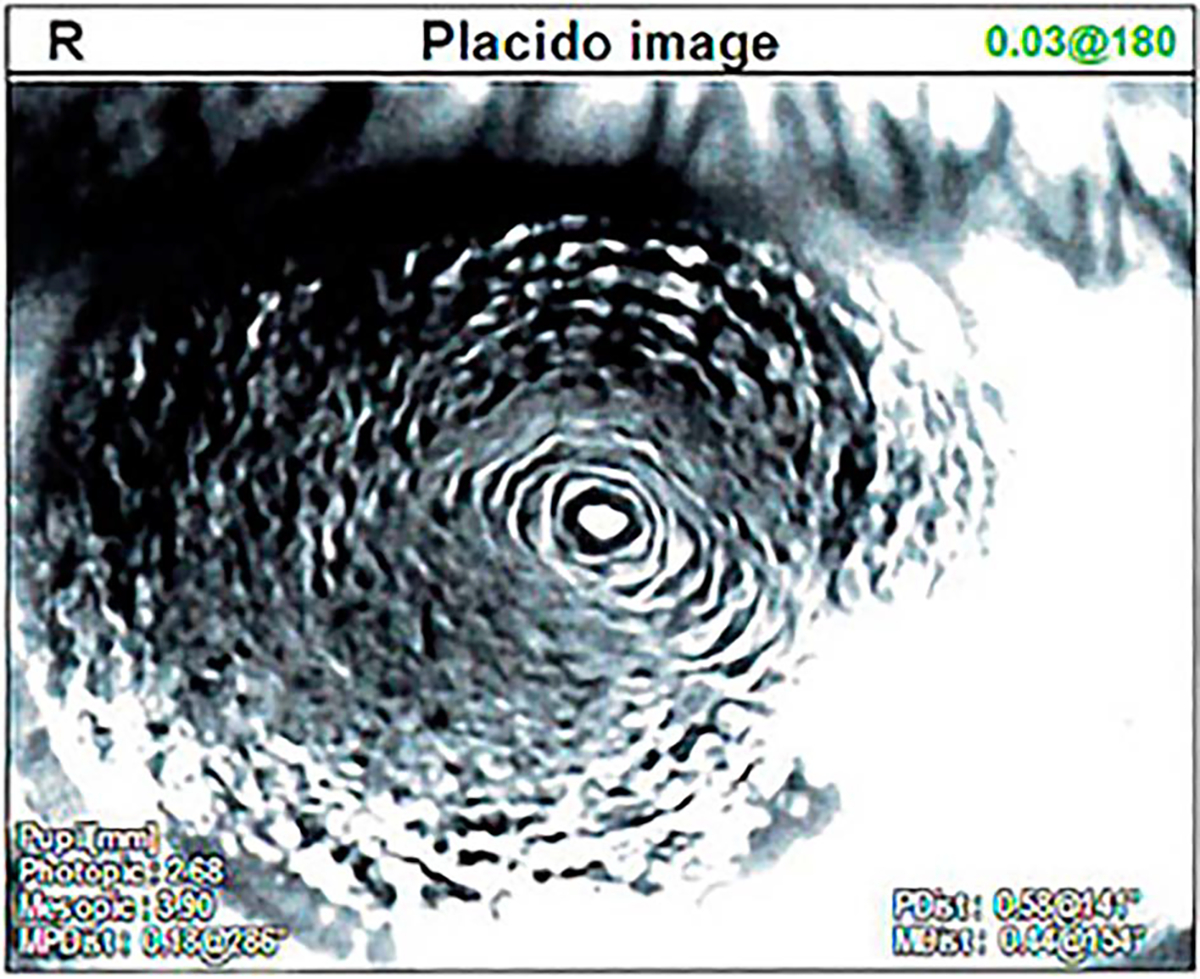
Placido image of the right eye on day of initial consultation.
Footnotes
Disclosures: J.J. Kang is a consultant to Sanofi. The other author has no financial or proprietary interest in any material or method mentioned.
Disclosures: The author has no financial or proprietary interest in any material or method mentioned.
Disclosures: The author has no financial or proprietary interest in any material or method mentioned.
Disclosures: The author has no financial or proprietary interest in any material or method mentioned.
Contributor Information
Majid Moshirfar, Satlt Lake City, Utah.
Carter Payne, Satlt Lake City, Utah.
Jenna Tauber, Bronx, New York.
Joann J. Kang, Bronx, New York.
Mark D. Mifflin, Salt Lake City, Utah.
Neel Pasricha, San Francisco, California.
Ali Djalilian, Chicago, Illinois.
Mohammad Soleimani, Chicago, Illinois.
REFERENCES
- 1.Kim KH, Mian SI. Diagnosis of corneal limbal stem cell deficiency. Curr Opin Ophthalmol 2017;28:355–362 [DOI] [PubMed] [Google Scholar]
- 2.Rossen J, Amram A, Milani B, Park D, Harthan J, Joslin C, McMahon T, Djalilian A. Contact lens-induced limbal stem cell deficiency. Ocul Surf 2016;14:419–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deng SX, Kruse F, Gomes JAP, Chan CC, Daya S, Dana R, Figueiredo FC, Kinoshita S, Rama P, Sangwan V, Slomovic AR, Tan D; and the International Limbal Stem Cell Deficiency Working Group. Global consensus on the management of limbal stem cell deficiency. Cornea 2020;39:1291–1302 [DOI] [PubMed] [Google Scholar]
- 1.Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye (Lond) 2003;17:989–995 [DOI] [PubMed] [Google Scholar]
- 2.Basti S, Macsai MS. Ocular surface squamous neoplasia: a review. Cornea 2003;22:687–704 [DOI] [PubMed] [Google Scholar]
- 3.Banayan N, Georgeon C, Grieve K, Borderie VM. Spectral-domain optical coherence tomography in limbal stem cell deficiency. A case-control study. Am J Ophthalmol 2018;190:179–190 [DOI] [PubMed] [Google Scholar]
- 4.Thomas BJ, Galor A, Nanji AA, et alet al. Ultra high-resolution anterior segment optical coherence tomography in the diagnosis and management of ocular surface squamous neoplasia. Ocul Surf 2014;12:46–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moshirfar M, Thomson AC, Ronquillo Y. Limbal epithelial transplant. In: StatPearls. Treasure Island, FL: StatPearls Publishing LLC; 2022. </Reference> [PubMed] [Google Scholar]
- 1.Kim BY, Riaz KM, Bakhtiari P, et alet al. Medically reversible limbal stem cell disease: clinical features and management strategies. Ophthalmology. 2014; 121:2053–2058</Reference> [DOI] [PMC free article] [PubMed] [Google Scholar]


