Abstract
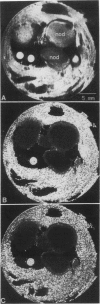
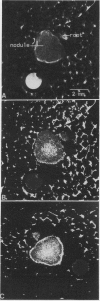
The effects of selected gas perfusion treatments on the spinlattice relaxation times (T1) of the soybean (Glycine max) nodule cortex and inner nodule tissue were studied with 1H high resolution magnetic resonance microscopy. Three gas treatments were used: (a) perfusion with O2 followed by N2; (b) O2 followed by O2; and (c) air followed by N2. Soybean plants with intact attached nodules were placed into the bore of a superconducting magnet and a selected root with nodules was perfused with the gas of interest. Magnetic resonance images were acquired with repetition times from 50 to 3200 ms. The method of partial saturation was used to calculate T1 times on selected regions of the image. Calculated images based on T1 showed longer T1 values in the cortex than in the inner nodule during all of the gas perfusions. When nodules were perfused with O2-O2, there was no significant change in the T1 of the nodule between the two gas treatments. When the nodule was perfused with O2-N2 or air-N2, however, the T1 of both the cortex and inner nodule increased. In these experiments, the increase in T1 of the cortex was 2- to 3-fold greater than the increase observed in the inner nodule. A similar change in T1 was found in detached live nodules, but there was no change in T1 with selective gas perfusion of detached dead nodules. These observations suggest that cortical cells respond differently to selected gas perfusion than the inner nodule, with the boundary of T1 change sharply delineated at the interface of the inner nodule and the inner cortex.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. M., Johnson G. A., Kramer P. J. In Vivo Magnetic Resonance Microscopy of Changing Water Content in Pelargonium hortorum Roots. Plant Physiol. 1986 Dec;82(4):1158–1160. doi: 10.1104/pp.82.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton G. D., Potter J. L., Dornbluth N. C. NMR relaxation of protons in tissues and other macromolecular water solutions. Magn Reson Imaging. 1982;1(4):209–226. doi: 10.1016/0730-725x(82)90172-2. [DOI] [PubMed] [Google Scholar]
- Gomori J. M., Grossman R. I., Yu-Ip C., Asakura T. NMR relaxation times of blood: dependence on field strength, oxidation state, and cell integrity. J Comput Assist Tomogr. 1987 Jul-Aug;11(4):684–690. [PubMed] [Google Scholar]
- Hunt S., King B. J., Canvin D. T., Layzell D. B. Steady and nonsteady state gas exchange characteristics of soybean nodules in relation to the oxygen diffusion barrier. Plant Physiol. 1987 May;84(1):164–172. doi: 10.1104/pp.84.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. A., Brown J., Kramer P. J. Magnetic resonance microscopy of changes in water content in stems of transpiring plants. Proc Natl Acad Sci U S A. 1987 May;84(9):2752–2755. doi: 10.1073/pnas.84.9.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B. J., Hunt S., Weagle G. E., Walsh K. B., Pottier R. H., Canvin D. T., Layzell D. B. Regulation of o(2) concentration in soybean nodules observed by in situ spectroscopic measurement of leghemoglobin oxygenation. Plant Physiol. 1988 Jun;87(2):296–299. doi: 10.1104/pp.87.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzell D. B., Hunt S., Palmer G. R. Mechanism of Nitrogenase Inhibition in Soybean Nodules : Pulse-Modulated Spectroscopy Indicates that Nitrogenase Activity Is Limited by O(2). Plant Physiol. 1990 Apr;92(4):1101–1107. doi: 10.1104/pp.92.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFall J. R., Wehrli F. W., Breger R. K., Johnson G. A. Methodology for the measurement and analysis of relaxation times in proton imaging. Magn Reson Imaging. 1987;5(3):209–220. doi: 10.1016/0730-725x(87)90022-1. [DOI] [PubMed] [Google Scholar]
- Pfeffer P. E., Rolin D. B., Kumosinski T. F., Macfall J. S., Schmidt J. H. P relaxation responses associated with n(2)/o(2) diffusion in soybean nodule cortical cells and excised cortical tissue. Plant Physiol. 1992 Dec;100(4):1682–1690. doi: 10.1104/pp.100.4.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair T. R., Goudriaan J. Physical and morphological constraints on transport in nodules. Plant Physiol. 1981 Jan;67(1):143–145. doi: 10.1104/pp.67.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz P. R., Sinclair T. R. Regulation of soybean nitrogen fixation in response to rhizosphere oxygen: I. Role of nodule respiration. Plant Physiol. 1987 Jul;84(3):900–905. doi: 10.1104/pp.84.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg J. B. Facilitated oxygen diffusion. The role of leghemoglobin in nitrogen fixation by bacteroids isolated from soybean root nodules. J Biol Chem. 1974 Jul 10;249(13):4057–4066. [PubMed] [Google Scholar]