Abstract
Passiflora edulis Var. flavicarpa (passion fruit) generates vast waste (60–70%) in the form of peel and seed after the juice extraction. The study aimed to isolate Scirpusin B (SB) from passion fruit (PF) seed waste collected from Northeast India and to analyse its anti-radical, antibacterial, anti-diabetic, and anti-oral cancer activities. Scirpusin B was isolated following hydro-alcoholic extraction, fractionation, and column chromatography. The isolated fraction was further identified through NMR and mass spectroscopy. SB exhibited significant antiradical activity against six standard antioxidant compounds, indicating its commercial application. SB inhibited α-amylase (IC50 Value: 76.38 ± 0.25 µg/mL) and α-glucosidase digestive enzymes (IC50 Value: 2.32 ± 0.04 µg/mL), signifying its antidiabetic properties. In addition, SB showed profound antibacterial activity against eight gram-positive and gram-negative bacteria reported for the first time. Furthermore, SB inhibited SAS and TTN oral cancer cell proliferation up to 95% and 83%, respectively. SB significantly inhibited colonies of SAS and TTn cells in the clonogenic assay, attributing to its anticancer properties. The PI-FACS assay confirmed the ability of SB (75 µM) to kill SAS and TTn cells by 40.26 and 44.3% in 72 h. The mechanism of SB inhibiting oral cancer cell proliferation was understood through western blot analysis, where SB significantly suppressed different cancer hallmark proteins, such as TNF-α, survivin, COX-2, cyclin D1, and VEGF-A. The present study suggests that SB isolated from PF seed can add noteworthy value to the waste biomass for various industrial and medical applications.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-023-03876-6.
Keywords: Passiflora edulis Var. flavicarpa seed, Scirpusin B, Preparative HPLC, Antibacterial agent, Oral squamous cell carcinoma, Northeast India
Introduction
Excessive production of agricultural waste has become a severe concern for environmental safety. The agro-waste enhances microbial contamination, releases tremendous amounts of greenhouse gases, damages aquatic life, and releases a foul odour. Therefore, different methods for waste handling have been highly studied over the last decade. The reuse of agro-waste to produce commercial products is growing day by day. As per the literature, extracts from agro-waste and vegetable waste have many valuable bioactive components with promising applications in the food, pharmaceutical, cosmeceutical, and bio-refinery industries (Vorobyova and Skiba 2022).
Passiflora edulis Var. flavicarpa (Yellow passion fruit) is an unpopular fruit found in India, confined specifically to Southern India and Northeast India. It is believed that the migration of Passion fruit (PF) into southern India (state of Tamilnadu and Kerala) is through Sri Lanka. A statistical report from the Indian horticulture department stated that PF cultivation is growing yearly. About 85,000 metric tons of PF was cultivated in 2019, of which Northeast India alone contributed 70% of the total production (Purohit et al. 2021a). Juice is the only edible portion of this fruit because of its flavor, aroma, and nutritional content. However, more than 60% of the total fruit, including peel and seeds, are discarded by the beverage companies after the juice extraction. The amount of waste generated from PF agriculture can be estimated using statistical data. Recently, many groups tried to valorize the waste PF peel, seeds, and leaves for sustainability. For instance, PF peel was systematically mixed with olive oil to enhance the total carotenoid content of the later (Chutia and Mahanta 2021). The peel has also been used as a fermentable carbohydrate source for increasing folate in soymilk (Albuquerque et al. 2017) and for quality improvement in yogurt formulations (Arias-Lamos et al. 2019). Leaves of PF have been explored for the formulation of silver nanoparticles (Santhoshkumar et al. 2019), isolating polyphenols, such as chlorogenic acid, rutin, caffeic acid, and many more (Shanmugam et al. 2019). Purple passion fruit (Passiflora edulis) seed contained many phytochemicals, such as Piceatannol, Scirpusin A, Scirpusin B, Astringin, Naringenin-7-O-glucoside, etc., reported by Xavier et al. (2022). PF seeds are mostly explored for their oil because of their high unsaturated fatty acids content, which has applications mainly in the food and cosmetic industries (Purohit et al. 2021b). Some recent studies reported the phytochemical composition of PF seed extracts from Northeast India (Purohit et al. 2021a; Sarkar et al. 2022). However, that study was conducted for raw extract and only reported the antioxidant and antibacterial activity of the raw extract. A similar work was also reported recently, where antidiabetic and antioxidant activity of PF seed extract and one isolated compound called Piceatannol was reported (Dos Santos et al. 2022a). Therefore, more insight towards isolation and identification of other chemical compounds and their bioactivity study to treat various diseases was due.
Cancer is one of the prevalent concerns apart from environmental pollution in India. Unhealthy lifestyles, surge in pollution, genetic mutation, and overuse of pesticides in food have increased the risk of cancer. Oral cancer (OC) is one of the most widespread cancers among various cancers (Subash et al. 2022). It affects mainly the buccal, head, and neck regions (Zhao et al. 2017). The possible risk factors of OC are chewing tobacco, betel nut, excessive use of alcohol, chronic oral infections, syphilis, ethnicity, genetic and hereditary predisposition, radiation, and unhealthy diets (Iglesias‐Velázquez et al. 2022). OC is highly widespread in Southeast Asian countries, which are mostly reported due to chewing tobacco and betel nuts (Saraswat et al. 2022). India reports the highest OC cases, accounting 35% of all cancers worldwide. Annually, 0.12 million cases of OC are only reported from India (Subash et al. 2022). Several reports suggest that in India, OC is mainly caused in males by tobacco chewing. Due to the ancient traditional history and cultural habits of chewing tobacco and betel nuts in different parts of India, including Northeast India (Chowdhury and Markus 2022), OC is increasing rapidly. Betel nut contains many alkaloids (arecoline, araciadine) whose metabolism takes place inside the buccal cavity that form by products such as nitrosamines which are carcinogenic (most cancerous nitrosamine found is methylnitrosaminopro-prionitrile) (Garg et al. 2014; Muthukumaran et al. 2023). Recent and advanced treatments of OC include immunotherapy, gene therapy, cancer vaccines, and targeted therapies (Deng et al. 2011; bin Umair et al. 2022; Sun et al. 2022). However, these treatments are associated with enormous adverse side effects, which further degrade the patient's quality of life. Therefore, there is a direct need to develop alternatives with minimal side effects to treat OC. The use of natural compounds isolated from plant extracts has a long history of management and treatment of various cancers, including breast cancer (Ben Menni et al. 2022), OC (Prakash et al. 2021; Banik et al. 2020; Kunnumakkara et al. 2018), etc. Scirpusin B isolated from waste PF seeds in treating OC has not been studied.
Reports suggest that the prevalence of co-morbidities such as type 2 diabetes and microbial infection with different cancers has become a more significant concern (Xiu et al. 2022). Type 2 diabetes is a lifestyle-related disease, where healthy food habits, exercise and certain hypoglycemic management drugs can manage the increased glycemic index. However, these tactics are sometimes not enough to maintain the glycemic level. It has been noted that an increase in the reacting oxygen species induces oxidative stress in the body to escalate diabetes. The mechanism of elevated oxidative stress in diabetes could be metabolic stress or non-enzymatic glycosylation (Sabu and Kuttan 2002). However, plant-based antioxidants can reduce oxidative stress and thereby reduce complications related to diabetes. Phytochemicals can also inhibit digestive enzymes such as α-amylase and α-glycosidase and further delay postprandial blood glycemic levels.
Therefore, the present study was designed to isolate SB from the waste PF seed extract of Northeast India and further analyze antiradical, antidiabetic, antibacterial, and anticancer (oral cancer) activity.
Materials and methods
Plant material and chemicals
Yellow passion fruits (YPF) were purchased from a local market in Manipur, India. The seeds of YPF (YPFS) were separated after extracting the juice, followed by drying at 50 °C, and stored in a closed plastic container. The YPF, YPFS, and target compound are depicted in Fig. 1. Solvents used for the extraction, reverse phase column chromatography (RPCC), fractionation, and high-performance liquid chromatography (HPLC) were procured from Nacalai Tesque Co. (Kyoto, Japan). Diaion HP20SS resin was purchased from Mitsubishi Chemical Co., Tokyo, Japan. Antioxidant standards were obtained from Sigma Aldrich. The Risazurin indicator was purchased from HiMedia. DMSO and MTT were purchased from Merck Life Science Pvt. Ltd. fetal bovine serum; Penstrep and DMEM were purchased from Gibco, USA. Propidium iodide was obtained from Sigma Aldrich. Antibodies used for Immunoblotting were procured from Cell Signalling Technologies, USA. All the chemicals and standards used for the study were of gradient-grade quality.
Fig. 1.
Scirpusin B from yellow passion fruit seed
Cell lines and bacterial strains
The SAS cell line was taken from NCCS (Pune, India), and Dr. Renu Wadhwa, AIST Japan, generously gifted the TTn cell line. DMEM medium supplemented with 10% fetal bovine serum and 1% Penstrep was used to maintain the cell lines. All the gram-positive and gram-negative bacteria used in the present study were purchased from the Institute of Microbial Technology (Chandigarh, India), and their strain designation is mentioned by Moges et al., (Moges et al. 2021).
Isolation and identification of SB from YPFS
Extraction and fractionation of YPFS
The seed sample (50 g) was initially subjected to a soxhlet apparatus using hexane for removing oil. Then, de-fatted seed residue was used for 80% methanolic extraction for 48 h in a soxhlet apparatus. Later, a rotary evaporator concentrated the YPFS hydro-alcoholic extract through solvent evaporation. Finally, the dried extract was stored in a 4 °C refrigerator for future use. Fractionation of YPFS was carried out in a separating funnel using different solvents, such as hexane, dichloromethane (DCM), ethyl acetate (EtOAc), and butanol (BuOH). At first, the extract (6.5 g) was mixed with water in a conical flask. Then, an equal volume of hexane was added to the flask and thoroughly shaken before transferring to the separating funnel and kept for an hour, followed by decantation of the water layer into the conical flask. The hexane layer was separately collected and passed through sodium sulfate for residual water entrapment. This step was repeated twice to recover hexane-soluble matter from the water layer. Similarly, the water layer was treated with two volumes of DCM, EtOAc, and BuOH one after another to fractionate the extracts. All the organic solvent fractions were evaporated to get the fractionated extracts except for the EtOAc fraction. The EtOAc fraction was subjected to base-acid treatment to enrich phenolic compounds in that fraction, as mentioned in Eq. 1. At first, the EtOAc fraction was treated with an equal volume of 1 M NaOH and kept in the separating funnel for 1 h with intermittent shaking. The basic layer is separated from the EtOAc layer. The basified EtOAc layer was evaporated and named the EtOAc base fraction. Furthermore, an equal amount of 1 M HCl was added to the polar layer until the solution was completely acidic (litmus paper turned orange). Then, the EtOAc acidic fraction was recovered by extracting the compounds three times with EtOAc. Finally, the EtOAc fraction was concentrated using a rotary evaporator. All the YPFS fractions were stored in a refrigerator at 4 °C for subsequent use.
Column chromatography and HPLC analysis
First, after drying the EtOAc and BuOH fractions, their solvent system optimization (needed for preparative HPLC) was carried out in a Jasco 4000 series HPLC unit (Jasco, Tokyo, Japan) coupled with a PDA detector and 5C18-MS-II Cosmosil Packed column (length- 150 mm, ID 4.6 mm, Nacalai Tesque Co., Kyoto, Japan) at a column temperature of 35 °C. The solvent system for gradient HPLC was 5–40% acetonitrile with 0.5% formic acid for 0–30 min. A 5 µL sample from 10 mg/mL stock was injected for the analysis, and peaks were detected at 254 nm and 330 nm. After assuming the tentative SB peak, both the fractions were subjected to reverse phase column chromatography (RPCC) using HP20SS ion exchange resin to separate and enrich SB from the entire fraction. The resin was regenerated five times (2-column volume) before loading the extract fraction.
Furthermore, the column was eluted twice, each with water, 20%, 50%, 80%, and 100% methanol. All the fractions were collected and further injected in the analytical HPLC to check the highest concentration of the assumed SB for its isolation through preparative HPLC. In addition, the optimized condition (30% methanol with 0.5% formic acid at 254 nm) was considered to isolate the purified compound using preparative HPLC. A Jasco 875 UV preparative HPLC coupled with a UV detector and Prep ODS Inertsil column (10 mm ID, 250 mm length, GL Sciences Inc., Tokyo, Japan) with a column temperature of 35 °C was used to purify SB. The enriched fraction containing SB was dissolved in DMSO and sonicated thoroughly before being injected into the instrument. The required sample was eluted as the recorder approached the peak, and finally, all the eluted fractions were mixed and evaporated to achieve the purified SB. The purity was further confirmed by injecting a measured volume of purified product into analytical HPLC, and then the purified sample was kept in refrigerator at − 20 °C until further use.
Identification of SB
The 1H spectrum of the purified compound was recorded on a JEOL ECA 500 spectrometer (JEOL, Tokyo, Japan) at 500 MHz, and 13C-NMR was carried out in a Bruker NMR spectrometer at 800 MHz. The sample was dissolved in CD3OD before being subjected to the NMR spectroscopy. The mass spectrum was obtained from a JMS-T100TD mass spectrometer (JEOL) on the negative mode of ESI (Electro Spray Ionization). Samples were dissolved in methanol before injection into the mass spectrometer.
In vitro analysis
Antiradical activity of SB
In vitro antioxidant activity of the purified SB was conducted by DPPH and ABTS methods as per protocol described by Purohit et al. (2021a) with some modifications. The sample concentration ranged from 2 to 10 µg/mL, and the IC50 values were further calculated. The purified compound results were compared with six standard polyphenols: gallic acid, caffeic acid, ferulic acid, ascorbic acid, p-coumaric acid, and quercetin.
Carbohydrate digestive enzyme inhibition activity of SB
The inhibition efficiency of SB on α-amylase and α-glucosidase digestive enzymes was carried out following the previous protocol (Swaraz et al. 2021) with some modifications. In brief, for α-amylase activity, 50 µL of five different concentrations (20–100 µg/mL) of SB were treated with 50 µL of α-amylase (0.5 g/mL in 0.1 M PBS, pH 6.8) for 15 min at 37 °C. Then, to the solution, 50 µL of starch solution (1% in PBS) was added, and the reaction mixture was further incubated for 20 min at 37 °C. After the incubation period, 100 µL of DNS solution (1 g DNS + 20 mL of 2 M NaOH + 50 mL of H2O + 30 g of KNaC4H4O6·4H2O) was added, and the mixture was boiled in the water bath for 15 min. Finally, the samples were diluted with 1 ml water, and the absorbance was recorded at 540 nm. Acarbose (1–5 µg/mL) was used as the standard drug for comparison. The background control (for sample and standard) did not have starch, whereas the background control (for the control group) had PBS instead of sample/standard and starch. The blank group contained only PBS. The inhibition percentage of the enzyme by the purified compound and standard drug was calculated from the following equation:
| 1 |
where Abs cont. means the absorbance of the control group; BG stands for absorbance of background; Abs samp is for absorbance of the sample/standard compound.
In a different experimental setup, enzymatic activity of SB on α-glucosidase was checked following the previous protocol (Swaraz et al. 2021) with minor modifications. Briefly, 50 µL of the sample (1–5 µg/mL) or standard (100–500 ng/mL) were mixed separately with 50 µL α-glucosidase enzyme (0.1U/ml in 0.1 M PBS) and the mixture was incubated for 15 min at 37 °C. After that, 50 µL of 1 mM PNPG (4-nitrophenyl-β-d-glucopyranoside) in PBS was added to the solution and further incubated for 20 min at 37 °C. Finally, 100 µL of Na2CO3 (0.1 M in PBS) was added to the mixture to stop the reaction, and absorbance was recorded at 405 nm. The inhibition percentage of α-glucosidase by SB and acarbose was calculated according to Eq. 1, where the background control (for samples) did not have PNPG and background control (for control) did not have sample/standard as well as PNPG substrate.
Antibacterial activity of SB
The antibacterial activity of SB was checked using eight bacterial strains (four of each gram positive and gram negative) by the minimum inhibitory concentration (MIC) and zone of inhibition (ZOI) study following the previous protocol (Purohit et al. 2021a). The concentration range of SB adapted for MIC was from 10 to 0.156 mg/mL. Based on the findings of MIC, different concentrations (2.5, 5, and 10 mg/mL) were chosen for ZOI analysis.
Bioactivity of SB against oral squamous cell carcinoma
Viability assay
The effect of SB on the viability of oral cancer cell lines was determined using an MTT assay. For this assay, 96 well plates were seeded with approx. 2000 cells in each well and then incubated at 37 °C for 24 h. Furthermore, the cells were treated with different concentrations of SB (0, 10, 25, and 50 µM) for 72 h. Then, 10 µL of MTT (5 mg/mL) solution was added to each well and incubated for 2 h. Then, the culture medium was taken out from each well, and 100 µL of DMSO was added and incubated at room temperature for 1 h to dissolve the formazan product. The absorbance of the colored solution was taken at 570 nm using a microtiter plate reader (Molecular Devices, Spectramax iD3). The percentage viability further determined the inhibition caused by SB on the proliferation of SAS and TTn cell lines (Bordoloi et al. 2019).
Colony formation assay
A colony formation assay determined the clonogenic potential of oral cancer cells treated with SB. The cells were seeded with 1500 cells/well in 6-well plates and treated with different concentrations of SB (0, 25, and 50 µM) for 10 days with media replacement on every alternate day. After this period, cell fixation by ethanol was done, followed by well cleaning using PBS, and finally stained with crystal violet (SRL Pvt. Ltd., India). Colonies were scanned for each concentration of SB, and the survival fraction was determined (Aswathy et al. 2021).
Propidium iodide flow cytometric assay
Propidium iodide (PI) flow cytometry was analyzed by seeding SAS and TTn cell lines in 6-well plates with a density of 5 × 104 cells per well and incubated for 24 h. The cells were treated with the indicated concentrations of SB (0, 25, 50, and 75 µM) for 72 h. Then, the cells were collected, washed with PBS, and centrifuged. The cell pellets were suspended in 495 µl PBS and incubated with 5 µl of PI (1 mg/ml) for 10 min. The effect of SB on the cells was analyzed using FACS Celesta™ (BD Biosciences) following the previous protocol (Khwairakpam et al. 2020).
Western blot analysis
To determine the effect of SB on different oncogenic proteins, whole cell lysates were prepared from the SAS and TTn cell lines treated with SB by exposing it to lysis buffer (20 mM HEPES, 2 mM EDTA, 250 mM NaCl, 0.1% NP40) in the presence of protease inhibitors (2 µg/mL Leupeptin hemisulfate, 2 µg/mL aprotinin, 1 mM PMSF, 1 mM DTT). The lysates were centrifuged at 13,000 g for 10 min to remove insoluble material, and the supernatants were collected and stored at − 20 °C. Furthermore, the lysates were resolved by SDS-PAGE, and the proteins were electrotransferred onto nitrocellulose membranes, blotted with relevant antibodies. The bands for different proteins were visualized with Clarity Western ECL Substrate (Bio-Rad, Hercules, CA, the USA) in a ChemiDoc™ XRS System (Bio-Rad). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was the loading control housekeeping gene (El-Naggar et al. 2021).
Statistical analysis
The antioxidant, antibacterial (ZOI) and antidiabetic activity results are presented in mean ± SD of two independent experiments in duplicates. In the anticancer activity section, the results of the MTT assay, PI-FACS, and colony-forming assays are presented in mean ± SE of three individual experiments in triplicate, and the p values for the PI-FACS and colony-forming assay were calculated using the student’s T test.
Results and discussion
Extraction and isolation of SB from YPFS
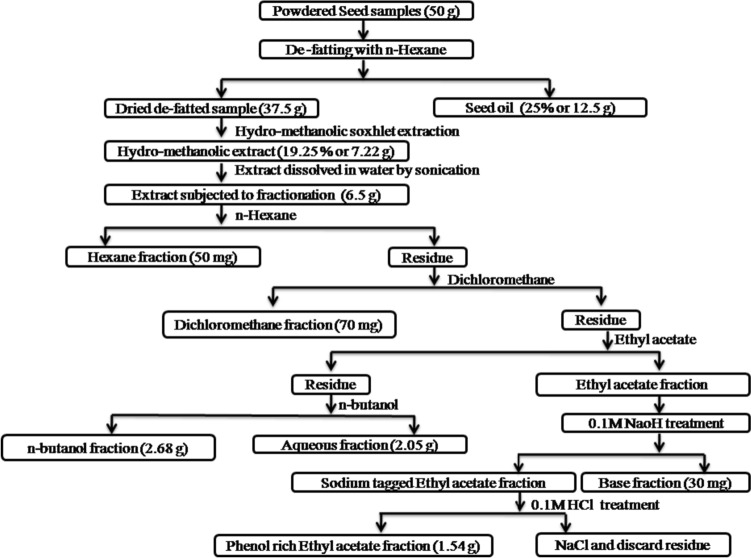
The process of extraction and isolation of SB from YPFS is shown in Fig. 2. The sample amount used for extraction, fractionation, and yield of isolated compound are presented in Table S1. From the gradient, HPLC chromatograms of the BuOH fraction, two stilbene molecules (piceatannol and scirpusin B) were presumed (supplementary file, Fig. S1). The EtOAc fraction was found to have a low concentration of stilbene compared to the BuOH fraction, which could be because of the lower solubility tendency of SB towards EtOAc. Acid–base treatment was applied to the fraction to enrich SB from the EtOAc fraction. Furthermore, both the EtOAc and BuOH fractions were passed through Diaion HP20SS resin for the maximum pull of SB in significant elution fractions using a gradient range of methanol with water, as mentioned in the methods. The HP20SS resin helps separate and elute smaller molecular weight compounds such as polyphenols, sugars, and chemicals for preparative or industrial applications. Both the major peaks were further resolved through an analytical HPLC, and the optimized solvent system was followed to isolate the compounds using a preparative HPLC (supplementary file, Fig. S2). The SB-rich eluted solvent from both the EtOAc and BuOH fractions was mixed and then evaporated to achieve a pale brown-colored amorphous powder of SB weighing approximately 98.52 mg (Supplementary file, Table S1). SB was first reported from the rhizome of Scirpus flaviatilis by Nakajima et al. (1978). This trans-stilbene molecule is a dimer of piceatannol, a hydroxylated resveratrol analog (Banik et al. 2020). Resveratrol is the abundant stilbene found naturally in plants with a wide array of biological activity (Wang and Yao 2016). Very few properties of SB have been reported so far, which include vaso relaxation (Sano et al. 2011), antioxidant (Xiang et al. 2005), anti-HIV (Yang et al. 2005), anti-adipogenesis and anti-obesity (Majeed et al. 2016) etc. As per the World Health Organisation’s report, more countries are now recognizing the utilization of plant-based pharmaceuticals. In this context, therapeutic SB from waste PF seed could be an ideal candidate for treating comorbidities. This study also reports maximum recovery (approximately 0.2% of total seed) of SB from PF seed of Northeast India for the first time, which further implies the commercial importance of the waste PF seeds.
Fig. 2.
Schematic diagram of extraction and fractionation of passion fruit seed. The de fatted passion fruit seed was subjected to hydro-methanolic extraction and further followed by fraction using hexane, dichloromethane, ethyl acetate and butanol. The amounts of different passion fruit fractioned extracts are presented in this table
Identification of SB
Identification of the isolated SB was carried out by 1H NMR, 13C NMR, and mass spectrometry in ESI negative mode, and the spectra are presented in Fig. S3 to S5 (supplementary file). The chemical shift of the 1H NMR (500 MHz, CD3OD) spectra are as follows δ: 4.38(d, J = 5.8 Hz, 1H), 5.33(d, J = 5.9 Hz, 1H), 6.20(d, J = 2.0 Hz, 2H) 6.21–6.23(m, 1H), 6.30(d, J = 1.8 Hz, 1H), 6.58(d, J = 16.1 Hz, 1H), 6.62(dd, J = 8.2 Hz, 1.8 Hz, 1H), 6.66(d, J = 1.9 Hz, 1H), 6.68(d, J = 8.1 Hz, 1H), 6.74(d, J = 1.8 Hz, 1H), 6.78(d, J = 8.1 Hz, 1H), 6.80(d, J = 1.9 Hz, 1H), 6.82(d, J = 6.3 Hz, 1H). 13C NMR (800 MHz, CD3OD) δ: 39.9. 49.4, 57.5, 94.3, 96.3, 101.7, 103.9, 106.8, 113.1, 113.5, 115.7, 115.8, 117.9, 119.3, 119.5, 123.1, 130.3, 130.4, 134.4, 136.5, 145.7, 145.8, 145.9, 146.0, 147.1, 159.1, 159.4, 162.3. The ESI− mass of SB was found to be 485.16 g/mol, and the actual mass was 486.16 g/mol.
In vitro analysis
Antioxidant activity
SB's in vitro antioxidant activity was estimated through DPPH and ABTS methods and compared with known polyphenols, such as hydroxybenzoic, hydroxycinnamic, ascorbic, and quercetin. The result of this analysis is presented as IC50 values (concentration needed to scavenge 50% of free radicals) in Table 1. SB showed superior antiradical activity against all the tested standard compounds except gallic acid. Two folds higher antioxidant activity of SB (5.19 ± 0.02 µg/mL) was observed than ferulic acid (9.80 ± 0.01 µg/mL) and p-Coumaric acid (10.19 ± 0.01 µg/mL) and remarkably better activity than caffeic acid (5.77 ± 0.04 µg/mL) and quercetin (7.85 ± 0.01 µg/mL) in DPPH assay. In the ABTS assay, the same trend was noticed. SB reduced the free radicals of DPPH and ABTS in a concentration-dependent manner. Both DPPH and ABTS assays work on the principle of electron transfer or hydrogen ion transfer mechanism (Purohit et al. 2021b). A recent report (dos Santos et al. 2022b) also furnished antioxidant activity of ethanolic extract of Passiflora cincinnata seed. However, they have not reported the possible phytochemicals responsible for the antiradical activity. The present work can fulfill that gap, that SB present in the seed of Passiflora genus possesses remarkable antioxidant activity. Free radicals receive electrons or hydrogen ions from the antioxidant molecule and get reduced, resulting in discoloration of the solution, indicating antiradical properties. Various reports suggest hydroxyl and methoxy groups have a significant tendency to donate electrons (Parcheta et al. 2021). Because of the abundant hydroxyl groups in SB, superior antioxidant activity can be correlated. Natural stilbenoids, the primary, secondary plant metabolites in large plant species, protect the plants from stress conditions, such as UV radiation, heat, microorganisms, etc. (Pecyna et al. 2020). The present study provides more comprehensive information on the usability of SB as an antioxidant over many known standard polyphenols (caffeic acid, ferulic acid, p-coumaric acid, ascorbic acid, and quercetin). To date, the commercialization of SB as a standard polyphenol has not yet been done by any pharmaceutical or chemical industries in India. However, different manufacturers are selling commercial Piceatannol (a monomer of SB) at a very high cost. A comparative table of the cost of various polyphenols is presented in Table S2 (supplementary file). From the present study, different approaches towards commercializing SB from PF seed into mainstream chemical world as an established natural antioxidant can be an interesting objective. Apart from this, antioxidant rich plant materials reduce the mucosal damage caused by cancer causing agents and further neutralize the free radicals found in mucosal lesions. They also interfere in the cellular signaling pathways to protect the healthy cells and induce apoptosis in carcinogenic cells (Prakash et al. 2021; Babu et al. 2003; Saleh et al. 2023). Therefore, antioxidant rich SB was also checked for its anti-cancer property which is mentioned in the later section.
Table 1.
Invitro antioxidant and antidiabetic activities by scirpusin B
| Activity | Scirpusin B | Acarbose | Gallic acid | Ferulic acid | Caffeic acid | p-Coumaric acid | Ascorbic acid | Quercetin |
|---|---|---|---|---|---|---|---|---|
| Antioxidant | ||||||||
| DPPH | 5.19 ± 0.02 | NA | 0.89 ± 0.01 | 9.80 ± 0.01 | 5.77 ± 0.04 | 10.19 ± 0.01 | 3.95 | 7.85 ± 0.01 |
| ABTS | 3.89 ± 0.03 | NA | 0.79 ± 0.01 | 6.02 ± 0.01 | 3.98 ± 0.03 | 6.36 ± 0.04 | 6.22 ± 0.04 | 6.40 ± 0.01 |
| Antidiabetic | ||||||||
| α-Amylase | 76.38 ± 0.25 | 0.65 ± 0.01 | NA | NA | NA | NA | NA | NA |
| α-Glycosidase | 2.32 ± 0.04 | 0.79 ± 0.03 | NA | NA | NA | NA | NA | NA |
Data are presented as IC50 value after taking the mean of two individual experiments in duplicate. All the values (µg/mL) of antidiabetic and antioxidant experiments are presented as mean ± SD. NA represents for not applicable
Carbohydrate digestive enzyme inhibition activity of SB
In vitro, antidiabetic potential of SB was evaluated by α-amylase and α-glucosidase inhibition assays. The IC50 values of SB on different enzymes are presented in Table 1. SB exhibited higher α-glucosidase activity (2.32 ± 0.04 µg/mL) compared with α-amylase activity (76.38 ± 0.25 µg/mL). A previous report (Tran et al. 2014) suggested that SB isolated from Cyperus rotundus rhizome could only inhibit α-glucosidase and not α-amylase. A recent report by Dos Santos et al., (Dos Santos et al. 2022a) suggested that Piceatannol (monomer of Scirpusin B) from passion fruit seed extract showed poor antidiabetic activity compared to our findings. Both the α-amylase and α-glucosidase inhibition activity of SB of the present study is significantly better than the published data. Inhibition of glucose absorption and, hence, delaying the postprandial glycemic level has been one of the approaches for treating diabetes (Jayawardana et al. 2022). Therefore, nowadays, inhibition of polysaccharide digestive enzymes such as α-amylase and α-glucosidase are taken into consideration to improve the glycemia level in the body. Several reports have proved stilbenoids (resveratrol and its analogs) to have antidiabetic activity. Most of the stilbene molecules, which are dimers, trimers, or oligomers, showed better activity (Dirir et al. 2021) because of the presence of maximum hydroxyl groups, which can help in increasing the bioavailability of the compound (Tran et al. 2014). The results of this study conferred that SB from YPFS can be explored as a promising hypoglycaemic agent for various preclinical and clinical studies.
Antibacterial activity
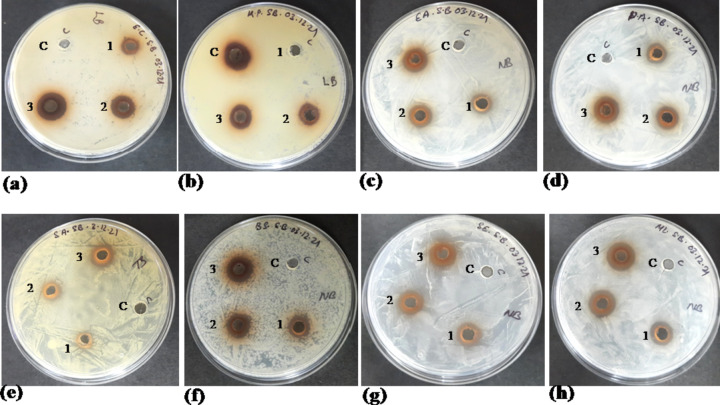
In vitro, antibacterial activity of SB was carried out following MIC and ZOI methods. The result for the MIC study is presented in Table 2 and Fig. S6 (supplementary material). Similarly, the result for the ZOI study is shown in Fig. 3 and Table S3 (supplementary material). Natural stilbenes are known to be potential antimicrobial agents (Gutierrez-Escobar et al. 2021; Kluska et al. 2023), but very few have been studied so far (Shih et al. 2021). Interestingly, there is no reporting on SB's antibacterial activity on food-borne and human pathogenic bacteria to date. Therefore, in this work, the antibacterial activity of SB was checked using eight different bacteria (four gram-positive and four gram-negative). Previously, the seed methanolic extract of YPF showed tremendous antibacterial activity against these pathogens (Purohit et al. 2021a). However, the compound responsible for the antibacterial properties of the seed extract was not confirmed. This present study reports for the first time that SB is one of the significant antibacterial agents found in the YPFS extract. SB manifested minimum inhibitory concentration ranging from 0.156 mg/mL to 1.25 mg/mL for all tested organisms. Gram-positive bacteria such as Staphylococcus epidermidis and Micrococcus luteus showed the best MIC (0.156 mg/mL), and this result can be attributed to their non-rigid cell wall structure into which SB can easily penetrate to cause cellular leakage and death. SB also inhibited the toughest gram-negative bacteria at a lower concentration of 0.625 to 1.25 mg/mL. This finding was further validated by the ZOI analysis. SB showed profound antibacterial activity even at the lowest tested concentration, i.e., 2.5 mg/mL for all the strains. At such low concentrations also, SB exhibited better ZOI in Escherichia coli (12 ± 1.41 mg/mL), Enterobacter aerogenes (11 ± 1.41 mg/mL), and Bacillus subtilis (13 mg/mL) compared to the previous study (at 10 mg/mL of seed methanolic YPF extract) (Purohit et al. 2021a). Overall, this study can support SB isolated from waste PF seed as a potent antibacterial agent and included in the natural broad-spectrum antibiotic class of drugs.
Table 2.
Minimum inhibitory concentration by scirpusin B
| Identified compound | Gram negative bacteria | Gram positive bacteria | ||||||
|---|---|---|---|---|---|---|---|---|
| EC | KP | EA | PA | SE | ML | SA | BS | |
| Scirpusin B | 1.25 | 1.25 | 0.625 | 0.625 | 0.156 | 0.156 | 1.25 | 1.25 |
Values are presented in mg/mL. EC (Escherichia coli), KP (Klebsiella pneumoniae), EA (Enterobacter aerogenes), PA (Pseudomonas aeruginosa), SE (Staphylococcus epidermidis), ML (Micrococcus luteus), SA (Staphylococcus aureus) and BS (Bacillus subtilis)
Fig. 3.
Zone of Inhibition study of Scirpusin B on different gram negative and gram positive bacteria. Control (C) and different concentrations of scirpusin B are represented as 1 (2.5 mg/mL), 2 (5 mg/mL) and 3 (10 mg/mL) inside the images Different gram negative bacteria used for the study are a E. Coli, b Klebsiella pneumonia, c Enterobacter aerogenes and d Pseudomonas aeruginosa. Different gram positive bacteria used for the study are e Staphylococcus aureus, f Bacillus subtilis, g Staphylococcus epidermidis and h Micrococcus luteus
Bioactivity of SB against oral squamous cell carcinoma
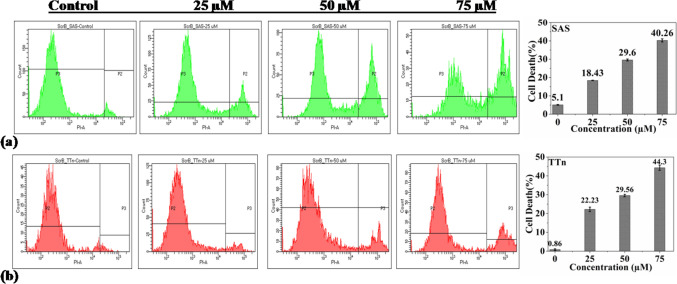
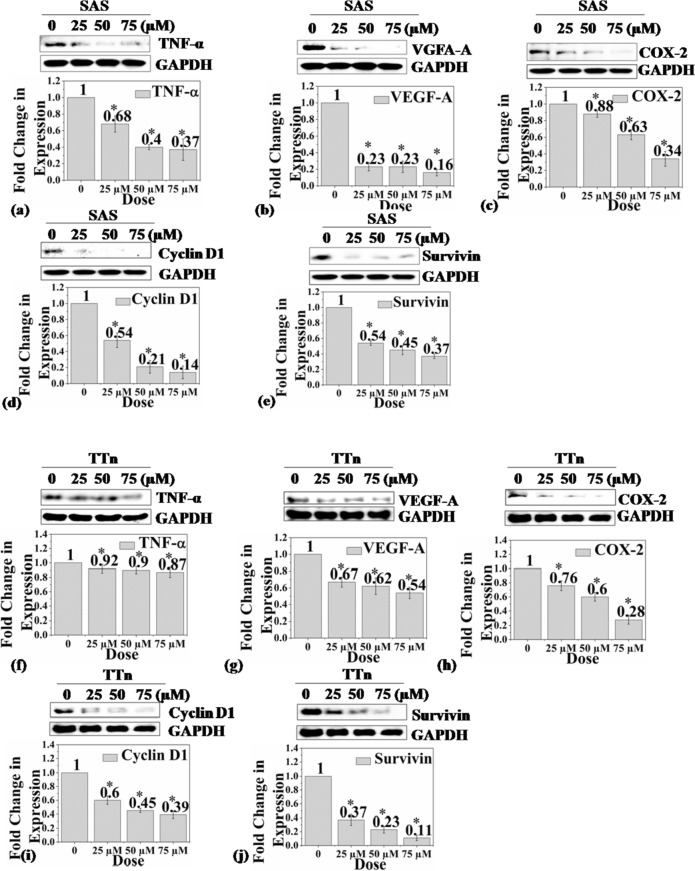
The anticancer activity of purified SB was determined using two different oral cancer cell lines in various in-vitro experiments. The results are presented in Figs. 4, 5, 6 and 7. First, the effect of SB on the viability of oral cancer cell lines (SAS and TTn) through MTT assay was checked. The result is presented in Fig. 4. In this experiment, SB reduced the viability of SAS and TTn cell lines in a dose-dependent manner. The IC50 for SAS and TTn cell lines were observed to be ≈27 µM and ≈32 µM, respectively. The results of the study can be compared with those reported by Yamamoto et al.; they observed the inhibition of cell proliferation in lung cancer cells (NCI-H522) (Yamamoto et al. 2019). Furthermore, the anticancer effect of SB on the killing of oral cancer cells was confirmed by colony formation assay (Fig. 5). Clonogenic assay is an in vitro survival assay that is used for determining the ability of a single cell to undergo unlimited cell division to form a colony (Hartmann et al. 2020). In this assay, the treatment of SAS and TTn cell lines with SB at 25 µM and 50 µM inhibited the colony-forming ability of SAS and TTn cell lines in a dose-dependent manner compared to control. In line with the present study, the previous investigation also demonstrated the anti-proliferative effect of SB in colon cancer (HCT-116) cell lines. SB's ability to inhibit oral cancer cells (SAS and TTn) is reported for the first time in this work. Furthermore, the PI-FACS analysis of the SB-treated oral cancer cells was carried out to check the percent cell death, and the result is presented in Fig. 6. The cell lines were treated with 25, 50, and 75 µM concentrations of SB for 72 h and it was observed that SB induced cell death in both the oral cancer cell lines in a dose-dependent manner. SB killed 40.26% SAS cells and 44.3% TTn cells in 72 h, confirming the anti-oral cancer efficiency of the compound. The mechanism of action of SB against different oral cancer cell lines was further understood by protein expression analysis (Fig. 7a–j). It is well-established that the pathogenesis of oral cancer is a multi-stage process (Rishabh et al. 2021). The risk factors of oral cancer, such as tobacco, areca nut, alcohol etc., are known to cause oxidative stress and inflammation in the oral mucosa through the expression of pro-inflammatory cytokines such as tumor necrosis factor (TNF-α), interleukin- 6 (IL-6), etc. and the induction of reactive oxygen species (ROS) which leads to oral submucosal fibrosis (Girisa et al. 2021). It is also well-known that oral sub-mucosal fibrosis has a high potential for malignant transformation to oral squamous cell carcinoma (Girisa et al. 2021; Ray et al. 2016; Divya et al. 2023). Therefore, the agents that can suppress pro-inflammatory cytokines and free radicals have high potential in preventing and treating oral cancer. The results showed that antioxidant SB significantly suppressed TNF-α expression and scavenged ROS (Fig. 7a, f) in SAS and TTn cells, suggesting its chemo-preventive potential against oral cancer. Furthermore, increasing lines of studies indicated that the formation of new blood vessels, known as angiogenesis, is very important for the growth of oral cancer, as it helps to provide oxygen and nutrition for the development of cancer cells and it is induced by a protein known as the vascular endothelial growth factor (VEGF) (Mărgăritescu et al. 2009; Rapone and Ferrara 2020). This factor secreted by oral cancer cells binds to its receptor VEGFR and induces tube formation in endothelial cells (Rapone and Ferrara 2020; Li et al. 2023). However, the present study showed that SB remarkably suppressed the expression of VEGF-A (Fig. 7b, g) of SAS and TTn cells in a concentration-dependent manner. Accumulating pieces of evidence suggest that proteins survivin, cyclooxygenase-2 (COX-2), and cyclin D1 are involved in the survival, proliferation, and chemoresistance of oral cancer cells (Bordoloi et al. 2019; Jaiswal et al. 2015; Lakshminarayana et al. 2018; Li et al. 2023). Interestingly, it was observed that SB inhibited the expression of these proteins (Fig. 7c–e, h–j), which helps to stop oxygen and nutrients supply to the oral cancer cells (Zirlik and Duyster 2018), leading to their death. From all of the above, results showed that SB is a potential anticancer agent for preventing and treating oral squamous cell carcinoma.
Fig. 4.
Inhibition of cell proliferation by scirpusin B on SAS (a) and TTn (a) oral squamous carcinoma cell. All the experiments carried out are presented as mean ± SD of three individual experiments in triplicate
Fig. 5.
Inhibition of clonogenic potential of scirpusin B on SAS (a) and TTn (b) cell lines. The dose versus survival fraction graph is presented adjacent to the respective SAS and TTn clonogenic images. All the experiments carried out are presented as mean ± SD of three individual experiments in triplicate. p < 0.05 vs control are reported for clonogenic assay
Fig. 6.
Scirpusin B induced cell death assay by PI-FACS on SAS (a) and TTn (b) cells. Cell death percentage by different concentrations of scirpusin B obtained from PI-FACS is presented in the adjacent graphs. All the experiments carried out are presented as mean ± SD of three individual experiments in triplicate. p < 0.05 vs control are reported for PI-FACS analysis
Fig. 7.
Inhibition of oral cancer hallmark proteins by scirpusin B on SAS and TTn cells by western blot analysis. Inhibition of different hallmark proteins in SAS cells by SB is presented from (a) to (e) (a; SAS–TNFα, b; SAS–VGFA-A, c; SAS–COX2, d; SAS-Cyclin D1 and e; SAS-Survivin). Similarly, Inhibition of different hallmark proteins in TTn cells by SB is presented from (f) to (j) (f; TTn-TNFα, g; TTn-VGFA-A, h; TTn-COX2, i; TTn-Cyclin D1 and j; TTn-Survivin)
Conclusion
Scirpusin B from the seeds of yellow passion fruit from Northeast India was isolated effectively, with a recovery of ~ 0.2% of the total seeds (98.52 mg/50 g). SB was found to have superior antiradical activity against many contemporary standard antioxidants, suggesting better commercial and therapeutic values. SB isolated from the Northeastern yellow passion fruit seed extract can inhibit both α-amylase and α-glucosidase enzymes. Novel antibacterial activity of SB can address the concern of dealing with multi-drug resistance caused by serious pathogenic bacteria. In addition, SB was also found to be a novel anti-oral cancer agent. It decreased cancer cell proliferation by suppressing many hallmark proteins, restricting the forming ability of cancer cells, etc. However, more evidence in preclinical and clinical studies are required to evaluate SB's pharmacokinetic and pharmacodynamic behavior for different cancer treatments. Using SB in the mainstream medicine world may aid in treating various life-concerning diseases, including diabetes, multi-drug resistance bacterial infection, and oral cancer. In addition, the valorisation of waste passion fruit seeds for isolating SB could add value to the waste product and help decrease environmental pollution, making the planet safer and greener.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors extend their gratitude to the School of Energy Science and Engineering, Indian Institute of Technology, Guwahati and United Graduate School of Agricultural Science, GIFU University for providing research laboratory facilities to conduct the work mentioned in this manuscript. The work presented in this article was financially supported by a research grant [vide Grant No. BT/311/NE/TBP/2012] from Department of Biotechnology (DBT), the Ministry of Science and Technology, New Delhi, India. A part of the research work was also carried out with the financial support of the Japan Student Services Organization (JASSO).
Author contributions
SP: conceptualization, investigation, methodology, data curation, writing—original draft, writing—review & editing; SG: formal analysis, writing—review & editing; YO: formal analysis, methodology; ABK: conceptualization, data curation, review and editing; LS: funding acquisition, supervision; EY: conceptualization, data curation, funding acquisition, supervision, review & editing; VVG: conceptualization, data curation, funding acquisition, project administration, resources, supervision, writing—review & editing.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest for this publication.
Contributor Information
Emiko Yanase, Email: e-yanase@gifu-u.ac.jp.
Vaibhav V. Goud, Email: vvgoud@iitg.ac.in
References
- Albuquerque MAC, Bedani R, LeBlanc JG, Saad SMI. Passion fruit by-product and fructooligosaccharides stimulate the growth and folate production by starter and probiotic cultures in fermented soymilk. Int J Food Microbiol. 2017;261:35–41. doi: 10.1016/j.ijfoodmicro.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Arias-Lamos D, Molina-Hernández JB, Andrade-Mahecha MM (2019) Evaluation of the potential use of passion fruit dehydrated epicarp (Passiflora edulis f. flavicarpa O. Deg.) in the formulation of yogurt. Revista UDCA Actualidad & Divulgación Científica 22 (1)
- Aswathy M, Banik K, Parama D, Sasikumar P, Harsha C, Joseph AG, Sherin DR, Thanathu MK, Kunnumakkara AB, Vasu RK. Exploring the cytotoxic effects of the extracts and bioactive triterpenoids from Dillenia indica against oral squamous cell carcinoma: a scientific interpretation and validation of indigenous knowledge. ACS Pharmacol Transl Sci. 2021;4(2):834–847. doi: 10.1021/acsptsci.1c00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu B, Jayram H, Nair M, Ajaikumar K, Padikkala J. Free radical scavenging, antitumor and anticarcinogenic activity of gossypin. J Exp Clin Cancer CR. 2003;22(4):581–589. [PubMed] [Google Scholar]
- Banik K, Ranaware AM, Harsha C, Nitesh T, Girisa S, Deshpande V, Fan L, Nalawade SP, Sethi G, Kunnumakkara AB. Piceatannol: a natural stilbene for the prevention and treatment of cancer. Pharmacol Res. 2020;153:104635. doi: 10.1016/j.phrs.2020.104635. [DOI] [PubMed] [Google Scholar]
- Ben Menni D, Belyagoubi-Benhammou N, Benmahieddine A, Ben Menni H, Gismondi A, Monteleone V, Di Marco G, D’Agostino A, Canini A, Benamar H (2022) Identification of Sterols from Anabasis articulata (Forssk.) Moq.(Chenopodiaceae) Growing in Algeria and Study of Their Potential Bioactivity. Waste and Biomass Valorization 1–13
- bin Umair M, Akusa FN, Kashif H, Butt F, Azhar M, Munir I, Ahmed M, Khalil W, Sharyar H, Rafique S. Viruses as tools in gene therapy, vaccine development, and cancer treatment. Arch Virol. 2022;167(6):1387–1404. doi: 10.1007/s00705-022-05432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordoloi D, Monisha J, Roy M, Padmavathi G, Banik K, Harsha C, Wang H, Kumar AP, Arfuso F, Kunnumakkara AB. An investigation on the therapeutic potential of butein, a tretrahydroxychalcone against human oral squamous cell carcinoma. Asian Pac J Cancer Prev APJCP. 2019;20(11):3437. doi: 10.31557/APJCP.2019.20.11.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury CR, Markus A. Level of oral cancer awareness among Indian rural population: a possible research model using knowledge, attitude and practice (KAP) intervention and its utilisation in low resource settings of LMICs. J Oral Biol Craniofac Res. 2022;12(1):154–160. doi: 10.1016/j.jobcr.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutia H, Mahanta CL. Green ultrasound and microwave extraction of carotenoids from passion fruit peel using vegetable oils as a solvent: optimization, comparison, kinetics, and thermodynamic studies. Innov Food Sci Emerg Technol. 2021;67:102547. doi: 10.1016/j.ifset.2020.102547. [DOI] [Google Scholar]
- Deng H, Sambrook P, Logan R. The treatment of oral cancer: an overview for dental professionals. Aust Dent J. 2011;56(3):244–252. doi: 10.1111/j.1834-7819.2011.01349.x. [DOI] [PubMed] [Google Scholar]
- Dirir AM, Daou M, Yousef AF, Yousef LF (2021) A review of alpha-glucosidase inhibitors from plants as potential candidates for the treatment of type-2 diabetes. Phytochemy Rev 1–31 [DOI] [PMC free article] [PubMed]
- Divya B, Vasanthi V, Ramadoss R, Kumar AR, Rajkumar K (2023) Clinicopathological characteristics of oral squamous cell carcinoma arising from oral submucous fibrosis: A systematic review. J Cancer Res Ther [DOI] [PubMed]
- Dos Santos FA, Xavier JA, da Silva FC, Merlin JJ, Goulart MO, Rupasinghe HV. Antidiabetic, antiglycation, and antioxidant activities of ethanolic seed extract of Passiflora edulis and piceatannol in vitro. Molecules. 2022;27(13):4064. doi: 10.3390/molecules27134064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos FAR, de Mendonça ELSS, da Silva FC, de Almeida XJ, Merlin JJ, Goulart MOF, Rupasinghe HV. Ethanol extract of Passiflora cincinnata seeds posses antidiabetic, antiglycant, and antioxidant activities in vitro. J Bioeng Technol Health. 2022;5(4):224–231. [Google Scholar]
- El-Naggar MH, Abdel Bar FM, Harsha C, Monisha J, Shimizu K, Kunnumakkara AB, Badria FA. Synthesis of new selective cytotoxic ricinine analogues against oral squamous cell carcinoma. Nat Prod Res. 2021;35(13):2145–2156. doi: 10.1080/14786419.2019.1663513. [DOI] [PubMed] [Google Scholar]
- Garg A, Chaturvedi P, Gupta PC. A review of the systemic adverse effects of areca nut or betel nut. Indian J Med Paediatr Oncol. 2014;35(01):3–9. doi: 10.4103/0971-5851.133702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girisa S, Kumar A, Rana V, Parama D, Daimary UD, Warnakulasuriya S, Kumar AP, Kunnumakkara AB. From simple mouth cavities to complex oral mucosal disorders—curcuminoids as a promising therapeutic approach. ACS Pharmacol Transl Sci. 2021;4(2):647–665. doi: 10.1021/acsptsci.1c00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Escobar R, Fernández-Marín MI, Richard T, Fernandez-Morales A, Carbu M, Cebrian-Tarancon C, Torija MJ, Puertas B, Cantos-Villar E. Development and characterization of a pure stilbene extract from grapevine shoots for use as a preservative in wine. Food Control. 2021;121:107684. doi: 10.1016/j.foodcont.2020.107684. [DOI] [Google Scholar]
- Hartmann L, Schröter P, Osen W, Baumann D, Offringa R, Moustafa M, Will R, Debus J, Brons S, Rieken S. Photon versus carbon ion irradiation: immunomodulatory effects exerted on murine tumor cell lines. Sci Rep. 2020;10(1):1–13. doi: 10.1038/s41598-020-78577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias-Velázquez Ó, López-Pintor RM, González-Serrano J, Casanas E, Torres J, Hernandez G. Salivary LDH in oral cancer and potentially malignant disorders: a systematic review and meta-analysis. Oral Dis. 2022;28(1):44–56. doi: 10.1111/odi.13630. [DOI] [PubMed] [Google Scholar]
- Jaiswal PK, Goel A, Mittal R. Survivin: a molecular biomarker in cancer. Indian J Med Res. 2015;141(4):389. doi: 10.4103/0971-5916.159250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawardana SAS, Samarasekera JKRR, Hettiarachchi GHCM, Gooneratne MJ (2022) Antidiabetic properties of finger millet (Eleusine coracana (L.) Gaertn.) varieties cultivated in Sri Lanka. J Herbal Med 100534
- Khwairakpam AD, Monisha J, Roy NK, Bordoloi D, Padmavathi G, Banik K, Khatoon E, Kunnumakkara AB (2020) Vietnamese coriander inhibits cell proliferation, survival and migration via suppression of Akt/mTOR pathway in oral squamous cell carcinoma. J Basic Clin Physiol Pharmacol 31(3) [DOI] [PubMed]
- Kluska M, Jabłońska J, Prukała W. Analytics, properties and applications of biologically active stilbene derivatives. Molecules. 2023;28(11):4482. doi: 10.3390/molecules28114482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnumakkara AB, Banik K, Bordoloi D, Harsha C, Sailo BL, Padmavathi G, Roy NK, Gupta SC, Aggarwal BB. Googling the Guggul (Commiphora and Boswellia) for prevention of chronic diseases. Front Pharmacol. 2018;9:686. doi: 10.3389/fphar.2018.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminarayana S, Augustine D, Rao RS, Patil S, Awan KH, Venkatesiah SS, Haragannavar VC, Nambiar S, Prasad K (2018) Molecular pathways of oral cancer that predict prognosis and survival: a systematic review. J Carcinog 17 [DOI] [PMC free article] [PubMed]
- Li K, Zeng X, Liu P, Zeng X, Lv J, Qiu S, Zhang P (2023) The role of inflammation-associated factors in head and neck squamous cell carcinoma. J Inflamm Res 4301–4315 [DOI] [PMC free article] [PubMed]
- Majeed M, Nagabhushanam K, Kalman D, Bhat B, Vaidyanathan P, Bani S, Pandey A (2016) Composition comprising scirpusin A and scirpusin B and anti-adipogenesis/anti-obesity potential thereof. Google Patents
- Mărgăritescu C, Pirici D, Simionescu C, Mogoantă L, Raica M, Stîngă A, Ciurea R, Stepan A, Ribatti D. VEGF and VEGFRs expression in oral squamous cell carcinoma. Roman J Morphol Embryol. 2009;50(4):527–548. [PubMed] [Google Scholar]
- Moges A, Barik CR, Purohit S, Goud VV. Dietary and bioactive properties of the berries and leaves from the underutilized Hippophae salicifolia D. Don grown in Northeast India. Food Sci Biotechnol. 2021;30(12):1555–1569. doi: 10.1007/s10068-021-00988-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaran RB, Bhattacharjee P, Bhowmick P, Zote L, Kumar NS, Jahau L, Cooke MS, Hu C-W, Chao M-R (2023) Genetic and epigenetic instability induced by betel quid associated chemicals. Toxicol Rep [DOI] [PMC free article] [PubMed]
- Nakajima K, Taguchi H, Endo T, Yosioka I. The constituents of Scirpus fluviatilis (Torr.) A. Gray. I.: the structures of two new hydroxystilbene dimers, scirpusin A and B. Chem Pharm Bull. 1978;26(10):3050–3057. doi: 10.1248/cpb.26.3050. [DOI] [Google Scholar]
- Parcheta M, Świsłocka R, Orzechowska S, Akimowicz M, Choińska R, Lewandowski W. Recent developments in effective antioxidants: the structure and antioxidant properties. Materials. 2021;14(8):198. doi: 10.3390/ma14081984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecyna P, Wargula J, Murias M, Kucinska M. More than resveratrol: new insights into stilbene-based compounds. Biomolecules. 2020;10(8):1111. doi: 10.3390/biom10081111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S, Kumar M, Kumari N, Thakur M, Rathour S, Pundir A, Sharma AK, Bangar SP, Dhumal S, Singh S. Plant-based antioxidant extracts and compounds in the management of oral cancer. Antioxidants. 2021;10(9):1358. doi: 10.3390/antiox10091358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit S, Barik CR, Kalita D, Sahoo L, Goud VV. Exploration of nutritional, antioxidant and antibacterial properties of unutilized rind and seed of passion fruit from Northeast India. J Food Meas Charact. 2021;15(4):3153–3167. doi: 10.1007/s11694-021-00899-6. [DOI] [Google Scholar]
- Purohit S, Kalita D, Barik CR, Sahoo L, Goud VV. Evaluation of thermophysical, biochemical and antibacterial properties of unconventional vegetable oil from Northeast India. Mater Sci Energy Technol. 2021;4:81–91. [Google Scholar]
- Rapone B, Ferrara E (2020) Vascular endothelial growth factor expression in the pathological angiogenesis in oral squamous cell carcinoma. In: Oral Diseases. IntechOpen
- Ray JG, Ranganathan K, Chattopadhyay A. Malignant transformation of oral submucous fibrosis: overview of histopathological aspects. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(2):200–209. doi: 10.1016/j.oooo.2015.11.024. [DOI] [PubMed] [Google Scholar]
- Rishabh K, Khadilkar S, Kumar A, Kalra I, Kumar AP, Kunnumakkara AB. MicroRNAs as modulators of oral tumorigenesis—a focused review. Int J Mol Sci. 2021;22(5):2561. doi: 10.3390/ijms22052561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabu M, Kuttan R. Anti-diabetic activity of medicinal plants and its relationship with their antioxidant property. J Ethnopharmacol. 2002;81(2):155–160. doi: 10.1016/S0378-8741(02)00034-X. [DOI] [PubMed] [Google Scholar]
- Saleh EAM, Al-Dolaimy F, Baymakov S, Ullah MI, Khlewee IH, Bisht YS, Alsaalamy AH (2023) Oxidative stress affects the beginning of the growth of cancer cells through a variety of routes. Pathol-Res Pract 154664 [DOI] [PubMed]
- Sano S, Sugiyama K, Ito T, Katano Y, Ishihata A. Identification of the strong vasorelaxing substance scirpusin B, a dimer of piceatannol, from passion fruit (Passiflora edulis) seeds. J Agric Food Chem. 2011;59(11):6209–6213. doi: 10.1021/jf104959t. [DOI] [PubMed] [Google Scholar]
- Santhoshkumar J, Sowmya B, Kumar SV, Rajeshkumar S. Toxicology evaluation and antidermatophytic activity of silver nanoparticles synthesized using leaf extract of Passiflora caerulea. S Afr J Chem Eng. 2019;29:17–23. [Google Scholar]
- Saraswat N, Prabhu N, Pillay R, Everett B, George A. Oral cancer risk behaviours of Indian immigrants in Australia: a qualitative study. Aust N Z J Public Health. 2022;46(1):87–94. doi: 10.1111/1753-6405.13183. [DOI] [PubMed] [Google Scholar]
- Sarkar T, Salauddin M, Roy A, Sharma N, Sharma A, Yadav S, Jha V, Rebezov M, Khayrullin M, Thiruvengadam M (2022) Minor tropical fruits as a potential source of bioactive and functional foods. Crit Rev Food Sci Nutr 1–45 [DOI] [PubMed]
- Shanmugam S, Murugaiyan I, dos Santos LB, Serafini MR, de Souza Araújo AA, Narain N, Quintans-Júnior LJ, Thangaraj P. HPLC–DAD–MS identification of polyphenols from Passiflora leschenaultii and determination of their antioxidant, analgesic, anti-inflammatory and antipyretic properties. Arab J Chem. 2019;12(6):760–771. doi: 10.1016/j.arabjc.2016.02.008. [DOI] [Google Scholar]
- Shih Y-H, Tsai P-J, Chen Y-L, Pranata R, Chen R-J. Assessment of the antibacterial mechanism of pterostilbene against Bacillus cereus through apoptosis-like cell death and evaluation of its beneficial effects on the gut microbiota. J Agric Food Chem. 2021;69(41):12219–12229. doi: 10.1021/acs.jafc.1c04898. [DOI] [PubMed] [Google Scholar]
- Subash A, Bylapudi B, Thakur S, Rao VU. Oral cancer in India, a growing problem: is limiting the exposure to avoidable risk factors the only way to reduce the disease burden? Oral Oncol. 2022;125:105677. doi: 10.1016/j.oraloncology.2021.105677. [DOI] [PubMed] [Google Scholar]
- Sun Z, Sun X, Chen Z, Du J, Wu Y. Head and neck squamous cell carcinoma: risk factors, molecular alterations, immunology and peptide vaccines. Int J Pept Res Ther. 2022;28:1–18. doi: 10.1007/s10989-021-10334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaraz A, Sultana F, Bari MW, Ahmed KS, Hasan M, Islam MM, Islam MA, Satter MA, Hossain MH, Islam MS. Phytochemical profiling of Blumea laciniata (Roxb.) DC. and its phytopharmaceutical potential against diabetic, obesity, and Alzheimer’s. Biomed Pharmacother. 2021;141:111859. doi: 10.1016/j.biopha.2021.111859. [DOI] [PubMed] [Google Scholar]
- Tran HHT, Nguyen MC, Le HT, Nguyen TL, Pham TB, Chau VM, Nguyen HN, Nguyen TD. Inhibitors of α-glucosidase and α-amylase from Cyperus rotundus. Pharm Biol. 2014;52(1):74–77. doi: 10.3109/13880209.2013.814692. [DOI] [PubMed] [Google Scholar]
- Vorobyova V, Skiba M (2022) Potential of tomato pomace extract as a multifunction inhibitor corrosion of mild steel. Waste Biomass Valoriz 1–25
- Wang X-F, Yao C-S. Naturally active oligostilbenes. J Asian Nat Prod Res. 2016;18(4):376–407. doi: 10.1080/10286020.2015.1094464. [DOI] [PubMed] [Google Scholar]
- Xavier JA, Santos JC, Nova MAV, Gonçalves CM, Borbely KS, Pires KS, dos Santos FA, Valentim IB, Barbosa JH, Silva FCd. Anti-Zika virus effects, placenta protection and chemical composition of Passiflora edulis seeds ethanolic extract. J Braz Chem Soc. 2022;33:701–714. [Google Scholar]
- Xiang T, Uno T, Ogino F, Ai C, Duo J, Sankawa U. Antioxidant constituents of Caragana tibetica. Chem Pharm Bull. 2005;53(9):1204–1206. doi: 10.1248/cpb.53.1204. [DOI] [PubMed] [Google Scholar]
- Xiu W, Huang Y, Li Y, Yu M, Gong Y. Comorbidities and mortality risk among extensive-stage small-cell lung cancer patients in mainland China: impacts of hypertension, type 2 diabetes mellitus, and chronic hepatitis B virus infection. Anticancer Drugs. 2022;33(1):80. doi: 10.1097/CAD.0000000000001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Sato A, Takai Y, Yoshimori A, Umehara M, Ogino Y, Inada M, Shimada N, Nishida A, Ichida R. Effect of piceatannol-rich passion fruit seed extract on human glyoxalase I–mediated cancer cell growth. Biochem Biophys Rep. 2019;20:100684. doi: 10.1016/j.bbrep.2019.100684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G-x, Zhou J-t, Li Y-z, Hu C-q. Anti-HIV bioactive stilbene dimers of Caragana rosea. Planta Med. 2005;71(06):569–571. doi: 10.1055/s-2005-864162. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Fu D, Xu C, Yang J, Wang Z. Identification of genes associated with tongue cancer in patients with a history of tobacco and/or alcohol use. Oncol Lett. 2017;13(2):629–638. doi: 10.3892/ol.2016.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirlik K, Duyster J. Anti-angiogenics: current situation and future perspectives. Oncol Res Treat. 2018;41(4):166–171. doi: 10.1159/000488087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.